Abstract
Peptide YY (PYY) is a 36-amino-acid peptide released from enteroendocrine cells upon food intake. The NH2 terminally truncated metabolite, PYY3–36, exerts anorexic effects and has received considerable attention as a possible antiobesity drug target. The kinetics and degradation products of PYY metabolism are not well described. A related peptide, neuropeptide Y, may be degraded from the COOH terminus, and in vivo studies in pigs revealed significant COOH-terminal degradation of PYY. We therefore investigated PYY metabolism in vitro after incubation in human blood and plasma and in vivo after infusion of PYY1–36 and PYY3–36 in eight young, healthy men. A metabolite, corresponding to PYY3–34, was formed after incubation in plasma and blood and during the infusion of PYY. PYY3–34 exhibited no agonistic or antagonistic effects on the Y2 receptor. PYY1–36 infused with and without coadministration of sitagliptin was eliminated with half-lives of 10.1 ± 0.5 and 9.4 ± 0.8 min (means ± SE) and metabolic clearance rates of 15.7 ± 1.5 and 14.1 ± 1.1 ml·kg−1·min−1 after infusion, whereas PYY3–36 was eliminated with a significantly longer half-life of 14.9 ± 1.3 min and a metabolic clearance rate of 9.4 ± 0.6 ml·kg−1·min−1. We conclude that, upon intravenous infusion in healthy men, PYY is inactivated by cleavage of the two COOH-terminal amino acids. In healthy men, PYY3–36 has a longer half-life than PYY1–36.
Keywords: peptide YY, kinetics, degradation
peptide yy (PYY) is a gastrointestinal peptide hormone, released into the circulation in response to food intake (3, 4, 8, 18), belonging to the pancreatic polypeptide family together with neuropeptide Y (NPY) and pancreatic polypeptide. These are all 36-amino-acid peptides characterized by a high content of tyrosine, proline, and arginine and a hairpin structure, the so-called “PP-fold” (17, 21).
PYY is synthesized and released from endocrine l-cells in the intestinal mucosa, often together with proglugacon-derived peptides, namely glucagon-like peptide-1 (GLP-1), GLP-2, oxyntomodulin, and glicentin. PYY immunoreactive cells are found primarily in ileum and colon with increasing density distally (4).
PYY is present in plasma in two major molecular forms, the 36-amino-acid form, PYY1–36, and the 34-amino-acid NH2-terminally truncated form, PYY3–36, which is formed by cleavage of the two NH2-terminal amino acids by the enzyme dipeptidyl peptidase-4 (DPP-4) (28). DPP-4 is present in both a soluble form and as a transmembrane protein in endothelial, epithelial, and lymphoid tissue. DPP-4 cleaves and inactivates, e.g., glucose-dependent insulinotropic polypeptide and GLP-1 with short plasma half-lives of 5 and 1–2 min, respectively (15, 23, 36).
Anorexigenic effects of PYY3–36 have been demonstrated in several studies with intravenous administration of PYY3–36 (6, 16, 32, 34), whereas infusions of PYY1–36 have no effect on food intake (34), suggesting that the cleavage of PYY1–36 to PYY3–36 is not as complete and rapid as for GLP-1 (34). The half-life of PYY1–36 has been determined as 9.1 min (5). Studies of PYY degradation in vitro have identified various enzymes involved in PYY metabolism besides the NH2-terminal truncation by DPP-4. PYY is also NH2 terminally degraded by aminopeptidase P, which cleaves off the NH2-terminal tyrosine residue, by the metalloendopeptidase meprin β, and neutral endopeptidase 24-11 (NEP 24.11) with major cleavage sites between residues 10 and 11 and 29–30, respectively (2, 28). PYY and NPY are COOH terminally amidated and therefore considered relatively protected from COOH-terminal degradation. However, COOH-terminal degradation of NPY, rendering the peptide inactive on the Y1- and Y2-receptors, has been demonstrated in vitro (1, 9, 22, 24). As the sequences of NPY and PYY are 70% homologous, we speculated that PYY might also be prone to COOH-terminal degradation. PYY3–36 infusions in pigs yielding near physiological concentrations showed PYY to be degraded COOH terminally to PYY3–34, which was not active on the Y2 receptor, and the peptide was degraded with a half-life (t½) of 3.6 min (35). Studies of NPY-Y1/Y2 receptor interactions showed that an intact COOH-terminal pentapeptide is crucial for the binding and activation of the receptors (27). The COOH-terminal pentapeptides are identical in PYY and NPY, indicating that COOH-terminal degradation of PYY will attenuate or ameliorate the binding to and activation of the Y2 receptor.
In this study, we investigated whether COOH-terminal degradation of PYY with a formation of PYY3–34 also occurs in humans and determined the elimination rates of PYY1–36 and PYY3–36 following intravenous administration of PYY1–36 and PYY3–36. Sitagliptin, a competitive DPP-4 inhibitor, was administered orally before one of the infusion days to inhibit NH2-terminal degradation of the peptide.
METHODS
Participants.
Eight normal-weight [BMI: 22.1 ± 0.4 kg/m2 (mean ± SE); age: 23.1 ± 1.2 yr (mean ± SE)], healthy, weight-stable, Caucasian men with no evidence of gastrointestinal or renal disease participated in a randomized, single-blinded, cross-over study with intravenous PYY infusions for 2 h on four separate days of either 1) 1.6 pmol·kg−1·min−1 PYY1–36, 2) 1.6 pmol·kg−1·min−1 PYY1–36 after DPP-4 inhibition, 3) 0.8 pmol·kg−1·min−1 PYY3–36, or 4) an equivalent volume of saline using a precision infusion pump (P2000; IVAC Medical Systems, Hampshire, United Kingdom). The doses were chosen to mimic postprandial PYY levels after gastric bypass and were similar to those used in previous human studies with PYY infusions (6, 7, 16, 26a, 32, 34, 37). The minimum washout period between study days was 1 wk. Written and oral informed consent was obtained from all participants, and, following approval by the Committees on Health Research Ethics for the Capital Region of Denmark and by the Danish Data Protection Agency, the study was conducted in accordance with the Declaration of Helsinki II. The trial was registered at clinicaltrials.gov as NCT 02493959.
Protocol.
Participants were instructed to fast from 22:00 the night before each test day but were allowed to drink water. Before one of the two PYY1–36 test days, participants were instructed to take 100 mg Januvia (sitagliptin) 10 h and 1 h before start of the infusion, respectively. On each test day, participants were weighed and placed in a reclined position in a hospital bed; two catheters were inserted into antecubital veins, one for infusion and one for blood sampling. After collection of two basal blood samples, the infusion was started and continued for 2 h. Blood was sampled at t = −15, 0, 60, 90, and 120 min relative to start of the infusion as well as frequently after the infusion was discontinued at t = 122, 125, 130, 135, 140, 150, 165, 195, and 240 min. At t = 0, 60, 90, 120, 135, 165, and 240 min 100-mm visual analog scales (VAS) were employed for rating of appetite, nausea, and abdominal pain, and blood pressure and heart rate were measured.
Infusions.
PYY peptides (>97% pure, structure and purity verified by mass spectrometry, sequence, and high-performance liquid chromatography analysis) were purchased from Bachem (Bubendorf, Switzerland), dissolved in sterilized water containing 2% human serum albumin (human albumin 20%; CSL Behring, Marburg, Germany), subjected to sterile filtration, and dispensed into vials under aseptic conditions. Vial content was tested for sterility and bacterial endotoxins (Central Pharmacy, Herlev Hospital, Denmark). Vials were kept at −20°C until the experimental days.
Blood samples and analysis.
Blood was collected 1) into chilled EDTA tubes containing a DPP-4 inhibitor (0.01 mmol/l valine-pyrrolidide, final concentration) and 500 KIE aprotinin (Trasylol, final concentration); they were immediately centrifuged and plasma stored at −20°C until analysis; and 2) into Eppendorf tubes containing EDTA, which were immediately centrifuged and the contents used for analysis of plasma glucose using YSI model 2300 STAT plus (YSI, Yellow Springs, OH).
Degradation of PYY1–36 in vitro.
Blood was collected from five healthy participants into chilled heparinized tubes. Synthetic human PYY1–36 (0.6 pmol/ml, final concentration, Bachem) was incubated in blood and plasma at 37°C. Samples were taken regularly (t = 0, 0.5, 1, 2, 3, 4, 6, 8, 21, and 24 h), mixed with EDTA, aprotinin (500 KIE/ml), and the DPP-4 inhibitor valine pyrrolidide (0.01 mmol/l, final concentration), and frozen instantly for the blood samples immediately after centrifugation and separation of plasma.
Peptide measurements.
Peptide levels in plasma samples from the infusion study were determined using three different in-house PYY radioimmunoassays (RIA). RIA of total PYY in plasma was performed using a monoclonal antibody MAB8500 (clone RPY-B12; Abnova, Taipei, Taiwan), which reacts equally well with PYY1–36 and PYY3–36. Synthetic human PYY3–36 (Bachem) was used as standard and 125I-labeled PYY1–36 (NEX341; Perkin Elmer, Waltham, MA) as tracer. Assay buffer was 80 mmol/l sodium phosphate buffer, pH 7.5, containing, in addition, 0.1% (wt/vol) human serum albumin (Calbiochem, San Diego, CA), 10 mmol/l EDTA, and 0.6 mmol/l thiomersal (Sigma, St. Louis, MO). PYY3–34 was measured with a COOH-terminal-specific antiserum (code no. 2940) raised in white rabbits against residues PYY23–34 coupled to keyhole limpet hemocyanin. Synthetic porcine PYY3–34 (Genscript, Piscataway, NJ) was used as standard, and the tracer was synthetic human PYY3–34, 125I labeled using stoichiometric chloramine-T method (20). The assay detects both PYY1–34 and PYY3–34 and shows negligible cross reaction (<1%) with p-PYY1/3–36. Assay buffer was 0.1 M Tris, pH 8.5, containing 0.2% (wt/vol) human serum albumin, 20 mmol/l EDTA, and 0.6 mmol/l thiomersal. PYY3–36 was measured using an NH2-terminal specific antibody (1067-HK; Millipore, Billerica, MA), 125I-labeled PYY3–36 (T-059-02; Phoenix Peptide, Belmont, CA) as tracer, and synthetic PYY3–36 (H-8585, Bachem) as standard. The assay buffer was 0.1 M Tris.
Free and bound moieties were separated with plasma-coated charcoal (E. Merck, Darmstadt, Germany). All plasma samples were extracted with 70% ethanol (final concentration) to remove unspecific, cross-reacting substances. The recovery of synthetic PYY3–36 and PYY3–34 added to plasma before extraction and assay was 71.5 ± 2.6% (mean ± SE) in the total PYY assay, 61.5 ± 5.5% in the NH2-terminal specific PYY3–36 assay, and 68.0 ± 3.1% in the COOH-terminal specific PYY3–34 assay. For all assays, the experimental detection limits were <5 pmol/l, and the intra-assay coefficient of variation was below 6% when evaluated at a concentration of 40 pmol/l.
Total PYY and PYY3–36 concentrations in samples from in vitro incubations were measured using two commercially available RIA kits (cat no. PYY-67HK and PYY-66HK, Millipore).
Transfections and phosphatidylinositol assay.
COS-7 cells were grown at 10% CO2 and 37°C in Dulbecco's modified Eagle's medium with GlutaMAX (Invitrogen, Taastrup, Denmark) to which was added 10% fetal bovine serum, 180 U/ml penicillin, and 45 μg/ml streptomycin (PenStrep). Cotransfection of the hNPY receptor and promiscuous chimeric G protein Gqi4myr (26) was performed using the calcium phosphate precipitation method, as previously described (25). The use of cotransfection with the Y2 receptor and the chimeric G protein Gqi4myr shuffles the Gai signal to a Gq signal and thereby enables measurement of activation (formation of IP3) rather than inhibition of cAMP. The cotransfected COS-7 cells were incubated for 24 h with 5 μCi/ml [myo3-H]inositol in growth medium, and the assay was carried out as described previously (29). Three independent experiments with duplicate measurements were performed. PYY3–36 was used as a positive control, and both agonistic as well as antagonistic effects of PYY1–34 and PYY3–34 were tested.
Calculations and statistics.
Descriptive data are stated as means ± SE, and P values of <0.05 are considered significant. All data analysis was done with Prism for Windows (version 6.05; GraphPad Software, San Diego, CA).
During PYY infusions, basal concentration was calculated as the mean of PYY determinations at t = −15 and 0 min, steady-state concentrations as mean of concentrations at t = 60, 90, and 120 min. PYY1–36 concentrations corrected for NH2-terminal degradation were estimated as total PYY immunoreactivity (IR) − PYY3–36 IR. On the PYY3–36 infusion day, intact PYY3–36 levels were estimated as total PYY IR − PYY3–34.
The t½ of PYY was calculated using the One-Phase Decay model of GraphPad Prism 6. Metabolic clearance rate (MCR) was calculated as the infused rate of PYY divided by incremental steady-state concentrations. The apparent distribution volume was calculated as Vdapp = MCR/k, where k was derived from k = ln2/t½. Differences between t½, MCR, and Vdapp values were evaluated using repeated-measures one-way ANOVA followed by Bonferroni correction for multiple comparisons.
The total area under the curve (AUC) during infusion for VAS ratings was calculated using the trapezoidal rule and analyzed using one-way ANOVA. AUCs for PYY infusions were compared with AUC for saline infusion using one-way ANOVA and Bonferroni correction for multiple comparisons.
Plasma glucose, heart rate, and blood pressure were analyzed using a repeated-measures two-way ANOVA. When treatment or treatment × time was found to be significant, PYY infusions were compared with placebo at each time point using post hoc testing with Bonferroni correction.
Half-lives of PYY in blood and plasma during in vitro incubation were determined using the One-Phase Decay model of GraphPad Prism 6. The rate of formation of PYY3–34 in plasma was fitted with linear regression and the slope tested for significance vs. zero. PYY3–36 formation was fitted with the One-Phase Association model using Prism 6.
Receptor assay.
Sigmoidal dose-response curves were fitted using GraphPad Prism 6.
RESULTS
Degradation of PYY in vivo.
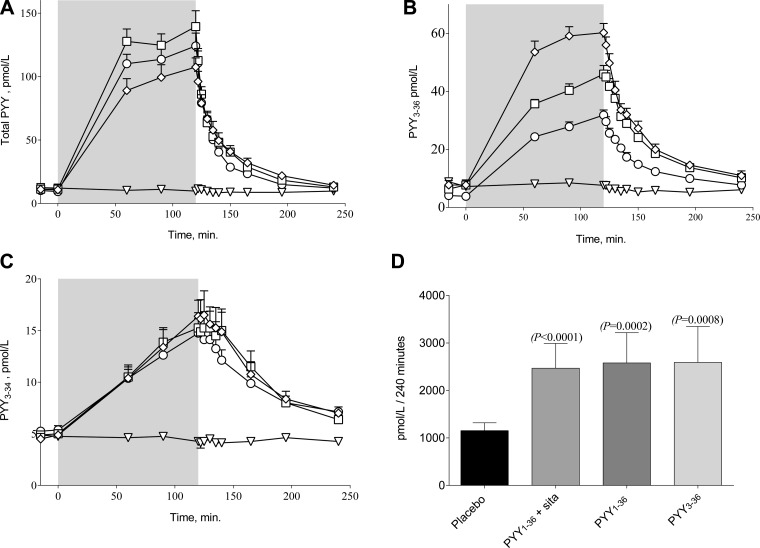
Infusion of PYY1–36 increased total PYY IR in plasma from 11.3 ± 1.2 to 130.6 ± 10.1 pmol/l, infusion of PYY1–36 + 2 × 100 mg sitagliptin from 9.8 ± 1.1 pmol/l to 116.0 ± 8.5 pmol/l, and PYY3–36 infusion from 10.9 ± 2.9 to 98.6 ± 8.3 pmol/l (steady-state concentrations, Fig. 1A). Measured with the NH2-terminal-specific PYY3–36 assay, the steady-state plasma concentration during PYY3–36 infusion was 57.7 ± 3.0 pmol/l. Infusion of PYY1–36 increased the COOH-terminal PYY3–34 concentrations from 5.3 ± 0.4 pmol/l to 14.8 ± 1.4 pmol/l with sitagliptin and from 4.9 ± 0.4 pmol/l to 15.3 ± 1.5 pmol/l without. At the PYY3–36 infusion day, COOH-terminal-specific PYY3–34 levels increased from 4.7 ± 0.5 pmol/l to 16.4 ± 1.6 pmol/l. AUCs for PYY3–34 were significantly different from placebo on all PYY infusion days (Fig. 1D).
Fig. 1.
Total peptide YY (PYY) (A), NH2-terminal-specific PYY3–36 (B), and COOH-terminal-specific PYY3–34 (C) are shown. Placebo (▽), PYY1–36 + sitagliptin (○), PYY1–36 (□), and PYY3–36 (◇) are shown. Total area under the curves (AUCs) of PYY3–34 concentrations during PYY1–36, PYY3–36, or saline infusions from t = 0 to t = 120 min are shown (D). Differences to placebo were evaluated using repeated-measures 1-way ANOVA followed by Bonferroni correction for multiple comparisons. Data are means ± SE.
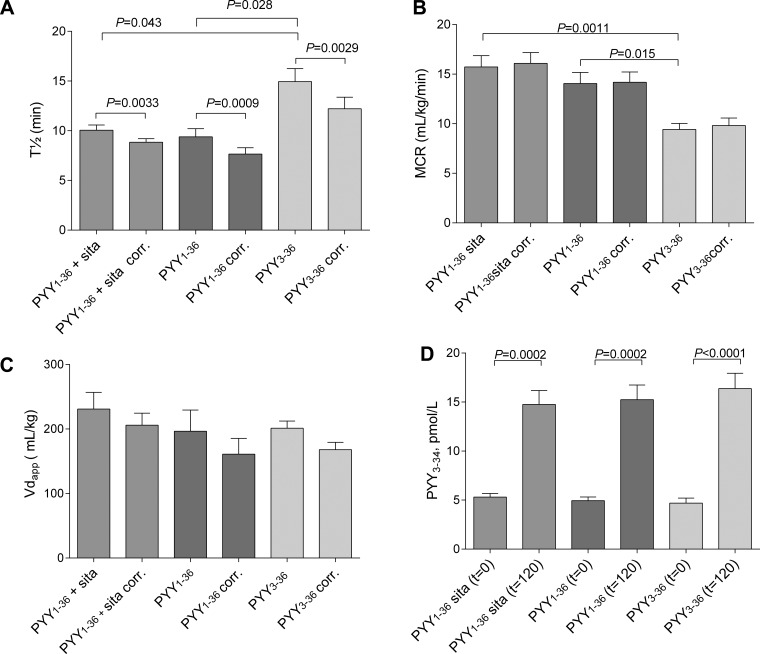
The elimination half-lives were similar after infusion of PYY1–36 with and without administration of 2 × 100 mg sitagliptin (10.1 ± 0.5 min and 9.4 ± 0.8 min, respectively). The half-life of PYY3–36 was significantly longer, 14.9 ± 1.3 min. The half-life of PYY3–36 determined with the NH2-terminal-specific PYY3–36 assay was 15.5 ± 0.8 min and not different from that determined with the total PYY assay.
When correcting for COOH-terminal degradation (by subtracting PYY3–34 levels from total PYY levels), the elimination half-lives of PYY1–36 were significantly shorter both with and without sitagliptin (8.8 ± 0.4 min and 7.7 ± 0.6 min, respectively). The half-life of PYY3–36 corrected for COOH-terminal degradation was 12.2 ± 1.2 min (Fig. 2A). The half-lives of PYY1–36 corrected for NH2-terminal degradation (calculated as PYY3–36 determination subtracted from total PYY) were 8.2 ± 0.5 when sitagliptin was coadministered and significantly lower (5.0 ± 0.3 min) without sitagliptin (P < 0.0001).
Fig. 2.
Half-lives (A), metabolic clearance rates (B), apparent volume of distribution (C), and PYY3–34 concentrations at t = 0 and t = 120 min (D) are shown. PYY corr (determined as PYY3–34 subtracted from total PYY) is shown. Data are means ± SE. Differences between groups evaluated using repeated-measures 1-way ANOVA followed by Bonferroni correction for multiple comparisons.
MCRs (using total PYY − PYY3–34 estimates of PYY concentration) were 16.1 ± 1.1 ml·kg−1·min−1 and 14.2 ± 1.0 ml·kg−1·min−1, respectively, when infusing PYY1–36 with and without administration of sitagliptin and significantly lower during PYY3–36 infusion (9.8 ± 0.7 ml·kg−1·min−1) (Fig. 2B). Vdapp was similar for the three different infusions (PYY1–36: 206.0 ± 18.6 ml/kg; PYY1–36 + sitagliptin: 160.9 ± 24.4 ml/kg; PYY3–36: 168.0 ± 11.5 ml/kg) (Fig. 2C).
VAS.
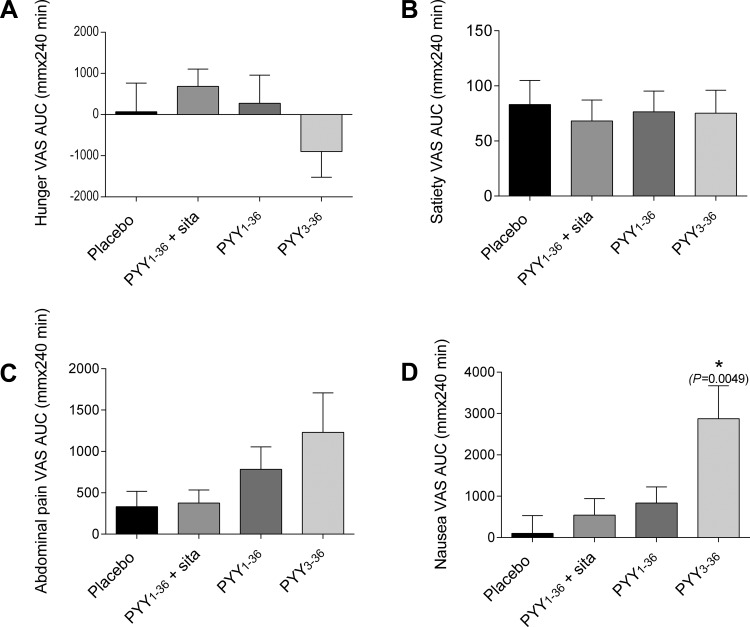
Infusions of PYY1–36 and PYY3–36 did not significantly influence VAS rating AUCs for hunger, satiety, or abdominal pain. On the PYY3–36 infusion day, participants felt nauseous, and 50% vomited. Consistent with this observation, the AUC of nausea VAS ratings for the PYY3–36 infusion was significantly greater than the corresponding AUC on the placebo day (P = 0.0049) (Figs. 3D and 4).
Fig. 3.
Visual analogue scale (VAS) ratings: AUC during infusion. Hunger (A), satiety (B), abdominal pain (C), and nausea (D) are shown. Data are means ± SE. AUC for PYY infusions were compared with AUC for saline infusion using 1-way ANOVA followed by post hoc tests with Bonferroni correction for multiple comparisons. *Different from placebo.
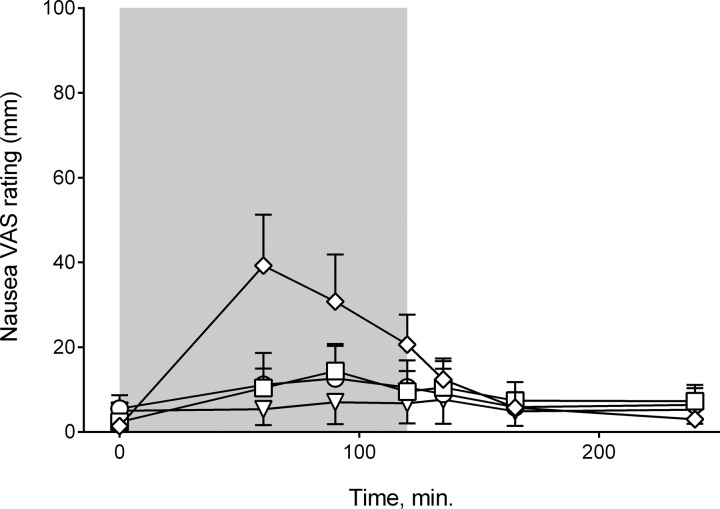
Fig. 4.
Nausea VAS ratings. Placebo (▽), PYY1–36 + sitagliptin (○), PYY1–36 (□), and PYY3–36 (◇) are shown. Data are means ± SE.
Glucose/pulse/blood pressure.
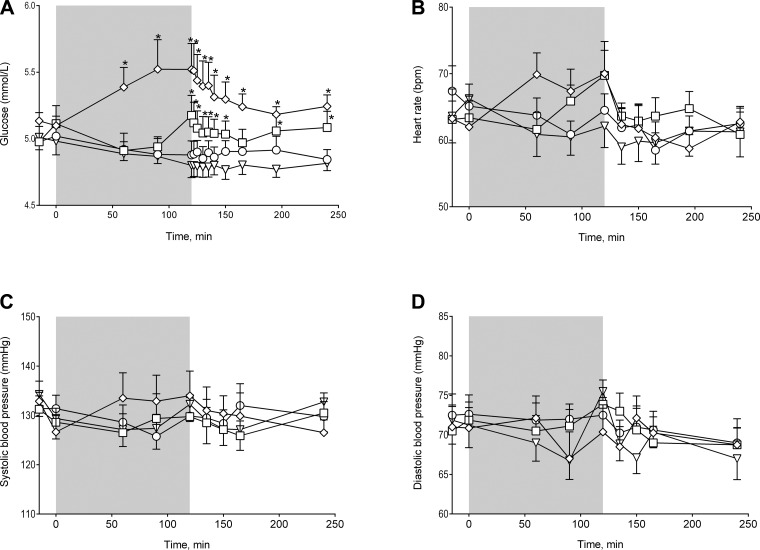
Plasma glucose concentrations were significantly higher during the infusion of PYY3–36 compared with placebo and also toward the end of the PYY1–36 infusion (Fig. 5A). Heart rate was not significantly different during any of the infusions (Fig. 5B), but there was a tendency for an increased heart rate during PYY3–36 infusion and toward the end of the PYY1–36 infusion (P = 0.091). Infusion of PYY did not alter systolic and diastolic blood pressure (Fig. 5, C and D).
Fig. 5.
A: plasma glucose time course (treatment × time effect, *P < 0.0001). B: heart rate (no treatment × time effect, P = 0.091). C and D: systolic and diastolic blood pressure (no treatment × time effect, P = 0.57 and P = 0.47, respectively). Placebo (▽), PYY1–36 + sitagliptin (○), PYY1–36 (□), and PYY3–36 (◇) are shown. Data are means ± SE. Comparisons were made using a 2-way repeated-measures ANOVA. If significant, PYY infusions were compared with placebo by post hoc testing with Bonferroni corrections for multiple comparisons.
Degradation of PYY in vitro.
In blood in vitro at 37°C, PYY, measured as total PYY, was degraded with t½ of 22.8 ± 8.0 h − 3.6 ± 0.6 h when correcting for NH2-terminal degradation. The concentrations of PYY3–36 and PYY3–34 peaked after 4 and 6 h, respectively, and hereafter the levels of the two PYY moieties declined.
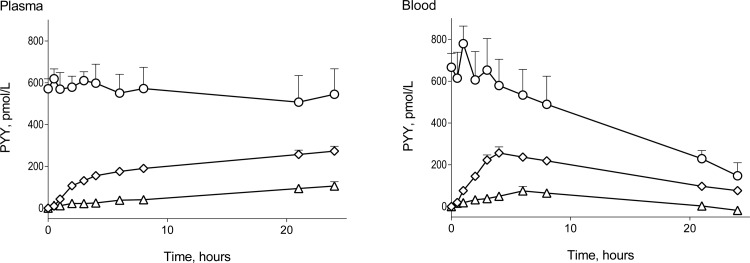
In plasma, no degradation of total PYY could be detected, but, when we corrected for NH2-terminal degradation, intact PYY1–36 had a t½ of 8.6 ± 3.6 h (Fig. 6). PYY3–34 formation followed a linear model with a slope of 4.08 ± 0.81 pmol·l−1·h−1 (significantly different from zero, P < 0.0001). PYY3–36 followed pseudo-first-order association kinetics with a rate constant of 0.22 ± 0.04 per h and a plateau of 268 ± 23.4 pmol/l.
Fig. 6.
In vitro incubation of PYY1–36 in plasma, n = 5 (left), and blood, n = 4 (right) is shown. Total PYY (○), PYY3–36 (◇), and PYY3–34 (△) are shown. Data are shown as means ± SE.
PYY signaling in COS-7 cells.
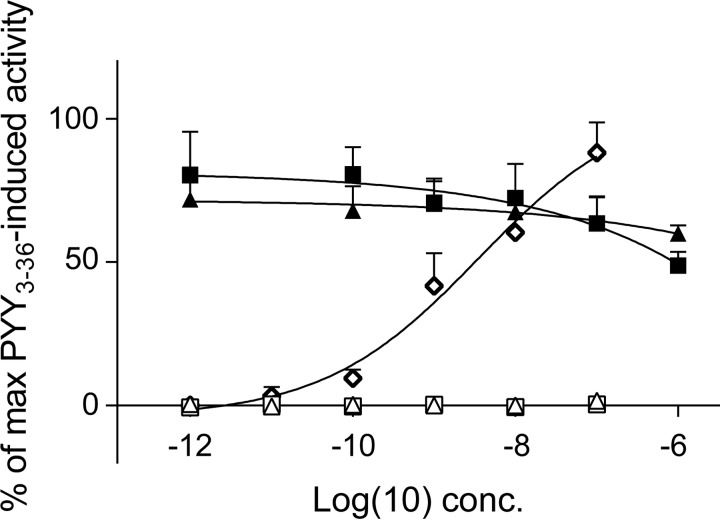
The biological activities of hPYY3–36, hPYY1–34, and hPYY3–34 were tested in COS-7 cells transiently transfected with the human Y2 receptor and the chimeric G protein Gqi4myr, using IP turnover measurements. The human Y2 receptor was activated by hPYY3–36 with an EC50 value of 3.5 nmol/l, but neither PYY1–34 nor PYY3–34 had agonistic or antagonistic activity on the receptor (Fig. 7).
Fig. 7.
Dose-response curves for IP3 generation in COS-7 cells cotransfected with the Y2 receptor and the chimeric G protein Gqi4myr. The curves are normalized to the maximal activation of the Y2 receptor (upon stimulation with PYY3–36). Stimulation with PYY3–36 (◇), PYY3–34 (△), and PYY1–34 (□) is shown. Antagonistic effects of PYY3–34 (▲) and PYY1–34 (■) are shown. Three individual experiments were carried out with duplicate detection. Data shown are means ± SE.
DISCUSSION
This study examined the pharmacokinetics and degradation of intravenously administered PYY1–36 and PYY3–36 in healthy normal-weight men. The results reveal that 1) both PYY moieties are degraded from the COOH terminus to form PYY1/3–34; 2) human PYY3–34 has no agonistic or antagonistic effects on the human Y2 receptor; and 3) PYY3–36 has a longer half-life than PYY1–36.
Previous studies have shown that in vitro incubations of NPY in human plasma resulted in cleavage of the COOH-terminal amino acid and dipeptide (1, 22). We have demonstrated that PYY3–36 is significantly degraded to PYY3–34 in the pig and also shown that the liver is involved in the COOH-terminal truncation (35). Here we extend these findings to humans and demonstrate formation of PYY1/3–34 after infusion of both PYY1–36 and PYY3–36, although to a somewhat lesser extent than in anesthetized pigs. At the PYY3–36 infusion day, COOH-terminal-specific PYY3–34 levels amounted to about 28% of NH2-terminal specific PYY3–36 levels during steady state. As we have not measured the intact COOH terminus of 1/3–36, we cannot exclude that the COOH terminus is degraded by other enzymes yielding different PYY moieties. Furthermore, as we cannot inhibit the COOH-terminal degradation, we cannot determine the role that COOH-terminal degradation plays in the overall inactivation of PYY. A method for exact measurements of active PYY3–36 levels (e.g., sandwich ELISA or LC-MS) would therefore be very valuable in the interpretation of the physiological effects of PYY. In vitro degradation of NPY in human plasma has indicated that NPY is COOH terminally cleaved to primarily NPY3–35, and only trace amounts of NPY3–34 have been identified (1). As in pig blood and plasma, PYY was COOH terminally degraded in human blood and plasma in vitro. As currently used assays are unlikely to discriminate between COOH-terminally degraded and intact PYY, PYY levels are probably generally overestimated. The only way to overcome this problem is to use a sandwich ELISA with COOH-terminal and NH2-terminal specific antibodies, but, to our best knowledge, such assays have not been developed so far.
PYY1–36 was infused at a double rate compared with PYY3–36, and we therefore expected higher plasma levels of PYY1/3–34 after PYY1–36 infusion. When measuring PYY3–34, it turned out that the plasma levels were comparable after PYY1–36 and PYY3–36 infusion. This discrepancy might be explained by the longer half-life of PYY3–36, or it could be that the NH2-terminal structure influences COOH-terminal degradation, as has been suggested to be the case for in vitro degradation of NPY (22). PYY3–34 was not active on the Y2 receptor, and the COOH-terminal degradation is therefore likely to represent an inactivation and elimination step; however, it cannot at the current stage be excluded that PYY3–34 exerts a physiological effect via a different receptor.
In vitro incubations in plasma and blood showed both COOH-terminal and NH2-terminal degradation of the peptide. The rates of the NH2-terminal dipeptide cleavage with t½ values of 8–9 h in plasma and 3–4 h in blood are similar to the half-lives of GLP-2 (which is also cleaved by DPP-4) in plasma and blood (19) but longer than those reported for GLP-1 (14).
The in vivo half-life of PYY (total PYY IR) was 10.1 ± 0.5 and 9.4 ± 0.8 min with and without sitagliptin, respectively, which is in accordance with an earlier determination of PYY half-lives of 9.1 ± 0.3 min (5). The half-life of PYY3–36 appeared to be significantly longer, 14.9 ± 1.3 min, suggesting that the change in tertiary conformation when the NH2-terminal dipeptide is cleaved off alters the affinity of the peptidases involved in PYY metabolism. In accordance with this finding, it was shown by in vitro incubations that PYY3–36 was not prone to degradation by NEP 24.11, whereas PYY1–36 is cleaved by NEP 24.11 with a major hydrolysis site between residues 29–30 (28).
Vd was determined as ∼180 ml/kg, a size equal to the extracellular volume. Adrian et al. (3) determined Vd to be considerably smaller (92 ml/kg), and also MCR (7.2 ml·kg−1·min−1) was lower than MCR found in this study. This difference is obviously due to differences in plasma peak levels in relation to the infused amount. Reported plasma peak concentrations after 0.8 pmol·kg−1·min−1 infusions of PYY vary between ∼35 pmol/l and 270 pmol/l (7, 16, 26a, 32, 34, 37), and this discrepancy probably reflects differences in the PYY assay performances and/or preparation of the infusion solutions.
The discrepancy between the PYY levels at the PYY3–36 infusion day determined with the total PYY assay and the PYY3–36 assay reveals an inconsistency. In accordance with this, recovery studies with the two assays showed a higher recovery when analyzing spiked plasma samples with the total PYY assay (71.5 ± 2.6%) compared with the PYY3–36 assay (61.5 ± 5.5%). Differences in antibody affinities or interassay variation may explain this discrepancy.
Blood glucose was significantly higher during the PYY3–36 infusion. Heart rate also tended to be elevated (P = 0.091). In vivo studies in mice (10, 12) and investigations in isolated perfused rat pancreas showed that PYY inhibits insulin secretion, and PYY knockout mice (11) and mice with a deletion of the Y1 receptor (13) were hyperinsulinemic. This points to PYY1–36 having a role in glucose homeostasis, but in our study we found no difference between plasma glucose on the saline vs. the PYY1–36 days. Other studies with PYY3–36 infusions in humans reported no effect on glucose levels (7, 27, 28), but Sloth et al. (34) found elevated heart rate during infusion. In our study, nausea VAS ratings were also significantly elevated during PYY3–36 infusion, and we speculate that the elevated blood glucose and heart rate reflect increased sympathoadrenal activity attributable to nausea. On the PYY3–36 infusion day, nausea ratings peaked at t = 60 min with a PYY3–36 concentration of ∼55 pM. Although the PYY3–36 concentration remained at about 55–60 pM during the last hour of infusion, the nausea VAS rating decreased, maybe attributable to tachyphylaxis. As the four subjects that vomited all had their first vomiting episode around t = 60 min, it could also be that emptying of the stomach (although they were fasted) relieved the symptoms.
During PYY1–36 infusion, some subjects experienced mild nausea. The nausea VAS rating peaked at t = 90 min with a PYY3–36 concentration of 40 pM. This could indicate that the threshold concentration for nausea is somewhere between 40 and 55 pM but could also reflect a decreased nausea response attributable to tachyphylaxis, as the PYY3–36 level increased at a slower rate during PYY1–36 infusion.
Nausea upon PYY3–36 infusion has been reported by others (16, 26b, 32, 34), and this is likely a confounder with regards to appetite sensations and previously reported reductions in food intake (26b, 34). However, studies of hypothalamic activity upon PYY3–36 administration in both humans and rodents have shown similar patterns as after food intake, indicating that PYY3–36 exerts anorexic effects by modulation of hypothalamic areas (17). Furthermore, these effects are absent in the Y2 receptor knockout mouse, pointing to PYY3–36 acting via the Y2 receptor (33). Degen et al.(16) demonstrated a dose relationship between PYY3–36 administration and food intake, and, during the lowest dose (0.2 pmol·kg−1·min−1), the subjects reduced their food intake and were free from adverse events, indicating that the therapeutic window for PYY3–36 as an obesity drug might be quite narrow.
In agreement with earlier findings, hunger and satiety ratings did not differ significantly at the PYY1–36 infusion days (34).
As a control, we performed PYY determinations on the infusate solutions (collected from the end of the infusion catheter). Unexpectedly, a degradation of PYY1–36 to PYY3–36 in the infusate was discovered. PYY3–36 amounted to ∼20% of the total PYY content. This finding could explain the rather high levels of PYY3–36 during the PYY1–36 infusion plus sitagliptin study day and impedes the calculation of a PYY1–36 to PYY3–36 conversion rate but does not influence determination of half-lives of total PYY, PYY3–36, and PYY1–36 (intact NH2 terminus). To investigate this unforeseen degradation, we incubated PYY1–36 with the infusate vehicle at room temperature and tested the albumin used for drug formulation for DPP-4 activity. This experiment revealed that PYY1–36 was degraded to PYY3–36 (∼20% of total PYY IR after 4 h at room temperature), and, surprisingly, DPP-4 activity was also detected in the albumin. When one formulates PYY and other DPP-4 substrates for infusion experiments, it is therefore essential to assess the stability of the peptide in the vehicle.
Conclusion.
A significant formation of the COOH-terminally truncated PYY metabolite, PYY3–34 has been described in pigs, and we here report that this also applies to humans. Human PYY3–34 is not active on the human Y2 receptor, but whether it has other effects remains to be investigated. The formation of this COOH-terminally truncated metabolite of PYY significantly shortens the survival time of PYY in the circulation. Therefore PYY levels determined by existing assays do not reflect concentrations of biologically active peptides, and assumptions regarding the physiological functions of PYY at these levels are also inaccurate. Furthermore, in healthy men, PYY3–36 has a longer half-life than PYY1–36.
Perspectives and Significance
The study presented here shows that PYY3–36 has a half-life of ∼15 min and is subjected to COOH-terminal degradation that eliminates activation of the Y2 receptor. The half-life of 15 min precludes a once-daily dosing regime, and modifications to prolong half-life are probably necessary to ensure compliance and effectiveness. Such modification may include protection (without losing the effect) of the COOH terminus because PYY, as shown here, is subjected to significant COOH-terminal degradation despite the COOH-terminal amidation.
GRANTS
The study was supported by a grant to J. J. Holst from the Novo Nordisk Foundation Center for Basic Metabolic Research and by a grant to S. Toräng from Aase and Ejnar Danielsens foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: S.T., K.N.B.-M., and J.J.H. conception and design of research; S.T., K.N.B.-M., and M.S.S. performed experiments; S.T. analyzed data; S.T., B.H., M.M.R., and J.J.H. interpreted results of experiments; S.T. prepared figures; S.T. drafted manuscript; S.T., K.N.B.-M., M.S.S., B.H., M.M.R., S.M., and J.J.H. edited and revised manuscript; S.T., K.N.B.-M., M.S.S., B.H., M.M.R., S.M., and J.J.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the technical assistance of Alis Andersen, Lone Bagger Thielsen, Lene Albæk, Sofie Pilgaard, and Maibritt Sigvardt Baggesen.
REFERENCES
- 1.Abid K, Rochat B, Lassahn PG, Stöcklin R, Michalet S, Brakch N, Aubert JF, Vatansever B, Tella P, Meester ID, Grouzmann E. Kinetic study of neuropeptide Y (NPY) proteolysis in blood and identification of NPY3-35. A new peptide generated by plasma kallikrein. J Biol Chem 284: 24715–24724, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addison ML, Minnion JS, Shillito JC, Suzuki K, Tan TM, Field BCT, Germain-Zito N, Becker-Pauly C, Ghatei MA, Bloom SR, Murphy KG. A role for metalloendopeptidases in the breakdown of the gut hormone, PYY3-36. Endocrinology 152: 4630–4640, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Adrian TE, Bacarese-Hamilton AJ, Smith HA, Chohan P, Manolas KJ, Bloom SR. Distribution and postprandial release of porcine peptide YY. J Endocrinol 113: 11–14, 1987. [DOI] [PubMed] [Google Scholar]
- 4.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89: 1070–1077, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Adrian TE, Sagor GR, Savage AP, Bacarese-Hamilton AJ, Hall GM, Bloom SR. Peptide YY kinetics and effects on blood pressure and circulating pancreatic and gastrointestinal hormones and metabolites in man. J Clin Endocrinol Metab 63: 803–807, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med 349: 941–948, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY[sub3-36] physiologically inhibits food intake. Nature 418: 650, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Batterham RL, Heffron H, Kapoor S, Chivers JE, Chandarana K, Herzog H, Le Roux CW, Thomas EL, Bell JD, Withers DJ. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 4: 223–233, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Beck-Sickinger AG, Weland HA, Wittneben H, Willim KD, Rudolf K, Jung G. Complete L-alanine scan of neuropeptide Y reveals ligands binding to Y1 and Y2 receptors with distinguished conformations. Eur J Biochem 225: 947–958, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Bertrand G, Gross R, Roye M, Ahrkn B, Ribes G. Evidence for a direct inhibitory effect of PYY on insulin secretion in rats. Pancreas 7: 595–600, 1992. [DOI] [PubMed] [Google Scholar]
- 11.Boey D, Lin S, Karl T, Baldock P, Lee N, Enriquez R, Couzens M, Slack K, Dallmann R, Sainsbury A, Herzog H. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia 49: 1360–1370, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Bottcher G, Ahren B, Lundquist I, Sundler F. Peptide YY: Intrapancreatic localization and effects on insulin and glucagon secretion in the mouse. Pancreas 4: 282–288, 1989. [PubMed] [Google Scholar]
- 13.Burcelin R, Brunner HR, Seydoux J, Thorensa B, Pedrazzini T. Increased insulin concentrations and glucose storage in neuropeptide Y Y1 receptor-deficient mice. Peptides 22: 421–427, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 80: 952–957, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Deacon CF, Nauck MA, Meier J, Hücking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab 85: 3575–3581, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, Beglinger C. Effect of peptide YY3-36 on food intake in humans. Gastroenterology 129: 1430–1436, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Glover ID, Barlow DJ, Pitts JE, Wood SP, Tickle IJ, Blundell TL, Tatemoto K, Kimmel JR, Wollmer A, Strassburger W, Zhang YS. Conformational studies on the pancreatic polypeptide hormone family. Eur J Biochem 142: 379–385, 1984. [DOI] [PubMed] [Google Scholar]
- 18.Greeley GH, Hashimoto T, Izukura M, Gomez G, Jeng J, Hill FLC, Lluis F, Thompson JC. A comparison of intraduodenally and intracolonically administered nutrients on the release of peptide-YY in the dog. Endocrinology 125: 1761–1765, 1989. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann B, Harr MB, Jeppesen PB, Wojdemann M, Deacon CF, Mortensen PB, Holst JJ. In vivo and in vitro degradation of glucagon-like peptide-2 in humans. J Clin Endocrinol Metab 85: 2884–2888, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Holst JJ, Bersani M. Assays for peptide products of somatostatin gene expression. In: Methods in Neurosciences, edited by Conn PM. New York, NY: Academic, 2015, p. 3–22. [Google Scholar]
- 21.Keire DA, Kobayashi M, Solomon TE, Reeve JR. Solution structure of monomeric peptide YY supports the functional significance of the PP-fold. Biochemistry 39: 9935–9942, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Khan IU, Reppich R, Beck-Sickinger AG. Identification of neuropeptide Y cleavage products in human blood to improve metabolic stability. Biopolymers 88: 182–189, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136: 3585–3596, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Kirby DA, Boublik JH, Rivier JE. Neuropeptide Y: Y1 and Y2 affinities of the complete series of analogues with single D-residue substitutions. J Med Chem 36: 3802–3808, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Kissow H, Hartmann B, Holst JJ, Viby NE, Hansen LS, Rosenkilde MM, Hare KJ, Poulsen SS. Glucagon-like peptide-1 (GLP-1) receptor agonism or DPP-4 inhibition does not accelerate neoplasia in carcinogen treated mice. Regul Pept 179: 91–100, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Kostenis E. Is Galpha16 the optimal tool for fishing ligands of orphan G-protein-coupled receptors? Trends Pharmacol Sci 22: 560–564, 2001. [DOI] [PubMed] [Google Scholar]
- 26a.le Roux CW, Batterham RL, Aylwin SJB, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147: 3–8, 2006. [DOI] [PubMed] [Google Scholar]
- 26b.le Roux CW, Borg CM, Murphy KG, Vincent RP, Ghatei MA, Bloom SR. Supraphysiological doses of intravenous PYY3-36 cause nausea, but no additional reduction in food intake. Ann Clin Biochem 45: 93–95, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Lindner D, Stichel J, Beck-Sickinger AG. Molecular recognition of the NPY hormone family by their receptors. Nutrition 24: 907–917, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros MD, Turner AJ. Processing and metabolism of peptide-YY: pivotal roles of dipeptidylpeptidase-IV, aminopeptidase-P, and endopeptidase-24.11. Endocrinology 134: 2088–2094, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Rosenkilde MM, Kledal TN, Schwartz TW. High constitutive activity of a virus-encoded seven transmembrane receptor in the absence of the conserved DRY motif (Asp-Arg-Tyr) in transmembrane helix 3. Mol Pharmacol 68: 11–19, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt JB, Gregersen NT, Pedersen SD, Arentoft JL, Ritz C, Schwartz TW, Holst JJ, Astrup A, Sjödin A. Effects of PYY3-36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am J Physiol Endocrinol Metab 306: E1248–E1256, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Shi YC, Lin Z, Lau J, Zhang H, Yagi M, Kanzler I, Sainsbury A, Herzog H, Lin S. PYY3-36 and pancreatic polypeptide reduce food intake in an additive manner via distinct hypothalamic dependent pathways in mice. Obesity (Silver Spring) 21: E669–E678, 2013. [DOI] [PubMed] [Google Scholar]
- 34.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab 292: E1062–E1068, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Toräng S, Veedfald S, Rosenkilde MM, Hartmann B, Holst JJ. The anorexic hormone Peptide YY3–36 is rapidly metabolized to inactive Peptide YY3–34 in vivo. Physiol Rep 3: e12455, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilsbøll T, Agersø H, Krarup T, Holst JJ. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab 88: 220–224, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Witte AB, Grybäck P, Holst JJ, Hilsted L, Hellström PM, Jacobsson H, Schmidt PT. Differential effect of PYY1-36 and PYY3-36 on gastric emptying in man. Regul Pept 158: 57–62, 2009. [DOI] [PubMed] [Google Scholar]