Abstract
Primary vagal afferent neurons express a multitude of thermosensitive ion channels. Within this family of ion channels, the heat-sensitive capsaicin receptor (TRPV1) greatly influences vagal afferent signaling by determining the threshold for action-potential initiation at the peripheral endings, while controlling temperature-sensitive forms of glutamate release at central vagal terminals. Genetic deletion of TRPV1 does not completely eliminate these temperature-dependent effects, suggesting involvement of additional thermosensitive ion channels. The warm-sensitive, calcium-permeable, ion channel TRPV3 is commonly expressed with TRPV1; however, the extent to which TRPV3 is found in vagal afferent neurons is unknown. Here, we begin to characterize the genetic and functional expression of TRPV3 in vagal afferent neurons using molecular biology (RT-PCR and RT-quantitative PCR) in whole nodose and isolated neurons and fluorescent calcium imaging on primary cultures of nodose ganglia neurons. We confirmed low-level TRPV3 expression in vagal afferent neurons and observed direct activation with putative TRPV3 agonists eugenol, ethyl vanillin (EVA), and farnesyl pyrophosphate (FPP). Agonist activation stimulated neurons also containing TRPV1 and was blocked by ruthenium red. FPP sensitivity overlapped with EVA and eugenol but represented the smallest percentage of vagal afferent neurons, and it was the only agonist that did not stimulate neurons from TRPV3−/−1 mice, suggesting FPP has the highest selectivity. Further, FPP was predictive of enhanced responses to capsaicin, EVA, and eugenol in rats. From our results, we conclude TRPV3 is expressed in a discrete subpopulation of vagal afferent neurons and may contribute to vagal afferent signaling either directly or in combination with TRPV1.
Keywords: vagus, calcium, autonomic reflexes, satiety, nodose ganglion
vagal afferent neurons relay peripheral information from visceral organs to the brain and initiate autonomic reflex pathways involved in the control of respiratory, cardiovascular, and gastrointestinal function (5, 31). Functional subtypes of vagal afferents are distinguished by their biophysical properties (including myelination and resulting conduction velocity), as well as their specific complement of ion channels. Similar to somatosensory afferents, vagal afferents express an abundance and multitude of transient receptor potential (TRP) ion channels. Transient receptor potential vanilloid subtype 1 (TRPV1), a calcium-permeable ion channel activated by heat (>40°C) and capsaicin (6), is selectively expressed on C-type and Aδ-type vagal afferent fibers (41). At the central terminals, TRPV1 mobilizes glutamate vesicles in response to capsaicin and warm temperatures (1, 30, 32). Furthermore, TRPV1 expression in vagal afferents provides mechanosensitivity to gastric tension (18). However, pharmacological block or genetic deletion of TRPV1 only reduces, but does not eliminate, these effects (13, 18, 30). Additionally, warm-range temperature-driven glutamate mobilization is maintained in the absence of TRPV1 (13). This suggests that additional thermosensitive TRP channels contribute to vagal afferent signaling. On the basis of these findings and reports, we hypothesize that heat-sensitive TRP channels that are similar and highly associated with TRPV1 may provide additional ionic conductances independent of TRPV1 to influence vagal afferent signaling.
Transient receptor potential vanilloid subtype 3 (TRPV3) is a thermosensitive, calcium-permeable, ion channel stimulated by warm temperature (33–39°C) and chemical compounds (28, 33, 39). The TRPV3 gene is located adjacent to TRPV1 on the same chromosome (chromosome 17 in humans and chromosome 11 in mice) and structurally homologous (28). TRPV3 also coexpresses with TRPV1 in small-unmyelinated sensory neurons and forms heterotetramers with TRPV1 when cotransfected into HEK cells (33). TRPV3 gene expression is distributed to many tissues, including expression in keratinocytes, where it contributes to temperature and possibly pain perception (9, 25, 28, 33, 39). Studies suggest that TRPV3, in coexpression or interaction with TRPV1, assists TRPV1-driven detection of noxious heat and pain (33). Mice with genetic deletion of TRPV3 show deficits in heat sensation (26); however, TRPV3 does not seem to be a major sensor for endogenous thermoregulation (16). One study has reported TRPV3 expression in nodose ganglia tissue of rats (43), but the importance of TRPV3 in vagally mediated reflexes and behaviors has not been elucidated. Pertinent to our study, TRPV3 is activated in the warm-temperature range (33–39°C) (13), and from this, we predict that TRPV3 may contribute to vagal afferent signaling in a similar capacity as TRPV1.
Here, we report initial experiments to investigate the role of TRPV3 in primary vagal afferent neurons. First, we demonstrated the relatively low abundance of TRPV3 message in relation to TRPV1 by performing RT-PCR and RT-quantitative PCR (qPCR) on nodose ganglia tissue. Next, we measured intracellular calcium concentrations in dissociated vagal afferent neurons treated with putative TRPV3 agonists [eugenol, ethyl vanillin (EVA), and farnesyl pyrophosphate (FPP)] to determine their functional expression. Agonist responses occurred in only a subset of capsaicin-sensitive cells and were attenuated or blocked by ruthenium red. FPP was the most TRPV3-selective, as it failed to stimulate neurons from TRPV3−/− mice. Single-cell RT-PCR on isolated vagal afferent neurons confirmed the distribution of TRPV3 within a subset of TRPV1-expressing neurons. Further, responses to capsaicin were significantly larger in neurons that also responded to FPP from rats but not mice. Our results suggest functional expression of TRPV3 within a small subset of vagal afferent neurons may enhance signaling, perhaps via interactions with TRPV1.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (120–250 g; Simonsen Laboratories), male C57BL/6 mice (20–30 g), male TRPV1−/− knockout mice (20–30 g) (B6.129X1-Trpv1tm1Jul/J; Jackson Laboratories), and male TRPV3−/− knockout mice (20–30 g) (B6.129-TRPV3tm1Apat/J; Jackson Laboratories) were used under procedures approved by the Institutional Animal Care and Use Committee at Washington State University. Animals were housed under 12:12-h light-dark conditions and fed standard pellet chow ad libitum. Transgenic mice were genotyped using the protocol for separated PCR and primer pairs suggested by Jackson Laboratories (Tables 1 and 2). We isolated genomic DNA from tail fragments using NaOH extraction. Genotyping of DNA from mouse tail fragments confirmed the identity of wild-type and transgenic-null mice.
Table 1.
Primer sequences and protocol for genotyping B6.129X1-TrpV1tm1Jul/J mice
| Primer Type | Sequence | PCR |
|---|---|---|
| Wild-type | F–CCT GCT CAA CAT GCT CAT TG | 94°C for 3 min |
| Common | R–TCC TCA TGC ACT TCA GGA AA | [94°C for 30 s, 63°C for 1 min, 72°C for 1 min (35 times)] |
| Mutant | F–TGG ATG TGG AAT GTG TGC GAG | 72°C for 2 min 4°C, hold |
Mutant = 450 bp; Common = 450 bp and 984 bp; Wild type = 984 bp.
Table 2.
Primer sequences and protocol for genotyping B6.129-TrpV3tm1Apat/J mice
| Primer Type | Sequence | PCR |
|---|---|---|
| Common | F–GGC CCT CAG AGG AGC C | 94°C for 2 min |
| Wild-type | R–CAG GTA CTG TGT CGC CCC | [94°C for 20 s, 65°C for 15 s (↓1.5°C per cycle), 68°C for 10 s (10 times)] |
| Mutant | R–TCT ATG GCT TCT GAG GCG G | [94°C for 15 s, 50°C for 15 s, 72°C for 10 s (28 times)] |
| 72°C for 2 min, 10°C, hold |
Common = 182 bp and 304 bp; Mutant = 182 bp; Wild-type = 304 bp.
Nodose ganglia isolations and primary neuronal cultures.
For primary rat and mouse neuronal cultures, we removed and pooled both the left and right nodose using aseptic surgical protocols previously reported (19). All surgeries and euthanasia were performed under a deep plane of anesthesia (ketamine, 25 mg/100 g; with Xylazine, 2.5 mg/100 g). Euthanasia was accomplished with an overdose of barbiturates and confirmed through the creation of a pneumothorax via thoracotomy. Once isolated, the connective tissue around the nodose ganglia was digested in Ca2+/Mg2+-free HBSS containing 1 mg/ml of both dispase II (Hoffmann-La Roche) and collagenase type 1A (Sigma-Aldrich, St. Louis, MO) (90 min at 37°C in 95% air-5% CO2). Following the enzymatic digestion, the neurons were dissociated by gentle trituration through silicanized pipettes, and then washed in DMEM supplemented with 10% FBS, and 1% penicillin-streptomycin. Dissociated neurons were plated onto polylysine-coated coverslips maintained in DMEM+10% FBS (37°C in 95% air-5% CO2) and used within 24–48 h of isolation for ratiometric fluorescent calcium measurements. These short-term cultures were not treated with growth factors, but maintained viability and TRPV1 expression consistent with previous results (36).
RT-quantitative PCR.
Nodose ganglia were removed and then placed into Qiazol (Qiagen, Germantown, MD) for storage at −80°C until final processing. Samples were homogenized via a TissueLyser, and RNA was extracted using the RNeasy mini kit and the QIACube robotic workstation (Qiagen). RNA was converted into cDNA using the Ambion DNA-free kit (Life Technologies, Grand Island, NY) and QuantiTect reverse transcription kit (Qiagen). Reverse-transcribed cDNA was dissolved in RNase-free water and stored at −20°C. To detect rat or mouse GAPDH, TRPV1, and TRPV3 cDNA, we used published primers or designed primers and performed PCR, according to cycling parameters detailed in Tables 3 and 4. We used RT-qPCR to quantify the amounts of TRPV3 transcript relative to TRPV1 in rat and mouse nodose ganglia. RT-qPCR was performed using Fast SYBR Green PCR Master Mix (Life Technologies) coupled with the 7500 fast real-time PCR system by Applied Biosystems (Life Technologies). Reactions were run with a total volume of 20 μl:10 μl of Fast SYBR Green Master Mix, 2 μl of cDNA, 6 μl of H2O, and 1 μl each of forward and reverse primer (0.5 μM). Each reaction was run in triplicate with the following cycling protocol: 95°C for 20 s, then 95°C for 3 s, followed by 60°C for 30 s 40 times. Water was run as a negative control, and GAPDH was run as a normalization control in every experiment for comparative analysis. All primers produced amplification curves, plotted as a normalized measure of fluorescence (Rn) against cycle number, within the accurately measurable ranges (Fig. 1C). Amplification curves for each gene were constructed using average ΔRn (Rn PCR reaction – Rn baseline) ± SE values (n = 6 rats, 1 animal per run, all samples run in triplicate). For each run, we calculated the cycle thresholds (Ct) from the amplification curves. Values are expressed as average Ct ± SE (Fig. 1D). From these data, we calculated ΔCt relative to GAPDH for TRPV1 and TRPV3. Values are expressed as ΔCt ± SE. TRPV3 amplification had a significantly higher Δ cycle threshold from GAPDH compared with TRPV1 (n = 6 rats, P < 0.001, using a t-test) (Fig. 1E). We then converted the values to relative abundance for comparison. The calculated relative abundance of TRPV3 was significantly lower compared with TRPV1 (n = 6 rats, P = 0.03, using a t-test) (Fig. 1F).
Table 3.
Primer sequences and protocol used for amplifying cDNA products from rats
| Gene | Sequence | Size, bp | PCR |
|---|---|---|---|
| TRPV1* | F–GGT GTG CCT GCA CCT AGC | 107 | 95°C for 5 min |
| R–CTC TTG GGG TGG GGA CTC | |||
| TRPV3* | F–CAC CCC CAC CAA GAA GAG T | 84 | [95°C for 45 s, 60°C for 45 s, 72°C for 1 min (35 times)] |
| R–GGG GGA GAG GT TTG GTA AC | |||
| GAPDH | F–CGT GTT CCT ACC CCC AAT GT | 153 | 72°C for 10 min, 4°C, hold |
| R–ACA GGA GAC AAC CTG GTC CT |
Primers obtained from Ref. 42.
Table 4.
Primer sequences and protocol for amplifying cDNA products from mice
| Gene | Sequence | Size (bp) | PCR |
|---|---|---|---|
| TRPV1* | F–CCT GCT CAA CAT GCT CAT TG | 134 | 94°C for 3 min |
| R–TCC TCA TGC ACT TCA GGA AA | |||
| TRPV3 | F–CGT CAT GAT CCA GAA GGT CA | 119 | [94°C for 30 s, 63°C for 1 min, 72°C for 1 min (35 times)] |
| R–TGT CCT TGG AGC ACT TCT CA | |||
| GAPDH | F–TGT GAA CGG ATT TGG CCG TA | 250 | 72°C for 2 min 4°C, hold |
| R–GGC CTC ACC CCA TTT GAT GT |
Primer obtained from Jackson Laboratories.
Fig. 1.
Vagal afferent neurons express TRPV3 mRNA in low abundance relative to TRPV1. A: agarose gel showing cDNA amplicons of TRPV1, TRPV3, and GAPDH from nodose ganglia tissue. Lane 1: (100 bp ladder), lane 2: (TRPV1 ∼107 bp), lane 3: (TRPV3 ∼84 bp), and lane 4: (GAPDH ∼153 bp). B: mRNA transcript of TRPV1 and TRPV3 from nodose ganglia tissue of WT, TRPV1−/−, and TRPV3−/− mice. C: RT-quantitative PCR (qPCR)-averaged amplification curves for GAPDH, TRPV1, and TRPV3 cDNA amplicons reverse transcribed from rat vagal afferent neurons. D: calculated cycle threshold values from amplification curves. E: TRPV3 amplification had a significantly higher Δ cycle threshold from GAPDH compared with TRPV1 (n = 6 rats, ***P < 0.001, t-test). F: calculated relative abundance of TRPV3 was significantly lower compared with TRPV1 (n = 6 rats, *P = 0.03, t-test). G: RT-qPCR-averaged amplification curves for GAPDH, TRPV1, and TRPV3 cDNA amplicons reverse transcribed from vagal afferent neurons taken from wild-type and TRPV3−/− mice. H: TRPV1 Δ cycle threshold compared with GAPDH was similar between wild-type and TRPV3−/− mice (n = 6 mice/group, P = 0.61, t-test), while TRPV3 amplification from the TRPV3−− mice was similar to the water (H2O) control and significantly greater in the wild-type mice (n = 6 mice/group, ***P < 0.001, t-test). I: calculated relative abundance of TRPV1 was not different between wild-type and TRPV3−/− mice (P = 0.63, t-test); however, the TRPV3 relative abundance was essentially absent from the TRPV3−/− mice (*P < 0.05, ***P < 0.001, t-test). Relative abundance of TRPV3 was also lower than TRPV1 in WT mice (P < 0.05, t-test). J: RT-qPCR-averaged amplification curves for GAPDH, TRPV1, and TRPV3 cDNA amplicons reverse transcribed from vagal afferent neurons taken from wild-type and TRPV1−/− mice. K: TRPV3 Δ cycle threshold compared with GAPDH was similar between wild-type and TRPV1−/− mice (n = 6 mice/group, P = 0.13, t-test), while TRPV1 amplification from the TRPV1−/− mice was similar to the water (H2O) control and significantly greater in the wild-type mice (n = 6 mice/group, ***P < 0.001, t-test). L: calculated relative abundance of TRPV3 was not different between wild-type and TRPV1−/− mice (P = 0.13, t-test); however, the TRPV1 relative abundance was essentially absent from the TRPV1−/− mice (n = 6 mice/group, **P = 0.002, t-test). Data are presented as averages ± SE.
Single-cell collection and RT-PCR.
Dissociated rat nodose neurons plated on coverslips were individually collected with sterile glass pipettes into 11 μl of lysis buffer and RNase-free PBS. Single-cell RNA was converted into cDNA and further amplified using the REPLI-g WTA single cell kit (Qiagen). Reverse transcribed cDNA was then purified using the QIAquick PCR purification kit (Qiagen). Conventional PCR was used to identify cells that expressed TRPV1 (∼118 bp) and TRPV3 (∼184 bp) using the primer sets in Table 5.
Table 5.
Primer sequences and protocol for RT-PCR of single-cell rat nodose neurons
| Gene | Sequence | Size, bp | PCR |
|---|---|---|---|
| TRPV1 | F–GGC TGT CTT CAT CAT CCT GTT A | 118 | 95°C for 3 min |
| R–GTT CTT GCT CTC TTG TGC AAT C | |||
| TRPV3 | F–GTG ACT CCA GAG GTC AGT AGA A | 184 | [95°C for 30 s, 60°C for 45 s, 72°C for 1 min (45 times)] |
| R–TGG CCC TAA CAA CAG AAC ATA G | |||
| GAPDH | F–CGT GTT CCT ACC CCC AAT GT | 153 | 72°C for 10 min, 4°C, hold |
| R–ACA GGA GAC AAC CTG GTC CT |
Ratiometric fluorescent calcium imaging.
Intracellular calcium concentrations were monitored using the fluorescent Ca2+ indicator Fura-2 AM (Molecular Probes) on an inverted Nikon Eclipse Ti microscope (Nikon Instruments) with an Andor Zyla digital camera (Andor Technology). Neurons previously plated on coverslips were loaded with Fura-2 AM (1 μM) for an hour, rinsed, and then mounted onto a closed chamber and constantly perfused with a physiological bath (in mM: 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 6 glucose, 10 HEPES with pH adjusted to 7.4 with Tris base). Fluorescence ratios were collected by alternatively exciting neurons containing Fura-2 with 340 and 380 nm light and monitoring fluorescence at 510 nm. Data points were collected with Nikon Elements software at 6-s time intervals, and the ratios of fluorescence intensity were converted to calcium concentrations using a standard curve. The calcium calibration curve was determined previously using a sequential calcium titration with Fura-2 in the bath. The resulting fluorescence ratios were fit to determine binding parameters using protocols previously summarized (15). The calibration curve is then saved in the imaging software for conversion of fluorescence ratios to calcium concentrations. Drugs were perfused via a common manifold, and all manipulations were performed at room temperature. Neuron viability was confirmed through depolarization with a high potassium bath, where 55 mM KCl was used with an equimolar reduction of NaCl to 90 mM.
Statistical analysis.
Data for each experiment were collected from a minimum of 2–3 nodose ganglion cell cultures. When possible, we designed within-subject protocols and analyzed data using repeated-measures ANOVA followed by post hoc comparisons against control. Dose-response parameters were determined by using a sigmoid fit of the data. For antagonist studies (ruthenium red), we compared all neurons that received each treatment using within-subject t-tests. Data are expressed as the average ± SE with all statistical analysis performed using SigmaStat software (Systat Software).
Reagents.
The following reagents were purchased from Sigma-Aldrich: eugenol (E51791), ethyl vanillin (EVA) (W246409), farnesyl pyrophosphate (FPP) (F6892), capsaicin (CAP) (no. M2028), and ruthenium red (RuR) (no. R2751).
RESULTS
TRPV3 RNA expression in vagal afferent neurons.
TRPV3 gene expression has been localized to multiple tissue types, including coexpression with TRPV1 in dorsal root and trigeminal sensory neurons in humans and nonhuman primates (33). Here, we probed for TRPV3 mRNA expression in vagal afferent neurons by performing RT-PCR. TRPV3 message was detected in rat and mouse nodose ganglia but in smaller quantities than TRPV1 (Fig. 1, A and B) and absent from nodose taken from TRPV3−/− mice as expected (Fig. 1B). Using RT-qPCR, we determined that nodose expression of TRPV3 was significantly lower compared with TRPV1 in rats (n = 6 rats, P = 0.03, using a t-test) (Fig. 1, C–F). We also quantified the amounts of TRPV3 transcript relative to TRPV1 in wild-type and TRPV3−/− mice. As predicted GAPDH and TRPV1 transcripts amplified similarly in nodose from wild-type and TRPV3−/− mice (n = 6 mice/group, P = 0.61, t-test), and we confirmed the lack of TRPV3 amplification selectively in the TRPV3−/− animals (n = 6 mice/group, P < 0.001, t-test) (Fig. 1G–I). Similar to the rat nodose ganglia, the TRPV3 gene was expressed in lower abundance relative to TRPV1 in wild-type control mice (P < 0.001, t-test). As a control, we also compared TRPV1 and TRPV3 expression in WT and TRPV1−/− mice, to determine whether TRPV1 absence influenced TRPV3 expression. GAPDH and TRPV3 transcripts amplified similarly in nodose from wild-type and TRPV1−/− mice (n = 6 mice/group, P = 0.13, t-test) (Fig. 1J), and we confirmed the lack of TRPV1 amplification selectively in the TRPV1−/−1 animals (n = 6 mice/group, P = 0.002, t-test) (Fig. 1, K and L).
Pharmacological identification of TRPV3.
Because of the lack of agonist selectivity, studying TRPV3 in native tissue is challenging, considering multiple TRP channels are commonly expressed. Because of this issue of selectivity, we chose three commercially available putative TRPV3 agonists [eugenol, ethyl vanillin (EVA), and farnesyl pyrophosphate (FPP)] to pharmacologically identify TRPV3 activity in primary cultured rat vagal afferent neurons (3, 38). Using ratiometric calcium imaging, we measured increases in intracellular calcium concentration to determine agonist activity. We found that all three agonists concentration-dependently increased intracellular calcium in a subset of neurons (Fig. 2). Eugenol and EVA activation required high micromolar to low millimolar concentrations to exhibit maximal activation (Fig. 2, A–D) but activated the largest subset of neurons (eugenol: 26 of 37, 70% and EVA: 26 of 60, 43%; P < 0.001, ANOVA). Of the three agonists, FPP activated the smallest subset of neurons (FPP: 11 of 118, 9%) but had the lowest EC50 value (eugenol vs. EVA: P < 0.001; eugenol vs. FPP: P < 0.001; EVA vs. FPP: P < 0.001, corrected t-tests) (Fig. 2H), suggesting relatively high-affinity binding compared with eugenol and EVA. The calculated slopes for all agonists were similar, although EVA was statistically lower than eugenol but not different from FPP (eugenol vs. EVA: P = 0.006; eugenol vs. FPP: P = 0.09; EVA vs. FPP: P = 0.88, corrected t-tests) (Fig. 2I). These results suggest that these three putative TRPV3 agonists are capable of producing transient increases in cytosolic calcium, which may be mediated by TRPV3.
Fig. 2.
Characterization of TRPV3 agonist pharmacology in rat vagal afferent neurons. Putative TRPV3 agonists eugenol, ethyl vanillin (EVA), and farnesyl pyrophosphate (FPP) concentration-dependently increased intracellular calcium levels in subpopulations of cultured vagal afferent neurons. A, C, and E: representative traces of intracellular calcium concentrations from individual neurons activated by eugenol, EVA, and FPP, respectively. B, D, and F: average concentration-response relationships for agonist-induced increases in intracellular calcium: eugenol (n = 26/37 neurons (70%), P < 0.001, ANOVA, *P < 0.05, Tukey's post hoc test); EVA (n = 26/60 neurons (43%), P < 0.001, ANOVA, *P < 0.05, Tukey's post hoc test), and FPP (n = 11/118 neurons (9%), P < 0.001, ANOVA, *P < 0.05, Tukey's post hoc test). G: averaged normalized concentration-response curves across FPP, eugenol, and EVA-activated neurons. Values are normalized to the last agonist challenge and expressed as average ± SE. Black bars denote eugenol, gray bars denote EVA, and green bars denote FPP. H: comparison of EC50 concentrations across agonists with FPP exhibiting the highest affinity of the three compounds (eugenol vs. EVA: P < 0.001; eugenol vs. FPP: P < 0.001; EVA vs. FPP: P < 0.001; corrected t-tests). I: average slope across all agonists. H and I: lowercase letters refer to differences or similarities between values corresponding to eugenol, EVA, and FPP. Values are very similar for all compounds, although the slope for EVA was statistically lower than eugenol but not different from that of FPP (eugenol vs. EVA: P = 0.006; eugenol vs. FPP: P = 0.09; EVA vs. FPP: P = 0.88; corrected t-tests).
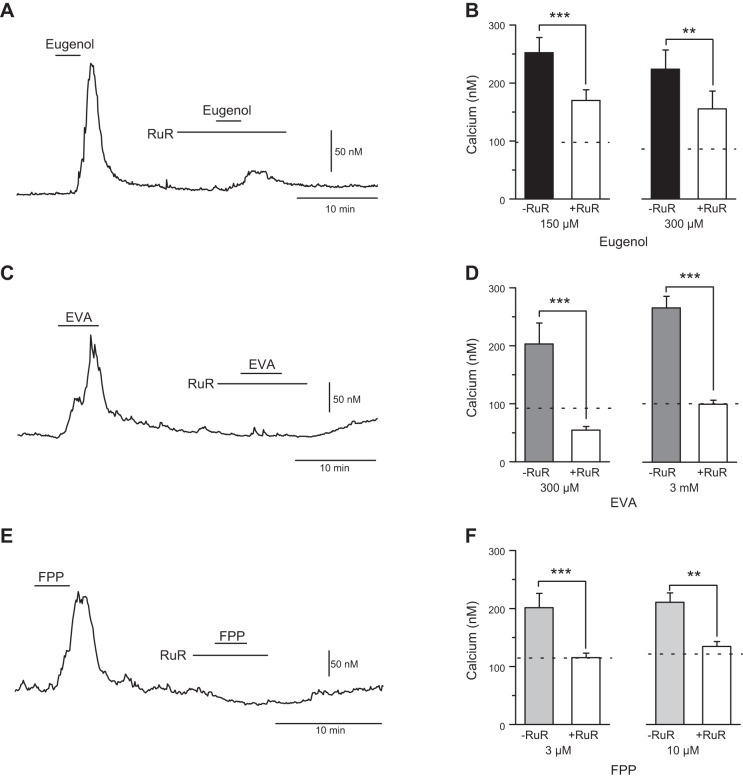
TRPV3 is sensitive to the broad-spectrum TRP channel blocker ruthenium red (RuR), and, as such, any suspected agonist activation of TRPV3 should be eliminated with RuR pretreatment. We tested the calcium responses to eugenol, EVA, and FPP in the absence and presence of RuR (1 μM) using a within-subjects design in rat vagal afferent neurons (Fig. 3). We found RuR only attenuated the response to eugenol at 150 μM eugenol (n = 21 neurons, P < 0.001, paired t-test) and 300 μM eugenol (n = 15 neurons, P < 0.01, paired t-test) (Fig. 3, A and B), while completely blocking the responses to EVA at 300 μM EVA (n = 14 neurons, P < 0.001, using a paired t-test) and 3 mM EVA (n = 21 neurons, P < 0.001, paired t-test) (Fig. 3, C and D) and FPP at 0.1 μM FPP (n = 16 neurons, P < 0.001, paired t-test) and 10 μM FPP (n = 9 neurons, P = 0.004, paired t-test) (Fig. 3, E and F). These experiments were conducted at two different concentrations of each agonist to confirm the effect of RuR. From these findings, we conclude that eugenol is activating both RuR-sensitive and RuR-insensitive conductances (17). The blockade of EVA and FPP responses by RuR is consistent with activation of a subset of TRP channels, including TRPV3.
Fig. 3.
Ruthenium red (RuR) attenuates the eugenol response and blocks the EVA and FPP response in rat vagal afferent neurons. Agonist activity at TRPV3 should be eliminated with RuR (1 μM) pretreatment. A: representative calcium trace of an individual neuron treated with eugenol (150 μM) before and after pretreatment RuR (1 μM). B: average response of eugenol-induced increase in cytosolic calcium before and after pretreatment with RuR at 150 μM eugenol (n = 21 neurons, ***P < 0.001, paired t-test) and 300 μM eugenol (n = 15 neurons, **P < 0.01). C: representative calcium trace showing EVA (300 μM) response before and after pretreatment with RuR (1 μM). D: average response to EVA-induced increase in cytosolic calcium before and after pretreatment with RuR at 300 μM EVA (n = 14 neurons, ***P < 0.001, paired t-test), and 3 mM EVA (n = 21, P < 0.001, paired t-test). E: representative calcium trace showing FPP (0.1 μM) response before and after pretreatment with RuR (1 μM). F: average response to FPP-induced increase in cytosolic calcium before and after pretreatment with RuR at 0.1 μM FPP (n = 16 neurons, ***P < 0.001, paired t-test) and 10 μM FPP (n = 9, **P = 0.004, paired t-test). Group data are expressed as average ± SE.
The antagonist isopentenyl pyrophosphate has activity at TRPV3, but also at transient receptor potential ankyrin 1 (TRPA1), thus confounding the determination of selective agonists (4). As such, we next examined the agonist activity in cultured vagal afferent neurons taken from wild-type (WT) control mice and TRPV3−/− mice. In the concentration-response study, we found eugenol activated a subset of neurons from both mouse strains at a concentration similar to what we found in rat vagal afferent neurons (Fig. 4A). Thus, the loss of TRPV3 did not alter the concentration-response relationship to eugenol (WT: n = 10 neurons, P < 0.001, using ANOVA and TRPV3−/−: n = 10 neurons, P < 0.001, using ANOVA) (Fig. 4B). However, the percentage of responsive neurons was somewhat lower in the control mouse compared with the rat. Surprisingly, deletion of TRPV3 seemed to increase the percentage of eugenol-responsive neurons (Fig. 4C). While eugenol may activate TRPV3, it does not appear to be doing so selectively in these neurons, likely due to the coexpression of other eugenol-activated TRP channels (38, 40). On average, EVA was also able to activate a subset of neurons in both mouse strains (Fig. 4D) at a concentration similar to that found in rat vagal afferent neurons (WT: n = 21 neurons, P < 0.001, ANOVA and TRPV3−/−: n = 13 neurons, P < 0.001, ANOVA) (Fig. 4E). While overall fewer cells responded to EVA than to eugenol, deletion of TRPV3 only reduced, but did not eliminate, the response (Fig. 4F). Similar to eugenol and EVA, the percentage of neurons that responded to FPP in the WT mouse was lower than in the rat (n = 9 of 170 neurons, 5%). However, the deletion of TRPV3 eliminated any responses to FPP (n = 100 neurons, P < 0.11, ANOVA) (Fig. 4, G and I). These results are consistent with FPP selectively activating TRPV3 in vagal afferent neurons.
Fig. 4.
FPP responses are absent in TRPV3−/− mice. Pharmacological profiling of putative TRPV3 agonists in wild-type and TRPV3−/− mice. A: representative traces of intracellular calcium concentrations from WT (left) and TRPV3−/− (right) mice that respond to eugenol. B: average concentration response relationship for eugenol-induced increases in intracellular calcium (WT: n = 10 neurons, P < 0.001, ANOVA, *P < 0.05, Tukey's post hoc test and TRPV3−/−: n = 10 neurons, P < 0.001, ANOVA, *P < 0.05, Tukey's post hoc test). C: percentage of vagal afferent neurons that responded to eugenol across animals tested. D: representative traces of intracellular calcium concentrations from WT (left) and TRPV3−/− (right) mice that respond to EVA. E: average concentration response relationship for EVA-induced increases in intracellular calcium (WT: n = 21 neurons, P < 0.001, ANOVA, *P < 0.05, Tukey's post hoc test and TRPV3−/−: n = 13 neurons, P < 0.001, ANOVA, *P < 0.05, Tukey's post hoc test). F: percentage of cells that respond to EVA in rat, WT mice, and TRPV3−/− mice. G: representative traces of intracellular calcium concentrations from WT (left) and TRPV3−/− (right) mice that respond to FPP. H: average concentration response relationship for FPP-induced increases in intracellular calcium at FPP (WT: n = 8 neurons, P < 0.017, ANOVA, *P < 0.05, Tukey's post hoc test and TRPV3−/−: n = 100 neurons, P < 0.11, ANOVA). I: percentage of cells that respond to FPP in rat, WT mice, and TRPV3−/− mice. Group data are expressed as average ± SE.
TRPV3 is known to be coexpressed with TRPV1 in dorsal root and trigeminal ganglia sensory neurons in humans and nonhuman primates (33). Consistent with these reports, we observed an overlap of responses between the TRPV3 agonists and capsaicin, a marker for the presence of TRPV1 (Fig. 5). We found that eugenol-activated neurons were nearly all responsive to CAP (all except for 1 eugenol responsive neuron in the rat) (Fig. 5, A and B). This relationship was conserved between rat, wild-type mice, and TRPV3−/− mice (rat: n = 37 neurons; WT mouse: n = 26 neurons; TRPV3−/− mice: n = 17 neurons) (Fig. 5B). The overlap of EVA responses to CAP was similar to eugenol and also maintained across species (rat: n = 60 neurons; WT mouse: n = 78 neurons; TRPV3−/− mice: n = 68 neurons) (Fig. 5, C and D). Responses to FPP also only occurred in a subset of CAP-responsive neurons from rats and wild-type mice and were absent from the TRPV3−/− animals, as reported above (rats: n = 180 neurons; WT mouse: n = 170 neurons; TRPV3−/− mice: n = 100 neurons) (Fig. 5, E and F). In a separate experiment, we determined the overlap of eugenol, EVA, and FPP responses in individual neurons from rats (Fig. 5, G and H). We confirmed the observation that eugenol activated the largest proportion of neurons (n = 83 of 150, 55%), followed by EVA (n = 59 of 150, 39%), and that FPP activated a small subset of neurons (n = 16 of 150, 11%). FPP responders were nearly always activated by both eugenol and EVA (with only one exception), suggesting FPP may be the TRPV3 agonist with the highest selectivity and that eugenol and EVA may produce more calcium responses via activation of additional TRP channels (38, 40).
Fig. 5.
Overlapping distribution of TRPV3 agonist responses with TRPV1 in vagal afferent neurons from rat, WT mice, and TRPV3−/− mice. Eugenol, EVA, and FPP stimulate a subset of TRPV1 expressing (CAP sensitive) vagal afferent neurons. A: calcium trace showing the typical profile of a eugenol and CAP-sensitive rat vagal afferent neuron. B: percentage of neurons sensitive to eugenol (EUG) and CAP (rat: n = 37 neurons; WT mouse: n = 26 neurons; TRPV3−/− mice: n = 17 neurons). C: representative calcium trace of an EVA and CAP-sensitive rat vagal afferent neuron. D: percentage of neurons sensitive to EVA and CAP (rat: n = 60 neurons; WT mouse: n = 78 neurons; TRPV3−/− mice: n = 68 neurons). E: representative calcium trace showing a typical profile of a FPP and CAP-sensitive rat vagal afferent neuron. F: percentage of neurons sensitive to FPP and CAP (rats: n = 180 neurons; WT mouse: n = 170 neurons; TRPV3−/− mice: n = 100 neurons). G: FPP-EVA-Eugenol overlapping population of rat vagal afferent neurons. H: percentage of neurons sensitive to FPP, EVA, EUG (n = 150 neurons).
To confirm the distribution and overlap of TRPV3 with TRPV1, we performed single-cell RT-PCR on dissociated rat vagal afferent neurons. We probed for GAPDH, TRPV1, and TRPV3 from each cell (Fig. 6A). With this method, we determined that out of 25 neurons examined; 18 cells were positive for TRPV1 message, two of which were also positive for TRPV3 message (Fig. 6B). These results align with the percentage of cells that were responsive to FPP and CAP, using the calcium imaging.
Fig. 6.
Single-cell RT-PCR analysis of TRPV1 and TRPV3 expression in primary rat vagal afferent neurons. A: representative agarose gel showing cDNA amplicons of GAPDH (∼153 bp), TRPV1 (∼118 bp), and TRPV3 (∼184 bp) from eight selected dissociated vagal afferent neurons. Gels are shown between ∼300 bp and 100 bp, as determined from a 100-bp DNA ladder. B: count of single neurons containing or lacking TRPV1 and TRPV3+, as determined via RT-PCR (n = 25 neurons examined). Percentage of total population shown above bars.
FPP is predictive of enhanced responses to CAP, EVA, and eugenol.
While TRPV3 is coexpressed with TRPV1 and known to form V1:V3 heterotetramers (7, 8), the extent to which TRPV3 activity interacts with TRPV1 signaling in native tissue is not well characterized, presumably, in part, due to the lack of selective agonists available. To explore this potential relationship, we compared CAP (100 nM)-induced responses in neurons that are sensitive to FPP (10 μM) (FPP responders) or not (FPP nonresponders). We observed that FPP-responsive neurons had greater peak and integrated CAP responses compared with FPP-nonresponsive vagal afferent neurons taken from rats (peak: P < 0.001, unpaired t-test; AUC: P < 0.001, unpaired t-test) (Fig. 7A, summarized in 7C). In separate experiments, we characterized EVA (300 μM) and eugenol (300 μM) responses following FPP (0.1 μM) exposure to identify FPP-responsive and FPP-nonresponsive neurons also taken from rats (Fig. 7B). We observed that in FPP-responsive neurons, EVA induced larger peak and integrated calcium responses compared with FPP-nonresponsive neurons (peak: P < 0.001, unpaired t-test; AUC: P < 0.001, unpaired t-test) (Fig. 7D). Although the average peak response to eugenol was greater in FPP-responsive neurons, it did not reach statistical difference from FPP-nonresponsive neurons. However, there was a clear and statistically significant increase in the integrated calcium response with eugenol between the FPP-responsive and FPP-nonresponsive neurons (peak: P = 0.11, unpaired t-test; AUC: P < 0.001, unpaired t-test) (Fig. 7E). In contrast, we found no difference in the CAP response between vagal afferent neurons in mice that were FPP-responsive, FPP-nonresponsive, or from TRPV3−/− mice (peak: P > 0.05, corrected unpaired t-tests; AUC: P > 0.05, corrected unpaired t-tests) (Fig. 7, F and G). These observations together suggest FPP is predictive of larger responses to other agonists, but there may exist important species-specific differences.
Fig. 7.
TRPV3 predicts enhanced responses to CAP, EUG, and EVA in the rat. FPP-responsive vagal afferent neurons taken from rats exhibited larger calcium responses to CAP (100 nM), EUG (300 μM), and EVA (300 μM). A: calcium traces showing CAP activation in neurons FPP-nonresponsive (left) and FPP-responsive (right), as determined using 10 μM FPP. B: calcium traces showing EVA and EUG-induced activation from neurons FPP-nonresponsive (left) and FPP-responsive (right), as determined using 0.1 μM FPP. C: average peak and integrated (AUC, area under the curve) CAP-induced intracellular calcium responses from FPP-responsive (n = 26) and FPP-nonresponsive (n = 78 neurons) neurons (peak: ***P < 0.001, and AUC: ***P < 0.001, unpaired t-test). D: average peak and integrated (AUC) EVA-induced intracellular calcium responses from FPP-responsive (n = 15) and FPP-nonresponsive (n = 44 neurons) neurons (peak: ***P < 0.001, unpaired t-test; AUC: ***P < 0.001, unpaired t-test). E: average peak and integrated (AUC) EUG-induced intracellular calcium responses from FPP-responsive (n = 16) and FPP-nonresponsive (n = 67 neurons) neurons (peak: P = 0.11 and AUC: P < 0.001, unpaired t-test). F: calcium traces showing CAP induced activation in vagal afferent neurons from wild-type (WT) mice (FPP-nonresponsive, left; and FPP-responsive, middle) and from TRPV3−/− mice (right). G: average CAP-induced intracellular calcium responses from FPP-responsive (n = 8 neurons), FPP-nonresponsive (n = 65 neurons), and TRPV3−/− (n = 38 neurons) (peak: P > 0.05 and AUC: P > 0.05, corrected unpaired t-tests). Group data are expressed as averages ± SE.
DISCUSSION
The contributions of thermosensitive TRP channels in somatosensory afferents are well established (11), although their role in visceral afferent neurons, which are in a region of little temperature variation, remains more enigmatic. In this study, we confirmed the expression of TRPV3 in primary vagal afferent neurons and examined the responses of three agents that are purported to be agonists on this subtype of TRP channel. Of the three agents, we conclude that FPP was the most selective agonist to detect functional TRPV3 activity in this experimental system. Despite the relatively small responses, FPP identified TRPV3 expression in a small subset of vagal afferent neurons and was predictive of enhanced activity of coexpressed TRP channels, including TRPV1 and EVA and eugenol-sensitive calcium influx pathways in the rat. In contrast, vagal afferent neurons lacking FPP responses exhibited smaller responses to CAP, EVA, and eugenol. This difference in agonist-induced calcium influx suggests TRPV3 may exert effects on vagal afferent signaling via interactions with other TRP channels, such as TRPV1. However, given the discrete expression of TRPV3, we predict it likely plays a minor role in vagal afferent signaling in general, but may importantly contribute to the function of specific subpopulations of vagal afferents.
TRP channels and vagal afferent neuronal signaling.
Our current findings and the published literature suggest that the neurophysiological contributions of TRP channels are complex and dependent on the specific complement of channels expressed within a neuron. Primary vagal afferent neurons express a multitude of TRP channels, many of them thermosensitive (37, 43). TRP channels seem indiscriminately expressed throughout the neuron (in specialized peripheral endings, along extensive unmyelinated axons, in cell bodies, and at central terminations in the brain stem) (14, 41, 43). Although some TRP channels can directly serve as effectors at the peripheral terminals (22), the physiological and neurophysiological function for most of these channels is not generally understood. Further, not all vagal afferent neurons express all TRP channel isoforms (37). In fact, many, like TRPV3, seem to be expressed in relatively low abundance across the whole population, while present in subsets of individual neurons (37, 43). The single-cell RT-PCR suggests that even when present, the TRPV3 mRNA levels are still relatively low compared with TRPV1. This variability in TRP channel abundance may reflect the physiological relevance of TRP channel function and the complexity of the organism, as TRP channel expression differs between species (33). Although we speculate that this small population of vagal afferent neurons expressing functional TRPV3 (∼10%) may contribute to translating temperature fluctuations to neurophysiological changes, the extent to which TRPV3 impacts vagal afferent signaling endogenously remains undetermined.
At the central terminals, we and others have previously characterized the contribution of TRPV1 to control quantal forms of glutamate release (30, 32, 34). TRPV1 is one of the most abundant TRP channels in the vagus and broadly distinguishes small unmyelinated (C-fibers) and lightly myelinated (Aδ-fibers) from heavily myelinated, large-diameter A-fibers (12, 29). While these TRPV1-augmented forms of release may contribute to the differential strength of C-fiber-mediated reflex function, even complete removal of TRPV1 signaling (pharmacologic or genetic) does not eliminate these release pathways (13, 30). Furthermore, the frequency of spontaneous events at temperatures ranges of 33–37°C is also maintained in the absence of TRPV1 (13). From these observations, we rationalized that the remaining control of TRPV1-independent glutamatergic events may come from other warm-sensing thermosensitive TRP channels (such as TRPV3) with the same structural homology and coexpressed with TRPV1. Our current findings characterize the genetic expression of TRPV3 in vagal afferent neurons and provide functional and pharmacological evidence of signaling at the membrane. Further, we provide initial details of a potential influence on TRPV1 and possibly other TRP channels in a subset of primary vagal afferent neurons that also express TRPV3. While these findings substantiate the possibility that TRPV3 may participate in vagal afferent signaling, the exact mechanism by which this occurs remains mysterious because of the paucity of selective pharmacologic tools (agonists and antagonists) available and the challenge of investigating the specific contribution of a low-abundance protein.
Pharmacology of FPP at TRPV3.
Achieving selective pharmacological activation of TRP channels in native systems is challenging due to off-target effects of many TRP channel agonists. FPP has been classified as a TRPV3-selective agonist when characterized in transfected HEK cells (3); however, whether this selectivity is maintained in native tissue is less well characterized. FPP is also reported to stimulate G protein-coupled receptor 92 (GPR92) and serve as an endogenous intermediate in the cholesterol synthesis pathway (20, 27), both of which may produce nonspecific effects. While GPR92 has been shown to be in afferent neurons of the spinal sensory system (27), its expression in vagal afferent neurons is unknown. We demonstrated a complete block of FPP with RuR, suggesting that a RuR-sensitive TRP channel was the likely effector. If present in vagal afferent neurons, GPR92 could couple through a RuR-sensitive TRP channels to increase cytosolic calcium, as reported with other G protein-coupled receptors (35). The lack of FPP effect following deletion of TRPV3 supports our conclusion of selectivity; however, experimental exclusion of GPR92 is necessary to confirm these results.
Many other TRPV3 agonists are known to have extensive nonspecific activity. For example, TRPV3 and TRPA1 share many agonists, including cinnamaldehyde, eugenol, and camphor (2, 24, 38). In addition, menthol targets transient receptor potential melastatin 8 (TRPM8) at lower concentrations but at higher concentrations also target TRPV3 (23, 24). We used eugenol, EVA, and FPP to identify TRPV3 activity in native nodose ganglion tissue. Given the high affinity of activation, complete block by RuR, lack of response in TRPV3−/− mice, we concluded that FPP was the most selective TRPV3 agonist for this experimental system. Furthermore, the distribution of rat single cells identified as expressing TRPV3 mRNA is consistent with the percentage of dissociated rat neurons that respond to FPP. In addition, we noticed FPP-sensitive cells occasionally exhibited a sensitizing profile following repeated exposures or with increasing concentrations, characteristic of TRPV3 channel sensitization via gating hysteresis (21).
Possible cellular interactions of TRPV3 and TRPV1 in vagal afferent neurons.
The association between TRPV3 and TRPV1 has been well documented in the literature. Both TRP channels are structurally similar, stimulated by heat (TRPV3 > ∼33°C and TRPV1 >∼42°C), and the genes are located on the same chromosome (28, 33, 39). There is also evidence of functional coexpression of TRPV3 and TRPV1 in dorsal root and trigeminal sensory neurons in primates and functional formation of TRPV3 and TRPV1 heterotetramers, which have distinct properties from TRPV1 or TRPV3 homomers in terms of heat sensitivity, activation threshold, and heat induced sensitization (7, 8, 33). In this system, this phenomenon seems to be present to some extent but was inconsistent across experiments. FPP responses were predictive of larger CAP responses in vagal afferent neurons from rats, but not mice. This species-specific difference may reflect: 1) the difference in pharmacological activation of TRPV1 by CAP between rat and mouse (19); 2) that FPP is less specific in the rat and activates additional targets, including other TRP channels; or 3) there exists a difference in coupling between rat and mouse TRPV3 and TRPV1. In rat vagal afferent neurons these putative interactions seemed to extend to other RuR-sensitive channels considering FPP was also predictive of larger EVA and eugenol intracellular calcium responses in rats. As such, TRPV3 may influence TRP channels stimulated by EVA and eugenol, which certainly includes TRPV1, but may also represent TRPA1 (10, 38). However, eugenol and EVA concentration responses curves were not altered between wild type and TRPV3−/− mice, consistent with a disconnect in FPP signaling between species. We predict that discrepancies between results from these native neuronal systems will be resolved with more precise pharmacological tools.
Perspectives and Significance
We conclude that TRPV3 is functionally expressed in a small subgroup of vagal afferent neurons and may interact with other TRP channels, such as TRPV1. From this, we speculate that even given the relatively low abundance, TRPV3 may be able to leverage its functional impact on vagal afferent signaling via suspected interactions with other higher-abundance TRP channels. Future work should focus on refining the pharmacological tools necessary to investigate TRPV3 signaling, identify and characterize the small subpopulation of vagal afferent neurons that contain TRPV3, and directly determine its impact on autonomic neurophysiology in vivo.
GRANTS
This work was supported by the National Institutes of Health with Grant DK-092651 (to J. H. Peters).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.W. and J.H.P. conception and design of research; S.W., J.E.M.L., and J.H.P. performed experiments; S.W., J.E.M.L., and J.H.P. analyzed data; S.W. and J.H.P. interpreted results of experiments; S.W. and J.H.P. prepared figures; S.W. and J.H.P. drafted manuscript; S.W., J.E.M.L., and J.H.P. edited and revised manuscript; S.W., J.E.M.L., and J.H.P. approved final version of manuscript.
REFERENCES
- 1.Andresen MC, Hofmann ME, Fawley JA. The unsilent majority-TRPV1 drives “spontaneous” transmission of unmyelinated primary afferents within cardiorespiratory NTS. Am J Physiol Regul Integr Comp Physiol 303: R1207–R1216, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J Biol Chem 285: 19,362–19,371, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang S, Yoo S, Yang TJ, Cho H, Hwang SW. Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain 152: 1156–1164, 2011. [DOI] [PubMed] [Google Scholar]
- 5.Berthoud HR. The vagus nerve, food intake and obesity. Regul Pept 149: 15–25, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Cheng W, Yang F, Liu S, Colton CK, Wang C, Cui Y, Cao X, Zhu MX, Sun C, Wang K, Zheng J. Heteromeric heat-sensitive transient receptor potential channels exhibit distinct temperature and chemical response. J Biol Chem 287: 7279–7288, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng W, Yang F, Takanishi CL, Zheng J. Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J Gen Physiol 129: 191–207, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng X, Jin J, Hu L, Shen D, Dong XP, Samie MA, Knoff J, Eisinger B, Liu ML, Huang SM, Caterina MJ, Dempsey P, Michael LE, Dlugosz AA, Andrews NC, Clapham DE, Xu H. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141: 331–343, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung G, Im ST, Kim YH, Jung SJ, Rhyu MR, Oh SB. Activation of transient receptor potential ankyrin 1 by eugenol. Neuroscience 261: 153–160, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Doyle MW, Bailey TW, Jin YH, Andresen MC. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci 22: 8222–8229, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenwick AJ, Wu SW, Peters JH. Isolation of TRPV1 independent mechanisms of spontaneous and asynchronous glutamate release at primary afferent to NTS synapses. Front Neurosci 8: 6, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glazebrook PA, Schilling WP, Kunze DL. TRPC channels as signal transducers. Pflügers Arch 451: 125–130, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Helmchen F. Calibration of fluorescent calcium indicators. Cold Spring Harbor Protoc 2011: 923–930, 2011. [DOI] [PubMed] [Google Scholar]
- 16.Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain 7: 37, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato A, Reisert J, Ihara S, Yoshikawa K, Touhara K. Evaluation of the role of g protein-coupled receptor kinase 3 in desensitization of mouse odorant receptors in a Mammalian cell line and in olfactory sensory neurons. Chem Senses 39: 771–780, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kentish SJ, Frisby CL, Kritas S, Li H, Hatzinikolas G, O'Donnell TA, Wittert GA, Page AJ. TRPV1 channels and gastric vagal afferent signalling in lean and high-fat diet-induced obese mice. PLoS One 10: e0135892, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinch DC, Peters JH, Simasko SM. Comparative pharmacology of cholecystokinin induced activation of cultured vagal afferent neurons from rats and mice. PLoS One 7: e34755, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SH, Raboune S, Walker JM, Bradshaw HB. Distribution of endogenous farnesyl pyrophosphate and four species of lysophosphatidic acid in rodent brain. Int J Mol Sci 11: 3965–3976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu B, Yao J, Zhu MX, Qin F. Hysteresis of gating underlines sensitization of TRPV3 channels. J Gen Physiol 138: 509–520, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu BY, Lin YJ, Lee HF, Ho CY, Ruan T, Kou YR. Menthol suppresses laryngeal C-fiber hypersensitivity to cigarette smoke in a rat model of gastroesophageal reflux disease: the role of TRPM8. J Appl Physiol (1985) 118: 635–645, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Sherkheli MA, Gisselmann G, Vogt-Eisele AK, Doerner JF, Hatt H. Menthol derivative WS-12 selectively activates transient receptor potential melastatin-8 (TRPM8) ion channels. Pak J Pharm Sci 21: 370–378, 2008. [PubMed] [Google Scholar]
- 24.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci 32: 335–343, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, Patapoutian A, Fukumi-Tominaga T, Mizumura K, Tominaga M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflügers Arch 458: 1093–1102, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 307: 1468–1472, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Oh DY, Yoon JM, Moon MJ, Hwang JI, Choe H, Lee JY, Kim JI, Kim S, Rhim H, O'Dell DK, Walker JM, Na HS, Lee MG, Kwon HB, Kim K, Seong JY. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J Biol Chem 283: 21,054–21,064, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peier AM, Reeve AJ, Andersson DA, Moqrich A, Earley TJ, Hergarden AC, Story GM, Colley S, Hogenesch JB, McIntyre P, Bevan S, Patapoutian A. A heat-sensitive TRP channel expressed in keratinocytes. Science 296: 2046–2049, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Peters JH, McDougall SJ, Fawley JA, Andresen MC. TRPV1 marks synaptic segregation of multiple convergent afferents at the rat medial solitary tract nucleus. PLoS One 6: e25015, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron 65: 657–669, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci 25: 433–469, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Shoudai K, Peters JH, McDougall SJ, Fawley JA, Andresen MC. Thermally active TRPV1 tonically drives central spontaneous glutamate release. J Neurosci 30: 14,470–14,475, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418: 186–190, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Smith SM, Chen W, Vyleta NP, Williams C, Lee CH, Phillips C, Andresen MC. Calcium regulation of spontaneous and asynchronous neurotransmitter release. Cell Calcium 52: 226–233, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veldhuis NA, Poole DP, Grace M, McIntyre P, Bunnett NW. The G protein-coupled receptor-transient receptor potential channel axis: molecular insights for targeting disorders of sensation and inflammation. Pharmacol Rev 67: 36–73, 2015. [DOI] [PubMed] [Google Scholar]
- 36.Winter J. Brain derived neurotrophic factor, but not nerve growth factor, regulates capsaicin sensitivity of rat vagal ganglion neurones. Neurosci Lett 241: 21–24, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Wu SW, Fenwick AJ, Peters JH. Channeling satiation: a primer on the role of TRP channels in the control of glutamate release from vagal afferent neurons. Physiol Behav 136: 179–184, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 9: 628–635, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, Ge P, Lilly J, Silos-Santiago I, Xie Y, DiStefano PS, Curtis R, Clapham DE. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418: 181–186, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Yang BH, Piao ZG, Kim YB, Lee CH, Lee JK, Park K, Kim JS, Oh SB. Activation of vanilloid receptor 1 (VR1) by eugenol. J Dent Res 82: 781–785, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Jones S, Brody K, Costa M, Brookes SJ. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol 286: G983–G991, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Zhao H, Simasko SM. Role of transient receptor potential channels in cholecystokinin-induced activation of cultured vagal afferent neurons. Endocrinology 151: 5237–5246, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol 298: G212–G221, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]