Abstract
On account of its poor prognosis and deficiency of therapeutic stratifications, triple negative breast cancer continues to form the causative platform of an incommensurate number of breast cancer deaths. Aiming at the development of potent anticancer agents as a continuum of our previous efforts, a novel series of 2-((benzimidazol-2-yl)thio)-1-arylethan-1-ones 5a–w was synthesized and evaluated for its anti-proliferative activity towards triple negative breast cancer (TNBC) MDA-MB-468 cells. Compound 5k was the most active analog against MDA-MB-468 (IC50 = 19.90 ± 1.37 µM), with 2.1-fold increased activity compared to 5-fluorouracil (IC50 = 41.26 ± 3.77 µM). Compound 5k was able to induce apoptosis in MDA-MB-468, as evidenced by the marked boosting in the percentage of florecsein isothiocyanate annexin V (Annexin V–FITC)-positive apoptotic cells (upper right (UR) + lower right (LR)) by 2.8-fold in comparison to control accompanied by significant increase in the proportion of cells at pre-G1 (the first gap phase) by 8.13-fold in the cell-cycle analysis. Moreover, a quantitative structure activity relationship (QSAR) model was established to investigate the structural requirements orchestrating the anti-proliferative activity. Finally, we established a theoretical kinetic study.
Keywords: synthesis, X-ray, anti-proliferative, breast cancer MDA-MB-468 cells, apoptosis
1. Introduction
Pertaining to the frequency of diagnosis worldwide, breast cancer is regarded as the second most frequently diagnosed cancer and the most frequently diagnosed tumor among women. Also, it is regarded as the fifth leading cause of cancer mortality [1]. In 2012, an estimated 1.67 million newly diagnosed breast cancer cases and 522,000 breast cancer deaths occurred worldwide [1]. The etiology of breast cancer is still unknown, although different risk factors have been established—to name just a few, first-degree relative’s breast cancer family history, mammographic density, benign breast disease, younger age at menarche, low parity, older age at first birth, older age at menopause, high postmenopausal body mass index, low premenopausal body mass index, and endogenous hormone levels have been established as risk factors for breast cancer [2,3].
Breast cancer is regarded as a diverse group of diseases with multiple intrinsic tumor subtypes that have various treatment modalities and long-term survival probabilities. The immunohistochemical expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER-2) forms the platform of characterization of clinically defined breast cancer subtypes [4]. In approximately 15%–20% of globally diagnosed breast cancers, the tumors do not express ER, PR, or HER-2. Such malignancies are designated as triple-negative breast cancer (TNBC) [5].
In the current medical era, TNBC, among all the breast cancer subgroups, has stood out as the greatest clinical challenge as these tumors have no clinically validated molecularly targeted therapy, are prevalent in younger women, associated with the worst prognosis, and often relapse rapidly. Also, TNBC are highly proliferative, poorly differentiated, often grade III carcinomas, genetically unstable, and preferentially metastasize to the brain and lungs [6,7,8,9,10]. Therefore, there is a critical need to develop potent and effective novel therapies to improve the outcomes of TNBC treatment.
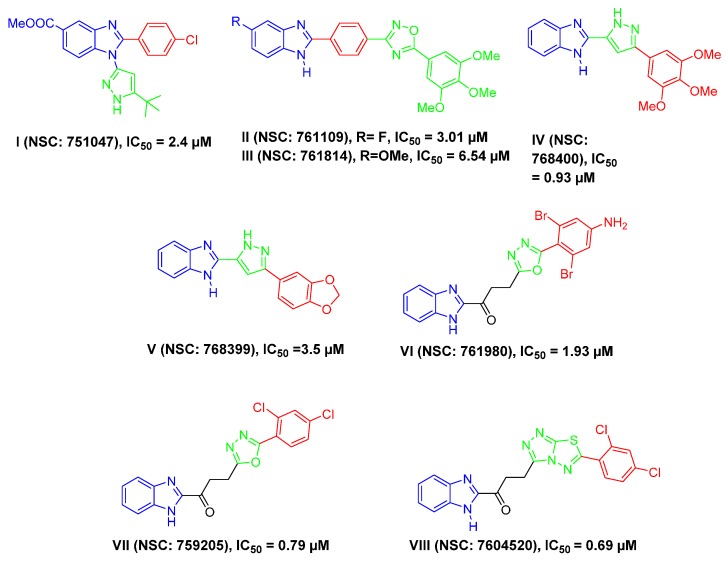
Because of their similarity to some naturally occurring nucleotides and their existence in several naturally occurring compounds, benzimidazole derivatives possess a wide range of biological activities and therapeutic effects [11,12,13]. In the field of medicinal chemistry, benzimidazole represents a highly privileged scaffold and has been copiously explored as an anti-proliferative agent targeting different breast cancer cells [14,15,16,17,18,19,20,21]. Surveying the literature revealed that different benzimidazole-based scaffolds were developed with significant activity toward the TNBC MDA-MB-468 cells in the anticancer drug screening program of the U.S. National Cancer Institute (NCI), according to their applied protocol against full NCI 60 human cell lines panel (Figure 1).
Figure 1.
Structures of some reported benzimidazoles I–VIII, by other research groups, with anti-proliferative activity against triple-negative breast cancer MDA-MB-468 cells [22,23,24,25,26,27].
Many researchers have reported the utility of 2-aryl benzimidazole derivatives as anti-proliferative agents against TNBC. Attaching a heterocyclic moiety, 5-tert-butyl-1H-pyrazol-3-yl, in position 1 and an aryl moiety, 4-chlorophenyl, in position 2 of benzimidazole core resulted in compound I (NSC: 751047) with good anti-proliferative activity against MDA-MB-468 (IC50 = 2.4 µM) [22] (Figure 1), while substitution of position 2 of 5-flouro and 5-methoxybenzimidazole with 1,2,4-oxadiazole moiety through a phenyl ring afforded compounds II and III, respectively, (NSC: 761109 and 761814) with IC50 values of 3.01 and 6.54 µM, respectively, against MDA-MB-468 [23] (Figure 1). Moreover, introduction of different aryl groups through a pyrazole linker at position 2 of the benzimidazole core, as in compounds IV and V (NSC: 768400 and 768399), achieved significant efficacy against MDA-MB-468 (IC50 values of 0.93 and 3.5 µM, respectively) [24] (Figure 1). Interestingly, linking different aryl moeities, through variable heterocyclic groups, via a three-atom linker, namely a propan-1-one group to position 2 of the benzimidazole core led to compounds VI–VIII (NSC: 761980, NSC: 759205 and NSC: 7604520) with low or sub-micromolar anti-proliferative activity against MDA-MB-468 (IC50 = 1.93, 0.79 and 0.69 µM, respectively) [25,26,27] (Figure 1).
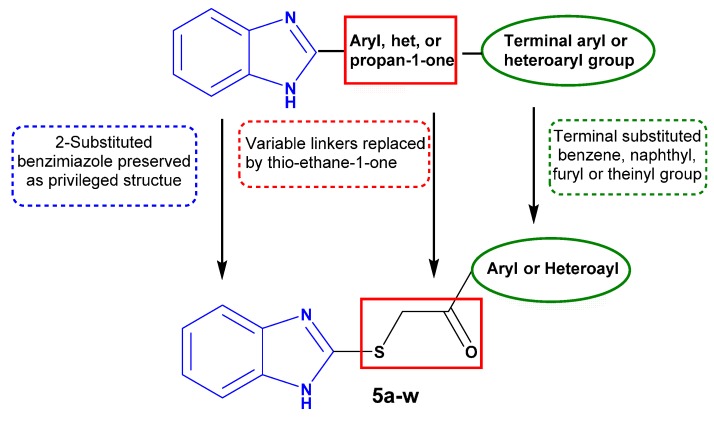
In addition, we recently introduced an efficacious benzimidazole-based scaffold as for development of potent antitumor agents that prove to have anti-proliferative activity not only toward the cancer stem cells but also toward the bulk of tumor cells of the colon HT-29 cell line [28]. The design of such a scaffold relies on linking different aryl or heteroaryl groups to position 2 of the benzimidazole core through a thio ethan-2-one linker. From the findings reported above, we came to the conclusion that linking the 2-position of benzimidazole scaffold to a terminal aryl or heteroaryl group directly or via variable spacers—an aryl, a heteroaryl, or a propan-1-one group—affords promising molecules that have significant anti-proliferative activity against TNBC.
Regarding these points and as a continuation of our research program on the design and synthesis of effective antitumor candidates [29,30,31,32,33,34,35], it was thought worthwhile to extend our investigations around our study [28] to probe for benzimidazole derivatives having anti-proliferative activity towards TNBC. Our structure-based design was three-fold: (i) preserving benzimidazole structure with subsitution at 2-position; (ii) maintaining a terminal lipophilic group; and (iii) establishing a three-atom thio ethane-1-one linker to afford more flexibility for the designed molecules (Figure 2). Thus, the present work reports the synthesis of benzimidazoles 5a–w and their in vitro anti-proliferative activity against the TNBC MDA-MB-468 cell line. Moreover, the most active member in this study, 5k, was selected to be further investigated regarding its effects on cell cycle progression and potential apoptotic effect in the MDA-MB-468 cells, to acquire perception of the mechanism of the anti-proliferative activity of the prepared compounds. Eventually, a theoretical kinetic study was constituted.
Figure 2.
Structure-based design of target benzimidazoles 5a–w as anti-triple-negative breast cancer (TNBC) agents.
2. Results
2.1. Synthetic Approach to Prepare the Target Derivatives
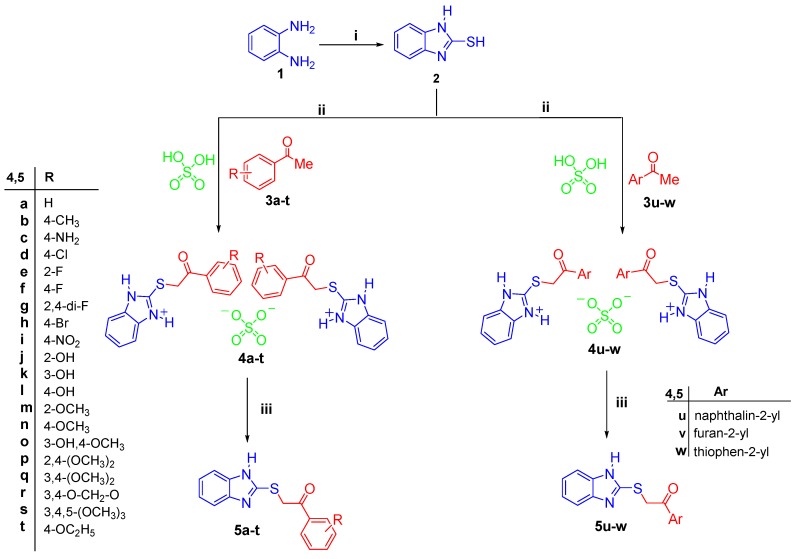
The target compounds were prepared following our recently published procedure [28] via the reaction of compound 2 with different aromatic ketones 3a–w in glacial acetic acid in the presence of two equivalents conc. H2SO4 to afford a quantitative yield from the sulfate salts 4a–w. The prepared sulfate salts 4a–w were subsequently neutralized to afford the target 2-((benzimidazol-2-yl)thio)-1-arylethan-1-ones 5a–w in an excellent yield of 82%–96% (Scheme 1).
Scheme 1.
Synthesis of the target 2-((benzimidazol-2-yl)thio)-1-arylethan-1-ones 5a–w and their corresponding sulfate salts 4a–w. Reagents and conditions: (i) CS2/KOH/ethanol/reflux 2 h; (ii) Glacial acetic acid/reflux 0.5 h; (iii) Aqueous Na2CO3/r.t. (room temperature) 2 h.
Infrared (IR) spectra for compounds 5a–w displayed absorption bands attributable for the NH group in the range 3324–3460 cm−1, also a (C=O) band in the range of 1654–1690 cm−1. Also, their 1H-nuclear magnetic resonance (NMR) spectra displayed one singlet D2O-exchangeable signal due to the NH proton in the range of δ 12.52–12.80 ppm, whereas (–CH2–) protons appeared as singlet signals within δ 5.00 ppm. Furthermore, the 13C-NMR spectra of compounds 5a–w showed signals resonating in the region δ 182.16–193.90 ppm due to the carbon of carbonyl group, whereas the carbons of the (–CH2–) group appeared in the region of δ 38.46–43.97 ppm.
2.2. Single Crystal Analysis of Compounds 4u and 5v
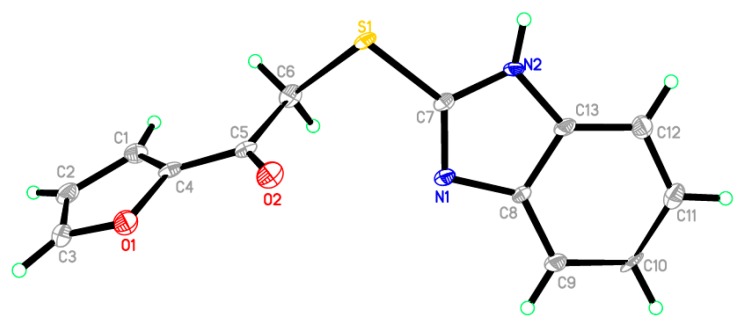
Crystals of compounds 4u and 5v were selected to analyze their single-crystal X-ray crystallographic after slow evaporation from solutions of ethanol. The instrument used is Bruker SMART APEX II D8 Venture diffractometer (Bruker, Karlsruhe, Germany) with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at 100 and 150 K, respectively. A direct method was applied to solve the structures that were subsequently refined with SHELXTL [36]. The positions of all the non-H-atoms were provided by E-maps. Using anisotropic temperature factors, the full-matrix least-squares refinement was carried out on F2’s for all non-H-atoms. Crystallographic data was deposited in the Cambridge Crystallographic Data Center and assigned the following deposition numbers: CCDC 1058838 and 1455648 for compounds 4u and 5v, respectively.
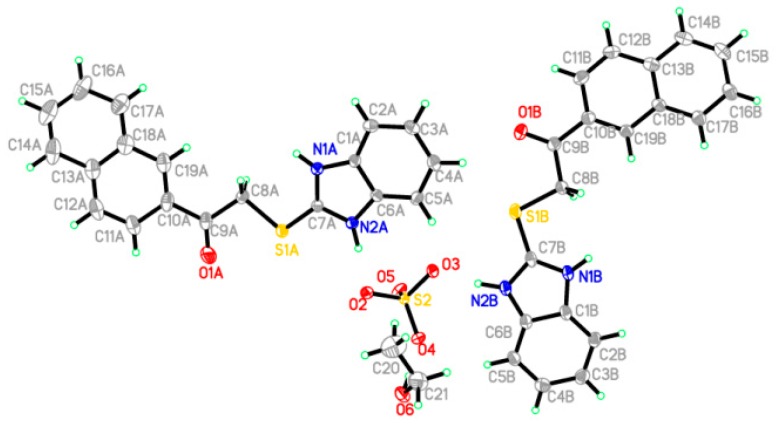
In Figure 2 and Figure 3, the crystallographic structures of compounds 4u and 5v are represented, respectively. The exact structure is unambiguously defined by the single crystal X-ray study on both derivatives. The crystal structure of 4u confirmed two crystallographically independent cation molecules with one sulfate anion, in the presence of one molecule of ethanol in its asymmetric unit, as shown in Figure 3. The asymmetric unit of 5v contains one molecule only, as depicted in Figure 4. Table 1 listed the crystallographic data and the refinement for the crystals. Table 2 and Table 3 summarized some selected geometric parameters for 4u and 5v, respectively. Also, Figure S1 (in Supplementary Materials) displayed the molecular packing of compound 5v, while Table S1 showed the hydrogen-bond geometry (Å, °) for compound 5v.
Figure 3.
An ORTEP diagram of the final X-ray structure of compound 4u.
Figure 4.
An ORTEP diagram of the final X-ray structure of compound 5v.
Table 1.
Crystallographic data and refinements for compounds 4u and 5v.
| Compound | 4u | 5v |
|---|---|---|
| Crystal data | ||
| Chemical formula | 2(C19H15N2OS) C2H6O·SO4 | C13H10N2O2S |
| Mr | 780.94 | 258.29 |
| Crystal system, space group | Orthorhombic, P212121 | Monoclinic, P21/c |
| Temperature (K) | 100 | 100 |
| a, b, c (Å) | 14.2409 (8), 15.8339 (9), 16.2043 (8) | 11.4097 (7), 10.8336 (7), 10.1256 (7) |
| β (°) | 90.00 | 114.165 (2) |
| V (Å3) | 3653.9 (3) | 1141.93 (13) |
| Z | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| µ (mm−1) | 0.26 | 0.28 |
| Crystal size (mm) | 0.44 × 0.26 × 0.16 | 0.47 × 0.36 × 0.11 |
| Data collection | ||
| Diffractometer | CCD area detector diffractometer | Bruker APEX-II D8 venture diffractometer |
| Absorption correction | multi-scan, SADABS | multi-scan, SADABS |
| Tmin, Tmax | 0.90, 0.92 | 0.89, 0.93 |
| Number of measured, independent and observed [I > 2σ(I)] reflections | 11,220, 11,220, 8647 | 9364, 1999, 1689 |
| R int | 0.090 | |
| Refinement | ||
| R[F2 > 2ó(F2)], wR(F2), S | 0.050, 0.121, 1.05 | 0.086, 0.250, 1.10 |
| Number of reflections | 11,220 | 1999 |
| Number of parameters | 494 | 163 |
| Number of restraints | 0 | 0 |
| H-atom treatment | by a mixture of independent and constrained refinement | by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e·Å−3) | 0.76, −0.37 | 1.12, −0.87 |
| CCDC number | 1,058,838 | 1,455,648 |
Table 2.
Selected geometric parameters (Å, °) for compound 4u.
| Bond Distances | |||
| S1A–C7A | 1.722 (3) | O6–C21 | 1.437 (6) |
| S1A–C8A | 1.811 (3) | N1A–C7A | 1.333 (4) |
| S1B–C7B | 1.724 (3) | N1A–C1A | 1.406 (4) |
| S1B–C8B | 1.804 (3) | N2A–C6A | 1.387 (4) |
| S2–O3 | 1.476 (3) | N2A–C7A | 1.338 (4) |
| S2–O5 | 1.479 (3) | N1B–C7B | 1.342 (4) |
| S2–O2 | 1.478 (3) | N1B–C1B | 1.398 (4) |
| S2–O4 | 1.467 (2) | N2B–C7B | 1.339 (4) |
| O1A–C9A | 1.203 (6) | N2B–C6B | 1.381 (4) |
| Bond Angles | |||
| C7A–S1A–C8A | 102.76 (15) | N1A–C7A–N2A | 109.8 (3) |
| C7B–S1B–C8B | 99.02 (15) | S1A–C7A–N1A | 130.1 (2) |
| O3–S2–O5 | 108.55 (14) | S1A–C8A–C9A | 105.5 (2) |
| O4–S2–O5 | 111.31 (14) | O1A–C9A–C10A | 121.0 (4) |
| O3–S2–O4 | 110.49 (14) | O1A–C9A–C8A | 120.6 (3) |
| O2–S2–O3 | 108.54 (16) | N1B–C1B–C2B | 131.1 (3) |
| O2–S2–O4 | 109.46 (15) | N1B–C1B–C6B | 106.5 (3) |
| O2–S2–O5 | 108.43 (14) | N2B–C6B–C5B | 131.8 (3) |
| C1A–N1A–C7A | 108.2 (3) | N2B–C6B–C1B | 107.0 (3) |
| C6A–N2A–C7A | 108.7 (3) | S1B–C7B–N1B | 128.7 (3) |
| C1B–N1B–C7B | 108.0 (3) | S1B–C7B–N2B | 121.3 (2) |
| C6B–N2B–C7B | 108.7 (3) | N1B–C7B–N2B | 109.9 (3) |
| N1A–C1A–C2A | 130.9 (3) | S1B–C8B–C9B | 107.8 (2) |
| N1A–C1A–C6A | 106.5 (3) | O1B–C9B–C10B | 122.0 (3) |
| N2A–C6A–C1A | 106.9 (3) | O1B–C9B–C8B | 120.4 (3) |
| N2A–C6A–C5A | 131.1 (3) | O6–C21–C20 | 111.9 (4) |
Table 3.
Selected geometric parameters (Å, °) for compound 5v.
| Bond Distances | |||
| Cl1–C13 | 1.7361 (12) | N1–C1 | 1.3831 (13) |
| S1–C7 | 1.7521 (10) | N1–C7 | 1.3686 (12) |
| S1–C8 | 1.7949 (10) | N2–C6 | 1.3947 (13) |
| S1–C8 | 1.7949 (10) | N2–C6 | 1.3947 (13) |
| O1–C9 | 1.2146 (12) | N2–C7 | 1.3157 (12) |
| Bond Angles | |||
| C6–S1–C7 | 102.2 (2) | S1–C6–C5 | 113.5 (4) |
| C3–O1–C4 | 106.5 (4) | S1–C7–N2 | 117.3 (3) |
| C7–N1–C8 | 103.8 (4) | N1–C7–N2 | 114.4 (5) |
| C7–N2–C13 | 106.7 (4) | S1–C7–N1 | 128.4 (4) |
| O1–C3–C2 | 111.2 (5) | N1–C8–C9 | 130.2 (4) |
| O1–C4–C5 | 116.2 (4) | N1–C8–C13 | 109.6 (4) |
| O1–C4–C1 | 109.8 (4) | N2–C13–C8 | 105.5 (5) |
| O2–C5–C6 | 123.4 (5) | N2–C13–C12 | 132.2 (5) |
2.3. Biological Evaluation of the Target Derivatives as Anti-Cancer Agents
2.3.1. In Vitro Anti-Proliferative Activity against MDA-MB-468
The WST-1 assay, as described by Ngamwongsatit et al. [37], was adopted to evaluate the anti-proliferative activity of the synthesized 2-((benzimidazol-2-yl)thio)-1-arylethan-1-ones 5a–w against human breast cancer cell line MDA-MB-468. 5-FU (florouracil) was selected as a positive control due to its broad spectrum of anticancer activity. The anti-proliferative activity was expressed as growth inhibitory concentration (IC50) values, which represent the compound concentrations required to produce a 50% inhibition of cell growth after 48 hours of incubation compared to untreated controls (Table 4).
Table 4.
In vitro anti-proliferative activity of compounds 5a–w against breast MDA-MB-468 cancer cell line.
| Compound | Aryl | IC50 (µM) a |
|---|---|---|
| 5a | C6H5 | 22.31 ± 2.04 |
| 5b | 4-Me–C6H4 | 74.25 ± 6.23 |
| 5c | 4-NH2–C6H4 | 53.79 ± 5.02 |
| 5d | 4-Cl–C6H4 | 31.97 ± 3.07 |
| 5e | 2-F–C6H4 | NA b |
| 5f | 4-F–C6H4 | 24.96 ± 2.55 |
| 5g | 2,4-di-F–C6H3 | 50.78 ± 5.11 |
| 5h | 4-Br–C6H4 | 53.83 ± 6.53 |
| 5i | 4-NO2–C6H4 | 32.80 ± 3.17 |
| 5j | 2-OH–C6H4 | 22.41 ± 2.13 |
| 5k | 3-OH–C6H4 | 19.90 ± 1.37 |
| 5l | 4-OH–C6H4 | 21.98 ± 1.91 |
| 5m | 2-OCH3–C6H4 | 22.32 ± 2.06 |
| 5n | 4-OCH3–C6H4 | 25.35 ± 2.15 |
| 5o | 3-OH-4-OCH3–C6H3 | 22.06 ± 1.93 |
| 5p | 2,4-(OCH3)2–C6H3 | 26.60 ± 2.24 |
| 5q | 3,4-(OCH3)2–C6H3 | 24.30 ± 2.13 |
| 5r | 3,4-O-CH2–O–C6H3 | 22.79 ± 1.87 |
| 5s | 3,4,5-(OCH3)3–C6H2 | 22.72 ± 2.03 |
| 5t | 4-OC2H5–C6H4 | 23.91 ± 2.19 |
| 5u | naphthalin-2-yl | 66.45 ± 7.12 |
| 5v | furan-2-yl | 30.34 ± 3.01 |
| 5w | thiophen-2-yl | 28.17 ± 2.24 |
| 5-Fluorouracil | 41.26 ± 3.77 |
a IC50 values are the mean ± S.D. of three separate experiments; b NA: Compounds having IC50 value > 100 µM.
The obtained results of the tested benzimidazole derivatives 5a–w indicated that most of the prepared compounds showed good to moderate anti-proliferative activity against the tested MDA-MB-468 cancer cell line. Compound 5k merged as the most potent member against MDA-MB-468 (IC50 = 19.90 ± 1.37 µM) as it was 2.1 times more potent and efficacious than 5-fluorouracil (IC50 = 41.26 ± 3.77 µM). Moreover, analogs 5a, 5f, and 5j–t showed superior anti-proliferative activity (IC50 values ranging from 21.98 ± 1.91 to 26.60 ± 2.24 µM) compared to 5-fluorouracil, the reference drug, (IC50 = 41.26 ± 3.77 µM). In addition, compounds 5d, 5i, 5v, and 5w with IC50 = 31.97 ± 3.07, 32.80 ± 3.17, 30.34 ± 3.01 and 28.17 ± 2.24 µM, respectively, displayed good activity against MDA-MB-468. On the other hand, compounds 5b, 5c, 5g, 5h, and 5u were moderately active against MDA-MB-468 with IC50 values ranging from 50.78 ± 5.11 to 74.25 ± 6.23 µM.
2.3.2. Structure Activity Relationship Study (SAR Study) of the Target Compounds
Observing the results in Table 4, valuable data could be extracted regarding the structure activity correlation of our compounds. Foremost, the effect of grafting diverse substituents on the terminal aryl moiety on the activities of the synthesized compounds 5a–t was closely investigated. Compound 5a bearing unsubstituted phenyl group showed good activity (IC50 = 22.31 ± 2.04 µM) in comparison to 5-fluorouracil (IC50 = 41.26 ± 3.77 µM), implying a doubling of the anti-proliferative activity.
Introduction of fluorine atom, a classical bioisostere of the hydrogen atom, at the 4-position as in compound 5f resulted in comparable activity to the unsubstituted analogue 5a (IC50 = 24.96 ± 2.55 and 22.31 ± 2.04 µM, respectively). Interestingly, transferring the fluorine atom from the 4-position to the 2-position, 5e, resulted in an inactive derivative. Again, di-substitution with two fluorine atoms in the 2- and 4-positions, 5g, was not favorable to the activity (IC50 = 50.78 ± 5.11 µM) compared to the mono 4-F substituted derivative 5f (IC50 = 24.96 ± 2.55 µM). Incorporation of more bulky halogens as chlorine and bromine led to compounds 5d and 5h, respectively, with decreased activity (IC50 = 31.97 ± 3.07 and 53.83 ± 6.53 µM, respectively), suggesting that incorporation of a small halogen fluorine atom only in the 4-position is markedly advantageous to the activity. The order of activities of the halogenated derivatives 5d–h decreased in the order of 4-F > 4-Cl > 2,4-di-F > 4-Br > 2-F. Also, grafting an electron-withdrawing nitro group as in compound 5i resulted only in moderate improvement of the activity (IC50 = 32.80 ± 3.17 µM) compared to 5-FU (IC50 = 41.26 ± 3.77 µM), while introduction of methyl or amino groups, electron-donating groups, reduced the activity against MDA-MB-468, as shown in 5b and 5c analogs (IC50 = 74.25 ± 6.23 and 53.79 ± 5.02 µM, respectively). Contrariwise, substitution with electron-donating hydroxyl, methoxy, or ethoxy groups as in compounds 5j–t maintained the activity in the good range of activity regardless of their positions or numbers (IC50 = (19.90 ± 1.37)–(26.60 ± 2.24) µM).
On the other hand, scrutinizing the anti-proliferative activity of compounds 5u–w gave us insight about the effect of exchanging the phenyl group of 5a for other aryl or heteroaryl moieties. Replacement of the phenyl ring of 5a with a naphthalin-2-yl group in compound 5u decreased the activity (IC50 = 66.45 ± 7.12 µM). Moreover, bioisosteric replacement of the phenyl moiety with 2-furyl or 2-thienyl groups (compounds 5v and 5w) moderately reduced the activity (IC50 = 30.34 ± 3.01 and 28.17 ± 2.24 µM, respectively). In conclusion, we can assume that incorporation of an unsubstituted phenyl group or its substitution with electron-donating hydroxyl, methoxy, or ethoxy groups is beneficial for activity against the MDA-MB-468 cell line, while introduction of heterocycles, such as 2-furyl or 2-thienyl, could not effectively replace the phenyl ring.
2.3.3. Cell-Cycle Analysis and Apoptotic Changes Investigation of Compound 5k
Cell reproduction necessitates DNA replication with a concomitant nuclear division followed by cytoplasmic partitioning to sporulate two daughter cells. Such a successive routine is known as the “cell cycle” and involves four distinguishable phases. The G1 phase is a gap integrated between the M phase (nuclear division) and the S phase (DNA synthesis); another gap called G2 phase also occurs between S and M. These gaps permit the repair of DNA damage and replication errors [38].
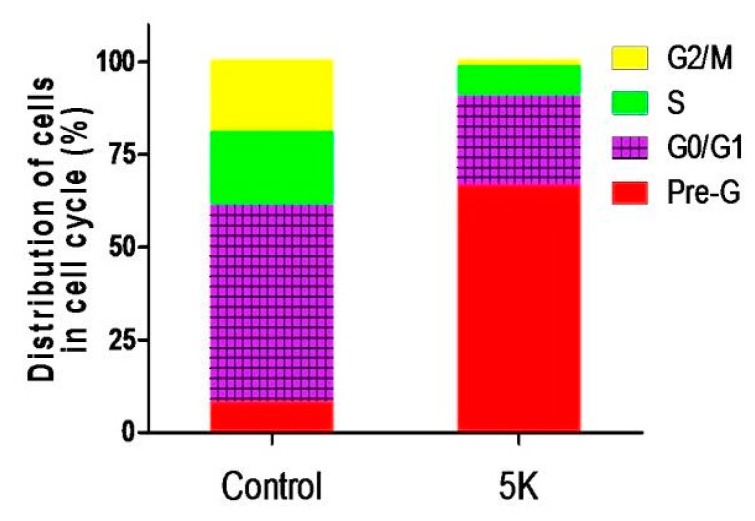
To understand the mechanism behind the tumor suppression activity of the prepared compounds, the most active member in this study, 5k, was selected to be further investigated regarding its effects on cell cycle progression and its potential apoptotic effects in the MDA-MB-468 cell line. The MDA-MB-468 cells were treated with IC50 concentration of compound 5k for 24 h and its effect on the normal cell cycle was detected by fluorescence-activated cell sorting (FACS) analysis (Figure 5, Figure S2). Interestingly, exposure of MDA-MB-468 cells to 5k induced a remarkable augmentation in the proportion of cells at pre-G1 phase by 8.13-fold. The increase was accompanied by concomitant noteworthy mitigation in the percentage of cells at the G0/G1, S, and G2/M phases by 2.21-, 2.43-, and 11.83-fold in comparison to the control, respectively.
Figure 5.
Bar chart shows percentage of MDA-MB-468 cells at each phase of cell cycle in control cells and cells treated with compound 5k.
2.3.4. Evaluation of the Apoptotic Effect of Compound 5k by Fluorescein Isothiocyanate (FITC)-Labeled Annexin V (Annexin V–FITC) Assay
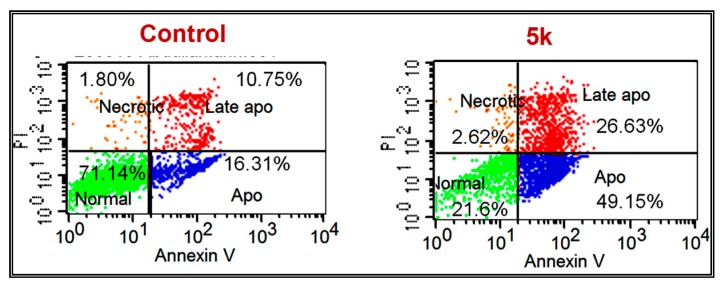
The apoptotic effect of 5k was further evaluated by Annexin VFITC/PI (AV/PI) dual staining assay to examine the occurrence of phosphatidylserine externalization and also to understand whether it is due to physiological apoptosis or nonspecific necrosis.
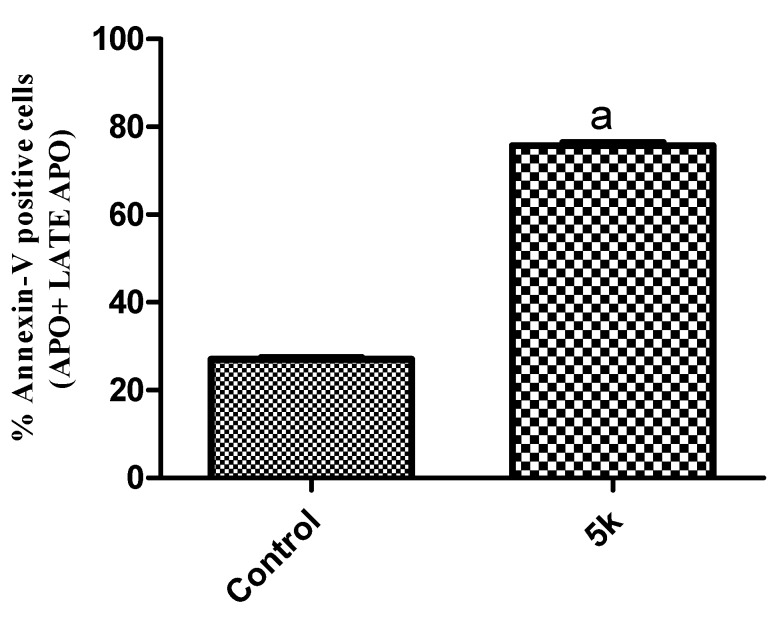
In this study MDA-MB-468 cells were treated with compound 5k for 48 h at 19.9 µM (IC50) to examine the apoptotic effect. It was observed that 5k showed significant apoptosis against MDA-MB-468 cells, as shown in Figure 6 and Figure 7. Results indicated that 5k showed 75.78% of apoptosis at 19.9 µM whereas 27.06% of apoptosis was observed in the control (untreated cells), comprising a 2.8-fold improvement compared to the control. This experiment suggests that 5k significantly induces apoptosis in MDA-MB-468 cells.
Figure 6.
Fluorescein isothiocyanate (FITC)-labeled annexin V (Annexin V–FITC) staining. The cells were treated with dimethylsulfoxide (DMSO) as a control or with compound 5k at IC50 concentration for 24 h. The experiment was done in triplicate.
Figure 7.
Effect of compound 5k on the percentage of annexin V–FITC-positive staining in MDA-MB-468 cells. Data are presented as means ± S.D. a Indicates statistical difference from control at p < 0.0001.
2.4. 2 Dimensional-Quantitative Structure Activity Relationship (2D-QSAR) Analysis for the Anti-Proliferative Activity of the Prepared Derivatives 5a–w
2.4.1. Elaboration of QSAR Model
QSAR analysis of the anti-tumor activity of the prepared derivatives 5a–w was performed to establish a correlation between the biochemical data and the compound structures; moreover, it aids us in identifying the positive and negative structural features within the three scaffolds. DS 2.5 software (Discovery Studio 2.5, Accelrys, Co., Ltd., Accelrys, San Diego, CA, USA) was used to run the analysis.
A set of 21 synthesized derivatives (5a–d, 5f–I, and 5k–w) was applied as a training set with their experimentally detected logIC50 against the MDA-MB-468 cancer cell line in the QSAR modeling. The two remaining synthesized members were used as an external test set to assess the predictive power and validate the established QSAR model. Various molecular descriptors for the training set molecules were calculated using the “Calculate Molecular Properties” module. 2D Descriptors entangled: topological descriptors, molecular properties, molecular property counts, AlogP, surface area, and volume. As for the 3D descriptors: dipole, principal moments of inertia, jurs descriptors, surface area, and volume, and shadow indices. To search for the best QSAR regression equation, genetic function approximation (GFA) was utilized, i.e., multiple linear regression modeling (MLR).
2.4.2. QSAR Study Results
The best performing QSAR model is represented by Equation (1);
Potency (LogIC50) against MDA-MB-468 cell line
| LogIC50 = 4.1269 + 0.0599 Num_ExplicitAtoms − 0.0139 Molecular_SAVol + 3.0316 CHI_V_3_C − 0.0920 Jurs_RPCS | (1) |
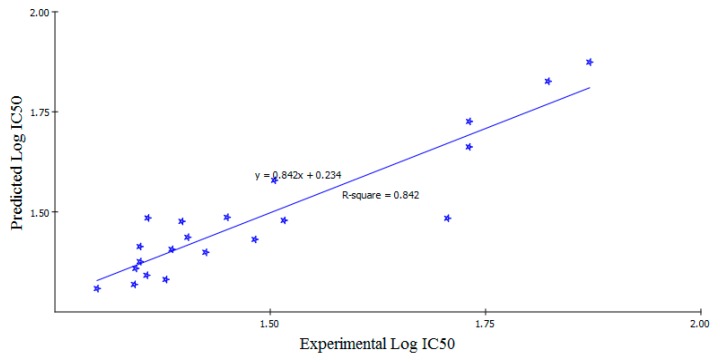
Adopting Equation (1), QSAR model was graphically represented. This was accomplished by plotting the experimental values against the predicted bioactivity values logIC50 for the training set compounds, as shown in Figure 8. Also, the estimated and experimental activities data and the calculated descriptors of the training set compounds were summarized in Table 5. The Least-Squares method was used to build the models, r2 = 0.842, r2 (adj) = 0.803, r2 (pred) = 0.795, Least-Squared error = 0.004 for model 1, where r2 (adj) is r2 adjusted for the number of terms in the model; r2 (pred) is the prediction r2, equivalent to q2 from a leave-one-out cross-validation.
Figure 8.
Predicted versus experimental logIC50 of the tested compounds against MDA-MB-468 cell line according to Equation (1) (r2 = 0.842).
Table 5.
Estimated IC50 data of the training set against MDA-MB-468 cell line and calculated descriptors governing IC50 according to Equation (1).
| Compound | Experimental Activity (LogIC50) | Predicted Activity (LogIC50) | Residual | Num_ExplicitAtoms | Molecular_SAVol | CHI_V_3_C | Jurs_RPCS |
|---|---|---|---|---|---|---|---|
| 5a | 1.3485 | 1.4134 | −0.0649 | 31 | 414.53 | 0.429 | 0.817 |
| 5b | 1.8707 | 1.8742 | −0.0035 | 34 | 430.44 | 0.596 | 0.834 |
| 5c | 1.7307 | 1.6624 | 0.0683 | 33 | 426.68 | 0.525 | 0.736 |
| 5d | 1.5047 | 1.5789 | −0.0742 | 31 | 443.37 | 0.618 | 0.860 |
| 5f | 1.3972 | 1.4763 | −0.0791 | 31 | 423.74 | 0.492 | 0.807 |
| 5g | 1.7057 | 1.4841 | 0.2216 | 31 | 432.96 | 0.536 | 0.752 |
| 5h | 1.7310 | 1.7260 | 0.0050 | 31 | 462.96 | 0.756 | 0.844 |
| 5i | 1.5159 | 1.4788 | 0.0371 | 33 | 441.92 | 0.541 | 0.928 |
| 5k | 1.2989 | 1.3084 | −0.0095 | 32 | 423.66 | 0.504 | 3.675 |
| 5l | 1.3420 | 1.3186 | 0.0234 | 32 | 423.66 | 0.504 | 3.563 |
| 5m | 1.3487 | 1.3758 | −0.0271 | 35 | 445.34 | 0.477 | 0.720 |
| 5n | 1.4040 | 1.4366 | −0.0326 | 35 | 445.34 | 0.497 | 0.727 |
| 5o | 1.3436 | 1.3587 | −0.0151 | 36 | 454.48 | 0.553 | 2.662 |
| 5p | 1.4249 | 1.3990 | 0.0259 | 39 | 476.16 | 0.545 | 0.630 |
| 5q | 1.3856 | 1.4065 | −0.0209 | 39 | 476.16 | 0.547 | 0.616 |
| 5r | 1.3577 | 1.4848 | −0.1271 | 34 | 444.90 | 0.528 | 0.633 |
| 5s | 1.3564 | 1.3416 | 0.0148 | 43 | 506.98 | 0.579 | 0.296 |
| 5t | 1.3786 | 1.3311 | 0.0475 | 38 | 465.86 | 0.497 | 0.710 |
| 5u | 1.8225 | 1.8264 | −0.0039 | 38 | 452.50 | 0.628 | 1.671 |
| 5v | 1.4820 | 1.4312 | 0.0508 | 28 | 396.29 | 0.405 | 0.637 |
| 5w | 1.4498 | 1.4865 | −0.0367 | 28 | 417.77 | 0.523 | 0.655 |
2.4.3. QSAR Validation
Two of the prepared compounds (5e and 5j) were utilized to carry out the external validation of the determined QSAR equation. 5e and 5j were chosen as they exhibit mild and excellent activities. The observed activities versus those provided by QSAR study are presented in Table 6.
Table 6.
External validation of the established QSAR model.
| Compound | Experimental Activity (LogIC50) | Predicted Activity (LogIC50) | Residual | Num_ExplicitAtoms | Molecular_SAVol | CHI_V_3_C | Jurs_RPCS |
|---|---|---|---|---|---|---|---|
| 5e | 2.1497 | 2.1497 | 0.00 | 31 | 423.74 | 0.473 | 0.795 |
| 5j | 1.3504 | 1.3504 | 0.00 | 32 | 423.66 | 0.482 | 3.313 |
2.5. Theoretical Kinetic ADME Study of the Target Derivatives 5a–w
A theoretical kinetic study carried out by Discovery Studio 2.5 software (Accelrys) was adopted to predict the ADME of the prepared derivatives 5a–w, Table 7. The lipophilicity was evaluated by calculating AlogP98, whereas the PSA_2D descriptor was adopted to estimate the polar surface area. Moreover, solubility level was predicted where all members of this study seemed to possess low solubility. In accordance with this anticipation, absorption levels implicate that they are well absorbed. Also, they are predicted to be non-inhibitors of CYP2D not to mention compounds 5b, 5q, and 5s, which are expected to inhibit CYP2D. LogP for compound 5k was determined experimentally and found to equal 3.75. It is worth mentioning that all compounds passed Lipinski’s rule of five.
Table 7.
Computer-aided ADME study of the prepared derivatives.
| Compound | AlogP98 a | PSA_2D b | Solubility c | Solubility Level d | Absorption Level e | CYP2D6 f | CYP2D6 Probability g |
|---|---|---|---|---|---|---|---|
| 5a | 3.684 | 43.616 | −4.830 | 2 | 0 | 0 | 0.316 |
| 5b | 4.171 | 43.616 | −5.324 | 2 | 0 | 1 | 0.554 |
| 5c | 2.938 | 70.156 | −4.307 | 2 | 0 | 0 | 0.376 |
| 5d | 4.349 | 43.616 | −5.560 | 2 | 0 | 0 | 0.376 |
| 5e | 3.890 | 43.616 | −5.158 | 2 | 0 | 0 | 0.336 |
| 5f | 3.890 | 43.616 | −5.152 | 2 | 0 | 0 | 0.376 |
| 5g | 4.095 | 43.616 | −5.478 | 2 | 0 | 0 | 0.326 |
| 5h | 4.433 | 43.616 | −5.636 | 2 | 0 | 0 | 0.287 |
| 5i | 3.579 | 86.440 | −5.034 | 2 | 0 | 0 | 0.257 |
| 5j | 3.442 | 64.432 | −4.355 | 2 | 0 | 0 | 0.326 |
| 5k | 3.442 | 64.432 | −4.363 | 2 | 0 | 0 | 0.336 |
| 5l | 3.442 | 64.432 | −4.366 | 2 | 0 | 0 | 0.386 |
| 5m | 3.668 | 52.547 | −4.911 | 2 | 0 | 0 | 0.376 |
| 5n | 3.668 | 52.547 | −4.887 | 2 | 0 | 0 | 0.396 |
| 5o | 3.426 | 73.362 | −4.486 | 2 | 0 | 0 | 0.425 |
| 5p | 3.652 | 61.477 | −4.959 | 2 | 0 | 0 | 0.306 |
| 5q | 3.652 | 61.477 | −4.949 | 2 | 0 | 1 | 0.524 |
| 5r | 3.453 | 61.477 | −5.112 | 2 | 0 | 0 | 0.346 |
| 5s | 3.635 | 70.407 | −5.013 | 2 | 0 | 1 | 0.554 |
| 5t | 4.017 | 52.547 | −5.102 | 2 | 0 | 0 | 0.366 |
| 5u | 3.287 | 28.624 | −4.870 | 2 | 0 | 0 | 0.306 |
| 5v | 3.080 | 56.171 | −4.348 | 2 | 0 | 0 | 0.099 |
| 5w | 3.410 | 43.616 | −4.643 | 2 | 0 | 0 | 0.425 |
a Lipophilicity descriptor; b Polar surface area; c Solubility parameter. (0–−2 = optimal, −2–−4 = good, −4–−6 = low, −6–−8 = very low); d Solubility level. (0 = extremely low, 1 = very low but possible, 2 = low, 3 = good, 4 = optimal); e Absorption level. (0 = good, 1 = moderate, 2 = low, 3 = very low); f CYP2D inhibition. (0 = non inhibitor, 1 = inhibitor); g CYP2D6 Probability: 0–0.5 = non inhibitor; 0.5–1 = inhibitor.
3. Discussion
In summary, a novel series of 2-((benzimidazol-2-yl)thio)-1-arylethan-1-one derivatives 5a–w has been synthesized. Their anti-proliferative activity against triple-negative breast cancer MDA-MB-468 cells was evaluated. Compound 5k was found to be the most active compound in this study with IC50 value of 19.90 ± 1.37 µM as it was 2.1 times more potent and efficacious than 5-fluorouracil (IC50 = 41.26 ± 3.77 µM). Also, analogs 5a, 5f, and 5j–t possessed excellent anti-proliferative activity with IC50 values ranging from 21.98 ± 1.91 to 26.60 ± 2.24 µM, which are better than the used reference drug. The preliminary SAR study showed that incorporation of unsubstituted phenyl group or its substitution with electron-donating hydroxyl, methoxy, or ethoxy groups are essential elements for the anti-tumor activity against MDA-MB-468, while introduction of heterocycles, such as 2-furyl or 2-thienyl, could not effectively replace the phenyl ring. In a cell-cycle analysis, compound 5k increased the percentage of MDA-MB-468 cells at pre-G1 by 8.13-fold and G2/M phase by 11.83-fold. Furthermore, treatment of MDA-MB-468 cells with 5k led to a marked increase in the percentage of annexin V–FITC-positive apoptotic cells (UR + LR) by 2.8-fold compared to the control. In addition, a QSAR model was established to investigate the structural requirements controlling activity against MDA-MB-468. Of note, the anticipated activities by the QSAR model were very near to the experimentally determined activities. Accordingly, this model could be conveniently applied for the prediction of more effective hits bearing the same structural framework. A theoretical kinetic study was constituted to anticipate the ADME of the prepared benzimidazoles. Moreover, single crystal X-ray diffraction has been included for compounds 4u and 5v.
Through this work we planned to add a scientific contribution for the treatment of the resistant type; TNBC by exploring different 2-benzimidazole derivatives. Our design was inspired by previously reported active scaffolds. Among the designed and synthetized molecules, many of them showed 2-fold increase in activity compared to 5-FU; this led us to develop a fruitful SAR analysis that will be a guideline for our future work.
4. Experimental
4.1. Chemistry
4.1.1. General
Melting points were determined using a Gallenkamp melting point apparatus (WeissTechnik, Loughborough, UK) and are uncorrected. Infrared (IR) Spectra were recorded as KBr disks using the Perkin Elmer FT-IR Spectrum BX apparatus (PerkinElmer, Boston, MA, USA). Mass spectra were measured on an Agilent TripleQuadrupole 6410 QQQ LC/MS equipped with an ESI (electrospray ionization) source (Agilent Technologies, Santa Clara, CA, USA). NMR Spectra were recorded on a Bruker NMR spectrometer (Bruker, Karlsruhe, Germany). 1H spectrum was run at 500 MHz and 13C spectrum was run at 125 MHz in deuterated dimethyl sulfoxide (DMSO-d6). Chemical shifts are expressed in δ values (ppm) using the solvent peak as internal standard. All coupling constant (J) values are given in hertz. The abbreviations used are as follows: s, singlet; d, doublet; m, multiplet. Elemental analyses were carried out at the Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt. Analytical thin layer chromatography (TLC) on silica gel plates containing UV indicator (Merck KGaA, Darmstadt, Germany) was employed routinely to follow the course of reactions and to check the purity of products. All reagents and solvents were purified and dried by standard techniques. Compounds 4 & 5a, b, f, h, 4 & 5l–o, 4 & 5q, r, t, v, and w, are previously reported [28].
4.1.2. Benzoimidazole-2-Thiol 2
Prepared according to the reported procedures [39].
4.1.3. General Procedures for Synthesis of Sulfate Salts 4a–w
Prepared according to the reported procedures [28].
2-((2-(4-Aminophenyl)-2-oxoethyl)thio)-1H-benzo[d]imidazol-3-ium sulfate (4c). White crystals, (yield 97%), m.p. 210–213 °C; IR (KBr, ν cm−1): 3450 (NH) and 1684 (C=O); 1H-NMR (DMSO-d6) δ ppm: 5.23 (s, 2H, CH2), 6.65 (d, 2H, H-3 and H-5 of 4-NH2C6H4, J = 8.5 Hz), 6.70 (s, 2H, NH2), 7.49–7.51 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.71–7.73 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 7.87 (d, 2H, H-2 and H-6 of 4-NH2C6H4, J = 8.5 Hz), 10.55 (s, 1H, NH), 12.64 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 41.55 (CH2), 113.40, 113.59, 114.66, 125.76, 130.92, 131.62, 132.74, 151.68, 189.11, 195.74 (C=O); ESI MS m/z: 665 [M + 1]+; Anal. Calcd. for C30H28N6O6S3: C, 54.20; H, 4.25; N, 12.64; Found C, 54.28; H, 4.21; N, 12.75.
2-((2-(4-Chlorophenyl)-2-oxoethyl)thio)-1H-benzo[d]imidazol-3-ium sulfate (4d). White crystals, (yield 98%), m.p. 227–230 °C; IR (KBr, ν cm−1): 3400 (NH) and 1683 (C=O); 1H-NMR (DMSO-d6) δ ppm: 5.29 (s, 2H, CH2), 7.37–7.40 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.62–7.65 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 7.68 (d, 2H, H-3 and H-5 of 4-ClC6H4, J = 8.5 Hz), 8.08 (d, 2H, H-2 and H-6 of 4-ClC6H4, J = 8.5 Hz), 10.55 (s, 1H, NH), 12.61 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 56.50 (CH2), 113.84, 120.85, 122.77, 124.68, 129.53, 130.89, 134.12, 134.86, 139.48, 150.64, 192.07 (C=O); ESI MS m/z: 703 [M + 1]+, 704 [M + 2]+; Anal. Calcd. for C30H24Cl2N4O6S3: C, 15.21; H, 3.44; N, 7.96; Found C, 15.30; H, 3.49; N, 8.02.
2-((2-(2-Fluorophenyl)-2-oxoethyl)thio)-1H-benzo[d]imidazol-3-ium sulfate (4e). White crystals, (yield 97%), m.p. 219–222 °C; IR (KBr, ν cm−1): 3435 (NH) and 1669 (C=O); 1H-NMR (DMSO-d6) δ ppm: 5.15 (s, 2H, CH2), 7.37–7.40 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.41–7.48 (m, 2H, Ar–H), 7.60–7.62 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 7.75–7.79 (m, 1H, Ar–H), 7.94 (t, 1H, H-6 of 2-FC6H4, J = 7.5 Hz), 10.64 (s, 1H, NH), 12.73 (s, 1H, NH). ESI MS m/z: 671 [M + 1]+; Anal. Calcd. for C30H24F2N4O6S3: C, 53.72; H, 3.61; N, 8.35; Found C, 53.67; H, 3.65; N, 8.38.
2-((2-(2,4-Difluorophenyl)-2-oxoethyl)thio)-1H-benzo[d]imidazol-3-ium sulfate (4g). White crystals, (yield 96%), m.p. 206–209 °C; IR (KBr, ν cm−1): 3468 (NH) and 1680 (C=O); 1H-NMR (DMSO-d6) δ ppm: 5.27 (s, 2H, CH2), 7.30 (t, 1H, Ar–H, J = 8.5 Hz), 7.43–7.46 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.52 (t, 1H, Ar–H, J = 9.0 Hz), 7.65–7.69 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 8.03 (t, 1H, Ar–H, J = 9.0 Hz),10.52 (s, 1H, NH), 12.67 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 44.06 (CH2), 105.70, 105.91, 106.12, 113.09, 113.27, 113.76, 120.81, 125.25, 133.50, 133.85, 150.71, 189.03 (C=O). ESI MS m/z: 707 [M + 1]+; Anal. Calcd. for C30H22F4N4O6S3: C, 50.99; H, 3.14; N, 7.93; Found C, 50.91; H, 3.18; N, 8.01.
2-((2-(2-Hydroxyphenyl)-2-oxoethyl)thio)-1H-benzo[d]imidazol-3-ium sulfate (4j). White crystals, (yield 98%), m.p. 238–240 °C; IR (KBr, ν cm−1): 3466 (NH) and 1684 (C=O); 1H-NMR (DMSO-d6) δ ppm: 5.30 (s, 2H, CH2), 7.02–7.11 (m, 4H, Ar–H), 7.38–7.42 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.67–7.70 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 9.59 (s, 1H, OH), 10.60 (s, 1H, NH), 12.87 (s, 1H, NH). ESI MS m/z: 667 [M + 1]+, 668 [M + 2]+; Anal. Calcd. for C30H26N4O8S3: C, 54.04; H, 3.93; N, 8.40; Found C, 54.12; H, 3.91; N, 8.46.
2-((2-(3-Hydroxyphenyl)-2-oxoethyl)thio)-1H-benzo[d]imidazol-3-ium sulfate (4k). White crystals, (yield 97%), m.p. 228–230 °C; IR (KBr, ν cm−1): 3418 (NH) and 1670 (C=O); 1H-NMR (DMSO-d6) δ ppm: 5.34 (s, 2H, CH2), 7.11 (d, 1H, H-4 of 3-OHC6H4, J = 8.0 Hz), 7.40–7.44 (m, 2H, H-2 and H-3 of 3-OHC6H4), 7.47–7.49 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.54 (d, 1H, H-6 of 3-OHC6H4, J = 8.0 Hz), 7.70–7.72 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 9.73 (s, 1H, OH), 10.48 (s, 1H, NH), 12.61 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 41.58 (CH2), 113.69, 115.09, 120.03, 121.82, 125.59, 130.55, 133.19, 136.46, 151.08, 158.23, 192.52 (C=O). ESI MS m/z: 667 [M + 1]+; Anal. Calcd. for C30H26N4O8S3: C, 54.04; H, 3.93; N, 8.40; Found C, 54.13; H, 4.03; N, 8.46.
2-((2-(2,4-Dimethoxyphenyl)-2-oxoethyl)thio)-1H-benzo[d]imidazol-3-ium sulfate (4p). White crystals, (yield 98%), m.p. 235–237 °C; IR (KBr, ν cm−1): 3377 (NH) and 1680 (C=O); 1H-NMR (DMSO-d6) δ ppm: 3.88 (s, 3H, OCH3), 4.00 (s, 3H, OCH3), 5.08 (s, 2H, CH2), 6.67 (d, 1H, H-5 of 2,4-(OCH3)2C6H3, J = 9.0 Hz), 6.74 (s, 1H, H-3 of 2,4-(OCH3)2C6H3), 7.43–7.44 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.65–7.66 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 7.79 (d, 1H, H-6 of 2,4-(OCH3)2C6H3, J = 9.0 Hz), 10.46 (s, 1H, NH), 12.37 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 45.31 (CH2), 56.33 (OCH3), 56.73 (OCH3), 98.92, 107.24, 113.37, 117.65, 121.38, 125.12, 133.06, 151.57, 162.02, 165.95, 191.78 (C=O). ESI MS m/z: 755 [M + 1]+; Anal. Calcd. for C34H34N4O10S3: C, 54.10; H, 4.54; N, 7.42; Found C, 54.16; H, 4.59; N, 7.38.
2-((2-(3,4,5-Trimethoxyphenyl)-2-oxoethyl)thio)-1H-benzo[d]imidazol-3-ium sulfate (4s). White crystals, (yield 98%), m.p. 213–215 °C; IR (KBr, ν cm−1): 3427 (NH) and 1675 (C=O); 1H-NMR (DMSO-d6) δ ppm: 3.77 (s, 6H, 2OCH3), 3.89 (s, 12H, 4OCH3), 5.29 (s, 4H, 2CH2), 7.40–7.67 (m, 12H, Ar–H), 10.67 (s, 2H, 2NH), 12.49 (s, 2H, 2NH). ESI MS m/z: 815 [M + 1]+, 816 [M + 2]+; Anal. Calcd. for C36H38N4O12S3: C, 53.06; H, 4.70; N, 6.88; Found C, 52.96; H, 4.63; N, 6.80.
2-((2-(Naphthalen-2-yl)-2-oxoethyl)thio)-1H-benzo[d]imidazol-3-ium sulfate (4u). White crystals, (yield 96%), m.p. 246–250 °C; IR (KBr, ν cm−1): 3412 (NH) and 1670 (C=O); 1H-NMR (DMSO-d6) δ ppm: 5.38 (s, 2H, CH2), 7.31–7.33 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.57–7.60 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 7.66–7.74 (m, 2H, H-6 and H-7 of naphthalene), 8.04 (d, 2H, Ar–H, J = 8.5 Hz), 8.08 (d, 1H, Ar–H, J = 8.5 Hz), 8.17 (d, 1H, Ar–H, J = 8.0 Hz), 8.85 (s, 1H, H-1 of naphthalene), 10.54 (s, 1H, NH), 12.68 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 56.49 (CH2), 113.96, 123.93, 124.13, 127.72, 128.26, 129.01, 130.14, 131.27, 132.56, 132.83, 135.79, 136.26, 150.58, 193.14 (C=O). ESI MS m/z: 735 [M + 1]+; Anal. Calcd. for C38H30N4O6S3: C, 62.11; H, 4.11; N, 7.62; Found C, 62.18; H, 4.13; N, 7.57.
4.1.4. General Procedure for Preparation of the Target Derivatives 5a–w
An aqueous solution (10 mL) of sodium bicarbonate was added to a stirred suspension of the adequate sulfate salts 4a–w (4 mmol) in water (20 mL). The mixture was stirred for 2 h at room temperature. The obtained solid was collected by filtration, washed several times with water, then dried and recrystallized from ethanol to furnish compounds 5a–w.
2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(4-aminophenyl)ethan-1-one (5c). White crystals (yield 85%), m.p. 175–178 °C; IR (KBr, ν cm−1): 3412 (NH) and 1680 (C=O); 1H-NMR (DMSO-d6) δ ppm: 4.87 (s, 2H, CH2), 6.21 (s, 2H, NH2), 6.59 (d, 2H, H-3 and H-5 of 4-NH2C6H4, J = 9.0 Hz), 7.10–7.14 (m, 4H, H-4, H-5, H-6 and H-7 of 2-mercaptobenzimidazole), 7.76 (d, 2H, H-2 and H-6 of 4-NH2C6H4, J = 8.5 Hz), 12.53 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 40.57 (CH2), 109.93, 113.03, 121.82, 122.78, 123.27, 131.42, 132.70, 150.41, 154.72, 190.66 (C=O); ESI MS m/z: 284 [M + 1]+; Anal. Calcd. for C15H13N3OS: C, 63.58; H, 4.62; N, 14.83; Found C, 63.81; H, 4.60; N, 14.89.
2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(4-chlorophenyl)ethan-1-one (5d). White crystals (yield 90%), m.p. 179–181 °C (reported: 189–191 °C [40]); IR (KBr, ν cm−1): 3410 (NH) and 1675 (C=O); 1H-NMR (DMSO-d6) δ ppm: 5.04 (s, 2H, CH2), 7.09–7.13 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.40–7.42 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 7.64 (d, 2H, H-3 and H-5 of 4-ClC6H4, J = 8.0 Hz), 8.08 (d, 2H, H-2 and H-6 of 4-ClC6H4, J = 8.5 Hz), 12.64 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 40.49 (CH2), 112.45, 121.87, 129.41, 130.82, 134.67, 139.06, 149.82, 193.09 (C=O); ESI MS m/z: 302.9 [M]+, 304.9 [M + 2]+.
2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(2-fluorophenyl)ethan-1-one (5e). White crystals (yield 89%), m.p. 145–148 °C; IR (KBr, ν cm−1): 3420 (NH) and 1654 (C=O); 1H-NMR (DMSO-d6) δ ppm: 4.93 (s, 2H, CH2), 7.09–7.12 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.37–7.49 (m, 4H, Ar–H), 7.70–7.74 (m, 1H, Ar–H), 8.08 (t, 1H, Ar–H, J = 7.5 Hz), 12.66 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 43.11 (CH2), 109.23, 117.29, 118.50, 121.69, 122.18, 124.61, 125.38, 131.09, 136.01, 149.80, 157.31, 160.50, 162.52, 191.90 (C=O); ESI MS m/z: 287 [M + 1]+, 288 [M + 2]+; Anal. Calcd. for C15H11FN2OS: C, 62.92; H, 3.87; N, 9.78; Found C, 63.09; H, 3.90; N, 9.84.
2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(2,4-difluorophenyl)ethan-1-one (5g). White crystals (yield 94%), m.p. 116–120 °C; IR (KBr, ν cm−1): 3420 (NH) and 1675 (C=O); 1H-NMR (DMSO-d6) δ ppm: 4.91 (s, 2H, CH2), 7.08–7.12 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.26 (t, 1H, Ar–H, J = 8.5 Hz), 7.39–7.41 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 7.47 (t, 1H, Ar–H, J = 9 Hz), 8.00 (q, 1H, Ar–H, J = 8.5 Hz), 12.61 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 42.95 (CH2), 105.54, 105.75, 105.96, 112.89, 113.04, 121.88, 133.35, 149.73, 190.71 (C=O); ESI MS m/z: 304 [M]+, 305 [M + 1]+; Anal. Calcd. for C15H10F2N2OS: C, 59.20; H, 3.31; N, 9.21; Found C, 59.46; H, 3.29; N, 9.32.
2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(2-hydroxyphenyl)ethan-1-one (5j). White crystals (yield 85%), m.p. 205–208 °C (reported: 201 °C [41]); IR (KBr, ν cm−1): 3408 (NH) and 1670 (C=O); 1H-NMR (DMSO-d6) δ ppm: 4.91 (s, 2H, CH2), 6.99–7.15 (m, 4H, Ar–H), 7.38–7.41 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 7.83–7.95 (m, 2H, Ar–H), 9.37 (s, 1H, OH), 12.53 (s, 1H, NH); ESI MS m/z: 285 [M + 1]+.
2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(3-hydroxyphenyl)ethan-1-one (5k). White crystals (yield 87%), m.p. 228–230 °C (reported: 224–227 °C [42]); IR (KBr, ν cm−1): 3336 (NH) and 1660 (C=O); 1H-NMR (DMSO-d6) δ ppm: 5.01 (s, 2H, CH2), 7.08–7.10 (m, 3H, H-5, H-6 of 2-mercaptobenzimidazole and H-4 of 3-OHC6H4), 7.37–7.45 (m, 4H, H-4, H-7 of 2-mercaptobenzimidazole and H-5, H-6 of 3-OHC6H4), 7.54 (s, 1H, H-2 of 3-OHC6H4), 9.89 (s, 1H, OH), 12.61 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 40.47 (CH2), 114.96, 119.86, 121.28, 121.61, 130.43, 137.23, 150.01, 158.14, 193.72 (C=O); ESI MS m/z: 285.
2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(2,4-dimethoxyphenyl)ethan-1-one (5p). White crystals (yield 92%), m.p. 208–211 °C; IR (KBr, ν cm−1): 3413 (NH) and 1655 (C=O); 1H-NMR (DMSO-d6) δ ppm: 3.92 (s, 3H, OCH3), 3.96 (s, 3H, OCH3), 4.80 (s, 2H, CH2), 6.65 (d, 1H, H-5 of 2,4-(OCH3)2C6H3, J = 9.0 Hz), 6.71 (s, 1H, H-3 of 2,4-(OCH3)2C6H3), 7.06–7.09 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.40–7.41 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 7.73 (d, 1H, H-6 of 2,4-(OCH3)2C6H3, J = 8.5 Hz), 12.60 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 43.97 (CH2), 56.20 (OCH3), 56.60 (OCH3), 98.88, 104.25, 106.96, 110.65, 114.19, 118.27, 121.63, 132.88, 150.15, 161.52, 165.36, 193.07 (C=O); ESI MS m/z: 329 [M + 1]+; Anal. Calcd. for C17H16N2O3S: C, 62.18; H, 4.91; N, 8.53; Found C, 62.40; H, 4.94; N, 8.45.
2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(3,4,5-trimethoxyphenyl)ethan-1-one (5s). White crystals (yield 88%), m.p. 233–235 °C; IR (KBr, ν cm−1): 3405 (NH) and 1670 (C=O); 1H-NMR (DMSO-d6) δ ppm: 3.77 (s, 3H, OCH3), 3.86 (s, 6H, OCH3), 5.04 (s, 2H, CH2), 7.12–7.43 (m, 6H, Ar–H), 12.68 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 39.37 (CH2), 56.60 (OCH3), 60.67 (OCH3), 106.58, 121.92, 131.12, 142.73, 149.87, 153.29, 192.94 (C=O); Anal. Calcd. for C18H18N2O4S: C, 60.32; H, 5.06; N, 7.82; Found C, 60.41; H, 5.03; N, 7.75.
2-((1H-Benzo[d]imidazol-2-yl)thio)-1-(naphthalen-2-yl)ethan-1-one (5u). White crystals (yield 92%), m.p. 160–162 °C; IR (KBr, ν cm−1): 3415 (NH) and 1673 (C=O); 1H-NMR (DMSO-d6) δ ppm: 5.20 (s, 2H, CH2), 7.10–7.13 (m, 2H, H-5 and H-6 of 2-mercaptobenzimidazole), 7.41–7.43 (m, 2H, H-4 and H-7 of 2-mercaptobenzimidazole), 7.64–7.72 (m, 2H, Ar–H), 8.02–8.07 (m, 3H, Ar–H), 8.15 (d, 1H, Ar–H, J = 8.0 Hz), 8.85 (s, 1H, H-1 of naphthalene), 12.80 (s, 1H, NH); 13C-NMR (DMSO-d6) δ ppm: 40.42 (CH2), 121.92, 124.19, 127.59, 128.20, 128.91, 129.42, 130.12, 131.11, 132.59, 133.19, 135.69, 149.99, 193.90 (C=O); ESI MS m/z: 319 [M + 1]+; Anal. Calcd. for C19H14N2OS: C, 71.68; H, 4.43; N, 8.80; Found C, 71.81; H, 4.40; N, 8.73.
4.2. Biological Evaluation
4.2.1. In Vitro Evaluation of the Anti-Proliferative Activity
The synthesized derivatives 5a–w was evaluated for their anti-proliferative activity via the Stem Cell Therapy and Tissue Reengineering Program in the King Faisal Specialized Hospital and Research Center, Riyadh, Saudi Arabia. In vitro anti-proliferative activity was measured by the cell growth inhibition assay. This assay was conducted using a WST-1 reagent (Sigma-Aldrich Chemie Gmbh, Munich, Germany) for determination of the IC50 for each compound and the results are given in Table 4. MDA-MB-468 breast cancer cell line was purchased from the American Type Culture Collection (Manassas, Virginia, USA). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich Chemie Gmbh), supplemented with 10% FBS (Lonza, Visp, Switzerland), 100 IU/mL penicillin, 100 mg/mL streptomycin, and 2 mmol/L l-glutamine (Sigma). Cells were seeded into 96-well plates at 0.4 × 104/well and incubated overnight. The medium was replaced with a fresh one containing the desired concentrations of the test compounds. After 48 h, 10 µL of the WST-1 reagent were added to each well and the plates were re-incubated for 4 h at 37 °C. The amount of formazan was quantified using an ELISA reader (Thermo Fisher Scientific, Waltham, MA, USA) at 450 nm. The IC50 values were calculated according to the equation for Boltzmann sigmoidal concentration response curve using the nonlinear regression models (GraphPad, Prism Version 5, San Diego, CA, USA). The results reported are means of at least three separate experiments. Significant differences were analyzed by one-way analysis of variance (ANOVA) wherein the differences were considered to be significant at p < 0.05.
4.2.2. Cell Cycle Analysis
The MDA-MB-468 cells were subjected to treatment with 19.90 µM of compound 5k for 24 h. Consequently, the cells were washed twice with ice-cold phosphate buffered saline (PBS). The treated cells were collected by centrifugation, fixed in ice-cold 70% (v/v) ethanol, washed with PBS, re-suspended with 0.1 mg/mL RNase, stained with 40 mg/mL PI, and analyzed by flow cytometry using FACScalibur (Becton Dickinson, BD, San Jose, CA, USA). The cell cycle distributions were calculated using CellQuest software (Becton Dickinson).
4.2.3. Annexin V–FITC Apoptosis Assay
The MDA-MB-468 cells were seeded as described above and then incubated with 19.90 µM of compound 5k for 24 h. Cells were harvested, washed twice with PBS, and centrifuged. In brief, 105 of cells were treated with annexin V–FITC and propidium iodide (PI) using the apoptosis detection kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s protocol. Annexin V–FITC and PI binding were analyzed by flow cytometry on FACScalibur (BD Biosciences) without gating restrictions using 10,000 cells. Data were collected using logarithmic amplification of both the FL1 (FITC) and the FL2 (PI) channels. Quadrant analysis of co-ordinate dot plots was performed with CellQuest software. Unstained cells were used to adjust the photomultiplier voltage and for compensation setting adjustment to eliminate spectral overlap between the FL1 and the FL2 signal.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University (Riyadh, Saudi Arabia) for its funding of this research through the Research Group Project no. PRG-1436-038.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/17/8/1221/s1.
Author Contributions
Hatem A. Abdel-Aziz and Wagdy M. Eldehna conceived and designed the experiments; Wagdy M. Eldehna, Hazem Ghabbour, Ghada H. Al-Ansary carried out the experiments; Wagdy M. Eldehna and Ghada H. Al-Ansary analyzed and interpreted the data; Hazem Ghabbour carried out the single crystal analysis of compounds 4u and 5v and interpreted their data; Hatem A. Abdel-Aziz, Wagdy M. Eldehna and Ghada H. Al-Ansary prepared the manuscript; Areej M. Assaf and Abdullah Al-Dhfyan performed the biological screening. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.International Agency for Research on Cancer . IGlobocan: Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2012. IARC; Lyon, France: 2014. [Google Scholar]
- 2.Adami H., Hunter D., Trichopoulos D. Textbook of Cancer Epidemiology. Oxford University Press; New York, NY, USA: 2002. pp. 301–373. [Google Scholar]
- 3.Barnard M.E., Boeke C.E., Tamimi R.M. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim. Biophys. Acta. 2015;1856:73–85. doi: 10.1016/j.bbcan.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson R.L., Yang W.T., Rosen D.G., Landis M.D., Wong H., Lewis M.T., Creighton C.J., Sexton K.R., Hilsenbeck S.G., Sahin A.A., et al. Cancer stem cell markers are enriched in normal tissue adjacent to triple negative breast cancer and inversely correlated with DNA repair deficiency. Breast Cancer Res. 2013;15:R77. doi: 10.1186/bcr3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahman M., Davis S.R., Pumphrey J.G., Bao J., Nau M.M., Meltzer P.S., Lipkowitz S. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res. Treat. 2009;113:217–230. doi: 10.1007/s10549-008-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rakha E.A., Reis-Filho J.S., Ellis I.O. Basal-like breast cancer: A critical review. J. Clin. Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- 7.Fadare O., Tavassoli F.A. Clinical and pathologic aspects of basal-like breast cancers. Nat. Clin. Pract. Oncol. 2008;5:149–159. doi: 10.1038/ncponc1038. [DOI] [PubMed] [Google Scholar]
- 8.Smid M., Wang Y., Zhang Y., Sieuwerts A.M., Yu J., Klijn J.G., Foekens J.A., Martens J.W. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 9.Maire V., Baldeyron C., Richardson M., Tesson B., Vincent-Salomon A., Gravier E., Marty-Prouvost B., De Koning L., Rigaill G., Dumont A., et al. TTK/hMPS1 is an attractive therapeutic target for triple-negative breast cancer. PLoS ONE. 2013;8:1221. doi: 10.1371/journal.pone.0063712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cleator S., Heller W., Coombes R.C. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 11.Bansal Y., Silakari O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012;20:6208–6236. doi: 10.1016/j.bmc.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Fei F., Zhou Z. New substituted benzimidazole derivatives: A patent review (2010–2012) Expert Opin. Ther. Pat. 2013;23:1157–1179. doi: 10.1517/13543776.2013.800857. [DOI] [PubMed] [Google Scholar]
- 13.Yadav G., Ganguly S. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem. 2015;97:419–443. doi: 10.1016/j.ejmech.2014.11.053. [DOI] [PubMed] [Google Scholar]
- 14.Gümüş F., Algül Ö., Eren G., Eroğlu H., Diril N., Gür S., Özkul A. Synthesis, cytotoxic activity on MCF-7 cell line and mutagenic activity of platinum (II) complexes with 2-substituted benzimidazole ligands. Eur. J. Med. Chem. 2003;38:473–480. doi: 10.1016/S0223-5234(03)00058-8. [DOI] [PubMed] [Google Scholar]
- 15.Bielawski K., Wolczynski S., Bielawska A. Inhibition of DNA topoisomerase I and II, and growth inhibition of MDA-MB-231 human breast cancer cells by bis-benzimidazole derivatives with alkylating moiety. Pol. J. Pharmacol. 2004;56:373–378. [PubMed] [Google Scholar]
- 16.Thimmegowda N.R., Swamy S.N., Kumar C.S.A., Kumar Y.C.S., Chandrappa S., Yip G.W., Rangappa K.S. Synthesis, characterization and evaluation of benzimidazole derivative and its precursors as inhibitors of MDA-MB-231 human breast cancer cell proliferation. Bioorg. Med. Chem. Lett. 2008;18:432–435. doi: 10.1016/j.bmcl.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 17.Taher A.T., Khalil N.A., Ahmed E.M. Synthesis of novel isatin-thiazoline and isatin-benzimidazole conjugates as anti-breast cancer agents. Arch. Pharmacal Res. 2011;34:1615–1621. doi: 10.1007/s12272-011-1005-3. [DOI] [PubMed] [Google Scholar]
- 18.Romero-Castro A., León-Rivera I., Ávila-Rojas L.C., Navarrete-Vázquez G., Nieto-Rodríguez A. Synthesis and preliminary evaluation of selected 2-aryl-5(6)-nitro-1H-benzimidazole derivatives as potential anticancer agents. Arch. Pharmacal Res. 2011;34:181–189. doi: 10.1007/s12272-011-0201-5. [DOI] [PubMed] [Google Scholar]
- 19.Bonham S., O’Donovan L., Carty M.P., Aldabbagh F. First synthesis of an aziridinyl fused pyrrolo[1,2-a]benzimidazole and toxicity evaluation towards normal and breast cancer cell lines. Org. Biomol. Chem. 2011;9:6700–6706. doi: 10.1039/c1ob05694h. [DOI] [PubMed] [Google Scholar]
- 20.Rahim A.S.A., Salhimi S.M., Arumugam N., Pin L.C., Yee N.S., Muttiah N.N., Keat W.B., Hamid S.A., Osman H., Mat I.B. Microwave-assisted synthesis of sec/tert-butyl 2-arylbenzimidazoles and their unexpected antiproliferative activity towards ER negative breast cancer cells. J. Enzym. Inhib. Med. Chem. 2013;28:1255–1260. doi: 10.3109/14756366.2012.729828. [DOI] [PubMed] [Google Scholar]
- 21.Yoon Y.K., Ali M.A., Wei A.C., Choon T.S., Osman H., Parang K., Shirazi A.N. Synthesis and evaluation of novel benzimidazole derivatives as sirtuin inhibitors with antitumor activities. Bioorg. Med. Chem. 2014;22:703–710. doi: 10.1016/j.bmc.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Abonia R., Cortés E., Insuasty B., Quiroga J., Nogueras M., Cobo J. Synthesis of novel 1,2,5-trisubstituted benzimidazoles as potential antitumor agents. Eur. J. Med. Chem. 2011;46:4062–4070. doi: 10.1016/j.ejmech.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Kamal A., Reddy T.S., Vishnuvardhan M.V.P.S., Nimbarte V.D., Rao A.V.S., Srinivasulu V., Shankaraiah N. Synthesis of 2-aryl-1,2,4-oxadiazolo-benzimidazoles: Tubulin polymerization inhibitors and apoptosis inducing agents. Bioorg. Med. Chem. 2015;23:4608–4623. doi: 10.1016/j.bmc.2015.05.060. [DOI] [PubMed] [Google Scholar]
- 24.Kamal A., Shaik A.B., Polepalli S., Kumar G.B., Reddy V.S., Mahesh R., Garimella S., Jain N. Synthesis of arylpyrazole linked benzimidazole conjugates as potential microtubule disruptors. Bioorg. Med. Chem. 2015;23:1082–1095. doi: 10.1016/j.bmc.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Husain A., Rashid M., Mishra R., Parveen S., Shin D.-S., Kumar D. Benzimidazole bearing oxadiazole and triazolo-thiadiazoles nucleus: Design and synthesis as anticancer agents. Bioorg. Med. Chem. Lett. 2012;22:5438–5444. doi: 10.1016/j.bmcl.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Rashid M., Husain A., Mishra R. Synthesis of benzimidazoles bearing oxadiazole nucleus as anticancer agents. Eur. J. Med. Chem. 2012;54:855–866. doi: 10.1016/j.ejmech.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 27.Husain A., Rashid M., Shaharyar M., Siddiqui A.A., Mishra R. Benzimidazole clubbed with triazolo-thiadiazoles and triazolo-thiadiazines: New anticancer agents. Eur. J. Med. Chem. 2013;62:785–798. doi: 10.1016/j.ejmech.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Aziz H.A., Ghabbour H.A., Eldehna W.M., Al-Rashood S.T.A., Al-Rashood K.A., Fun H.-K., Al-Tahhan M., Al-Dhfyan A. 2-((Benzimidazol-2-yl)thio)-1-arylethan-1-ones: Synthesis, crystal study and cancer stem cells CD133 targeting potential. Eur. J. Med. Chem. 2015;104:1–10. doi: 10.1016/j.ejmech.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Aboul-Fadl T., Kadi A., Abdel-Aziz H.A. Novel N,N′-Hydrazino-bis-isatin Derivatives with Selective Activity Against Multidrug-Resistant Cancer Cells. 20120252860. U.S. Patent. 2012 Oct 4;
- 30.Abdel-Aziz H.A., Ghabbour H.A., Eldehna W.M., Qabeel M.M., Fun H.-K. Synthesis, crystal structure and biological activity of cis/trans amide rotomers of (Z)-N′-(2-oxoindolin-3-ylidene)formohydrazide. J. Chem. 2014;2014:760434. doi: 10.1155/2014/760434. [DOI] [Google Scholar]
- 31.Fares M., Eldehna W.M., Abou-Seri S.M., Abdel-Aziz H.A., Aly M.H., Tolba M.F. Design, synthesis and in vitro antiproliferative activity of novel isatin-quinazoline hybrids. Arch. Pharm. Chem. Life Sci. 2015;348:144–154. doi: 10.1002/ardp.201400337. [DOI] [PubMed] [Google Scholar]
- 32.Eldehna W.M., Ibrahim H.S., Abdel-Aziz H.A., Farrag N.N., Youssef M.M. Design, synthesis and in vitro antitumor activity of novel N-substituted-4-phenyl/benzylphthalazin-1-ones. Eur. J. Med. Chem. 2015;89:549–560. doi: 10.1016/j.ejmech.2014.10.064. [DOI] [PubMed] [Google Scholar]
- 33.Eldehna W.M., Altoukhy A., Mahrous H., Abdel-Aziz H.A. Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential anti-proliferative agents. Eur. J. Med. Chem. 2015;90:684–694. doi: 10.1016/j.ejmech.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Alafeefy A.M., Ahmad R., Abdulla M., Eldehna W.M., Al-Tamimi A.S., Abdel-Aziz H.A., Al-Obaid O., Carta F., Al-Kahtani A.A., Supuran C.T. Development of certain new 2-substituted-quinazolin-4-yl-aminobenzenesulfonamide as potential antitumor agents. Eur. J. Med. Chem. 2016;109:247–253. doi: 10.1016/j.ejmech.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Eldehna W.M., Fares M., Ibrahim H.S., Aly M.H., Zada S., Ali M.M., Abou-Seri S.M., Abdel-Aziz H.A., Abou El Ella D.A. Indoline ureas as potential anti-hepatocellular carcinoma agents targeting VEGFR-2: Synthesis, in vitro biological evaluation and molecular docking. Eur. J. Med. Chem. 2015;100:89–97. doi: 10.1016/j.ejmech.2015.05.040. [DOI] [PubMed] [Google Scholar]
- 36.Sheldrick G.M. A short history of SHELX. Acta Crystallogr. Sect. A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 37.Ngamwongsatit P., Banada P.P., Panbangred W., Bhunia A.K. WST-1-based cell cytotoxicity assay as a substitute for MTT-based assay for rapid detection of toxigenic Bacillus species using CHO cell line. J. Microbiol. Methods. 2008;73:211–215. doi: 10.1016/j.mimet.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 39.Mavrova A.T., Denkova P., Tsenov Y.A., Anichina K.K., Vutchev D.I. Synthesis and antitrichinellosis activity of some bis(benzimidazol-2-yl)amines. Bioorg. Med. Chem. 2007;15:6291–6297. doi: 10.1016/j.bmc.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Loghmani-Khouzani H., Hajiheidari D. Synthesis of difluorinated β-ketosulfones and novel gem-difluoromethylsulfone-containing heterocycles as fluorinated building blocks. J. Fluor. Chem. 2010;131:561–569. doi: 10.1016/j.jfluchem.2009.12.022. [DOI] [Google Scholar]
- 41.Turan-Zitouni G., Demirayak S., Erol K., Ozdemir M. Synthesis of some 2-[(benzazole-2-yl)thoiacetyl]phenol derivatives and preliminary investigation on their vasodilatory activity. Farmaco. 1994;40:755–757. [PubMed] [Google Scholar]
- 42.Madsen P., Knudsen L.B., Wiberg F.C., Carr R.D. Discovery and structure-activity relationship of the first non-peptide competitive human glucagon receptor antagonists. J. Med. Chem. 1998;41:5150–5157. doi: 10.1021/jm9810304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.