Abstract
The Cancer/Testis Antigens (CTAs) are a group of so-called tumor antigens that exhibit provocative expression patterns in most types of cancer. However, because most CTAs appear to be intrinsically disordered, they are recalcitrant to classical structural studies. Thus, the functions of most, if not all, CTAs have remained elusive and their potential as pharmacological targets in cancer has remained largely unexplored. In this viewpoint, we suggest that perhaps, an integrative approach applying dynamical systems theory and a system-level perspective rather a merely reductionist view, may shine new light on these enigmatic molecules.
Because of their restricted expression, the Cancer/Testis Antigens (CTAs) have received considerable attention as potential biomarkers and targets for cancer immunotherapy.1 However, since the functions of most, if not all, CTAs have remained elusive, their role in tumorigenesis and thus their therapeutic potential has been underappreciated.
In a recent study, employing a functional genomics approach, Maxfield et al.2 demonstrated that the CTAs play an obligatory role in multiple hallmarks of cancer. Despite numerous reports in the literature on the CTAs in a given type of cancer, the authors are to be lauded for the sheer scope of their endeavor and for elucidating the role of several CTAs that appear to fulfill a “pan-obligatory” role across cancer types including prostate cancer (PCa). In particular, two observations from this study are especially significant. First, FATE1, a key survival factor in multiple oncogenic backgrounds that prevents the accumulation of the stress-sensing BH3-only protein, BIK, is an intrinsically disordered protein (IDP) and second ZNF165 promotes TGFβ signaling by directly suppressing negative feedback loops. Unfortunately, however, the authors2 not only failed to acknowledge previous work demonstrating that both FATE1 as well as ZNF185 are predicted to be IDPs,3 but also failed to appreciate the significance of their own work uncovering the role of IDPs in regulating information flow via feedback loops. Thus, the main intent of this brief missive is to put the intrinsically disordered characteristic of the CTAs in perspective.
A striking characteristic of the CTAs as a group that could shed new light on their role in tumorigenesis is that >90% of the CTAs are predicted to be IDPs.3 IDPs lack a rigid 3D structure and exist as conformational ensembles instead. Furthermore, although the ensembles exhibit conformational preferences, they are highly dynamic and malleable, and can be remodeled extensively in response to environmental changes such as posttranslational modifications and stressful conditions. For example, the CTA PAGE4 that is upregulated in PCa is a stress-response protein that potentiates the transcriptional activity of the oncogene c-Jun.4 The latter heterodimerizes with members of the Fos family of transcription factors to form the AP-1 complex. However, phosphorylation of PAGE4 at predominantly a single residue by the stress-response kinase HIPK15 perturbs the local and long-range conformational preferences of the ensemble, but does not induce transition from disorder to order.6 In contrast, multisite phosphorylation of an intrinsically disordered region in 4E-BP2, another stress-response protein that binds the translation initiation factor eIF4E and suppresses cap-dependent translation initiation, induces its folding.7 Thus, structural flexibility and plasticity of IDPs is a major functional advantage for the IDPs enabling them to interact with a broad range of binding partners. However, because of their inherent flexibility, IDPs are prone to initiate promiscuous molecular interactions when overexpression resulting in toxicity/pathology.8
Being IDPs, the structural plasticity of CTAs could also contribute significantly to “noise” in the PINs, especially when they are overexpressed.9 Recent progress has revealed that many biological processes are driven by probabilistic events underscoring the importance of “noise” in biological systems.10 Just as transcriptional noise plays an important role in probabilistic differentiation and adaptation, it has been postulated that noise inherent in protein interactions may underlie the activation of latent pathways and cellular transformation. Therefore, it is quite plausible that noise in PINs is contributed by the conformational dynamics intrinsic to the CTAs (IDPs). Stochasticity allows the system to search through numerous iterations of network configurations to rewire the PIN and select those that impact fitness of the cancer cell.9 Thus, the ability of ZNF165 to regulate critical feedback loops, i.e., rewiring the PIN is of particular significance that needs to be underscored. Furthermore, it should be noted that ZNF165 is also predicted to be an IDP.3
Yet another aspect that can contribute to the obligatory role of CTAs in tumorigenesis is the fact that living systems are dynamical systems. Therefore, the loss/gain of negative/positive feedback loops in key regulatory pathways due to CTA (IDP) overexpression as demonstrated by Maxfield et al.2 can lead to the activation of certain oscillators and death of other oscillators as the dynamical system crosses bifurcation points. Such bifurcations may play a profound role in specifying cell fate; for example, switch from a normal to a cancerous phenotype.11 Of note, the CTA cyclin A1, which is also predicted to be an IDP (Figure 1), is a component of the cyclin/cdk oscillator which is important in cell cycle regulation. Furthermore, such phenotypic switching can be reversed across multiple phenotypes suggesting that information specifying cell fate may not reside in the genome alone. Rather, it seems likely that it resides in the PINs and may eventually be transferred to the genome for transgenerational inheritance.9 Of particular note, the CTAs (IDPs) play an important role in many of these phenotypic switching events.12 Thus, we trust that these observations on the CTAs will cast them in new light and highlight them as being important players in cancer.
Figure 1.
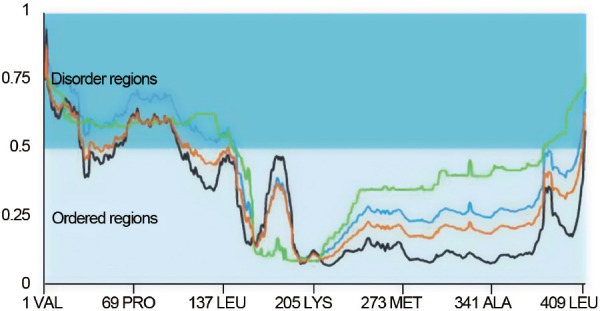
The human cancer/testis antigen cyclin A1 is an intrinsically disordered protein. The sequence of the full-length cyclin A1 (411 amino acids) was obtained from NCBI, and the extent of disorder was determined employing the metaPrDOS algorithm (http://iimcb.genesilico.pl/metadisorder). The X-axis denotes amino acid residues and the Y-axis represents disorder tendency. Although 18 different algorithms are applied for the computation, the outputs of only four of them, MetaDisorderMD2 (blue), MetaDisorder (black), MetaDisorder3D (green), and MetaDisorderMD (orange) are displayed for clarity.
COMPETING INTERESTS
The authors declare no competing financial or other conflicts of interests.
REFERENCES
- 1.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–25. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 2.Maxfield KE, Taus PJ, Corcoran K, Wooten J, Macion J, et al. Comprehensive functional characterization of cancer-testis antigens defines obligate participation in multiple hallmarks of cancer. Nat Commun. 2015;6:8840. doi: 10.1038/ncomms9840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajagopalan K, Mooney SM, Parekh N, Getzenberg RH, Kulkarni P. A majority of the cancer/testis antigens are intrinsically disordered proteins. J Cell Biochem. 2011;112:3256–67. doi: 10.1002/jcb.23252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajagopalan K, Qiu R, Mooney SM, Rao S, Shiraishi T, et al. The Stress-response protein prostate-associated gene 4, interacts with c-Jun and potentiates its transactivation. Biochim Biophys Acta. 2014;1842:154–63. doi: 10.1016/j.bbadis.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mooney SM, Qiu R, Kim JJ, Sacho EJ, Rajagopalan K, et al. Cancer/testis antigen PAGE4, a regulator of c-Jun transactivation, is phosphorylated by homeodomain-interacting protein kinase 1, a component of the stress-response pathway. Biochemistry. 2014;53:1670–9. doi: 10.1021/bi500013w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Chen Y, Mooney SM, Rajagopalan K, Bhargava A, et al. Phosphorylation-induced conformational ensemble switching in an intrinsically disordered cancer/testis antigen. J Biol Chem. 2015;290:25090–102. doi: 10.1074/jbc.M115.658583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bah A, Vernon RM, Siddiqui Z, Krzeminski M, Muhandiram R, et al. Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch. Nature. 2015;519:106–9. doi: 10.1038/nature13999. [DOI] [PubMed] [Google Scholar]
- 8.Vavouri T, Semple JI, Garcia-Verdugo R, Lehner B. Intrinsic protein disorder and interaction promiscuity are widely associated with dosage sensitivity. Cell. 2009;138:198–208. doi: 10.1016/j.cell.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoudabadi G, Rajagopalan K, Getzenberg RH, Hannenhalli S, Rangarajan G, et al. Intrinsically disordered proteins and conformational noise: implications in cancer. Cell Cycle. 2013;12:26–31. doi: 10.4161/cc.23178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldar A, Elowitz MB. Intrinsically disordered proteins and conformational noise: implications in cancer. Nature. 2010;467:167–73. [Google Scholar]
- 11.Rangarajan N, Fox Z, Singh A, Kulkarni P, Rangarajan G. Disorder, oscillatory dynamics and state switching: the role of c-Myc. J Theor Biol. 2015;386:105–14. doi: 10.1016/j.jtbi.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Yang P, Huo Z, Liao H, Zhou Q. Cancer/testis antigens trigger epithelial-mesenchymal transition and genesis of cancer stem-like cells. Curr Pharm Des. 2015;21:1292–300. doi: 10.2174/1381612821666141211154707. [DOI] [PubMed] [Google Scholar]


