Abstract
Ischemic preconditioning (IP) has been shown to improve exercise performance and to delay fatigue. However, the precise mechanisms through which IP operates remain elusive. It has been hypothesized that IP lowers the sensation of fatigue by reducing the discharge of group III and IV nerve endings, which also regulate hemodynamics during the metaboreflex. We hypothesized that IP reduces the blood pressure response during the metaboreflex. Fourteen healthy males (age between 25 and 48 yr) participated in this study. They underwent the following randomly assigned protocol: postexercise muscle ischemia (PEMI) test, during which the metaboreflex was elicited after dynamic handgrip; control exercise recovery session (CER) test; and PEMI after IP (IP-PEMI) test. IP was obtained by occluding forearm circulation for three cycles of 5 min spaced by 5 min of reperfusion. Hemodynamics were evaluated by echocardiography and impedance cardiography. The main results were that after IP the mean arterial pressure response was reduced compared with the PEMI test (means ± SD +3.37 ± 6.41 vs. +9.16 ± 7.09 mmHg, respectively). This was the consequence of an impaired venous return that impaired the stroke volume during the IP-PEMI more than during the PEMI test (−1.43 ± 15.35 vs. +10.28 ± 10.479 ml, respectively). It was concluded that during the metaboreflex, IP affects hemodynamics mainly because it impairs the capacity to augment venous return and to recruit the cardiac preload reserve. It was hypothesized that this is the consequence of an increased nitric oxide production, which reduces the possibility to constrict venous capacity vessels.
Keywords: cardiac preload, blood pressure, myocardial contractility, stroke volume, fatigue
ischemic preconditioning (IP) classically refers to the capacity of brief episodes of sublethal ischemia to render cardiac cells more resistant to subsequent, more sustained ischemic insults leading to infarction (52). The protective effects of IP against myocardial ischemia have been confirmed in many mammal species, including humans (6, 15, 18, 28, 46, 58, 60, 64, 68). Along with the effects on the myocardium, recent research has reported positive effects of IP on muscle functions. In particular, enhanced exercise performance has been found when muscle IP was applied before various kinds of effort such as handgrip, cycling, running, and swimming (8, 9, 24, 27, 41, 44, 59). Although some authors found uncertain or no effect (13, 34, 36, 67), or even a negative effect (56), of IP on exercise performance, the majority of the reported research suggests that IP induces some mild improvements in the capacity to exercise. However, the mechanisms through which IP positively influences exercise remain largely speculative, and several hypotheses have been formulated about the putative effects of IP on exercise performance. Previous studies have demonstrated that these positive effects occurred independently from a cardiac response (7, 8, 24, 27). Thus the mechanisms are probably related to a local phenomenon at the muscle and vascular level and/or to the perceived sensation of fatigue. For instance, muscle IP has been demonstrated to locally improve vascular function and perfusion by increasing nitric oxide (NO) formation and by attenuating sympathetic vasoconstriction (7, 8, 37). Moreover, the IP maneuver can promote an ATP-sparing effect, thereby producing more efficient muscle contraction (1, 57). Finally, it has been hypothesized that IP lowers the sensation of fatigue by reducing the discharge of afferent group III and IV nerve endings in the muscle (24). Feedback from these nervous afferents inhibits central motor drive and facilitates central fatigue, thus limiting exercise performance (3, 32).
It is important to highlight that type III and IV nerve endings also exert an essential contribution to the cardiovascular regulation during exercise. In particular, they activate the exercise pressor reflex, which, along with central command, is responsible for the increased sympathetic activity during exercise (2, 4, 54). In detail, type III and IV nerve endings operate as mechano- and metaboreceptors within the muscle and reflexively modulate sympathetic tone on the basis of the mechanical and metabolic conditions of the working muscle, with type III acting mainly as mechanoreceptors and type IV as metaboreceptors (54). The stimulation of these latter nerve endings activates the so-called “metaboreflex,” a reflex that elicits a substantial increase in sympathetic tone and that regulates the main hemodynamic modulators (i.e., heart rate, cardiac contractility, preload, and afterload) (20, 21, 23, 39, 48–50, 54, 55).
Hence, type III and IV nerve endings have a twofold role: on the one hand, they ensure effective cardiovascular adjustments to exercise; on the other, these afferents induce the sensation of fatigue. Inasmuch as IP can reduce type III and IV activity and fatigue, it is then possible that IP can also reduce the hemodynamic response to the metaboreflex activation. However, it is also considered that blocking these afferent nerve endings leads to an impairment in the hemodynamic and respiratory responses to exercise (2–4). In other words, the positive effect of their inhibition may be outweighed by the negative effects on the cardiovascular and respiratory regulation.
In accordance with these concepts, the present study was aimed to investigate the effect of IP on the hemodynamics during metaboreflex recruitment. In detail, we hypothesized that IP reduced the blood pressure response during the metaboreflex by reducing the response of one or more of the main hemodynamic modulators: heart rate, cardiac contractility, preload, and afterload. A second aim was to verify whether or not this IP effect was accompanied by a reduction in the perception of fatigue.
METHODS
Study Population
Fourteen healthy Caucasian males aged 25–48 yr (means ± SD 33.2 ± 6.4 yr), whose height and mass were 175.4 ± 7.6 cm and 71.5 ± 7.2 kg, respectively, agreed to participate in this study. All were judged healthy on the basis of a preliminary medical examination (see Experimental Design). None had any history of cardiac or respiratory disease or were taking any medication at the time of the experiment. All subjects were normotensive and nonsmokers and were blinded about the nature of the experiment. Subjects were asked to refrain from alcoholic beverage and caffeine for at least 24 h before the experimental sessions. The study was performed according to the Declaration of Helsinki and was approved by the local ethics committee. Written informed consent was obtained from all participants.
Experimental Design
Preliminary test.
Subjects underwent a general medical examination with electrocardiogram (ECG) and echocardiography to exclude any cardiovascular problem. Afterward, a cardiopulmonary test (CPX) with a gas analyzer (ULTIMA CPX, MedGraphics, St. Paul, MN) on an electromagnetically braked cycle ergometer with ECG monitoring (CUSTO Med, Ottobrunn, Germany) was conducted to assess their physical capacity.
Test for metaboreflex function assessment.
After the preliminary visit (interval 4–7 days), the subjects performed a test to measure their maximum voluntary contraction (MVC) during handgrip, which was assessed in the seated position. Subjects squeezed a hydraulic dynamometer (MAP 1.1, Kern, Balingen, Germany) to the maximal force for 1 s. MVC was considered as the peak reached during 5 maximal compressions, with at least 30-s interval. Handgrip was performed by the dominant arm. The subjects then underwent the following protocol, randomly assigned, with sessions spaced by at least 4 days interval (within 4–9 days), to study their cardiovascular response to metaboreflex activation and to asses the effects of IP on hemodynamics during the metaboreflex:
POSTEXERCISE MUSCLE ISCHEMIA SESSION SESSION.
The postexercise muscle ischemia session (PEMI) session encompassed 3 min of resting, followed by 3 min of exercise, consisting of rhythmic (30 compressions/min dictated by a metronome) handgrip on the same dynamometer used for MVC assessment and in the same seated position. The workload was set at 30% of MVC, which was by means of visual feedback. The exercise period was followed by 3 min of PEMI on the exercised arm. Ischemia was induced by rapidly (in <3 s) inflating at the cessation of exercise an upper arm biceps cuff to 50 mmHg above peak exercise systolic pressure. The cuff was kept inflated for 3 min. An additional 3 min of recovery were allowed after the cuff was deflated, for a total of 6 min of recovery. Thus the total session duration was 12 min. This protocol, although conducted at mild workload, has been found be able to trap muscle metabolites in the exercising limb and to maintain stimulation of the metaboreceptors thereby eliciting substantial hemodynamic responses in terms of cardiac contractility, preload, and afterload modulation (19, 22, 23, 48, 50, 61). Moreover, this protocol allows for the metaboreflex-mediated cardiovascular response to be isolated from that arising from central command and mechanoreflex activation, since during PEMI these two cardiovascular reflexes no longer operate (10, 21, 54).
CONTROL EXERCISE RECOVERY SESSION SESSION.
The same rest-exercise protocol used for PEMI was used in the control exercise recovery session (CER) session, but the recovery was conducted without tourniquet inflation.
PEMI AFTER IP SESSION.
Participants underwent the same protocol as described for the PEMI session in the PEMI after IP (IP-PEMI) session, but it was preceded by the IP maneuver obtained by occluding forearm circulation with one pneumatic cuff positioned around the arm, at the level of the biceps muscle, and inflated 50 mmHg above the subject's systolic blood pressure. Circulatory occlusion lasted 5 min and was performed three times, each separated by 5 min of reperfusion. The exercise test started 5 min after the IP maneuver. This protocol had already been used in similar studies (24, 45).
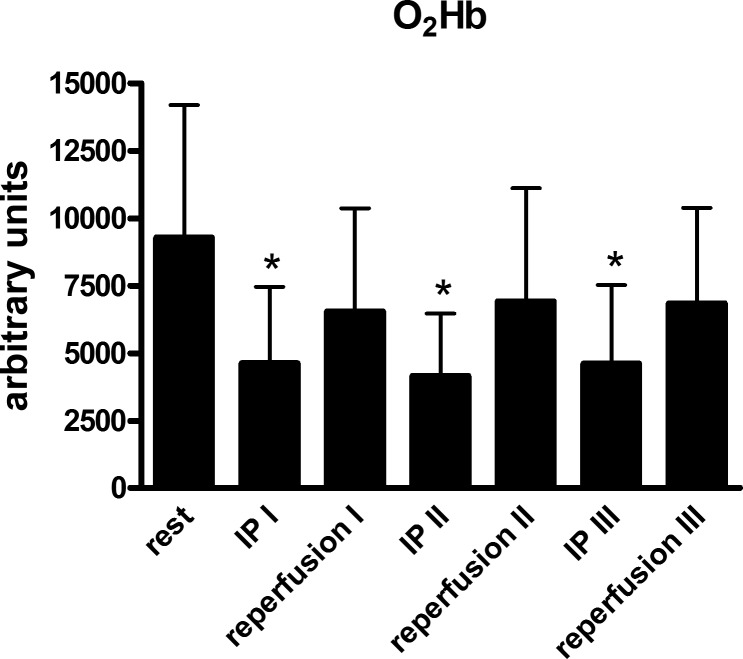
During the preconditioning phases of the IP-PEMI session, the absence or presence of pulsatile arterial flow was assessed using an Echo-Doppler ultrasound device (M5 Diagnostic Ultrasound System, Mindray Bio-Medical Electronics, Shenzen, China) equipped with a linear-array transducer at 5.0 MHz in the pulsed-wave mode. The transducer was positioned at the level of anatomical snuff box to visualize the radial artery. Blood velocity was imaged with an insonation angle of 60°. Furthermore, during the preconditioning maneuvers, the probe of a portable near-infrared spectroscopy (NIRS) apparatus (PortaMon, Atrinis Medical Systems, Elst, Holland) was placed medially on the volar forearm, at the widest girth of the forearm. The probe was covered with a dark cloth to minimize the intrusion of ambient light, and it was secured with elastic straps to avoid motion. This device has a dual wavelength continuous wave system, which simultaneously uses the modified Beer-Lambert law and spatially resolved spectroscopy (29, 42). The PortaMon is able to assess changes in tissue oxyhemoglobin (O2Hb) by using the difference in absorption characteristics of wavelengths at 760 and 850 nm. During testing, the NIRS system was connected to a laptop by Bluetooth for data acquisition and analog-to-digital conversion at a sampling rate of 10 Hz. The NIRS analysis was conducted to verify whether or not the IP-PEMI session led to any reduction in muscle oxygenation to further confirm the presence of ischemia.
Task failure test.
Before the PEMI and the control exercise recovery session (CER) tests and after the preconditioning maneuvers of IP-PEMI test (the interval after the IP maneuver was between 20 and 30 min), each participant performed handgrip contraction with a rhythm of 30 contractions/min (1 s contraction followed by 1 s relaxation) in the same position, with the same arm, and with the same dynamometer described for the MVC protocol. They were asked to maintain a force of 45% of the MVC until exhaustion, which was considered as the point when they were unable to maintain the target power output for at least three consecutive contractions. A similar task failure protocol had already been used to investigate on the effect of IP on fatigue (9). Subjects were encouraged to exert for as long as possible. The time taken to achieve the task failure was gathered with a hand chronometer. Participants and operators were blinded to the task failure timing. The task failure test was employed to test whether or not the IP maneuver delayed fatigue during dynamic handgrip. Others have found beneficial effects of IP during handgrip (9), although conflicting results have been reported on the capacity of IP to improve exercise with larger muscle mass (13, 34, 36, 67).
All experiments were carried out in a temperature-controlled, air-conditioned room (22°C, relative humidity at 50%).
Hemodynamic assessment during metaboreflex activation.
Throughout sessions of metaboreflex activation, subjects' hemodynamics were collected by impedance cardiography (NCCOM 3, BoMed, Irvine, CA). The impedance method allows for continuous noninvasive hemodynamic evaluation and has been previously utilized in similar experimental settings (19–23). The data acquisition procedure is described in detail in previous works by our group (19–23). Briefly, analog traces of electrocardiogram, thorax impedance (Z0), and Z0 first derivative obtained with the impedance cardiograph were collected and stored by means of a digital chart recorder (ADInstruments, PowerLab 8sp, Castle Hill, Australia) at a sampling rate of 500 Hz. Stored traces were then used to calculate beat-to-beat heart rate (HR) and stroke volume (SV). In particular, HR was calculated as the reciprocal of the electrocardiogram R-R interval, whereas SV was obtained using the Sramek-Bernstein equation (11). Cardiac output (CO) was calculated as SV·HR. The preejection period (PEP) and the left ventricular ejection time (LVET) were also assessed from impedance traces, as shown in detail by previously published papers (17, 23). Diastolic time (DT) was measured by subtracting the sum of PEP and LVET from the cardiac cycle total period. The ventricular filling rate (VFR), which is a measure of the mean rate of diastolic blood flux, was obtained dividing SV by DT (23, 35, 48, 50).
A standard manual sphygmomanometer was employed for systolic (SBP) and diastolic (DBP) blood pressure assessment, which was performed in the nondominant arm by the same physician throughout all protocol sessions. The formula by Moran and coworkers (51), which takes into account changes in the diastolic and systolic periods, was utilized to calculate mean arterial blood pressure (MAP). Systemic vascular resistance (SVR) was derived by multiplying the MAP-to-CO ratio by 80, where 80 is a conversion factor to change units to standard resistance units.
Left ventricular measures were also collected by utilizing two-dimensional echocardiography (M5 Diagnostic Ultrasound System, Mindray Bio-Medical Electronics). A hand-held 3.5-MHz ultrasound probe was employed and end-diastolic volume (EDV) and end-systolic volume (ESV) were measured in the apical four chamber position. Volumes were calculated automatically by software using a conventional formula: 8A2/3πL, where A was the left ventricular area and L was ventricular longest length (12). The ventricular area was determined by tracing along the inner edge of the endocardial targets, and the length was obtained by measuring the distance from the left ventricular apex to the midpoint of the mitral annulus. Echocardiography images were taken at rest and during the last minute of the PEMI period and the corresponding periods of the CER and the IP-PEMI (i.e., at the third minute of recovery). As previously stated, subjects were in the sitting position. If images were considered to be of good quality by the operator, a 6-s frame was recorded and then analyzed offline by another operator who was blinded to the study protocol. For each analysis at least 3 beats were taken into consideration (range 3–6 beats). Individual values in each beat were calculated as the average of three trials of the same beat; i.e., each beat value was the average from 3 measures. Left ventricular ejection fraction (EF) was considered as (EDV − ESV/EDV)·100.
In the same beats utilized for left ventricular volumes assessment, early and atrial transmitral filling peak velocities (E vel and A vel, respectively) and their ratio (E/A) were collected by pulse-wave Doppler (PWD) recording. Measures were obtained from the apical four-chamber view with a 5-mm PWD sample volume placed distal to the mitral anulus, between the mitral leaflets. The interrogation beam was aligned with mitral flow. These transmitral measures are considered to be well correlated with left ventricular diastolic functions (14, 33).
Data analysis
Data are shown as means ± SD. Hemodynamic data collected by means of impedance cardiography during the metaboreflex tests were averaged over 1 min. Values at rest, at the third minute of exercise, at the third minute of PEMI (when a steady state in metaboreflex activity was expected to be reached), and at the sixth minute of recovery were taken into consideration for statistical analysis. Hemodynamic parameters gathered by echocardiography (EDV, ESV, and EF) were collected only at rest and at the third minute of recovery because of the difficulties in obtaining reliable data during exercise and to have continuos monitoring during recovery. Thus these variables are shown only during these periods. Two-way analysis of variance (ANOVA) was utilized to compare hemodynamic data for the effects of settings (rest, exercise, and recovery) and conditions (PEMI, CER, and IP-PEMI test) followed by Bonferroni post-hoc when appropriate. To further assess metaboreflex activity the following procedure was employed: the difference in the level of variables between the postexercise ischemia phases of the PEMI and the IP-PEMI tests and the CER test at the third minute of recovery were calculated. This procedure enabled metaboreflex response to be assessed, i.e., the response due to the metaboreflex activity (25, 50). Differences in measured variables due to metaboreflex response were assessed by means of the t-test for paired data. To check differences in task failure tests, one-way ANOVA for repeated measures was applied, followed by Tukey post-hoc if appropriate. Data from the NIRS gathered during the preconditioning phase of the IP-PEMI test were averaged over 5 min. Differences in O2Hb level were calculated by one-way ANOVA for repeated measures, followed by Tukey post-hoc if appropriate. Statistical analysis was carried out by utilizing commercially available software (GraphPad Prism). Statistical significance was established as a P value of <0.05 in all cases.
RESULTS
The protocol was completed by all subjects and none of them complained of unbearable pain or discomfort during the periods of PEMI. No signs or symptoms of cardiac and/or respiratory diseases were detected during the preliminary medical examination or the incremental CPX test, and none of the subjects showed signs and/or symptoms of exercise intolerance. The maximum oxygen uptake reached by subjects was 43.5 ± 6.7 ml·min−1·kg−1. The values of data recorded during rest periods preceding hand-grip strains are reported in Table 1. Hemodynamic parameters were not different between conditions, therefore, subjects started tests for metaboreflex assessment at similar parameter levels. This fact indicates that IP was unable to affect variables value at rest.
Table 1.
Hemodynamic data values during rest periods preceding PEMI, CER, and IP-PEMI tests
| PEMI | CER | IP-PEMI | P Value | |
|---|---|---|---|---|
| HR, beats/min | 65.25 ± 10.09 | 67.74 ± 10.47 | 66.06 ± 8.78 | 0.79 |
| SV, ml | 85.88 ± 32.47 | 85.96 ± 27 | 84.35 ± 29.6 | 0.98 |
| CO, l/min | 5.57 ± 1.72 | 5.69 ± 1.48 | 5.56 ± 2.13 | 0.97 |
| MAP, mmHg | 80.68 ± 6.79 | 83.86 ± 5.91 | 79.92 ± 7.43 | 0.27 |
| SVR, dyne·s−1·cm−5 | 1280.59 ± 384.98 | 1267.03 ± 385.6 | 1283.57 ± 431.6 | 0.99 |
| VFR, ml/s | 165.05 ± 54.65 | 177.63 ± 54.87 | 168.9 ± 69.77 | 0.85 |
| EDV, ml | 143.59 ± 22.55 | 138.28 ± 19.12 | 146.65 ± 19.33 | 0.57 |
| ESV, ml | 57.7 ± 35.42 | 52.31 ± 32.58 | 62.30 ± 32.19 | 0.31 |
| EF, % | 60.48 ± 20.58 | 63.09 ± 20.50 | 58.08 ± 19.59 | 0.8 |
| E vel, cm/s | 61.7 ± 11.83 | 57.34 ± 14.49 | 57.88 ± 9.64 | 0.58 |
| A vel, cm/s | 38.3 ± 6.97 | 36.28 ± 9.36 | 40.35 ± 7.58 | 0.41 |
| E/A | 1.63 ± 0.34 | 1.65 ± 0.55 | 1.45 ± 0.24 | 0.35 |
Values are means ± SD; n = 14. PEMI, postexercise muscle ischemia;
CER, control exercise recovery; IP-PEMI, PEMI after IP; HR, heart rate; SVCO, cardiac output; MAP, mean arterial pressure; SVR, systemic vascular resistance; VFR, ventricular filling rate; EDV, end-diastolic volume; ESV, end-systolic volume; E vel, early transmitral filling peak velocity; A vel, atrial transmitral filling peak velocity.
The complete absence of any arterial flow during the IP maneuvers before the IP-PEMI test was confirmed by the Echo-Doppler ultrasound measurement, which detected no arterial flow during the application of a pressure of 50 mmHg higher than the subjects' SBP. Furthermore, the NIRS analysis highlighted that there was a reduction in O2Hb levels during each of the three cycles of 5 min arterial occlusion compared with rest (Fig. 1). During the reperfusion phases, this parameter increased toward rest level. The mean values of MVC reached by subjects was 33.1 ± 2.6 kg. Hence, participants performed the handgrip strains for metaboreflex activity assessment with the hand dynamometer set at 9.9 ± 0.7 kg, i.e., 30% of MVC, whereas the task failure session was conducted with the dynamometer set at 14.9 ± 1.1 kg, i.e., 45% of MVC.
Fig. 1.
Changes in the level of forearm oxyhemoglobin (O2Hb) during the periods of circulation blockage to induce ischemic preconditioning (IP) and reperfusion. Values are means ± SD. *P < 0.05 vs. rest.
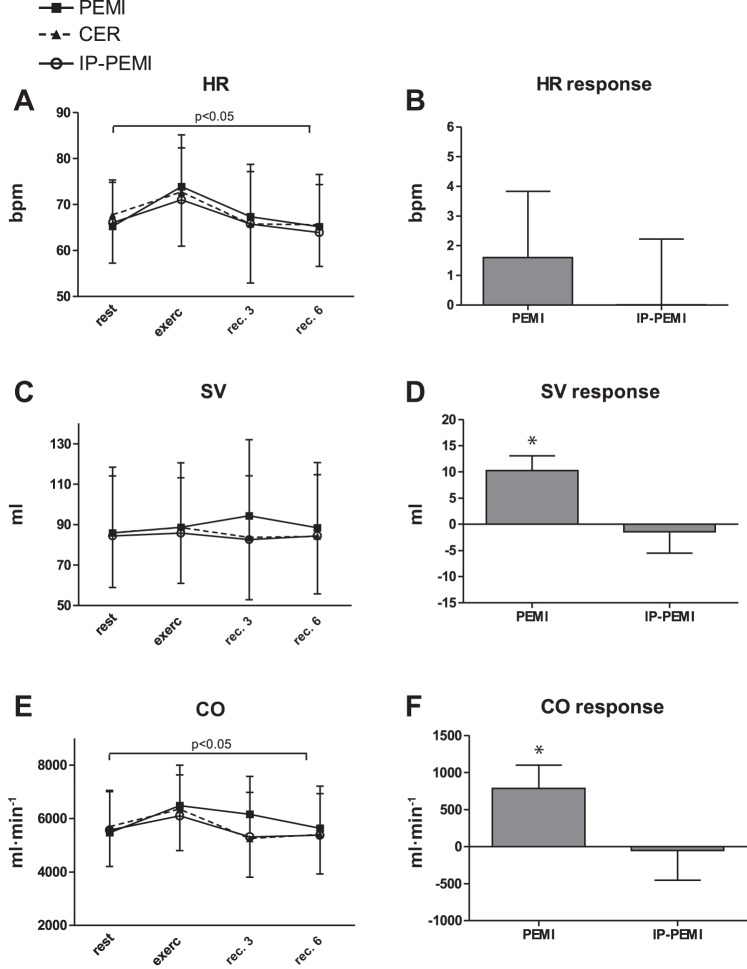
Figures 2–5 show the levels of hemodynamic parameters and responses collected during all sessions of the protocol. In detail, Fig. 2A illustrates that HR was not different between condition, while time significantly affected this variable. Figure 2B also shows that HR response was not significantly different between the PEMI and the IP-PEMI test. Stroke volume (Fig. 2C) was unaffected by time or condition. However, the PEMI test induced a higher SV response compared with the IP-PEMI test (Fig. 2D). HR and SV behavior resulted in a significant time effect upon CO time course (Fig. 2E), whereas condition did not exert any effect. Moreover, CO response was higher during the PEMI than during the IP-PEMI test (Fig. 2F).
Fig. 2.
Absolute values during the postexercise muscle ischemia (PEMI), the control exercise recovery (CER), and the postexercise muscle ischemia after ischemic preconditioning (IP-PEMI) tests and response in heart rate (HR, A and B), stroke volume (SV, C and D), and cardiac output (CO, E and F). Responses were calculated as the difference between the PEMI and the IP-PEMI tests and the CER test at the third minute of recovery (see text for further details). Values are means ± SD. The P value indicates the overall main effect of time (horizontal bracket). There was no interaction effect. *P < 0.05 vs. IP-PEMI.
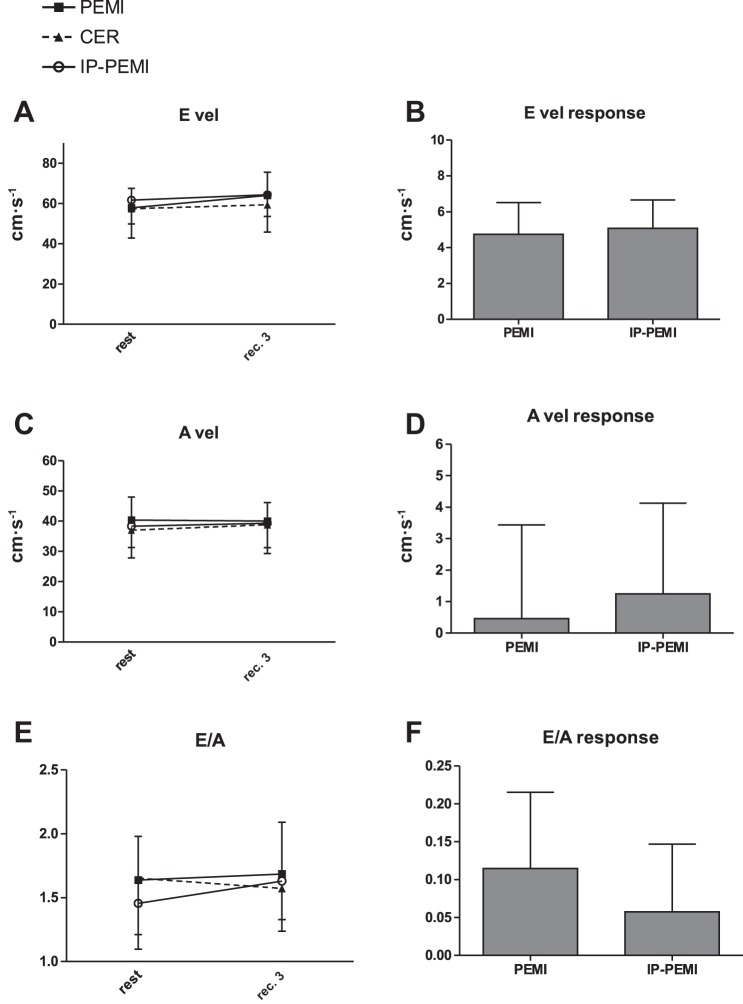
Fig. 5.
Absolute values during the postexercise muscle ischemia (PEMI), the control exercise recovery (CER), and the postexercise muscle ischemia after ischemic preconditioning (IP-PEMI) tests and response in E wave velocity (E vel, A and B), A wave velocity (A vel, C and D), and E/A ratio (E/A, E and F). Responses were calculated as the difference between the PEMI and the IP-PEMI tests and the CER test at the third minute of recovery (see text for further details).
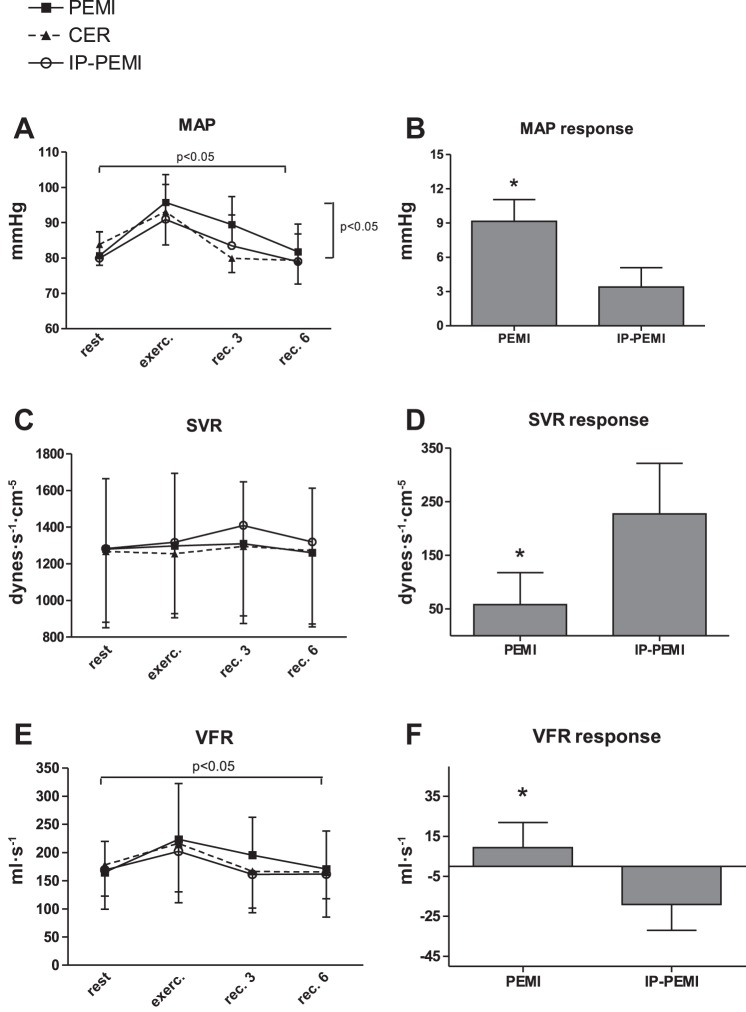
MAP was significantly influenced by time and condition, but there was no interaction effect (Fig. 3A). The response in MAP was higher during the PEMI than during the IP-PEMI test (Fig. 3B). Figure 3C shows that there was no effect due to time or condition upon SVR. However, Fig. 3D demonstrates that the response in this parameter was higher during the IP-PEMI compared with the PEMI test. Time significantly changed VFR time course (Fig. 3E) without any difference due to condition. The response in this parameter was more elevated during the PEMI compared with the IP-PEMI test (Fig. 3F).
Fig. 3.
Absolute values during the postexercise muscle ischemia (PEMI), the control exercise recovery (CER), and the postexercise muscle ischemia after ischemic preconditioning (IP-PEMI) tests and response in mean arterial pressure (MAP, A and B), systemic vascular resistance (SVR, C and D), and ventricular filling rate (VFR, E and F). Responses were calculated as the difference between the PEMI and the IP-PEMI tests and the CER test at the third minute of recovery (see text for further details). Values are means ± SD. The P value indicates the overall main effect of time (horizontal bracket) and condition (vertical bracket). There was no interaction effect. *P < 0.05 vs. IP-PEMI.
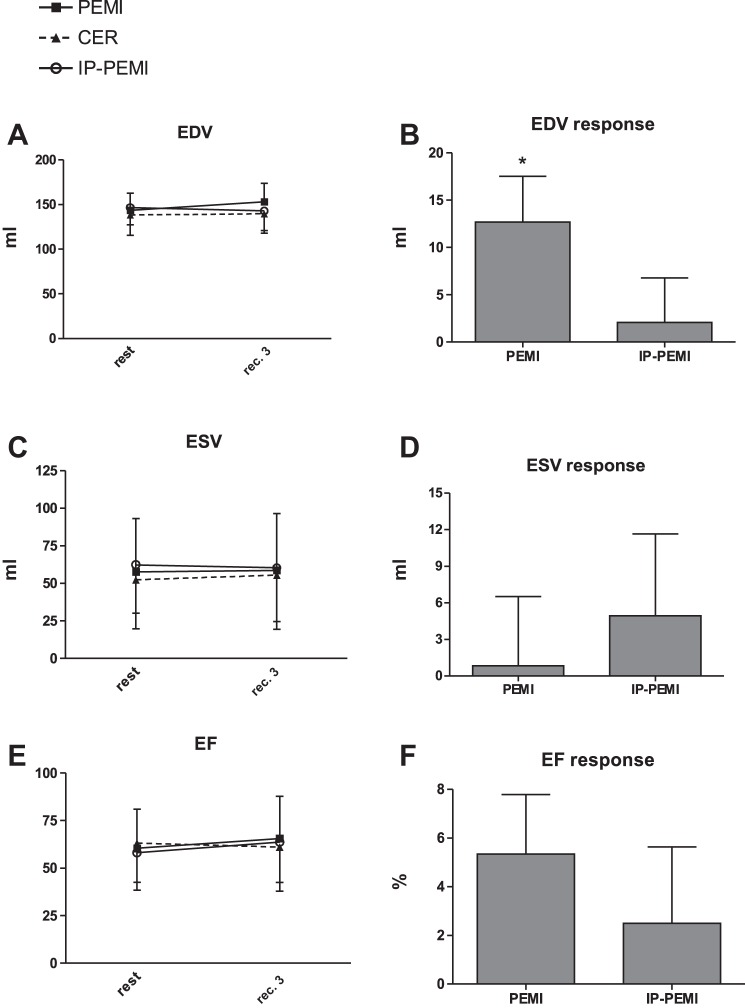
There was no difference between settings in terms of EDV, ESV, and EF time course (Fig. 4, A, C, and E, respectively). Responses in ESV and EF were not different between the PEMI and the IP-PEMI test (Fig. 4, D and F), whereas EDV response was higher during the PEMI than during the IP-PEMI test. Time courses and responses of Doppler-derived indexes of myocardial diastolic function were unaffected by protocol settings (Fig. 5).
Fig. 4.
Absolute values during the postexercise muscle ischemia (PEMI), the control exercise recovery (CER), and the postexercise muscle ischemia after ischemic preconditioning (IP-PEMI) tests and response in end-diastolic volume (EDV, A and B), end-systolic volume (ESV, C and D), and ejection fraction (EF, E and F). Responses were calculated as the difference between the PEMI and the IP-PEMI tests and the CER test at the third minute of recovery (see text for further details). Values are means ± SD. There was no interaction effect. *P < 0.05 vs. IP-PEMI.
Finally, there was no difference in time-to-task failure performed before each test. In detail, time-to-task failure was 118.1 ± 92.81, 132.22 ± 92.38, and 125.13 ± 85.51 s for the PEMI, the CER, and the IP-PEMI test, respectively (P = 0.42).
DISCUSSION
The main new finding of the present investigation was that ischemic preconditioning of the forearm was able to reduce the blood pressure response during the metaboreflex activation. After a classic preconditioning maneuver that caused a substantial muscular deoxygenation, we found that the typical blood pressure response, which is normally observed during postexercise muscle ischemia, was almost abolished. Actually, the MAP response was reduced during the IP-PEMI compared with the PEMI test. However, our data do not support the hypothesis that ischemic preconditioning successfully reduces the activity of type III and IV nerve afferents fibers of the muscle as an alternative hemodynamic explanation is more adherent to the hemodynamic scenario found.
It has been demonstrated that some of type III and IV afferences (especially type IV) act as “metaboreceptors” (5), because they are sensitive to the accumulation of metabolic by-products of muscle metabolism such as lactic acid, potassium, bradykinin, arachidonic acid products, ATP, diprotonated phosphate, and adenosine (5, 43, 53, 54). Together with the mechanoreflex, the metaboreflex participates in the “exercise pressor reflex,” whose activation is essential to achieve a normal hemodynamic adjustment to exercise (2, 4, 54, 66). When the metaboreflex is triggered, the sympathetic nervous system is activated and this activation regulates the main hemodynamic modulators (cardiac performance, preload, afterload, and HR), which are modified to achieve the target blood pressure. It has been demonstrated that during the metaboreflex, the sympathetic tone is enhanced (30, 40, 53) and, at least in healthy subjects, this fact increases myocardial performance (19, 23, 55, 62), supports cardiac preload by means of splanchnic and venous constriction (10, 47, 63, 65), and causes arteriolar constriction (23, 39, 49, 55). The effect on HR is variable, because it depends on the setting of metaboreflex activation: it normally raises when the metaboreflex is activated during exercise, whereas it does not vary if the metaboreflex is stimulated by means of PEMI. This phenomenon is explained by the fact that during PEMI the enhanced sympathetic tone is counteracted by the concomitantly increased parasympathetic outflow due to the loss of central command and the baroreflex resetting, which together buffer the increased sympathetic activity to the sinus node (16, 21, 30, 31). However, it should be considered that the PEMI-induced HR response depends also on the mass of muscle involved. If PEMI follows exercise conducted with larger muscle mass than during handgrip, HR may remain above normal recovery values, although previous results from our laboratory did not show any difference between the PEMI and the CER tests after running (19).
The hemodynamic response we found in our experimental setting was characterized by a blunted MAP response after IP. This effect was the consequence of a reduced SV response that took place in this condition. Since HR was similar between the PEMI and the IP-PEMI tests, the reduced SV also led to a diminished CO response. This SV response was the consequence of a different diastolic volume between the two settings. In detail, during the IP-PEMI test there was a lower EDV response compared with the PEMI test. Moreover, during the IP-PEMI test a reduction in VFR, a measure of diastolic blood flux, occurred. Taken together these two phenomena suggest that diastolic flux to the heart was negatively affected by IP. Ample evidence demonstrates that diastolic functions and the capacity to increase cardiac preload are important to achieve normal hemodynamics during the metaboreflex (10, 20, 48, 50). In particular, it has been reported both in animal as well as in human studies, that the capacity to centralize blood volumes by means of visceral and venous constriction supports ventricular performance by recruiting the Frank-Starling mechanism during the metaboreflex (10, 63, 65). Moreover, it has recently been reported that if this mechanism is impaired by inducing venous dilation, then the CO-mediated increment in MAP that normally takes place during PEMI is reduced (48). However, a recent investigation from our laboratory has highlighted the fact that, if cardiac compliance is impaired, such as in aging, then the preload reserve cannot be properly recruited and the SV response during PEMI is abolished (50). All these facts are in line with the concept that the possibility to increase venous return and cardiac preload together with diastolic functions are important to achieve normal cardiovascular response during the metaboreflex.
In our investigation, the observed reduced EDV and VFR after IP was coherent to our initial hypothesis that IP reduced cardiac preload. However, the mechanism is apparently different from what initially speculated, i.e., a reduced sympathetic-mediated venoconstriction due to a IP-induced dampening in type III and IV nerve endings activity. Although not directly assessed, sympathetic activity to vessels and to the heart was likely unaffected during the metaboreflex after IP since we did not observed any reduction in myocardial performance, as testified by EF, or in arteriolar tone, as indicated by the SVR increment. Notwithstanding sympathetic tone was unaffected by IP, EDV and VFR were reduced, thereby indicating the occurrence of venous dilation and blood pooling.
We cannot provide a definitive explanation as to why IP caused a reduction in these parameters. It is unlikely that this was the consequence of an altered diastolic proprieties of the heart as, to the best of our knowledge, nobody to date has reported that IP can negatively affect this function. Moreover, the indexes of diastolic functions we used (i.e., E and A waves velocity) were not influenced by IP. It is possible to hypothesize the reduced EDV was the consequence of an impaired capacity to induce venous constriction after IP. It is well known that IP can promote the production NO at the vascular level (26, 47). It has recently been shown that the administration of NO donors before the metaboreflex activation can impair cardiac preload by inducing venous capacity vessels dilation (48). This venous dilation reduced the ability to propel blood volumes toward the heart and to recruit the Frank-Starling mechanism during the metaboreflex. This phenomenon was in part responsible for the reported reduced SV and MAP response (48). It is then possible to speculate that, in our setting, the IP maneuvers before the metaboreflex could have augmented the NO production which in turn resulted in an impaired possibility to induce venous constriction. Coherently, this phenomenon could have resulted in a reduced capacity to increase SV and CO during the metaboreflex, and this fact caused the blunted MAP response. However, it must be acknowledged that this hypothesis remains speculative since we did not have the opportunity to gather a direct measure of NO production in our experimental setting.
An important point that deserves to be highlighted and that is in contrast to this hypothesis is that SVR was not reduced after the metaboreflex. This parameter would have dropped if an increase in the NO concentration had taken place as NO induces arteriolar dilation. Moreover, the increased SVR response found is in sharp contrast to our initial hypothesis that IP reduced type III and IV afferents activity, thereby reducing the blood pressure response during the metaboreflex. In fact, a reduced activity of these afferent could have caused a blunted sympathetic activation and a reduced SVR increment during the metaboreflex. Furthermore, the HR response should also have been blunted if this were the case. Neither the first nor the second phenomena occurred in our experimental setting. In our opinion, this fact does not support the hypothesis that the sympathetic tone was reduced after IP and that metaboreceptors were desensitized by the preconditioning maneuvers. A possible explanation for the increased SVR reported during the IP-PEMI test may be that the baroreflex could have been activated by the reduced MAP, thereby counteracting the lower CO response by recruiting the afterload reserve and inducing arteriolar constriction. This in turn partially corrected the MAP response in the IP-PEMI condition, which however remained at lower levels compared with the PEMI test. This phenomenon could also have masked any possible IP-induced arteriolar dilation due to the supposed increase in NO production. It is also to be considered that nitrates exert a venodilator effect while a quantitatively lesser effect on arteriolar resistance vessels has been reported (38, 48). The increased SVR could also have “trapped” blood on the arterial side of the circulation and led to reductions in venous return and ventricular filling, thereby partially explaining the reduced VFR and EDV.
Therefore, in our opinion, the blunted MAP response after IP was not the consequence of a reduction in the activity of the metaboreceptors, rather the main responsible mechanism was the capacity of the preconditioning maneuvers to induce venous dilation, thereby preventing the possibility to augment the diastolic flux to sustain EDV and SV. Our proposed hemodynamic scenario is that the normal flow-mediated mechanism that normally increases MAP during the metaboreflex was impaired after the IP because of the reduced capacity to properly increase cardiac preload. In this scenario, the baroreflex partially succeeded in maintaining blood pressure by inducing arteriolar constriction. This result is in line with the concept that the hemodynamic response during the metaboreflex is dependent on the possibility to regulate all the four main hemodynamic mediators (HR, cardiac performance, preload, and afterload) and that the possibility to enhance cardiac preload is a key factor in this scenario (10, 48, 63, 65).
Another finding of our experiment was that time to task failure was unaffected by IP. This fact speaks against any role of preconditioning in improving exercise capacity, at least when applied in limited muscle groups, such as in the forearm during handgrip. Thus it does not seem that IP can successfully reduce the sensation of fatigue by reducing type III and IV afferents activity in this setting. It should however be considered that, as described in the introduction, on the one hand, these afferents induce the sensation of fatigue, whereas on the other, they ensure effective cardiovascular adjustments to exercise. Thus blocking these fibers may inhibit the sensation of fatigue, but it also impairs the cardiovascular response, thereby limiting exercise performance (2, 4). In other words, the potential positive effect of reducing the sensation of fatigue is counterbalanced by the potential impairment of the cardiovascular regulation. In the present investigation a reduction in MAP during the metaboreflex after IP was evident. Another potential explanation for the lack of any improvement in task failure is that the beneficial effect of IP may appear only when the muscle masses being engaged are larger than in the present investigation. Actually, most of the studies reporting positive effects employed cycling, running, and even swimming as exercise mode (8, 24, 27, 41, 59). It could then be argued that during handgrip the muscle mass being recruited is too small to detect any beneficial effect of IP on fatigue.
Limitations of the study.
It should be recognized that the present study has some potential limitations. In detail, one limitation could be that we did not employ a sham test. We made this choice considering that it is virtually impossible to effectively have a sham that mimics the IP maneuvers. Classically, this is done by inflating the tourniquet to a level of 20 mmHg (67). However, subjects clearly recognize the difference between 20 and 50 mmHg above their systolic pressure (as used in the present study). Moreover, this can potentially block venous return and cause metabolite accumulation. This in turn potentially activates the IP cascade, thereby paradoxically further confounding results. Another potential limitation was that we did not collect data on NO production. The dosage of this metabolite would have confirmed our hypothesis that NO production was augmented after IP and that it was responsible for the reduced capacity to constrict large venous vessels and support cardiac preload during the metaboreflex. However, we consider this hypothesis very likely to have occurred since it has been reported on several occasions that IP increases NO production. Our experimental setting did not allow to assess whether or not the activity of the metaboreflex was blunted during exercise after the IP maneuver. The pressor response was not different between the different settings, but it should be considered that during exercise, along with the metaboreflex, the central command and the mechanoreflex are also activated and their hemodynamic effects superimpose to those due to the metaboreflex. Moreover, some redundancy exists between these reflexes and neural occlusion can be operative; that is, their effects do not sum (53) thereby rendering the contribution of each of them very difficult to be ascertained. Hence, it is to be underscored that without direct measurement of sympathetic nerve activity, it is difficult to make any conclusion about the exercise pressor reflex after IP. Finally, the study population of the present investigation was limited to young, healthy, Caucasian males. Therefore, results cannot be extended to a general population.
In conclusion, our data suggest that ischemic preconditioning successfully affect the hemodynamic response to metaboreflex activation, since in the present investigation it reduced the blood pressure response during the recruitment of this cardiovascular reflex, but this hemodynamic response was not linked to any ergogenic effect. However, contrary to our initial hypothesis, this effect was not achieved by reducing heart rate and arterial vascular resistance response, which was instead increased. Rather, it was the consequence of an impaired capacity to propel blood volumes toward the heart and to recruit the Frank-Starling mechanism, as testified by the impaired ventricular filling rate and EDV found in our experimental setting. This in turn resulted in an impaired capacity to support SV and CO during the metaboreflex, and this fact blunted arterial pressure response. We hypothesized that this was the consequence of an increase in NO production which reduced the possibility to constrict venous capacity vessels.
Perspectives and Significance
Results from the present study do not support the hypothesis that IP is of any aid for improving exercise performance in exercise with limited muscle mass. However, this investigation does support the hypothesis that IP affects hemodynamics. Further study is warranted to better characterize the phenomenon and to understand whether or not this effect is present also for exercise conducted with larger muscle mass and in patients with cardiovascular disease.
GRANTS
This study was supported by the University of Cagliari and the Italian Ministry of Scientific Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: G.M., G.S., S.M., R.M., and A.C. conception and design of research; G.M., G.S., S.M., G.P., N.M., A.O., S.R., E.M., R.M., and A.C. performed experiments; G.M., G.S., S.R., E.M., R.M., and A.C. analyzed data; G.M. and A.C. interpreted results of experiments; G.M., G.S., S.M., A.O., E.M., R.M., and A.C. prepared figures; G.M., G.S., S.M., G.P., N.M., S.R., E.M., R.M., and A.C. drafted manuscript; G.M., G.S., S.M., G.P., N.M., A.O., S.R., E.M., R.M., and A.C. edited and revised manuscript; G.M., G.S., S.M., G.P., N.M., A.O., S.R., E.M., R.M., and A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Barry Mark Wheaton for editorial assistance.
REFERENCES
- 1.Addison PD, Neligan PC, Ashrafpour H, Khan A, Zhong A, Moses M, Forrest CR, Pang CY. Noninvasive remote ischemic preconditioning for global protection of skeletal muscle against infarction. Am J Physiol Heart Circ Physiol 285: H1435–H1443, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF, Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J Appl Physiol 105: 1714–1724, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Walter Wray D, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreani CM, Hill JM, Kaufman MP. Responses of group III and IV afferents to dynamic exercise. J Appl Physiol 82: 1811–1817, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Asimakis GK, Innerd-McBridge K, Medellin G, Conti VR. Ischemic preconditioning attenuates acidosis and postischemic dysfunction in isolated rat heart. Am J Physiol Heart Circ Physiol 263: H887–H894, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Bailey TG, Birk GK, Cable NT, Atkinson G, Green DJ, Jones H, Thijssen DHJ. Remote ischemic preconditioning prevents reduction in brachial artery flow-mediated dilation after strenuous exercise. Am J Physiol Heart Circ Physiol 303: H533–H538, 2012. [DOI] [PubMed] [Google Scholar]
- 8.Bailey TG, Jones H, Gregson W, Atkinson G, Cable NT, Thijssen DHJ. Effect of ischemic preconditioning on lactate accumulation and running performance. Med Sci Sport Exerc 44: 2084–2089, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Barbosa TC, Machado AC, Braz ID, Fernandes IA, Vianna LC, Nobrega AC, Silva BM. Remote ischemic preconditioning delays fatigue development during handgrip exercise. Scand J Med Sci Sports 25: 256–364, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Bastos BG, Williamson JW, Harrelson T, Nôbrega ACL. Left ventricular volumes and hemodynamic responses to postexercise ischemia in healthy humans. Med Sci Sports Exerc 32: 1114–1118, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein DP. A new stroke volume equation for thoracic electrical bioimpedance: theory and rationale. Crit Care Med 14: 904–909, 1986. [DOI] [PubMed] [Google Scholar]
- 12.Christie J, Sheldahl LM, Tristani FE, Sagar KB, Ptacin MJ, Wann S. Determination of stroke volume and cardiac output during exercise: comparison of two-dimensional and Doppler echocardiography, Fick oxymetry, and thermodiluition. Circulation 76: 539–547, 1987. [DOI] [PubMed] [Google Scholar]
- 13.Clevidence MW, Mowery RE, Kushnick MR. The effects of ischemic preconditioning on aerobic and anaerobic variables associated with submaximal cycling performance. Eur J Appl Physiol 112: 3649–3654, 2012. [DOI] [PubMed] [Google Scholar]
- 14.Cohen GI, Pietrolungo JF, Thomas JD, Klein AL. A practical guide to assessment of ventricular diastolic function using Doppler echocardiography. J Am Coll Cardiol 27: 1753–1760, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Cohen MV, Baines CP, Downey JM. Ischemic preconditioning: from adenosine receptor to KATP channel. Ann Rev Physiol 62: 79–109, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Crisafulli A, Marongiu E, Ogho S. Cardiovascular reflexes activity, and their interaction during exercise. Biomed Res Int 2015, doi: 10.1155/2015/394183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crisafulli A, Melis F, Orrù V, Lener R, Lai C, Concu A. Impedance cardiography for non invasive assessment of systolic time intervals during exercise. Sports Med Train Rehab 10: 13–27, 2001. [Google Scholar]
- 18.Crisafulli A, Melis F, Tocco F, Santoboni UM, Lai C, Angioy G, Lorrai L, Pittau G, Concu A, Pagliaro P. Exercise-induced and nitroglycerin-induced myocardial preconditioning improves hemodynamics in patients with angina. Am J Physiol Heart Circ Physiol 287: H235–H242, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Crisafulli A, Milia R, Lobina A, Caddeo M, Tocco F, Concu A, Melis F. Hemodynamic effect of metaboreflex activation in men after running above and below the velocity of the anaerobic threshold. Exp Physiol 93: 447–457, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Crisafulli A, Milia R, Vitelli S, Caddeo M, Tocco F, Melis F, Concu A. Hemodynamic responses to metaboreflex activation: insights from spinal cord-injured humans. Eur J Appl Physiol 106: 525–533, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Crisafulli A, Piras F, Filippi M, Piredda C, Chiappori P, Melis F, Milia R, Tocco F, Concu A. Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. J Physiol Sci 61: 385–394, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJS, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sports Exerc 35: 221–228, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Crisafulli A, Tangianu F, Tocco F, Concu A, Mameli O, Mulliri G, Caria MA. Ischemic preconditioning of the muscle improves maximal exercise performance but not maximal oxygen uptake in humans. J Appl Physiol 111: 530–536, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Crisafulli A, Tocco F, Milia R, Angius L, Pinna M, Olla S, Roberto S, Marongiu E, Porcu M, Concu A. Progressive improvement in hemodynamic response to muscle metaboreflex in heart transplant recipients. J Appl Physiol 114: 421–427, 2013. [DOI] [PubMed] [Google Scholar]
- 26.Dawn B, Bolli R. Role of nitric oxide in myocardial preconditioning. Ann NY Acc Sci 962: 18–41, 2002. [DOI] [PubMed] [Google Scholar]
- 27.de Groot PCE, Thijssen DHJ, Sanchez M, Ellenkamp R, Hopman MTE. Ischemic preconditioning improves maximal performance in humans. Eur J Appl Physiol 108: 141–146, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deutsch E, Berger M, Kussmaul WG, Hirshfeld JW, Herrman HC, Laskey WK. Adaptation to ischemia during percutaneous transluminal coronary angioplasty: clinical, hemodynamic, and metabolic features. Circulation 82: 2044–2051, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Ferrari M, Muthalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans A Math Phys Eng Sci 369: 4577–45790, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Fisher JP, Adlan AM, Shantsila A, Secher F, Sørensen H, Secher NH. Muscle metaboreflex and autonomic regulation of heart rate in humans. J Physiol 591: 3777–3788, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher JP, Seifert T, Hartwich D, Young CN, Secher NH, Fadel PJ. Autonomic control of heart rate by metabolically sensitive skeletal muscle afferents in humans. J Physiol 588: 1117–1127, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Gardin JM, Dabestani A, Takenaka K, Rohan MK, Knoll M, Russell D, Henry WL. Effect of imaging view and sample volume location on evaluation of mitral flow velocity by pulsed Doppler echocardiogaphy. Am J Cardiol 57: 1335–1339, 1986. [DOI] [PubMed] [Google Scholar]
- 34.Gibson N, White J, Neish M, Murray A,. Effect of ischemic preconditioning on land-based sprinting in team-sport athletes. Int J Sprts Physiol Perform 8: 671–676, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Gledhill N, Cox D, Jamnik R. Endurance athletes' stroke volume does not plateau: major advantage is diastolic function. Med Sci Sports Exerc 26: 1116–1121, 1994. [PubMed] [Google Scholar]
- 36.Hittinger EA, Maher JL, Nash MS, Perry AC, Signorile JF, Kressler J, Jacobs KA. Ischemic preconditioning does not improve peak exercise capacity at sea level or simulated high altitude in trained male cyclists. Appl Physiol Nutr Metab 40: 65–71, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Horiuchi M, Endo J, Thijssen DH. Impact of ischemic preconditioning on functional sympatholysis during handgrip exercise in humans. Physiol Rep 22: 3. pii: e12304, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koole MAC, Aerts A, Praet J, Franken P, Dendale P, Block P. Venous pooling during nitrate-stimulated tilt testing in patients with vasovagal syncope. Europace 2: 343–345, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Ichinose MJ, Sala-Mercado JA, Coutsos M, Li ZH, Ichinose TK, Dawe E, O'Leary DS. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol Heart Circ Physiol 298: H245–H250, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Muscle metaboreflex contribution to sinus node regulation during static exercise. Circulation 100: 27–32, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Jean-St-Michel E, Manlhiot C, Li J, Tropak M, Michelsen MM, Schmidt MR, McCrindle BW, Wells GD, Redington AN. Remote preconditioning improves maximal performance in highly-trained athletes. Med Sci Sports Exerc 43: 1280–1286, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Jones B, Dat M, Cooper CE. Underwater near-infrared spectroscopy measurements of muscle oxygenation: laboratory validation and preliminary observations in swimmers and triathletes. J Biomed Opt 2014; 19: 127002. doi: 10.1117/1.JBO.19.12.127002. [DOI] [PubMed] [Google Scholar]
- 43.Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res, Suppl 61: 160–165, 1987. [PubMed] [Google Scholar]
- 44.Kjeld T, Rasmussen MR, Jattu T, Nielsen HB, Secher NH. Ischemic preconditioning of one forearm enhances static and dynamic apnea. Med Sci Sports Exerc 46: 151–155, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Lalonde F, Curnier DY. Can anaerobic performance be improved by remote ischemic preconditioning? J Strength Cond Res 29: 80–85, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Leesar MA, Stoddard MF, Xuan YT, Tang XL, Bolli R. Nonelectrocardiographic evidence that both ischemic preconditioning and adenosine preconditioning exist in humans. J Am Coll Cardiol 42: 437–445, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Marongiu E, Crisafulli A. cardioprotection acquired through exercise: the role of ischemic preconditioning. Curr Cardiol Rev 10: 336–348, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marongiu E, Piepoli MF, Milia R, Angius L, Pinna M, Bassareo P, Roberto S, Tocco F, Concu A, Crisafulli A. Effects of acute vasodilation on the hemodynamic response to muscle metaboreflex. Am J Physiol Heart Circ Physiol 305: H1387–H1396, 2013. [DOI] [PubMed] [Google Scholar]
- 49.McNully CL, Moody WE, Wagenmakers AJ, Fisher JP. Effect of muscle metaboreflex activation on central hemodynamics and cardiac function in humans. App Physiol Nutr Metab 39: 861–870, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Milia R, Roberto S, Mulliri G, Loi A, Marcelli M, Sainas G, Milia N, Marongiu E, Crisafulli A. Effect of aging on hemodynamic response to metaboreflex activation. Eur J Appl Physiol 115: 1693–1703, 2015. [DOI] [PubMed] [Google Scholar]
- 51.Moran D, Epstein Y, Keren G, Laor A, Sherez J, Shapir Y. Calculation of mean arterial pressure during exercise as a function of heart rate. Appl Human Sci 14: 293–295, 1995. [DOI] [PubMed] [Google Scholar]
- 52.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. [DOI] [PubMed] [Google Scholar]
- 53.Nishiyasu T, Ueno H, Nishiyasu M, Tan N, Morimoto K, Morimoto A, Deguchi T, Murakami N. Relationship between mean arterial pressure and muscle pH during forearm ischemia after sustained handgrip. Acta Physiol Scand 151: 143–148, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Nóbrega ACL, O'Leary DS, Silva BM, Marongiu E, Piepoli MF, Crisafulli A. Neural regulation of cardiovascular response to exercise: role of central command and peripheral afferents. Biomed Res Int 2014; article ID 478965, http://dx.doi.org/10.1155/2014/478965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Paixao RC, da Mota GR, Marocolo M. Acute effect of ischemic preconditioning is detrimental to anaerobic performance in cyclists. Int J Sports Med 35: 912–915, 2014. [DOI] [PubMed] [Google Scholar]
- 57.Pang CY, Yang RZ, Zhong A, Xu N, Boyd B, Forrest CR. Acute ischemic preconditioning protects against skeletal muscle infarction in the pig. Cardiovasc Res 29: 782–788, 1995. [PubMed] [Google Scholar]
- 58.Parratt J, Vegh A. Pronounced antiarrhythmic effects of ischemic preconditioning. Cardioscience 5: 9–18, 1994. [PubMed] [Google Scholar]
- 59.Patterson SD, Bezodis NE, Glaister M, Pattison JR. The effect of ischemic preconditioning on repeated sprint cycling performance. Med Sci Sports Exerc 47: 1652–1658, 2015. [DOI] [PubMed] [Google Scholar]
- 60.Peart JN, Headrick JP. Clinical cardioprotection and the value of conditioning responses. Am J Physiol Heart Circ Physiol 296: H1705–H1720, 2009. [DOI] [PubMed] [Google Scholar]
- 61.Roberto S, Marongiu E, Pinna M, Angius L, Olla S, Bassareo P, Tocco F, Concu A, Milia R, Crisafulli A. Altered hemodynamics during muscle metaboreflex in young, type 1 diabetes patients. J Appl Physiol 113: 1323–1331, 2012. [DOI] [PubMed] [Google Scholar]
- 62.Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O'Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006. [DOI] [PubMed] [Google Scholar]
- 63.Sheriff DD, Augstyniak RA, O'Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998. [DOI] [PubMed] [Google Scholar]
- 64.Shiki K, Hearse DJ. Preconditioning of ischemic myocardium: reperfusion-induced arrhythmias. Am J Physiol Heart Circ Physiol 253: H1470–H1476, 1987. [DOI] [PubMed] [Google Scholar]
- 65.Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P, Hughson RL. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J Appl Physiol 103: 228–233, 1995. [DOI] [PubMed] [Google Scholar]
- 66.Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, Saltin B. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol 470: 693–704, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tocco F, Marongiu E, Ghiani G, Sanna I, Palazzolo G, Olla S, Pusceddu M, Sanna P, Corona F, Concu A, Crisafulli A. Muscle ischemic preconditioning does not improve performance during self-paced exercise. Int J Sports Med 36: 9–15, 2015. [DOI] [PubMed] [Google Scholar]
- 68.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev 83: 1113–1151, 2003. [DOI] [PubMed] [Google Scholar]