Abstract
In this study, we investigated the essential criteria for late-onset hypogonadism (LOH) syndrome based on the presence of symptoms associated with low testosterone levels in Han Chinese men. Blood tests for total testosterone (TT) and sex hormone–binding globulin (SHBG) were performed, and the aging male symptoms (AMS) questionnaire was conducted in a randomly selected cohort composed of 944 Chinese men aged 40 to 79 years from nine urban communities. Three sexual symptoms (decreased ability/frequency of sexual activity, decreased number of morning erections, and decreased libido) were confirmed to be related to the total and free testosterone levels. The thresholds for TT were approximately 12.55 nmol l−1 for a decreased ability/frequency to perform sex, 12.55 nmol l−1 for decreased frequency of morning erections, and 14.35 nmol l−1 for decreased sexual desire. The calculated free testosterone (CFT) thresholds for these three sexual symptoms were 281.14, 264.90, and 287.21 pmol l−1, respectively. TT <13.21 nmol l−1 (OR = 1.4, 95%CI: 1.0–1.9, P = 0.037) or CFT <268.89 pmol l−1 (OR = 1.5, 95%CI: 1.1–20, P = 0.020) was associated with an increase in the aforementioned three sexual symptoms. The prevalence of LOH was 9.1% under the criteria, including all three sexual symptoms with TT levels <13.21 nmol l−1 and CFT levels <268.89 pmol l−1. Our results may improve the diagnostic accuracy of LOH in older men.
Keywords: diagnosis, hypogonadism, middle-aged and elderly male, reference values, sexual dysfunction, testosterone
INTRODUCTION
The world population is aging. In China, with an increasing elderly population, the health problems that affect this group are becoming important public health concerns. With aging, a significant percentage of men develop a gradual and moderate decrease in testicular function known as late-onset hypogonadism (LOH), which is defined as “a clinical and biochemical syndrome associated with advancing age and characterized by typical symptoms and a deficiency in testosterone levels. It may result in significant detriment in the quality-of-life and adversely affect the function of multiple organ systems.”1,2,3,4 As this definition implies, it is important to recognize and treat LOH appropriately if there are no contraindications. However, it was estimated that only 5%–35% of men experiencing LOH actually receive treatment for their condition2 primarily because many LOH symptoms are similar to other conditions or are physiologically associated with the aging process.2 No pathognomonic findings exist regarding specific androgen concentrations for clinically relevant subsets of older men.2,3,4,5 Therefore, the designation of a reliable testosterone threshold in aging males (below which hypogonadism-related symptoms emerge and adverse health outcomes ensue) is needed. Then, the use of arbitrary thresholds can be avoided.3
Recently, progress has been made in the study of male LOH. In an effort to define a syndrome of LOH, rather than a checklist of various symptoms, Wu et al.3 reported the results of the European male aging study (EMAS) and concluded that the presence of three sexual symptoms (poor morning erection, erectile dysfunction, or low sexual desire) and a morning total testosterone level <11 nmol l−1 can be considered as evidence-based criteria for the diagnosis of LOH. However, as with the EMAS, most of the data regarding the advantages of diagnostic criteria for LOH have primarily been obtained from studies on Western populations. Therefore, studies to determine better criteria for Asian men, who may have different serum testosterone levels than Western men, are needed. Iwamoto et al.6 reviewed the diagnostic criteria for LOH from several Asian countries and reported that the diagnostic methods for LOH in the International Society of Andrology (ISA), the International Society for the Study of Aging Male (ISSAM), and the European Association of Urology (EAU) recommendations are not necessarily feasible for men in Asian countries.
In a previous study,7 we investigated LOH in elderly community-dwelling Chinese men using symptom score evaluation systems and measurements of sex hormone levels. However, no essential criteria for the LOH syndrome have been defined. With an improved analysis strategy, we reanalyzed the data to characterize the clinical symptoms associated with low testosterone levels and identified the essential criteria for LOH syndrome based on the presence of symptoms associated with low testosterone levels.
METHODS
Study population
The participants were recruited by phone call invitations to form a cohort of randomly selected men between the ages of 40 and 79 years from nine urban communities. The mean age of the subjects was 59.3 (7.4) years. The participants, who gave written informed consent, underwent initial screening tests, including blood tests for biochemical and hormone measurements, and completed the aging male symptoms (AMS) questionnaire.8 The study was approved by the Ethical Committee of Changhai Hospital. Men who currently had a malignancy, liver cirrhosis, acute illness, hypothyroidism, or chronic alcoholism; or who were taking hormones, anti-androgen agents, antifungal agents, or steroidal agents; or who had undergone surgical or medical therapy for benign prostate hyperplasia (BPH) were excluded from this study. The study was open to the general population, and all the study subjects were members of the Chinese Han population.
Serum measurements of sex hormones
A total of 944 venous blood samples were collected between 09:00 and 11:00 h following a 12 h fast (to minimize the effects of diurnal variation).9 The subjects completed the questionnaires, and the concentrations of serum sex hormones were measured (including total testosterone [TT] and sex hormone–binding globulin [SHBG]). Each index was measured using a chemical or luminescence method (instruments and test agents manufactured by Beckmann Co., Bremen, Germany). The lower limit of the total testosterone measurement was 0.35 nmol l−1. The coefficients of variation of the testosterone measurements were 1.67%–3.93% within runs and 4.22%–7.08% between runs. The free testosterone level was derived from measurements of TT, SHBG, and albumin10 and is referred to as the calculated free testosterone (CFT).
Statistical analysis
The AMS scale includes 17 items to assess symptoms that may be associated with androgen decline in aging males.8 Each question is answered on a scale from 1 to 5. The 17 items comprise three subscales: psychological, somatic, and sexual symptoms – all of which were dichotomized into symptomatic (responses 3–5) and asymptomatic (response 1–2) categories based on differences in the IIEF-5 scores between sub-groups.
As a validation of the response dichotomization, the Mann–Whitney U-test was used to confirm whether the symptomatic group and the asymptomatic group had different distributions of TT or CFT values. Subsequently, all items were screened, and the positive items from the Mann–Whitney U-test were confirmed using ordinal logistic regression models (in which the hierarchy of standardized responses to an item provided ordinal response items) to identify those that were significantly associated with TT and CFT levels. Based on the results of the Mann–Whitney U-test and the ordinal logistic regression models, only items that were significantly associated with TT or CFT levels were selected.
Later, a locally weighted linear regression11 was used to identify the threshold levels of testosterone below which the probability of a symptom increased above the background prevalence in the overall study population (Figure 1). Finally, using the symptoms associated with testosterone decline coupled with lower TT or CFT thresholds, the minimum standard LOH levels were derived. From this standard level, the prevalence of LOH in Chinese middle-aged men was identified.
Figure 1.
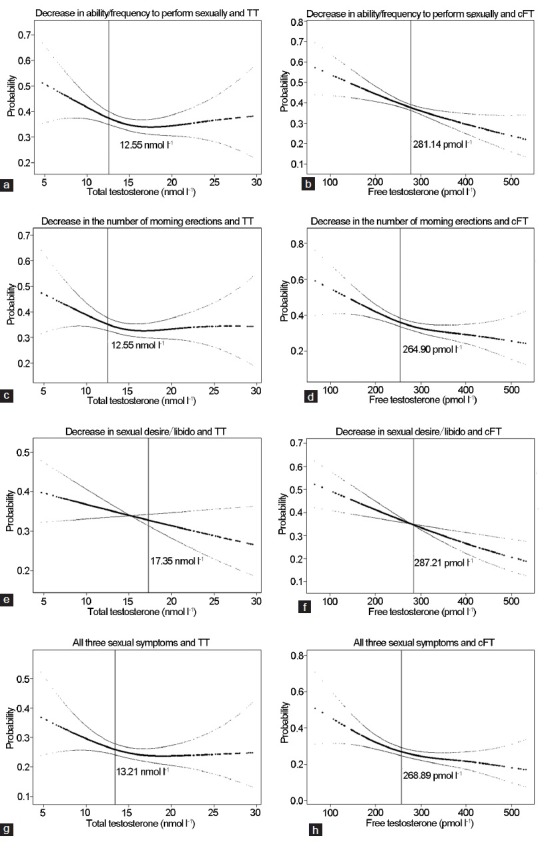
The relationship between symptoms and testosterone. (a) Perform sexually and testosterone (TT); (b) perform sexually and calculated free testosterone (CFT); (c) morning erections and TT; (d) morning erections and cFT; (e) sexual desire and TT; (f) sexual desire and cFT; (g) all sexual symptoms and TT; (h) all sexual symptoms and cFT.
All analyses were performed using Empower (R) (www.empowerstats.com, X and Y solutions, Inc., Boston, MA, USA) and R (http://www.R-project.org).
RESULTS
Study population
Among the 1000 study participants, 972 provided questionnaire data. Among these, 936 samples of venous blood were tested for TT, and 766 samples were tested for SHBG and calculated free testosterone. The characteristics of the male subjects who had complete TT and CFT tests were not significantly different from the subjects who had incomplete tests (Table 1).
Table 1.
Characteristics of study population

Associations between symptoms and testosterone levels
Of an initial pool of 17 candidate symptoms of testosterone deficiency that were explored in the AMS questionnaires, three sexual symptoms (decreased ability/frequency of sexual, decreased number of morning erections, and decreased sexual desire/libido) were confirmed to be related to the total or free testosterone levels. There were significant differences between the symptomatic group and the asymptomatic group (Table 2). The result was integrated from both the Mann–Whitney U-test and logistic regression models.
Table 2.
Comparisons of TT and FT levels between symptomatic and asymptomatic subjects in 17 symptoms of AMS

The probability of experiencing these symptoms increased with decreasing levels of testosterone (Figure 1). The thresholds for TT were approximately 12.55 nmol l−1 for a decreased ability and frequency to perform sexually (Figure 1a), 12.55 nmol l−1 for a decreased frequency of morning erections (Figure 1c), 14.35 nmol l−1 for a decreased sexual desire/libido, and 13.21 nmol l−1 for experiencing all three symptoms (Figure 1e and 1g). The CFT thresholds for these three sexual symptoms were 281.14, 264.90, and 287.21 pmol l−1, respectively, and 268.89 for experiencing all three symptoms (Figure 1b, 1d, 1f, and 1h). The six samples with extreme TT values and 12 samples with extreme CFT values, defined as three folds of standard deviations away from the mean values, were excluded from the analysis. A sensitivity analysis, which included theses extreme values, indicated similar results.
Criteria for hypogonadism
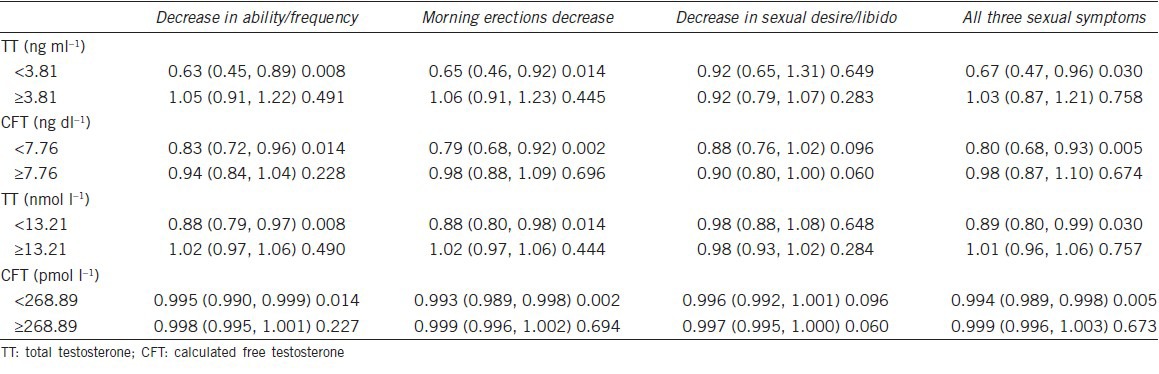
TT (13.21 nmol l−1 or 3.81 ng ml−1) and CFT thresholds (268.89 pmol l−1 or 7.76 ng dl−1) for experiencing all three symptoms (decrease in ability/frequency to perform sexually, decreased number of morning erections, and decreased sexual desire/libido), which were coincidentally similar to the levels recommended by the Endocrine Society clinical practice guidelines,1 were adopted.
A piece-wise linear regression indicated that when the TT level was <13.21 nmol l−1 (3.81 ng ml−1), the risk of experiencing all three symptoms increased with decreasing TT levels (OR = 0.67, 95%CI: 0.47, 0.96, P = 0.03). When the TT level was ≥13.21 nmol l−1 (3.81 ng ml−1), none of these associations were found (Table 3). When the CFT level was <268.89 pmol l−1 (7.76 ng dl−1), the risk of experiencing all three symptoms increased with decreasing CFT levels (OR = 0.80, 95%CI: 0.68, 0.93, P = 0.005). When the CFT level was ≥268.89 pmol l−1 (7.76 ng dl−1), none of these associations were found (Table 3). Therefore, the boundaries where the symptoms were significantly associated with a low testosterone level were a TT level of <13.21 nmol l−1 or a CFT level of <268.89 pmol l−1.
Table 3.
Two-piece-wise linear regression of sexual symptoms with TT (ng ml−1) (nmol l−1) and CFT (ng dl−1) (pmol l−1)
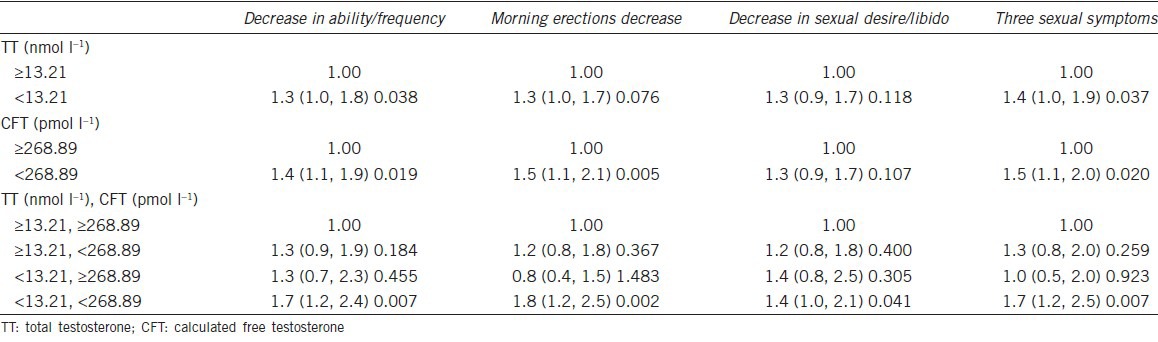
A logistic regression indicated that a TT <13.21 nmol l−1 or CFT <268.89 pmol l−1 was associated with increased frequency of the three sexual symptoms (OR = 1.4, 95%CI: 1.0–1.9, P = 0.037 for TT <13.21 nmol, and OR = 1.5, 95%CI: 1.1–20, P = 0.020 for CFT <268.89 pmol l−1) (Table 4).
Table 4.
Logistic regression of three sexual symptoms with dichotomized TT and CFT levels
Estimated prevalence of hypogonadism
In this sample, 37.4% of the subjects had decreased ability/frequency to perform sexually, 35.0% had decreased frequency of morning erections, and 34.4% had decreased sexual desire/libido. Furthermore, 44.9% of the subjects experienced one of the three sexual symptoms, and 26.0% of the subjects experienced all three sexual symptoms. In total, 35.4% of the subjects had TT levels <13.21 nmol l−1, and 49.4% of the subjects had CFT levels <268.89 pmol l−1. If late-onset hypogonadism syndrome is considered to include these three sexual symptoms and TT levels <13.21 nmol l−1, the prevalence of LOH was 10.8%. If late-onset hypogonadism syndrome is considered to include all three sexual symptoms and TT levels <13.21 nmol l−1, and FT levels <268.89 pmol l−1, the prevalence of LOH in this study was 9.1% (Figure 2).
Figure 2.
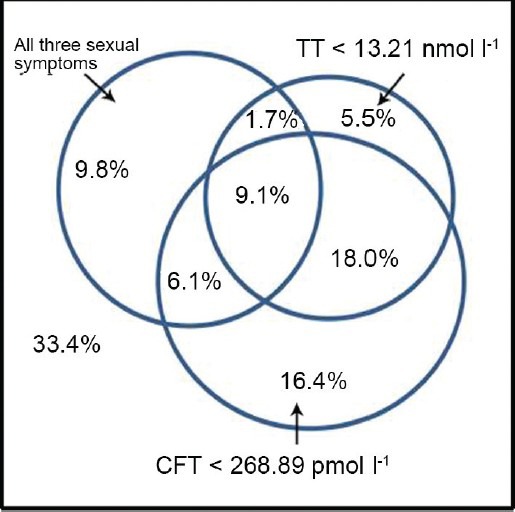
Estimated prevalence of hypogonadism. TT = testosterone; CFT = calculated free testosterone.
DISCUSSION
The diagnosis of LOH requires the presence of signs and symptoms suggesting testosterone deficiency.1,2,3,4 Several questionnaires, including the AMS scales and the androgen deficiency in aging males (ADAM) questionnaire, have been proposed to screen for androgen deficiency. However, because the symptoms of hypogonadism in middle-aged and older men are not specific to LOH and the results of these questionnaires may be affected by other chronic diseases, malignancy, or depressive disorders, a diagnosis may still be difficult in some men.12,13 For example, Yoshida et al. reported that a strong correlation was found between the AMS scale results and major depressive disorders.14 We excluded subjects with these conditions. In addition, there is a lack of consensus with respect to the testosterone threshold for an LOH diagnosis. Some studies have proposed that TT levels below 300 ng dl−1 can be considered as the androgen deficiency threshold.15,16 However, in our previous study on aging Chinese men,7 we found that TT levels were not related to aging, and LOH cannot be diagnosed merely based on serum TT levels (although the SHBG levels increased, and the FT and Bio-T decreased with aging).
In the present study, we reanalyzed the data using a reductive approach to produce parsimonious clinical and biochemical criteria for diagnosing LOH. Individual symptoms were evaluated by the well-known AMS scale. In our previously published work, neither the AMS nor ADAM questionnaires could obtain sufficient sensitivity and specificity. The AMS scores for each question had more possible responses (5-point scale) than a binary yes or no selection. We examined the correlations between the scores for individual questions and the serum TT and FT measures regardless of the cumulative scores for all items.17 To dichotomize the AMS responses into symptomatic and asymptomatic categories for the 17 items, a response of 1 or 2 was adopted as the asymptomatic cut-off point for each item. This approach is more consistent with a true asymptomatic response.
The AMS scale was initially developed to assess the symptoms of aging (irrespective of disease) between groups of men under different conditions to evaluate the severity of symptoms over time and to measure changes before and after androgen replacement therapy.8 The AMS scale is primarily intended to assess quality-of-life and was not designed to evaluate symptoms associated with LOH in aging men.18 However, many items in the AMS scale are common symptoms of androgen deficiency. A comparison with other scales for aging males or screening instruments for androgen deficiency revealed sufficiently good correlations indicating good criterion-oriented validity. The same is true of the comparison with the generic quality-of-life scale, SF-36, with which high correlation coefficients have also been calculated.8,19,20,21 Altogether, these reasons may explain why the scale has been translated into 31 languages thus far.22,23,24
During the analysis of TT or FT distributions between the symptomatic and asymptomatic groups, we observed that many candidate symptoms included in the AMS scale were not associated with decreased testosterone levels in older men. Even among the three selected sexual symptoms, the differences in mean testosterone levels between symptomatic and asymptomatic men were minimal (reflecting the weak overall association between symptoms and testosterone levels in this population).
Further analyses revealed nonlinear threshold relationships between all three sexual symptoms and TT at approximately 13.21 nmol l−1 (Figure 1g). Sexual symptoms are therefore of diagnostic importance in elderly men but should suggest androgen deficiency only when testosterone levels are clearly sub-normal.3,25 The presence of multiple symptom-specific testosterone thresholds indicates that different functional thresholds exist for different androgen-dependent targets.3,26,29,30,31 Our study suggests that some symptoms of LOH may manifest at higher concentrations of androgens than other symptoms (Table 2), which suggest that testosterone thresholds may be symptom-specific26 or that the phenotype upon which the symptoms of androgen deficiency are based does not apply in all respects to aging men.32 If true, individual and symptom-oriented treatments need to be established and applied in the future.26,27,28
In this study, we confirmed the boundaries for significant associations between symptoms and low testosterone levels and identified the minimum criteria necessary to diagnose LOH syndrome. Our suggested requirement of the presence of at least three sexual symptoms with a TT level <13.21 nmol l−1 and an FT level <268.89 pmol l−1 supports the recommendations of the latest practice guidelines1,4 (though with a slightly higher TT and FT threshold). As mentioned above, a diagnosis may still be difficult in some patients because the symptoms are nonspecific for LOH. Previous studies26,32,33,34 have also suggested that the prevalence of limited complaint profiles restricted to disturbances of sexual symptoms of androgen deficiency was relatively high among men with unequivocally normal testosterone levels. The prevalence of LOH in the general population is quite variable (ranging from 7% to 40%) according to the different criteria.35 In Asia, the prevalence of LOH is also different. Recently, a community-based study from Korea indicated that 25.6% of men over age 40 met the clinical and biochemical diagnostic criteria for LOH.36 However, the prevalence of symptomatic androgen deficiency in Taiwanese men was 12.0% using the criterion of TT <300 ng dl−1 and FT <5 ng dl−1.37 In our previously published work, the incidence of LOH according to the AMS questionnaires (without reference to hormone criteria) was 59.88%.7 Using the new criteria provided in our current study, the prevalence of LOH in 40–79 years old Shanghai men was much lower than previously reported (9.1%). Thus, measurements of serum testosterone levels should accompany the three sexual symptoms when diagnosing LOH. Our results highlight the importance of specifying the presence of three sexual symptoms as a diagnostic criterion, preferably with the lower TT/FT thresholds, to increase the probability of correctly diagnosing LOH.
According to the most recent Endocrine Society clinical practice guidelines,1 FT measurements should be performed in men in whom the TT concentrations are near the lower limit of the normal range. Some previous studies have suggested that androgen deficiency in patients with lower TT levels (below 12 nmol l−1) should be confirmed by testing the FT levels (below 220 pmol l−1) because the concentration of FT may correlate better with biological androgen activities.4,33,37 Our previous study7 also found that FT (measured by direct immunoassay) was more sensitive for evaluating LOH than TT. Although the production of testosterone decreases as a result of age-related testicular atrophy, there is no clear tendency toward decreased TT (attributable to a lower metabolic rate caused by increased SHBG levels). Decreased TT may occur at a later stage when LOH has advanced, and the decrease in FT occurs at a relatively early stage. From this point of view, FT is potentially a more sensitive indicator of LOH than TT.6,17 Therefore, we adopted the FT threshold as a helpful criterion in this study because it did not indicate a substantial difference with respect to the association between symptoms and testosterone levels in the present analytical approach. Also, the Mann–Whitney U-test revealed that the calculated FT level was more sensitive in the association between sexual symptoms and testosterone levels (Table 2). However, under the current circumstances in China, TT is used more universally than FT because the measurement of FT concentrations is still technically demanding in many hospitals.
This study had certain limitations. First, the data suggested that the participants are representative of elderly men in a highly developed city of China, a developing country, with respect to general characteristics but are markedly different from the general population concerning the prevalence and perception of symptoms. Considering the regional variability, a new study using a multi-center prospective cohort from a different region of China is being planned. Second, although the Access Immunoassay System (Beckman Coulter) is used for the detection of testosterone in many large hospitals, the variability and lack of accuracy of platform-based immunoassays should be considered.38 The proposed testosterone thresholds should be applied only with appropriate adjustments for the assay methods used in local laboratories. In addition, a comprehensive general assessment is required, including the improvement or development of optimal screening scales for the Chinese population and the evaluation of these scales with other sex hormones. Our results do not imply that a low testosterone level is the sole or foremost cause of LOH symptoms.3
CONCLUSION
Researchers in China are focusing more on the diagnosis of LOH, although little progress has been made. Based on data from a large population, our study indicated that the presence of three sexual symptoms combined with a TT level <13.21 nmol l−1 and an FT level <268.89 pmol l−1 can be the minimum criteria for the diagnosis of LOH in aging men. Our results may prevent the excessive diagnosis of hypogonadism in older men. Further, study using a multi-center prospective cohort from different regions of China is required to verify and improve these criteria for future use in the clinic.
AUTHOR CONTRIBUTIONS
ZYL and RYZ conceived of the study, XL drafted the manuscript and QSZ, HQW revised it. YHS participated in the design of the study. LZ participated in the design and coordination and critical revision of the manuscript. All authors read and approved the final manuscript.
COMPETING FINANCIAL INTERESTS
The authors declared no competing financial interests.
ACKNOWLEDGMENTS
This study was funded by a Major Scientific Research Proposal award of the Science and Technology Commission of Shanghai Municipality (No. 09DJ1400400).
The authors would like to thank Professor Shen Qian from the clinical laboratory of Changhai Hospital for arranging blood testing.
REFERENCES
- 1.Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, et al. International Society of Andrology (ISA); International Society for the Study of Aging Male (ISSAM); European Association of Urology (EAU); European Academy of Andrology (EAA); American Society of Andrology (ASA). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30:1–9. doi: 10.2164/jandrol.108.006486. [DOI] [PubMed] [Google Scholar]
- 2.Bassil N. Late-onset hypogonadism. Med Clin North Am. 2011;95:507–23. doi: 10.1016/j.mcna.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–35. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 4.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, et al. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010. doi: 10.1210/jc.2005-2847. [DOI] [PubMed] [Google Scholar]
- 5.Liverman CT, Blazer DG. Testosterone and Aging: Clinical Research Directions. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 6.Iwamoto T, Yanase T, Horie H, Namiki M, Okuyama A. Late-onset hypogonadism (LOH) and androgens: validity of the measurement of free testosterone levels in the diagnostic criteria in Japan. Int J Urol. 2009;16:168–74. doi: 10.1111/j.1442-2042.2008.02203.x. [DOI] [PubMed] [Google Scholar]
- 7.Sun K, Liang GQ, Chen XF, Ping P, Yao WL, et al. Survey for late-onset hypogonadism among old and middle-aged males in Shanghai communities. Asian J Androl. 2012;14:338–40. doi: 10.1038/aja.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinemann LA, Zimmermann T, Vermeulen A, Thiel C, Hummel W. A new aging males'symptoms (AMS) rating scale. Aging Male. 1999;2:105–14. [Google Scholar]
- 9.Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol (Oxf) 2003;58:710–7. doi: 10.1046/j.1365-2265.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- 10.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 11.Cleveland WS. Robust locally weighted fitting and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- 12.Benzécri JP. Correspondence Analysis Handbook. New York: Marcel Dekker; 1992. [Google Scholar]
- 13.Morales A, Spevack M, Emerson L, Kuzmarov I, Casey R, Tremblay R, et al. Adding to the controversy: pitfalls in the diagnosis of testosterone deficiency syndromes with questionnaires and biochemistry. Aging Male. 2007;10:57–65. doi: 10.1080/13685530701342686. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida NM, Kumano H, Kuboki T. Does the Aging Males’ Symptoms scale assess major depressive disorder. A pilot study? Maturitas. 2006;53:171–5. doi: 10.1016/j.maturitas.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract. 2006;60:762–9. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab. 2006;91:1336–44. doi: 10.1210/jc.2005-1830. [DOI] [PubMed] [Google Scholar]
- 17.Chen W, Liu ZY, Wang LH, Zeng QS, Wang HQ, et al. Are the Aging Male's Symptoms (AMS) scale and the Androgen Deficiency in the Aging Male (ADAM) questionnaire suitable for the screening of late-onset hypogonadism in aging Chinese men? Aging Male. 2013;16:92–6. doi: 10.3109/13685538.2013.805319. [DOI] [PubMed] [Google Scholar]
- 18.Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, et al. The Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol. 2004;46:80–7. doi: 10.1016/j.eururo.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Kratzik CW, Reiter WJ, Riedl AM, Lunglmayr G, Brandstätter N, et al. Hormone profiles, body mass index and aging male symptoms: results of the Androx Vienna Municipality study. Aging Male. 2004;7:188–96. doi: 10.1080/13685530412331284650. [DOI] [PubMed] [Google Scholar]
- 20.Heinemann LA, Saad F, Po¨lla¨nen P. Measurement of quality of life specific for aging males. In: Schneider HP, editor. Hormone Replacement Therapy and Quality of Life. London: Parthenon Publishing Group; 2002. pp. 63–83. [Google Scholar]
- 21.Daig I, Heinemann LA, Kim S, Leungwattanakij S, Badia X, et al. The Aging Males’ Symptoms (AMS) scale: review of its methodological characteristics. Health Qual Life Outcomes. 2003;1:77. doi: 10.1186/1477-7525-1-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinemann LA, Saad F, Thiele K, Wood-Dauphinee S. The Aging Males’ Symptoms (AMS) rating scale. Cultural and linguistic validation into English. Aging Male. 2001;3:14–22. [Google Scholar]
- 23.Heinemann LA, Saad F, Zimmermann T, Novak A, Myon E, et al. The Aging Males’ Symptoms (AMS) scale: update and compilation of international versions. Health Qual Life Outcomes. 2003;1:15. doi: 10.1186/1477-7525-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Convay K, Heinemann LA, Giroudet C, Johannes EJ, Myon E, et al. Harmonized French version of the Aging Males’ Symptoms scale. Aging Male. 2003;6:106–9. [PubMed] [Google Scholar]
- 25.Travison TG, Morley JE, Araujo AB, O’Donnell AB, McKinlay JB. The relationship between libido and testosterone levels in aging men. J Clin Endocrinol Metab. 2006;91:2509–13. doi: 10.1210/jc.2005-2508. [DOI] [PubMed] [Google Scholar]
- 26.Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91:4335–43. doi: 10.1210/jc.2006-0401. [DOI] [PubMed] [Google Scholar]
- 27.Delhez M, Hansenne M, Legros JJ. Andropause and psychopathology: minor symptoms rather than pathological ones. Psychoneuroendocrinology. 2003;28:863–74. doi: 10.1016/s0306-4530(02)00102-6. [DOI] [PubMed] [Google Scholar]
- 28.Tancredi A, Reginster JY, Schleich F, Pire G, Maassen P, et al. Interest of the Androgen Deficiency in Aging Males (ADAM) questionnaire for the identification of hypogonadism in elderly community-dwelling male volunteers. Eur J Endocrinol. 2004;151:355–60. doi: 10.1530/eje.0.1510355. [DOI] [PubMed] [Google Scholar]
- 29.Kshirsagar A, Seftel A, Ross L, Mohamed M, Niederberger C. Predicting hypogonadism in men based upon age, presence of erectile dysfunction, and depression. Int J Impot Res. 2006;18:47–51. doi: 10.1038/sj.ijir.3901369. [DOI] [PubMed] [Google Scholar]
- 30.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–88. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 31.Gray PB, Singh AB, Woodhouse LJ, Storer TW, Casaburi R, et al. Dose-dependent effects of testosterone on sexual function, mood, and visuospatial cognition in older men. J Clin Endocrinol Metab. 2005;90:3838–46. doi: 10.1210/jc.2005-0247. [DOI] [PubMed] [Google Scholar]
- 32.Gladh YM, Rahgozar M, Hammar ML, Fredriksson MG, Spetz AC. Prevalence of symptoms possibly related to PADAM, in a Swedish population aged 55, 65 and 75 years. Maturitas. 2005;50:161–6. doi: 10.1016/j.maturitas.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, et al. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–7. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 34.Travison TG, Araujo AB, Wruck LM, Kupelian V, O’Donnell AB, et al. Assessment of screening evaluations is not straightforward. Maturitas. 2006;54:305–8. doi: 10.1016/j.maturitas.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Buvat J, Maggi M, Guay A, Torres LO. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. J Sex Med. 2013;10:245–284. doi: 10.1111/j.1743-6109.2012.02783.x. [DOI] [PubMed] [Google Scholar]
- 36.Kang S, Park HJ, Park NC. Serum total testosterone level and identification of late-onset hypogonadism: a community-based study. Korean J Urol. 2013;54:619–23. doi: 10.4111/kju.2013.54.9.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu CC, Wu WJ, Lee YC, Wang CJ, Ke HL, et al. The prevalence of and risk factors for androgen deficiency in aging Taiwanese men. J Sex Med. 2009;6:936–46. doi: 10.1111/j.1743-6109.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- 38.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–13. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]


