Abstract
Chandlerella quiscali is a filarial nematode parasite of the common grackle (Quiscalus quiscula), a widespread bird species found throughout most of North America. Worms collected from wild-caught birds were morphologically identified as C. quiscali and tested for the presence of Wolbachia, an alphaproteobacterial endosymbiont required for reproduction and maturation by many filarial species. Although various methods, including PCR, in situ hybridization and immunohistology, were employed, we were unable to detect evidence of colonization with Wolbachia. Due to the widespread distribution of the grackle host, localization within the host, and high-infection rates, C. quiscali may be among the most easily obtainable of Wolbachia-free filarial species. Further studies of C. quiscali and other Wolbachia-free filarial species may help to shed light on the reason(s) that some filarial species require Wolbachia while others do not.
Filarial nematodes are a biologically diverse superfamily of parasitic worms that infect a wide array of vertebrates including amphibians, reptiles, birds, mammals, and humans. The causative agents of lymphatic filariasis (LF), Wuchereria bancrofti and Brugia malayi) and onchocerciasis (Onchocerca volvulus) infect approximately 150 million people in tropical and subtropical regions (Remme et al., 2006; 2009). New treatments are being sought to prevent and eliminate these infections, as they are important causes of long-term disability (2009).
Attention has recently shifted to Wolbachia endobacteria as a target for anti-filarial drugs. Wolbachia are common reproductive parasites of insects, but they behave as mutualists in many filarial nematode species (Werren et al., 2008). Some of the most important filarial pathogens such as B. malayi, W. bancrofti, Dirofilaria immitis, and O. volvulus, are dependent on Wolbachia endobacteria (order Rickettsiales, family Anaplasmataceae) for growth, fertility, and sometimes even survival (Bandi et al., 1999; Hoerauf et al., 1999; Casiraghi et al., 2002; Chirgwin et al., 2003). Other filarial species are naturally Wolbachia-free and thrive in the absence of a bacterial partner (Plenge-Bonig et al., 1995; Bandi et al., 1998; Chirgwin et al., 2002; Büttner et al., 2003; McGarry et al., 2003; Ferri et al., 2011). The biological mechanisms responsible for this disparity are poorly understood.
Phylogenetic studies may provide insight into the evolutionary history of Wolbachia-filaria relationships and improve understanding of the biological basis of Wolbachia dependence in some species. Early surveys focused mainly on medically and economically important and readily available parasites of humans and domestic animals. Most of these species are Wolbachia-dependent, but most also belong to just 2 of the 10 filarial subfamilies, the Onchocercinae and the Dirofilariinae (Bandi et al., 1998; Casiraghi et al., 2004). A recent study by Ferri et al. examined 35 species from 6 filarial subfamilies for Wolbachia infection, most of which were Wolbachia-free (Ferri et al., 2011). Their findings suggest that Wolbachia-dependent species may be in the minority in many subfamilies and that Wolbachia may be entirely absent in filarial parasites of lizards, frogs and birds.
Here we report results of studies to determine whether Wolbachia is present in Chandlerella quiscali (subfamily Splendidofilariinae), a parasite of the common grackle (Quiscalus quiscula) that was first described by Von Linstow in 1904 (Linstow, 1904). Grackles are widespread in North America east of the Rocky Mountains. This makes C. quiscali accessible throughout most of the United States and Canada. PCR studies, in situ hybridization and immunohistology were performed to detect evidence of colonization with Wolbachia endobacteria, but none was found. Our results offer C. quiscali as a readily available model in the study of Wolbachia-free filarial parasites.
MATERIALS AND METHODS
Parasite materials
Adult C. quiscali were dissected from the cerebral ventricles of euthanized Q. quiscula trapped in North Dakota, USA (Odetoyinbo, 1960). Trapping was conducted under USFW Permit MB072162, ND Game & Fish Permit GNF02344201, and University of North Dakota IACUC protocol 0705-3c. Adult B. malayi and Acanthocheilonema viteae were obtained from experimentally infected Mongolian gerbils as previously described (Ash et al., 1970; Lucius et al., 1995). Adult O. volvulus, adult Onchocerca flexuosa and W. bancrofti microfilariae (mf) were available from previous studies (Fischer et al., 1993; Weil et al., 2008; McNulty et al., 2010). Captive bred field crickets (Gryllus bimaculatus) were purchased from a pet shop in Hamburg, Germany.
For PCR studies, harvested worms were frozen in PBS or Trizol (Invitrogen, Carlsbad, California) at −80 C until use. For in situ hybridization and immunohistology, adult worms and crickets were fixed for 24–72 hr in 4% buffered formaldehyde, embedded in paraffin, and sectioned using standard histological procedures. For morphological examination, adult worms were killed with heated saline, preserved in 70% ethanol, and cleared in lactophenol. Worms were studied using an Olympus BX-51 compound light microscope equipped with DIC optics and a digital imaging system. Measurements were obtained using Rincon software (v. 7.1.2, Imaging Planet, Goleta, California).
DNA isolation
DNA was isolated from B. malayi, O. volvulus, O. flexuosa, A. viteae and C. quiscali adult worms, W. bancrofti mf and G. bimaculatus formalin-fixed, paraffin embedded histological sections. The DNeasy Blood and Tissue Kit was used to isolate DNA from adult worms according to the manufacturer’s suggested protocol (Qiagen). For G. bimaculatus, paraffin was removed from the tissue by 2, 30 minute incubations with 100% xylene, 2, 30 minute incubations with 100% ethanol, 2, 30 minute incubations with 75% ethanol, and 2, 15 minute incubations with 1× PBS. After removing the final PBS wash, tissue was re-suspended in 15 µl 1× PBS and subjected to alkaline lysis. The same alkaline lysis protocol was used to obtain DNA from W. bancrofti mf. Briefly, tissue was brought to a final volume of 15 µl in sterile water. Fifteen µl alkaline lysis buffer (400 mM KOH, 100 mM DTT and 10 mM EDTA) was added, and the mixture was heated to 95 C for 30 min before chilling on ice for 10 min. The solution was neutralized with 15 µl of neutralization buffer (400 mM HCl and 600 mM Tris-HCl pH 7.5). One µl of lysate or 10 ng purified DNA served as template in PCR reactions.
PCR reactions
Primers used to amplify the 5S ribosomal intergenic spacer were S2 5’-GTTAAGCAACGTTGGGCCTGG-3’ and S16 5’-TTGACAGATCGGACGAGATG-3’ (Fischer et al., 1998). Primers used to detect Wolbachia endobacteria are reported in Table I. Wolbachia primers are designed to match portions of the most highly conserved Wolbachia genes (Holman et al., 2009). The 5s intergenic spacer was cloned using the TOPO-TA cloning kit for sequencing (Invitrogen) and sequenced.
Table I.
PCR primer sequences used to detect Wolbachia endobacteria (5’-3’)
| 16s Universal | CCAGTGGCGAAGGCGTCTAT | CCCCGTCAATTCCTTTGAGTTT |
| RpoB/C | TTCTGGCTCTGGTGCTGTAG | AACCTTGCCAAGACATAAAAGC |
| FusA | GATGGTGCAGCTTCTATGGAT | GCAACTCCATCAAATACAGCAA |
| SucDyd | CAGGTGGATATGGACGTGTT | CCATATATTCCTGTTGGATGAAA |
In situ hybridization
A 424bp segment of the 16S rDNA gene of the Wolbachia endosymbiont of B. malayi was amplified from B. malayi genomic DNA using the following primers: 16sF 5’-CAGCTCGTGTCGTGAGATGT-3’ and 16sR ‘5-CCCAGTCATGATCCCACTT-3’ (Fischer et al., 2011). Biotinylated antisense and sense (negative control) RNA probes were constructed from this sequence as previously described (Fischer et al., 2011).
For hybridization, 5µ histological sections were deparaffinized, digested in pepsin HCl for 7 min and hybridized overnight at 37 C in humid chambers with 1 µg of probe in hybridization buffer (50% formamide, 5× SSC, 0.3 mg/ml yeast tRNA, 100 µg/ml heparin, 1× Denhardt’s solution, 0.1% CHAPS, and 5 mM EDTA). A stringency wash was performed at 60 C for 30 min, and detection was carried out using the ‘In situ Hybridization Detection System’ (Dako, Carpinteria, California). Sections were incubated with streptavidin-AP conjugate for 20 min at room temperature and with BCIP/NBT for 10–20 min to localize bound probe.
Immunohistochemistry
The alkaline phosphatase anti-alkaline phosphatase (APAAP) technique was used for immunostaining according to the recommendations of the manufacturer (Dako) and as described previously (Rao et al., 2009; Fischer et al., 2011). A monoclonal antibody directed against Wolbachia surface protein (WSP) of B. malayi was used as the primary antibody at a dilution of 1:100. A monoclonal antibody directed against human heat shock protein 60 (mAb HSP 60 LK2, Sigma, St. Louis, Missouri) was used as a positive control at a dilution of 1:5, as our prior studies have shown that it binds to Wolbachia and filarial mitochondria.
RESULTS
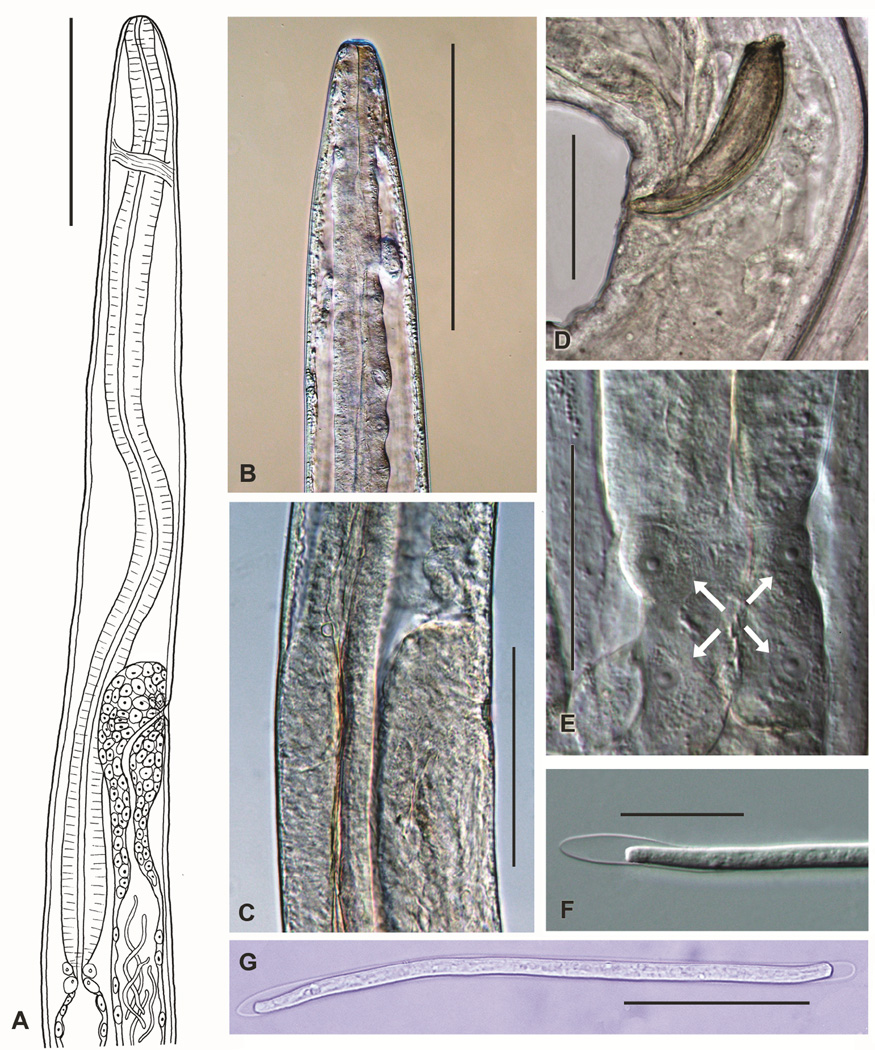
Based on morphological examination of adult and mf stages (Fig. 1), the grackle parasites that were used in this study belong to the genus Chandlerella. Details of adult morphology, such as the rounded shape of the anterior end (Fig. 1A,B), relative position of the nerve ring (Fig. 1A), position of the vulva relative to the end of the esophagus (Fig. 1A), presence of characteristic large cells at the junction between the esophagus and the intestine (Fig. 1E), and, most importantly, the configuration of the distal portion of the uterus (Fig. 1A,C), confirmed that the worms were C. quiscali. The distal portion of the uterus does not extend anteriorly beyond the vulva in C. quiscali. In contrast, the uterus extends anteriorly well beyond the vulva and then loops posteriorly to reach the vulva in closely related species,. Likewise, the length of spicules in males in our material (left spicule 75 µm, right spicule 82 µm) is identical to that of C. quiscali and different from the length of spicules in closely related species (Fig. 1D) Bartlett et al., 1980). Microfilariae in the blood were long (182–197 µm) and sheathed, with blunt, very slightly tapered tails (Fig. 1F,G), and this corresponds to the description of C. quiscali mf reported by Bartlett and Anderson (Bartlett et al., 1980). Localization of adult worms in the grackle brain also supports the identity of these parasites as C. quiscali.
FIGURE 1.
Morphological characteristics of C. quiscali. Figure depicts a line drawing of the anterior end of the female demonstrating the relative position of the vulva, esophagus and nerve ring (A), the anterior end of C. quiscali (B), the region of the vulva (C) (note that the uterus does not make a loop anterior to the vulva), the posterior end of male with spicules (D), the of junction between esophagus and intestine (E) with characteristic large cells indicated with arrows, and sheathed microfilaria of C. quiscali from blood of common grackle (F,G). Scale-bars: A, B = 250 µm, C = 150 µm, D, E, G = 50 µm, F = 25 µm.
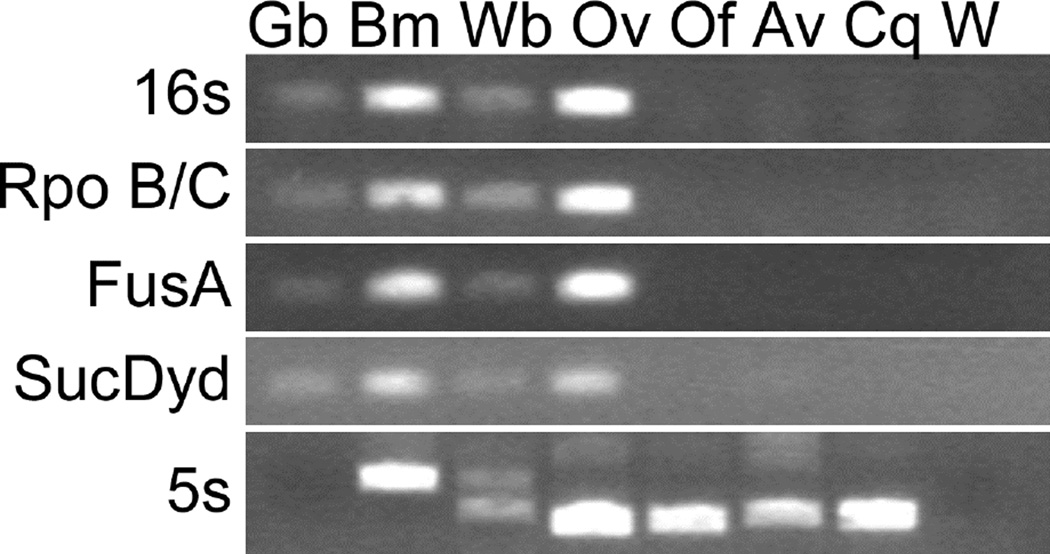
DNA samples from worms taken from separate birds were tested by PCR with primer sets targeting Wolbachia 16s rRNA and other highly conserved Wolbachia genes, including the DNA-directed RNA polymerase (Rpo B/C), translation elongation factor GTPase (FusA), and succinate dehydrogenase (SucDyd) (Fig. 2). Primer sets previously used to demonstrate the absence of Wolbachia in L. loa were also tested (data not shown) (McGarry et al., 2003). All primer sets employed in this study amplified Wolbachia sequences from infected species (crickets, B. malayi, W. bancrofti, and O. volvulus) but not from Wolbachia-free filarial species (O. flexuosa and A. viteae) (Plenge-Bonig et al., 1995; Bandi et al., 1998) or C. quiscali. The filarial 5s rRNA intergenic spacer was amplified and sequenced as a positive control to ensure the quality of the C. quiscali genomic DNA. This sequence was deposited in Genbank under the accession number HM641830.
FIGURE 2.
Results of a PCR-based search for evidence of colonization with Wolbachia endobacteria in various species. Template species are designated as follows: Gb, field cricket Gryllus bimaculatus; Bm, B. malayi; Wb, W. bancrofti; Ov, O. volvulus; Of, O. flexuosa; Av, A. viteae; Cq, C. quiscali; W, water only (a no template control).
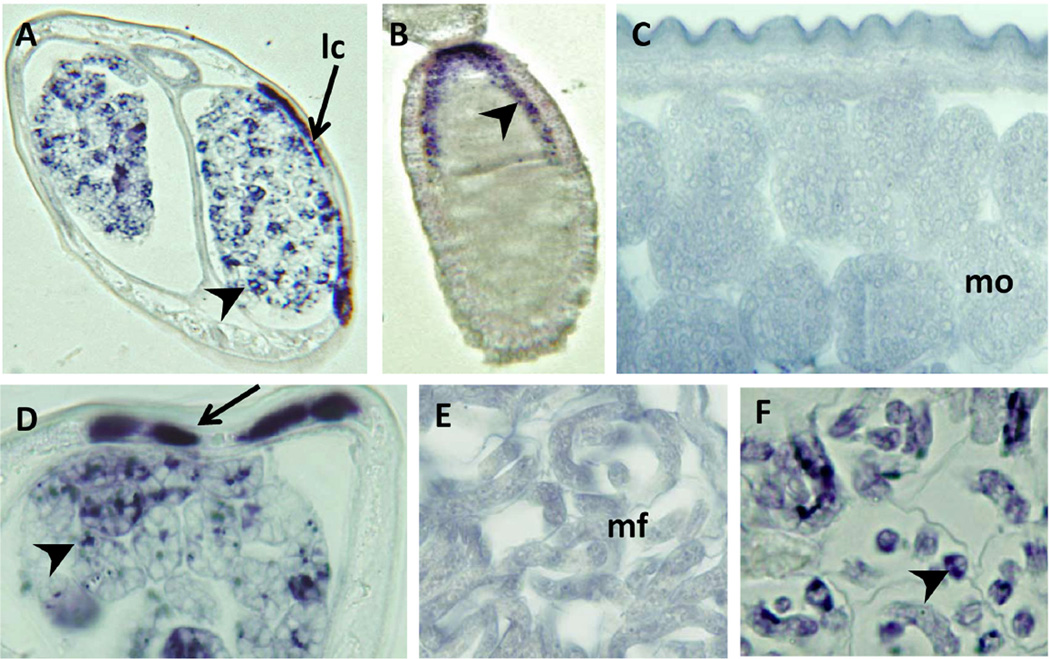
An in situ hybridization probe designed to bind highly conserved portions of Wolbachia 16s rRNA was also used to test for the presence of the endobacteria in C. quiscali (Fig. 3). This probe is able to detect a wide array of Wolbachia strains, including those in B. malayi (Figs. 3A, D, F) and in the cricket G. bimaculatus (Fig. 3B). Although dozens of sections were examined, no staining was detected in C. quiscali (Figs. 3C, E).
FIGURE 3.
In situ hybridization using a probe for the 16s rRNA of Wolbachia endobacteria. The 16s probe bound to Wolbachia in the lateral chords (A,D), early (A) and late (D) morulae, and microfilariae (F) of B. malayi. It also bound Wolbachia in the developing eggs of the cricket G. bimaculatus (B). No staining was detected in the late morulae (C) or microfilaria (E) of C. quiscali. Scale bars are all 25 µm. Species are designated within the figure as follows: Bm, B. malayi; Gb, G. bimaculatus; Cq, C. quiscali.
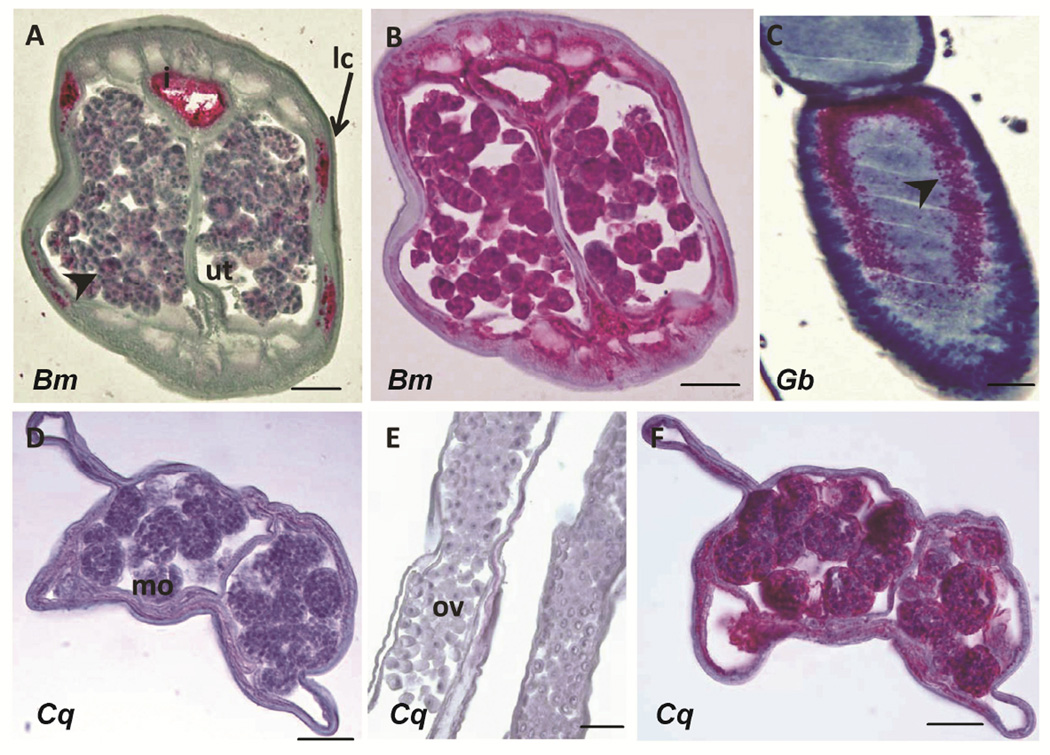
Likewise, a monoclonal antibody against a Wolbachia surface protein was used to stain the Wolbachia in B. malayi (Fig. 4A) and G. bimaculatus (Fig. 4C), but this antibody did not produce a signal in C. quiscali (Figs. 4D, E). A positive control antibody against hsp60 that labels filarial mitochondria did produce a signal in C. quiscali (Figs. 4B, F). This positive control indicates that the C. quiscali material was intact and suitable for antibody staining.
FIGURE 4.
Immunohistological detection of Wolbachia surface protein (WSP). WSP antibody labeled Wolbachia in the lateral chords (lc) and morula stage embryos in the uterus (ut) of B. malayi (A) and in developing eggs of G. bimaculatus (C). No staining was detected in morula stage (mo) embryos (D) or the ovaries (ov) (E) of C. quiscali. A positive control Hsp60 antibody labeled mitochondria in both B. malayi (B) and C. quiscali (F). Scale bars are all 25 µm. Species are designated within the figure as follows: Bm, B. malayi; Gb, G. bimaculatus; Cq, C. quiscali.
DISCUSSION
The worms used in this study were identified as C. quiscali based on their morphologic features, host species, and localization within the host (Odetoyinbo, 1960; Anderson et al., 1976; Bartlett et al., 1980). These characteristics place species of the genus Chandlerella in the subfamily Splendidofilariinae (Anderson et al., 1976). C. quiscali is one of only two species of this subfamily to have been formally examined for Wolbachia infection (Ferri et al., 2011).
PCR with different primer sets, in situ hybridization, and immunohistology all indicated that C. quiscali does not contain Wolbachia. The genes targeted by PCR were among the most highly conserved among all Wolbachia strains, as indicated by the fact that they were able to amplify Wolbachia sequences present in a diverse array of host species including both insects and filarial nematodes. The in situ probe against Wolbachia 16s rRNA and the antibody against WSP also detected highly conserved targets in a wide array of filarial and insect Wolbachia strains. Similar methods have been used to confirm the Wolbachia-free nature of other filarial species, including Loa loa and O. flexuosa (Plenge-Bonig et al., 1995; Büttner et al., 2003; McGarry et al., 2003).
Our understanding of the co-evolutionary dynamics of Wolbachia in filarial parasites will benefit from wider sampling of parasite species representing different subfamilies of Onchocercidae. To-date, 54 species of Onchocercid species have been examined for Wolbachia, of which 26 (48%) harbor Wolbachia (McLaren et al., 1975; Taylor et al., 1999; Casiraghi et al., 2004; Ferri et al., 2011). Most (72%) of the species examined to-date belong to a single subfamily, Onchocercinae. Because there is good representation within this subfamily, some patterns can be discerned when comparing parasite genera. For example, 13 of 14 (93%) species tested within the genus Onchocerca harbor Wolbachia, whereas only 1 of 9 (11%) species tested within the genus Cercopithifilaria harbor Wolbachia. This difference in proportions is statistically significant difference (Fisher's exact test, p=0.002). The two subfamilies that contain most avian filarioid species (Splendidofilarinae and Lemdaninae) have not been extensively examined for Wolbachia symbionts. Avian filarioids (ca. 15 genera) may be among the most widely distributed of the filarioid parasites because of their hosts' unique ability to fly, yet only two avian species, an unidentified Aproctella species (Ferri et al., 2011) and Chandlerella quiscali, both Splendidofilarinae, have been studied. Both of these species are Wolbachia free. More species will need to be examined in order to determine if this is true of all avian filaria.
Future studies should not only include examination of a wider array of filarial species, but a more in-depth examination of species that have already been characterized as Wolbachia-free. Our previous studies uncovered evidence of horizontal gene transfer from Wolbachia endobacteria to two species that are currently Wolbachia-free (O. flexuosa and A. viteae,). This indicates that their ancestors must have been infected with Wolbachia (McNulty et al., 2010). Genomic surveys of other Wolbachia-free species like C. quiscali will help to determine whether Wolbachia was present in their lineage in the past.
Now that C. quiscali has been experimentally shown to lack Wolbachia-endobacteria, it can be used as a model for studies of Wolbachia-free filarial parasites. C. quiscali has certain attributes that may make it an attractive model system for such studies. First, its host, the common grackle, is abundant and synanthropic, it is easy to trap, and it does well in captivity. Local infection rates, particularly in forested regions where vector [ornithophilic Culicoides] populations are high) can approach 100% (Welker, 1962; Granath, 1980; Johnson, 1984). Moreover, unlike many avian filarids whose adult stages are small and often difficult to locate within their hosts, C. quiscali adults are easily found within the ventricles of the brain. In depth studies of readily available Wolbachia-free species like C. quiscali may help to determine why some filarial nematodes require Wolbachia for fertility and maturation while others do not.
Acknowledgments
The authors thank Dr. Daojun Jiang (Washington University School of Medicine) for providing PCR primers for the amplification of the 16s rRNA in situ probe and Dr. Patrick Lammie (Center for Disease Control) for providing the anti-WSP antibody. This work was supported by NIH grant T32-AI007172 to S. McNulty and by Barnes-Jewish Hospital Foundation Grant No. 01251-060.
LITERATURE CITED
- Global programme to eliminate lymphatic filariasis. The Weekly Epidemiological Record. 2009;84:437–444. [PubMed] [Google Scholar]
- Anderson RC, Bain O. Keys to genera of the order Spirurida. Part 3. Diplotriaenoidea, Aproctoidea and Filarioidea. In: Anderson RC, Chabaud AG, Willmott S, editors. CIH keys to the nematode parasites of vertebrates. Vol. 3. Farnham Royal, UK: Commonwealth Agricultural Bureau; 1976. pp. 59–116. [Google Scholar]
- Ash LR, Riley JM. Development of subperiodic Brugia malayi in the jird, Meriones unguiculatus with notes on infections in other rodents. The Journal of Parasitology. 1970;56:969–973. [PubMed] [Google Scholar]
- Bandi C, Anderson TJC, Genchi C, Blaxte ML. Phylogeny of Wolbachia in filarial nematodes. Proceedings of the Royal Society of London Series B, Biological Sciences. 1998;265:2407–2413. doi: 10.1098/rspb.1998.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandi C, McCall JW, Genchi C, Corona S, Venco L, Sacchi L. Effects of tetracycline on the filarial worms Brugia pahangi and Dirofilaria immitis and their bacterial endosymbionts Wolbachia. International Journal for Parasitology. 1999;29:357–364. doi: 10.1016/s0020-7519(98)00200-8. [DOI] [PubMed] [Google Scholar]
- Bartlett CM, Anderson RC. Development of Chandlerella chitwoodae Anderson, 1961 (Filarioidea: Onchocercidae) in Culicoides Stilobezzioides Foote and Pratt and C. travisi Vargas (Diptera: Ceratopogonidae) Canadian Journal of Zoology. 1980;58:1002–1006. doi: 10.1139/z80-140. [DOI] [PubMed] [Google Scholar]
- Büttner DW, Wanji S, Bazzocchi C, Bain O, Fischer P. Obligatory symbiotic Wolbachia endobacteria are absent from Loa loa. Filaria Journal. 2003;2:10. doi: 10.1186/1475-2883-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casiraghi M, Bain O, Guerrero R, Martin C, Pocacqua V, Gardner SL, Franceschi A, Bandi C. Mapping the presence of Wolbachia pipientis on the phylogeny of filarial nematodes: Evidence for symbiont loss during evolution. International Journal for Parasitology. 2004;34:191–203. doi: 10.1016/j.ijpara.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Casiraghi M, McCall JW, Simoncini L, Kramer LH, Sacchi L, Genchi C, Werren JH, Bandi C. Tetracycline treatment and sex-ratio distortion: A role for Wolbachia in the moulting of filarial nematodes? International Journal for Parasitology. 2002;32:1457–1468. doi: 10.1016/s0020-7519(02)00158-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin SR, Nowling JM, Coleman SU, Klei TR. Brugia pahangi and Wolbachia: The kinetics of bacteria elimination, worm viability, and host responses following tetracycline treatment. Experimental Parasitology. 2003;103:16–26. doi: 10.1016/s0014-4894(03)00063-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin SR, Porthouse KH, Nowling JM, Klei TR. The filarial endosymbiont Wolbachia sp. Is absent from Setaria equina. The Journal of Parasitology. 2002;88:1248–1250. doi: 10.1645/0022-3395(2002)088[1248:TFEWSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ferri E, Bain O, Barbuto M, Martin C, Lo N, Uni S, Landmann F, Baccei SG, Guerrero R, Lima SDS, et al. New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PLoS One. 2011;6:e20843. doi: 10.1371/journal.pone.0020843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Beatty WL, Jiang D, Weil GJ, Fischer PU. Tissue and stage-specific distribution of Wolbachia in Brugia malayi. PLoS Neglected Tropical Diseases. 2011;5:e1174. doi: 10.1371/journal.pntd.0001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer PU, Buttner DW, Bamuhiiga J, Williams SA. Detection of the filarial parasite Mansonella streptocerca in skin biopsies by a nested polymerase chain reaction-based assay. The American Journal of Tropical Medicine and Hygiene. 1998;58:816–820. doi: 10.4269/ajtmh.1998.58.816. [DOI] [PubMed] [Google Scholar]
- Fischer PU, Kipp W, Bamuhiga J, Binta-Kahwa J, Kiefer A, Buttner DW. Parasitological and clinical characterization of Simulium neavei-transmitted onchocerciasis in western Uganda. Annals of Tropical Medicine and Parasitology. 1993;44:311–321. [PubMed] [Google Scholar]
- Granath WO. Fat of the wild avian filarial nematode Chandlerella quiscali (Onchocercidae: Filarioidea) in the domestic chicken. Poultry Science. 1980;59:996–1000. doi: 10.3382/ps.0590996. [DOI] [PubMed] [Google Scholar]
- Hoerauf A, Nissen-Pähle K, Schmetz C, Henkle-Dührsen K, Blaxter ML, Büttner DW, Gallin MY, Al-Qaoud KM, Lucius R, Fleischer B. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. The Journal of Clinical Investigation. 1999;103:11–18. doi: 10.1172/JCI4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman AG, Davis PJ, Foster JM, Carlow CK, Kumar S. Computational prediction of essential genes in an unculturable endosymbiotic bacterium, Wolbachia of Brugia malayi. BMC Microbiology. 2009;9:243. doi: 10.1186/1471-2180-9-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AA. Helminths of common grackles (Quiscalus quiscula-versicolor, vieillot) in central arkansas. Arkansas Acedemy of Science Proceedings. 1984;38:53–55. [Google Scholar]
- Linstow OFBV. Beobachtungen an nematoden und cestoden. Archiv für Naturgeschichte Berlin. 1904;70:297–309. [Google Scholar]
- Lucius R, Textor G. Acanthocheilonema viteae: Rational design of the life cycle to increase production of parasite material using less experimental animals. Applied Parasitology. 1995;36:22–33. [PubMed] [Google Scholar]
- McGarry HF, Pfarr K, Egerton G, Hoerauf A, Akue J-P, Enyong P, Wanji S, Kläger SL, Bianco AE, Beeching NJ, et al. Evidence against Wolbachia symbiosis in Loa loa. Filaria Journal. 2003;2:9. doi: 10.1186/1475-2883-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DJ, Worms MJ, Laurence BR, Simpson MG. Micro-organisms in filarial larvae (Nematoda) Transactions of the Royal Society of Tropical Medicine and Hygiene. 1975;69:509–514. doi: 10.1016/0035-9203(75)90110-8. [DOI] [PubMed] [Google Scholar]
- McNulty SN, Foster JM, Mitreva M, Hotopp JCD, Martin J, Fischer K, Wu B, Davis PJ, Kumar S, Brattig NW, et al. Endosymbiont DNA in endobacteria-free filarial nematodes indicates ancient horizontal genetic transfer. PLoS One. 2010;5:e11029. doi: 10.1371/journal.pone.0011029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odetoyinbo JA. Ph.D. Thesis. Ames, Iowa: Iowa State University of Science and Technology; 1960. Biology of Splendidofilaria quiscai (von linstow, 1904) n. Comb. (nematoda: Onchocercindae) [Google Scholar]
- Plenge-Bonig A, Kromer M, Buttner DW. Light and electron microscopy studies on Onchocerca jakutensis and O. flexuosa of red deer show different host-parasite interactions. Parasitology Research. 1995;81:66–73. doi: 10.1007/BF00932419. [DOI] [PubMed] [Google Scholar]
- Rao RU, Huang Y, Fischer K, Fischer PU, Weil GJ. Brugia malayi: Effects of nitazoxanide and tizoxanide on adult worms and microfilariae of filarial nematodes. Experimental Parasitology. 2009;121:38–45. doi: 10.1016/j.exppara.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Remme JHF, Feenstra P, Lever PR, Medici AC, Morel CM, Noma M, Ramaiah KD, Richards F, Seketeli A, Schmunis G, et al. Tropical diseases targeted for elimination: chagas disease, lymphatic filariasis, onchocerciasis, and leprosy. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrov P, editors. Disease control programs in developing countries. New York: Oxford University Press; 2006. [Google Scholar]
- Taylor MJ, Hoerauf A. Wolbachia bacteria of filarial nematodes. Parasitology Today. 1999;15:437–442. doi: 10.1016/s0169-4758(99)01533-1. [DOI] [PubMed] [Google Scholar]
- Weil GJ, Kastens W, Susapu M, Laney SJ, Williams SA, King CL, Kazura JW, Bockarie MJ. The impact of repeated rounds of mass drug administration with diethylcarbamazine plus albendazole on Bancroftian filariasis in Papua New Guinea. PLoS Neglected Tropical Diseases. 2008;2:e344. doi: 10.1371/journal.pntd.0000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker GW. Ph.D. Thesis. Columbus, Ohio: Ohio State University; 1962. Helminth parasites of the common grackle, Quiscalus quiscula versicolor veillot in indiana. [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: Master manipulators of invertebrate biology. Nature Reviews Microbiology. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]