Abstract
Steroid hormone receptors (SHRs) act in cell type- and gene-specific manner through interactions with coregulatory proteins to regulate numerous physiological and pathological processes at the level of gene regulation. Binding of steroid receptor modulator (SRM) ligand leads to allosteric changes in SHR to exert positive or negative effects on the expression of target genes. Due, in part, to the fact that current SRMs generally target ligand binding domain (LBD)/AF2 and neglect intrinsically disordered (ID) N-terminal domain (NTD)/AF1, clinically relevant SRMs lack selectivity and are also prone to the development of resistance over time. Therefore, to maximize the efficacy of SHR-based therapeutics, the possibility of developing unique modulators that act to control AF1 activity must be considered. Recent studies targeting androgen receptor's (AR's) ID AF1 domain for the castration-resistant prostate cancer has provided the possibility of therapeutically targeting ID NTD/AF1 surfaces by allosteric modulations to achieve desired effects. In this review article, we discuss how inter- and intra- molecular allosteric regulations controlled by AR's structural flexibility and dynamics particularly the ID NTD/AF1 is an emerging area of investigation, which could be exploited for drug development and therapeutic targeting of prostate cancer.
Keywords: allosteric regulation, coregulatory proteins, endocrine cancers, intrinsically disordered proteins, steroid hormone receptors, structural dynamics, transactivation activity
INTRODUCTION
The actions of androgens such as testosterone and dihydrotestosterone are mediated via the androgen receptor (AR), a ligand-activated intracellular transcription factor belonging to the family of steroid hormone receptors (SHRs).1,2 The SHRs act in cell type- and gene-specific manners to regulate numerous physiological and pathological processes including carbohydrate metabolism, lipid metabolism, inflammation, cancer, and cardiovascular disease.3,4,5 It is well known that steroid hormones exert most of their biological effects through their receptors at the level of gene regulation.6,7,8 The SHRs pass signals from a steroid/hormone to the target genes by interacting with specific response element DNA sequences and various coregulatory proteins that consist of activators and/or corepressors.9,10,11 Because the outcome of SHR transcriptional complexes is determined regarding the target genes expression, any dysregulation of SHR function may perturb normal homeostasis resulting into the development of malignant phenotypes.12,13,14 The structural organization of SHRs and its classical mechanisms of actions (Figure 1) suggests the presence of at least three major functional domains: N-terminal (NTD)-, DNA binding (DBD)-, and C-terminal ligand binding (LBD)- domain.15 Within the NTD lies a constitutively active, activation function-1 (AF1) and AF2 in the LBD that acts in a ligand-dependent manner. It is well established that the transcriptional activities of both AF1 and AF2 are regulated through their respective interactions with a specific set of coregulatory proteins including coactivators and corepressors.16,17,18,19 Although either AF1 or AF2 alone may be capable of regulating transcription to some extent, full transcriptional activation by SHRs usually requires functional synergy between them.20
Figure 1.
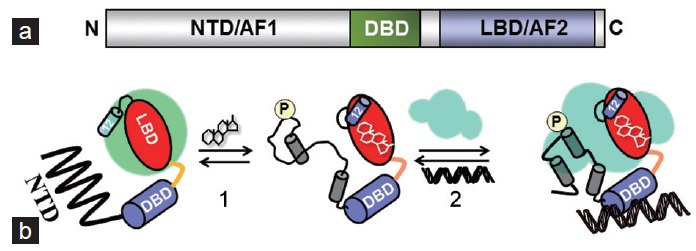
General dynamics and mechanism of SHR action. (a) Schematic representation of the domain organization of SHRs, showing the LBD, DBD and the structurally distinct NTD. (b) In the absence of hormone, the SHR is complexed with co-chaperone molecules (green) in the cytoplasm. The NTD/AF1 exists in an ID conformation, compared with the well-ordered DBD and LBD. Potential phosphorylation sites in the NTD indicated as (P). Other potential posttranslational modifications such as acetylation, methylation, and sumoylation are not shown. The binding of hormone (1) causes structural rearrangement leading to translocation to the nucleus and binding to DNA response elements and coregulatory proteins (light blue) (2). During this process, NTD/AF1 undergoes disorder-order transition, resulting in the folding of NTD/AF1. In this folded conformation, AF1/NTD surfaces are well suited for the interaction with specific coactivators, binding of which further influences the conformation of NTD/AF1 and facilitates the assembly of the transcription initiation complex in a promoter-specific manner.
There is still a major gap in our understanding of the regulatory mechanisms by which AR functions in a tissue- and gene-specific manner remain unclear, which has hampered drug discovery with improved tissue and target gene selectivity. The AR is an important therapeutic target in prostate cancers, and the “Holy Grail” for AR as therapeutic targets is the potential to confine their actions by small-molecule ligands/hormones to specific cell/tissue types and gene targets.21 Binding of agonistic or antagonistic ligands leads to different allosteric changes in AR, allowing them to exert positive or negative effects on the expression of target genes in a differential manner depending on the physiological and genetic context of the cell.22,23,24,25,26,27 The most widely used small-molecule ligands target the ligand binding pocket and exhibit varying degrees of agonist or antagonist activities associated AF2 orientation.28
However, current steroid receptor modulators (SRMs) generally lack this level of selectivity and have the additional problem of the development of resistance over time.29 This has emerged as a major clinical problem, requiring the development of novel agents to circumvent resistance in many cancer patients including castration-resistant prostate cancer. This, in part, is because rational structure-based design of SRMs has by necessity been limited to available crystal structures of LBD and thereby neglecting the highly flexible and intrinsically disordered (ID) NTD for which no refined three-dimensional structure is available as yet.30,31,32 This is despite the fact that NTD/AF1 is functionally important for tissue-specific activities of SHRs and has a critical role in allosteric modulation of intact receptors through its structural dynamics and flexibility.33 It is, therefore, critical to investigate the possibility of development of modulators that act to control NTD/AF1 activity. However, until recently, NTD/AF1 was thought to be an unattractive drug target, mainly due to its ID conformation.33 Recently, a small-molecule was found to interact with NTD/AF1 of the AR and differentially disrupt both AF1-coactivator binding and subsequent AF1-regulated target gene expression in castration-resistant prostate cancer.34,35 The significance of these findings lies in the possibility of therapeutically targeting ID NTD/AF1 surfaces directly or indirectly by allosteric modulations to achieve desired effects.36,37,38 Therefore, a better understanding of structure/function properties of the NTD/AF1 is essential for facilitating the development of improved therapeutic targeting of AR in prostate cancer. In this review article, we discuss how structural flexibility and dynamics of the AR, particularly the NTD/AF1, is an emerging area of investigation, which could be exploited for drug development and therapeutic targeting of prostate cancer.
ROLE OF ANDROGEN RECEPTOR IN PROSTATE CANCER
Prostate cancer contains phenotypically and functionally distinct cells, and this cellular heterogeneity poses clinical challenges as the distinct cell types likely respond differently to various therapies. The AR is present in many human tissues; and the AR-signaling pathway is essential for maintaining normal metabolic function, cell proliferation, and homeostasis and is an important component in the early pathogenesis of prostate cancer.39 Most of the biological effects of androgens are mediated through the androgen receptor (AR) at the level of gene regulation.40 The cellular growth of neoplastic prostate cells is dependent on the androgen-AR interaction, which plays a pivotal role in the progression of metastatic prostate cancer.41 This AR signaling axis is considered to be the main target for hormonal therapy and has extensively been exploited for the treatment of prostate cancer. Therefore, therapeutic targeting of AR has provided a useful remedial tool to develop hormone-based therapies for the management of prostate cancer and has extensively been exploited for the treatment of prostate cancer.42 Clinical studies have been investigated to determine the potential of AR as prognosis or diagnostic markers for prostate cancer.42,43,44,45,46 Dissection of the signaling pathways responsible for AR activities has made a major contribution to the treatment of prostate cancer, and small-molecule, SRMs have been used as therapeutic tools using their cell/tissue-specific partial agonist/antagonist activities.30,31,32
Similarly, the AR and the modulators of its activity play a critical role in prostate cancer development and progression,47,48 and various endocrine-based therapies are directed toward inhibiting AR activity.49,50 The androgen biosynthesis inhibitor abiraterone and the anti-androgen enzalutamide have been shown to prolong survival in randomized clinical trials both pre- and post-chemotherapy and are now in routine clinical uses. With the use of these drugs and other novel survival-prolonging therapeutics, patients with advanced prostate cancer are now living longer with better quality of life. However, there are reports suggesting that a significant proportion of prostate cancer may be resistant to androgen ablation for which the exact mechanism is still under investigation.51,52 It is well appreciated that AR heterogeneity becomes more pronounced in castration-resistant prostate cancers than in the primary tumors and activation of alternative AR signaling in prostate cancer cells may promote cell proliferation under androgen-deprived environment.53,54
ROLE OF INTRINSICALLY DISORDERED REGIONS/DOMAINS IN THE STRUCTURAL DYNAMICS OF THE AR
The structural analyses of SHR proteins illustrate the complexity of multiple mechanisms of allosteric regulation imparted upon SRMs, DNA, or coregulatory protein bindings. The consequences of such allosteric regulations can result into multiple SHR surfaces that can be engaged in protein-protein interactions in a selective and reversible manner. These interactions coupled with allosteric regulations seem to provide the possible means that could influence the SHRs’ target-specific transcriptional activity (Figure 2). The significance of these findings lies not just in providing evidence for tissue/cell-specific selectivity of AF1 and AF2, but also in illustrating the possibility of therapeutically targeting these receptor functions to achieve tissue-restricted effects. Until recently, the prevalent view in biology was that the specific function and its potential as a drug target of a given protein is determined by its unique globular structure, the so-called “lock and key” hypothesis. However, in recent years, we have learned that many important transcription factor proteins possess large stretches of amino acid sequences that do not automatically adopt a well-defined three-dimensional structure rather they exist as dynamic ensembles of interconverting conformers that collectively appear to be unstructured. These unstructured protein regions have been termed “intrinsically disordered” (ID) under specific physiological conditions (Figure 3).55,56,57,58,59,60 The significance of such ID domains/regions is that their conformational flexibility creates large protein surfaces that allow macromolecular interactions with high specificity and low affinity through coupled binding and folding, an important functional property of SHRs in gene regulation.55,56,57,58,59,60
Figure 2.
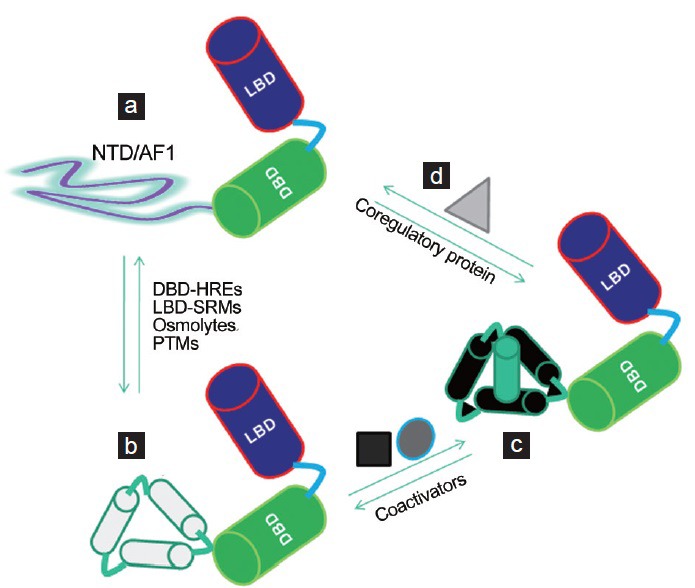
Factors affecting disorder-order transition of the SHRs’ AF1/NTD. (a) As such NTD/AF1 exists in an ID conformation compared to well-ordered DBD and LBD. (b) Under the influence of factors shown, NTD/AF1 undergoes disorder-order transition, resulting into the folding of NTD/AF1. (c) In this conformation, AF1/NTD surfaces are well suited for its interaction with specific coactivators, binding of which further influences the conformation of NTD/AF1 and facilitates the formation of AF1-coactitors assembly in a promoter-specific manner to regulate the expression of target gene(s). (d) In cells/tissues where there is an excess level of expression of an AF1 binding partner (e.g., proteins from basal transcription machinery), AF1 can directly interact with it and regulate the expression of target gene(s).
Figure 3.
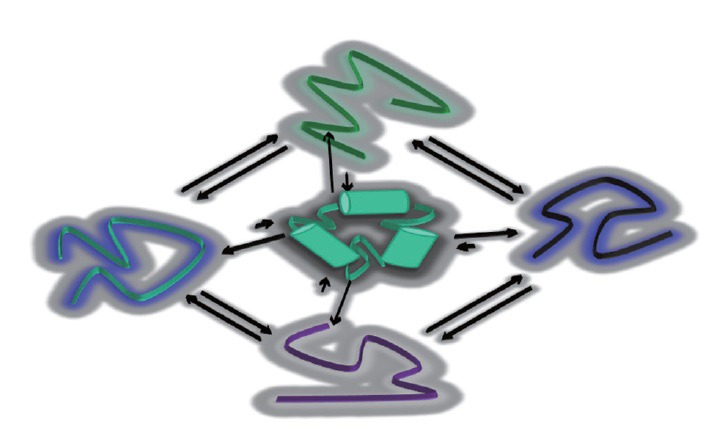
ID sequences exist in an ensemble of conformers, which collectively appear to be unstructured. Each conformer is in a reversible equilibrium with each other. Except for a very small fraction, which may be relatively well ordered (shown in center), all other conformers possess the characteristics of random coil. However, the equilibrium is always shifted towards random conformers.
It is now well-accepted fact that these ID regions/domains promote molecular recognition such that ID regions can undergo disorder-order transition through these interactions.55,56 It has been predicted that almost 75% of cancer-associated proteins possess long ID regions/domains, which play a critical role in cell cycle control, transcriptional and translational regulation, and signal transduction.61,62,63,64 The amino acid sequences of the NTD of the SHRs have poor sequence homology and are much less conserved than DBD and LBD.61 Despite this, NTD of all the SHRs, which contain AF1, are reported to exist as ID sequences.61 Due to their regulation through specific interactions, SHRs are tightly regulated through the allosteric coupling, and SHRs’ ID AF1/NTD generally allows allosteric modulations through inter- and intra-molecular interactions.33,61,62 As a result, SHRs can switch from one functional state to another by selective stabilization of different ID NTD/AF1 conformations. Identification of different binding events and subsequent allosteric coupling is likely to play an important role in developing improved therapeutic interventions for endocrine-based cancers. Therapeutic targeting of SHRs is presently restricted due to a limited knowledge of the function and structure of the ID NTD/AF1, and thereby missing the full signaling spectrum of the SHR activity, which is a critical component of cell/tissue-specific effects of the receptor. Recent studies have shown that highly flexible and structurally dynamic NTD/AF1 of SHRs can be exploited as drug targets for endocrine-related cancers, thereby opening unique opportunities for development of new and novel small-molecule that could block/inhibit SHR-coregulatory protein interactions outside the LBD/AF2.34,35,37,38
Like other SHRs, the AR NTD also exists as an ID protein and undergoes disorder/order transition under specific cellular conditions such that AR AF1's interaction with specific coregulatory proteins including p160 group of proteins and subsequent AR-mediated transcriptional activity is significantly enhanced.65 It is important to note that protein-protein interactions with the NTD/AF1 are essential for AR transcriptional activity.65 Therefore, small-molecule inhibitors that can disrupt essential protein-protein interactions from active transcriptional complexes involving AR NTD/AF1 could be a potential avenue to block/inhibit AR activity. Targeting ID regions of proteins by small-molecule to block protein-protein interactions is a rapidly evolving field, which provides potential for small-molecule inhibitors to have a sustained therapeutic effect. The AR NTD as a viable target for in vivo intervention was first suggested by application of decoy molecules that demonstrated AR specificity and antitumor activity.65 These studies not only provided much-needed proof of principle for developing inhibitors to target the ID AR AF1/NTD but also led to a novel concept in the off targeting SHRs beyond AF2/LBD surfaces.
STRUCTURAL DYNAMICS OF ANDROGEN RECEPTOR AND CASTRATION-RESISTANT PROSTATE CANCER
Prostate cancers are commonly called androgen-sensitive or androgen-dependent prostate cancers. Over time, however, prostate cancer tends to relapse and progresses into an incurable state which is refractory to androgen deprivation therapy. The AR is an important driver of prostate cancer, and while treatment for early-stage disease using combinations of androgen ablation or anti-androgen therapy is often successful, resistance almost inevitably occurs, and patients progress to advanced castration-resistant prostate cancer (CRPC) for which treatment options are limited. Preclinical and clinical data have demonstrated the requirement of AR in CRPC, yet how it functions under these conditions is not fully resolved.66,67,68,69 Several studies have shown that the mechanism in CRPC development involves AR amplification, AR mutation, and aberrant AR co-regulators activities in prostate cancer cells. One mechanism that has been identified for CRPC is the expression of AR splice variants, which are truncated within the LBD and therefore fail to bind to ligand yet are transcriptionally active (Figure 4). In recent years, the role of AR splice variant expression in the progression of CRPC has been extensively studied.70,71 These AR splice variants are generated through random RNA splicing, resulting into truncated AR proteins, some of which instead of losing function, activate AR pathway in the absence of androgens.70,71
Figure 4.
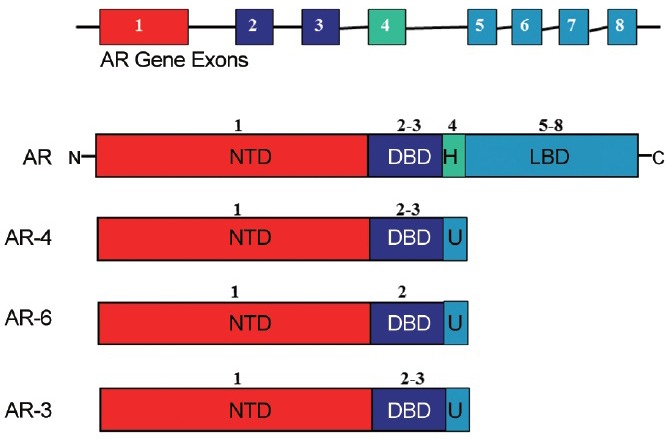
A schematic representation of various AR Splice Variants: Various forms of RNA splicing can rearrange the exons involved in the DNA binding domain, ligand binding domain, and NH2 terminal binding domain, resulting in constitutively active ARs that are unresponsive to androgens altogether.
The CRPC is associated with significant morbidity and mortality, and the majority of patients develop painful bone metastases. An enhanced understanding of the mechanisms behind the development of CRPC has led to the development of several new targeted agents that are now making their way into the clinic and have improved treatment outcomes for patients. The AR variants are believed to drive prostate tumorigenesis in the absence of hormone through activation of transcriptional networks regulated entirely by the ID NTD/AF1. The most widely observed splice variant, AR-V7 contains an intact AR NTD and DBD appended to a unique 16-residue sequence that replaces the LBD.70 The AR-V7 has been shown in wild-type AR-positive prostate cancer cells to be essential for proliferation in the absence of hormone, and in androgen-resistant tumor xenografts models to be a driver of relapsed tumors under castrate conditions.71 Therapy-resistant forms of AR lacking the LBD underscore the importance of targeting the NTD for the development of more effective prostate cancer therapies. Mechanisms proposed for the continued transcriptional activity of the AR in spite of castrate levels of circulating androgens include ligand-independent activation of the AR through its NTD/AF1 in the absence of ligand. Cell- and gene-specific regulation of AR results from its recruitment of different coregulatory proteins, and therefore AR variants lacking specific regions of the AR could be hypothesized to be devoid of protein interfaces or to have new interfaces for the recruitment of different subsets of coregulatory proteins.
The AR NTD/AF1 is the major activation domain for the AR's transcriptional activity irrespective of the presence or absence of ligands.34,35 Targeting the AR NTD with decoy molecules has been shown to possess antitumor activity in vivo.34,35 Therefore, there is a strong rationale for developing small molecule that specifically bind the AR NTD to block activity in CRPC. However, one challenge in developing small-molecule capable of blocking the AR NTD/AF1 activity has been its highly flexible ID conformation as discussed earlier, in general for all the SHRs, which in turn has precluded high throughput screening of small-molecule inhibitors. Recent studies have circumvented this problem by using cell-based screening protocols that led to the identification of one NTD antagonist, EPI-001.34,35 In these studies, it was found that the treatment of CRPC xenograft tumors with EPI-001 resulted into tumor regression34,35 by inducing apoptosis and reducing proliferation. EPI-001 was also found to inhibit constitutively active AR by binding to NTD but not to the LBD.72 Despite the ID nature of all SHRs’ NTD, EPI-001 was specific for inhibiting AR and had no effect on the activities of other related SHRs.72 Biophysical analysis revealed that EPI-001 altered the folding of AR's ID AF1 region such that AF1's interaction with specific coregulatory proteins, which are reported to have higher levels of expression in CRPC, was inhibited. Further, EPI-001 inhibited AR's N/C interaction and androgen-induced expression of PSA and TMPRSS2 androgen-responsive genes.72
SUMMARY AND FUTURE PERSPECTIVES
Since the cloning of the first SHR in the mid-80s, the scientific community has made phenomenal progress toward the understanding of the structure-function relationships of the SHRs, which has been of tremendous help in designing small-molecule drugs for SHR-based therapeutics. However, as we have started to learn more and more about the structural dynamics and allosteric regulations that govern the actions of SHRs in a cell/tissue- and gene- specific manner, it has become quite evident that without a deep understanding of how these factors influence the full spectrum of the SHRs’ activities, achieving target-specific inhibitors to block SHR functions in endocrine-related cancers will result into mixed outcomes. The success with solving the three-dimensional structure of some full-length nuclear receptors has given a much-needed boost toward these goals.73 Further recent developments of the functional and structural understandings of the ID NTD together with the allosteric coupling have opened up the possibility of investigating the drug targets beyond the LBD/AF2. Thus, the emerging point of view is that to access the entire SHR-signaling spectrum for the development of novel and potent therapeutic agents, we must gain further insights into how modulation of the structural dynamics of intact SHRs controls receptor function. In this context, identifying potential avenues that could modify the structural dynamics of the ID NTD/AF1 domain may provide opportunities to design more effective and target-specific SRMs for clinical applications.
COMPETING INTERESTS
The author does not have any competing interests in the manuscript.
ACKNOWLEDGMENTS
The author would like to acknowledge the important contributions of many other investigators to the field of prostate cancer whose work may not have been cited.
REFERENCES
- 1.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escriva H, Bertrand S, Laudet V. The evolution of the nuclear receptor superfamily. Essays Biochem. 2004;40:11–26. doi: 10.1042/bse0400011. [DOI] [PubMed] [Google Scholar]
- 3.Papi A, Orlandi M. Role of nuclear receptors in breast cancer stem cells. World J Stem Cells. 2016;8:62–72. doi: 10.4252/wjsc.v8.i3.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKenna NJ, Evans RM, O’Malley BW. Nuclear receptor signaling: a home for nuclear receptor and coregulator signaling research. Nucl Recept Signal. 2014;12:e006. doi: 10.1621/nrs.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–64. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 6.Lamont KR, Tindall DJ. Androgen regulation of gene expression. Adv Cancer Res. 2010;107:137–62. doi: 10.1016/S0065-230X(10)07005-3. [DOI] [PubMed] [Google Scholar]
- 7.Stanisic V, Lonard DM, O’Malley BW. Modulation of steroid hormone receptor activity. Prog Brain Res. 2010;181:153–76. doi: 10.1016/S0079-6123(08)81009-6. [DOI] [PubMed] [Google Scholar]
- 8.Zanchi NE, Filho MA, Felitti V, Nicastro H, Lorenzeti FM, et al. Glucocorticoids: extensive physiological actions modulated through multiple mechanisms of gene regulation. J Cell Physiol. 2010;224:311–5. doi: 10.1002/jcp.22141. [DOI] [PubMed] [Google Scholar]
- 9.Huang P, Chandra V, Rastinejad F. Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu Rev Physiol. 2010;72:247–72. doi: 10.1146/annurev-physiol-021909-135917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Malley BW, Kumar R. Nuclear receptor coregulators in cancer biology. Cancer Res. 2009;69:8217–22. doi: 10.1158/0008-5472.CAN-09-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto KR, Darimont BD, Wagner RL, Iñiguez-Lluhí JA. Building transcriptional regulatory complexes: signals and surfaces. Cold Spring Harb Symp Quant Biol. 1998;63:587–98. doi: 10.1101/sqb.1998.63.587. [DOI] [PubMed] [Google Scholar]
- 12.Lonard DM, O’malley BW. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell. 2007;27:691–700. doi: 10.1016/j.molcel.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Wu RC, O’Malley BW. Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer. 2009;9:615–30. doi: 10.1038/nrc2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Malley BW, Qin J, Lanz RB. Cracking the coregulator codes. Curr Opin Cell Biol. 2008;20:310–5. doi: 10.1016/j.ceb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar R, Thompson EB. Structure and functions of the nuclear hormone receptors. Steroids. 1999;64:310–9. doi: 10.1016/s0039-128x(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 16.Bevan CL, Hoare S, Claessens F, Heery DM, Parker MG. The AF1 and AF2 domains of the androgen receptor interact with distinct regions of SRC1. Mol Cell Biol. 1999;19:8383–92. doi: 10.1128/mcb.19.12.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benecke A, Chambon P, Gronemeyer H. Synergy between estrogen receptor activation functions AF1 and AF2 mediated by transcription intermediary factor TIF2. EMBO Rep. 2000;1:151–7. doi: 10.1093/embo-reports/kvd028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onate SA, Boonyaratanakornkit V, Spencer TE, Tsai SY, Tsai MJ, et al. The steroid receptor coactivator-1 contains multiple receptor interacting and activation domains that cooperatively enhance the activation function 1 (AF1) and AF2 domains of steroid receptors. J Biol Chem. 1998;273:12101–8. doi: 10.1074/jbc.273.20.12101. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R, Thompson EB. Transactivation functions of the N-terminal domains of nuclear hormone receptors: protein folding and coactivator interactions. Mol Endocrinol. 2003;17:1–10. doi: 10.1210/me.2002-0258. [DOI] [PubMed] [Google Scholar]
- 20.He B, Gampe RT, Jr, Kole AJ, Hnat AT, Stanley TB, et al. Structural basis for androgen receptor interdomain and coactivator interactions suggests a transition in nuclear receptor activation function dominance. Mol Cell. 2004;16:425–38. doi: 10.1016/j.molcel.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 21.Kumar R, McEwan IJ. Allosteric modulators of steroid hormone receptors: structural dynamics and gene regulation. Endocrine Rev. 2012;33:271–99. doi: 10.1210/er.2011-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–8. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 23.Berry M, Metzger D, Chambon P. Role of the two activating domains of the oestrogen receptor in the cell type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 1990;9:2811–8. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pike AC, Brzozowski AM, Hubbard RE. A structural biologist's view of the oestrogen receptor. J Steroid Biochem Mol Biol. 2000;74:261–8. doi: 10.1016/s0960-0760(00)00102-3. [DOI] [PubMed] [Google Scholar]
- 25.Szwarc MM, Lydon JP, O’Malley BW. Steroid receptor coactivators as therapeutic targets in the female reproductive system. J Steroid Biochem Mol Biol. 2015;154:32–8. doi: 10.1016/j.jsbmb.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ball LJ, Levy N, Zhao X, Griffin C, Tagliaferri M, et al. Cell type- and estrogen receptor-subtype specific regulation of selective estrogen receptor modulator regulatory elements. Mol Cell Endocrinol. 2009;299:204–11. doi: 10.1016/j.mce.2008.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moras D, Billas IM, Rochel N, Klaholz BP. Structure-function relationships in nuclear receptors: the facts. Trends Biochem Sci. 2015;40:287–90. doi: 10.1016/j.tibs.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Spillman MA, Manning NG, Dye WW, Sartorius CA, Post MD, et al. Tissue specific pathways for estrogen regulation of ovarian cancer growth and metastasis. Cancer Res. 2010;70:8927–36. doi: 10.1158/0008-5472.CAN-10-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardell SE, Kazmin D, McDonnell DP. Research resource: transcriptional profiling in a cellular model of breast cancer reveals functional and mechanistic differences between clinically relevant SERM and between SERM/estrogen complexes. Mol Endocrinol. 2012;26:1235–48. doi: 10.1210/me.2012-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson S, Koehler KF, Gustafsson JA. Development of subtype-selective oestrogen receptor-based therapeutics. Nat Rev Drug Discov. 2011;10:778–92. doi: 10.1038/nrd3551. [DOI] [PubMed] [Google Scholar]
- 31.McDonnell DP, Wardell SE. The molecular mechanisms underlying the pharmacological actions of ER modulators: implications for new drug discovery in breast cancer. Curr Opin Pharmacol. 2010;10:620–8. doi: 10.1016/j.coph.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shapiro DJ, Mao C, Cherian MT. Small molecule inhibitors as probes for estrogen and androgen receptor action. J Biol Chem. 2011;286:4043–8. doi: 10.1074/jbc.R110.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simons SS, Edwards DP, Kumar R. Dynamic structures of nuclear hormone receptors: new promises and challenges. Mol Endocrinol. 2014;28:173–82. doi: 10.1210/me.2013-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen RJ, Mawji NR, Wang J, Wang G, Haile S, et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell. 2010;17:535–46. doi: 10.1016/j.ccr.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 35.Myung JK, Banuelos CA, Fernandez JG, Mawji NR, Wang J, et al. An androgen receptor N-terminal domain antagonist for treating prostate cancer. J Clin Invest. 2013;123:2948–60. doi: 10.1172/JCI66398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu YL, Yang X, Ren Z, McDonnell DP, Norris JD, et al. Structural basis for an unexpected mode of SERM-mediated ER antagonism. Mol Cell. 2005;18:413–24. doi: 10.1016/j.molcel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Arnal JF, Fontaine C, Abot A, Valera MC, Laurell H, et al. Lessons from the dissection of the activation functions (AF-1 and AF-2) of the estrogen receptor alpha in vivo. Steroids. 2013;78:576–82. doi: 10.1016/j.steroids.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Arao Y, Hamilton KJ, Goulding EH, Janardhan KS, Eddy EM, et al. Transactivating function (AF) 2-mediated AF-1 activity of estrogen receptor alpha is crucial to maintain male reproductive tract function. Proc Natl Acad Sci U S A. 2012;109:21140–5. doi: 10.1073/pnas.1216189110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC. Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol. 2010;42:813–27. doi: 10.1016/j.biocel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinog. 2011;10:20. doi: 10.4103/1477-3163.83937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan ME, Li J, Xu HE, Melcher K, Yong E. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin. 2015;36:3–23. doi: 10.1038/aps.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmad N, Kumar R. Steroid hormone receptors in cancer development: a target for cancer therapeutics. Cancer Lett. 2011;300:1–9. doi: 10.1016/j.canlet.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Wardell SE, Marks JR, McDonnell DP. The turnover of estrogen receptor alpha by the selective estrogen receptor degrader (SERD) fulvestrant is a saturable process that is not required for antagonist efficacy. Biochem Pharmacol. 2011;82:122–30. doi: 10.1016/j.bcp.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graham J, Pitz M, Gordon V, Grenier D, Amir E, et al. Clinical predictors of benefit from fulvestrant in advanced breast cancer: a meta-analysis of randomized controlled trials. Cancer Treat Rev. 2016;45:1–6. doi: 10.1016/j.ctrv.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Wardell SE, Nelson ER, Chao CA, McDonnell DP. Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease. Clin Cancer Res. 2013;19:2420–31. doi: 10.1158/1078-0432.CCR-12-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kretzer NM, Cherian MT, Mao C, Aninye IO, Reynolds PD, et al. A noncompetitive small molecule inhibitor of estrogen-regulated gene expression and breast cancer cell growth that enhances proteasome-dependent degradation of estrogen receptor {alpha} J Biol Chem. 2010;285:41863–73. doi: 10.1074/jbc.M110.183723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pritchard CC, Nelson PS. Gene expression profiling in the developing prostate. Differentiation. 2008;76:624–40. doi: 10.1111/j.1432-0436.2008.00274.x. [DOI] [PubMed] [Google Scholar]
- 48.Graham L, Schweizer MT. Targeting persistent androgen receptor signaling in castration-resistant prostate cancer. Med Oncol. 2016;33:44. doi: 10.1007/s12032-016-0759-3. [DOI] [PubMed] [Google Scholar]
- 49.Winterhalder RC. Prostate cancer: a review of therapeutic strategies. Praxis. 2009;98:1149–53. doi: 10.1024/1661-8157.98.20.1149. [DOI] [PubMed] [Google Scholar]
- 50.Kung HJ, Evans CP. Oncogenic activation of androgen receptor. Urol Oncol. 2009;27:48–52. doi: 10.1016/j.urolonc.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tasseff R, Nayak S, Salim S, Kaushik P, Rizvi N, et al. Analysis of the molecular networks in androgen dependent and independent prostate cancer revealed fragile and robust subsystems. PLoS One. 2010;5:e8864. doi: 10.1371/journal.pone.0008864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Isaacs JT. New strategies for the medical treatment of prostate cancer. BJU Int. 2005;96:35–40. doi: 10.1111/j.1464-410X.2005.05945.x. [DOI] [PubMed] [Google Scholar]
- 53.Liu X, Chen X, Rycaj K, Chao HP, Deng Q, et al. Systematic dissection of phenotypic, functional, and tumorigenic heterogeneity of human prostate cancer cells. Oncotarget. 2015;6:23959–86. doi: 10.18632/oncotarget.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng Q, Tang DG. Androgen receptor and prostate cancer stem cells: biological mechanisms and clinical implications. Endocr Relat Cancer. 2015;22:T209–20. doi: 10.1530/ERC-15-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dunker AK, Uversky VN. Drugs for ‘protein clouds’: targeting intrinsically disordered transcription factors. Curr Opin Pharmacol. 2010;10:782–8. doi: 10.1016/j.coph.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Ferreon AC, Ferreon JC, Wright PE, Deniz AA. Modulation of allostery by protein intrinsic disorder. Nature. 2013;498:390–4. doi: 10.1038/nature12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan SH, Awasthi S, Guo C, Goswami D, Ling J, et al. Binding of the N-terminal region of coactivator TIF2 to the intrinsically disordered AF1 domain of the glucocorticoid receptor is accompanied by conformational reorganizations. J Biol Chem. 2012;287:44546–60. doi: 10.1074/jbc.M112.411330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar R, Moure CM, Khan SH, Callaway C, Grimm S, et al. Regulation of the structurally dynamic amino-terminal domain of progesterone receptor by protein induced folding. J Biol Chem. 2013;288:30285–99. doi: 10.1074/jbc.M113.491787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar R, Baskakov IV, Srinivasan G, Bolen DW, Lee JC, et al. Interdomain signaling in a two-domain fragment of the human glucocorticoid receptor. J Biol Chem. 1999;274:24737–41. doi: 10.1074/jbc.274.35.24737. [DOI] [PubMed] [Google Scholar]
- 60.Goswami D, Pascal B, Kumar R, Edwards DP, Griffin PR. Structural dynamics and inter domain crosstalk of PR-TBP interaction probed by hydrogen/deuterium exchange mass spectrometry. Structure. 2014;22:961–73. doi: 10.1016/j.str.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar R, Litwack G. Structural and functional relationships of the steroid hormone receptors’ N-terminal transactivation domain. Steroids. 2009;74:877–83. doi: 10.1016/j.steroids.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garza AS, Ahmad N, Kumar R. Role of intrinsically disordered protein regions/domains in transcriptional regulation. Life Sci. 2009;84:189–93. doi: 10.1016/j.lfs.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 63.Krasowski MD, Reschly EJ, Ekins S. Intrinsic disorder in nuclear hormone receptors. J Proteome Res. 2008;7:4359–72. doi: 10.1021/pr8003024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys. 2008;37:215–46. doi: 10.1146/annurev.biophys.37.032807.125924. [DOI] [PubMed] [Google Scholar]
- 65.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 66.Ahmed A, Ali S, Sarkar FH. Advances in androgen receptor targeted therapy for prostate cancer. J Cell Physiol. 2014;229:271–6. doi: 10.1002/jcp.24456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kobayashi T, Inoue T, Kamba T, Ogawa O. Experimental evidence of persistent androgen-receptor dependency in castration-resistant prostate cancer. Int J Mol Sci. 2013;14:15615–35. doi: 10.3390/ijms140815615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharifi N. Mechanisms of androgen receptor activation in castration-resistant prostate cancer. Endocrinology. 2013;154:4010–7. doi: 10.1210/en.2013-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharifi N. Minireview: androgen metabolism in castration-resistant prostate cancer. Mol Endocrinol. 2013;27:708–14. doi: 10.1210/me.2013-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo Z, Yang X, Sun F, Jiang R, Linn DE, et al. A novel androgen receptor splice variant is upregulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu Z, Chen S, Sowalsky AG, Voznesensky OS, Mostaghel EA, et al. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clin Cancer Res. 2014;20:1590–600. doi: 10.1158/1078-0432.CCR-13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sadar MD, Williams DE, Mawji NR, Patrick BO, Wikanta T, et al. Sintokamides A to E, chlorinated peptides from the sponge Dysidea sp. that inhibit transactivation of the N-terminus of the androgen receptor in prostate cancer cells. Org Lett. 2008;10:4947–50. doi: 10.1021/ol802021w. [DOI] [PubMed] [Google Scholar]
- 73.Rastinejad F, Ollendorff V, Polikarpov I. Nuclear receptor full-length architectures: confronting myth and illusion with high resolution. Trends Biochem Sci. 2015;40:16–24. doi: 10.1016/j.tibs.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


