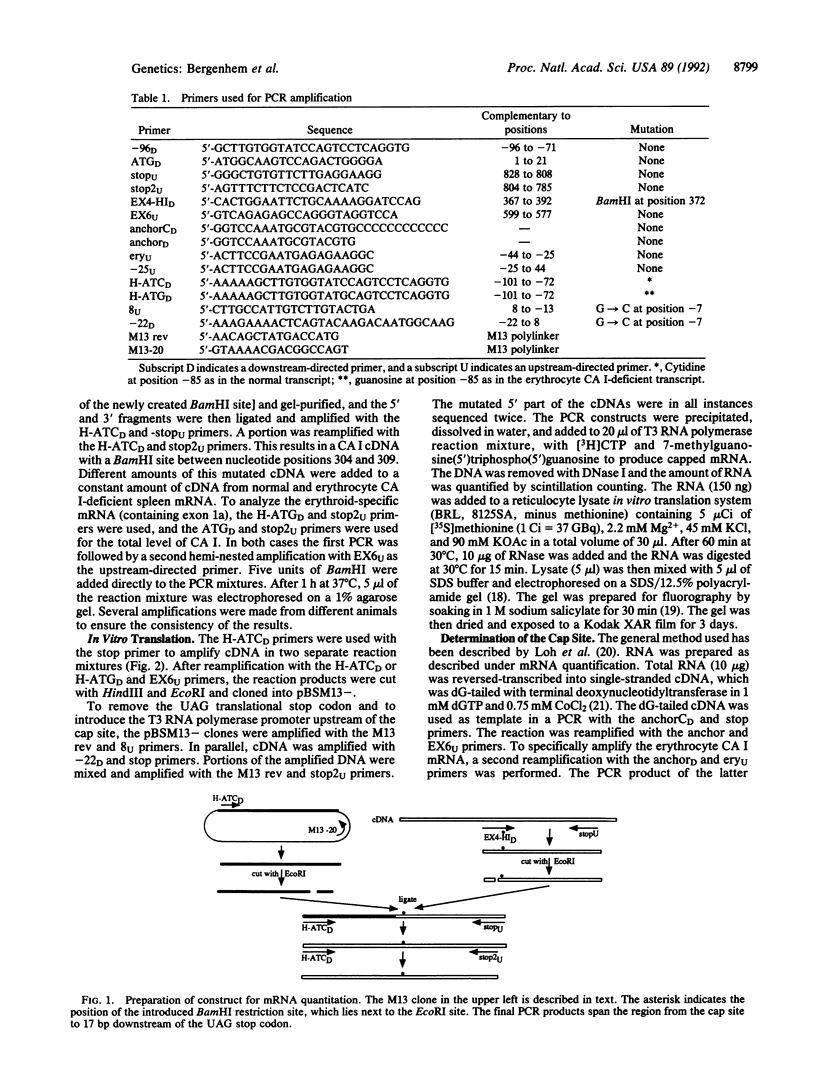
Abstract
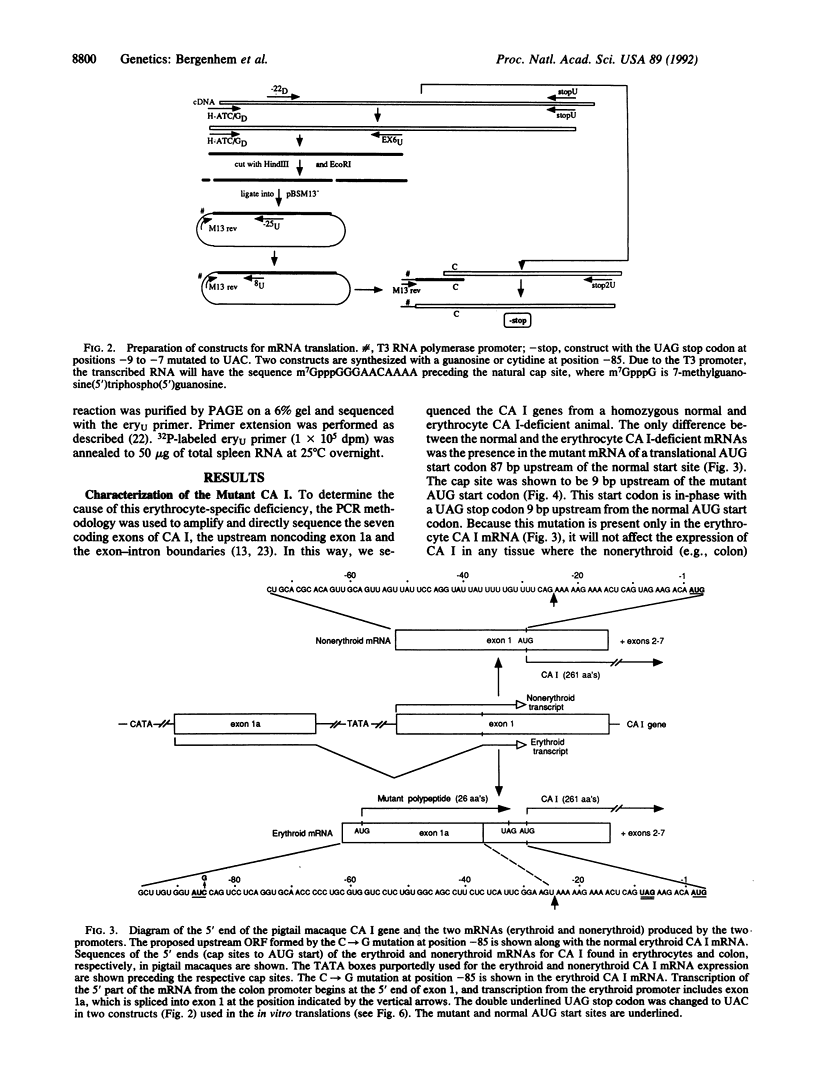
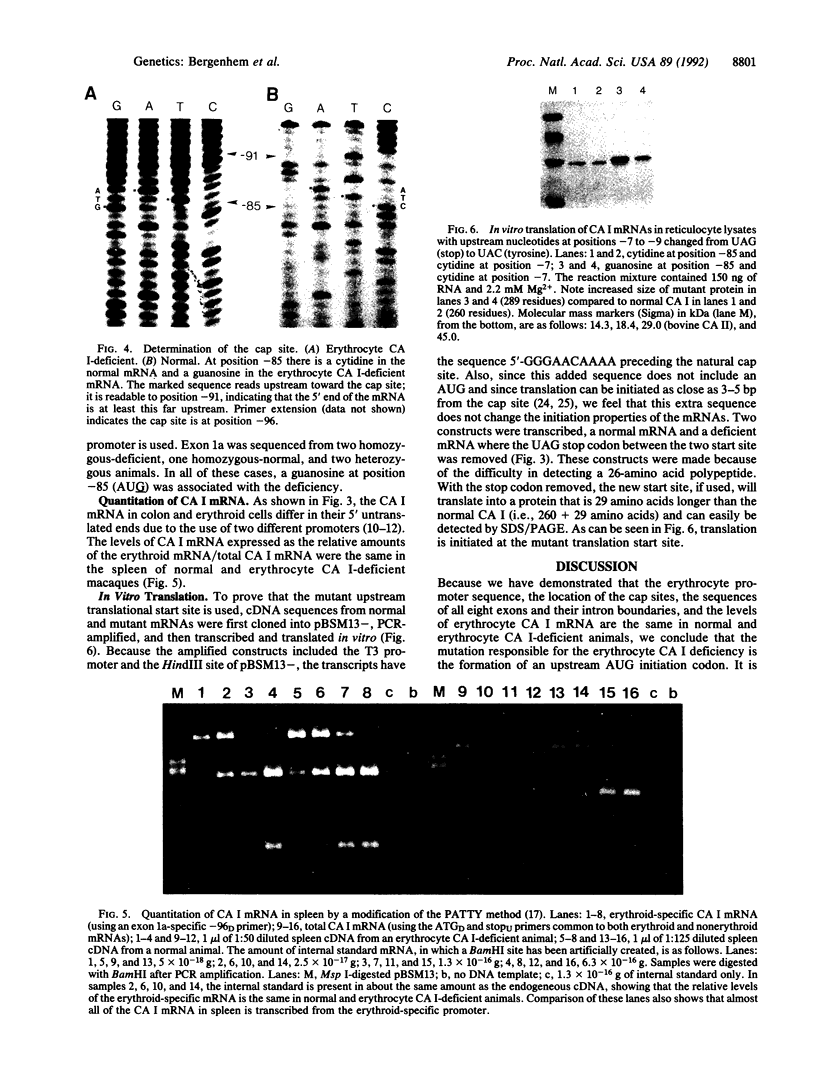
A variant allele at the CA I locus that produces a deficiency of erythrocyte-specific CA I occurs as a widespread polymorphism in pigtail macaques from southeast Asia. Sequence analyses revealed a C----G substitution 12 nucleotides downstream of the cap site in the variant erythrocyte CA I mRNA. This mutation forms a new AUG start site and an open reading frame coding for 26 amino acids that terminates 6 nucleotides before the normal AUG initiation codon for CA I. It appears that the presence of this upstream open reading frame greatly diminishes reinitiation of translation from the normal start site, resulting in trace levels of CA I in erythrocytes. Preferential use of the first AUG codon supports the scanning model for translation initiation in eukaryotes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B., Lüke W., Hunsmann G. Improvement of PCR amplified DNA sequencing with the aid of detergents. Nucleic Acids Res. 1990 Mar 11;18(5):1309–1309. doi: 10.1093/nar/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker-André M., Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 1989 Nov 25;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenhem N. C., Venta P. J., Hopkins P. J., Tashian R. E. Variation in coding exons of two electrophoretic alleles at the pigtail macaque carbonic anhydrase I locus as determined by direct, double-stranded sequencing of polymerase chain reaction (PCR) products. Biochem Genet. 1992 Jun;30(5-6):279–287. doi: 10.1007/BF02396217. [DOI] [PubMed] [Google Scholar]
- Brady H. J., Lowe N., Sowden J. C., Edwards M., Butterworth P. H. The human carbonic anhydrase I gene has two promoters with different tissue specificities. Biochem J. 1991 Aug 1;277(Pt 3):903–905. doi: 10.1042/bj2770903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- DeSimone J., Magid E., Tashian R. E. Genetic variation in the carbonic anhydrase isozymes of macaque monkeys. II. Inheritance of red cell carbonic anhydrase levels in different carbonic anhydrase I genotypes of the pig-tailed macaque, Macaca nemestrina. Biochem Genet. 1973 Feb;8(2):165–174. doi: 10.1007/BF00485544. [DOI] [PubMed] [Google Scholar]
- Deng G., Wu R. An improved procedure for utilizing terminal transferase to add homopolymers to the 3' termini of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4173–4188. doi: 10.1093/nar/9.16.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P., Cummings P., Curtis P. The mouse carbonic anhydrase I gene contains two tissue-specific promoters. Mol Cell Biol. 1989 Aug;9(8):3308–3313. doi: 10.1128/mcb.9.8.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch A. G. Involvement of an initiation factor and protein phosphorylation in translational control of GCN4 mRNA. Trends Biochem Sci. 1990 Apr;15(4):148–152. doi: 10.1016/0968-0004(90)90215-w. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Murtha P., Davies M. V. Translational efficiency of polycistronic mRNAs and their utilization to express heterologous genes in mammalian cells. EMBO J. 1987 Jan;6(1):187–193. doi: 10.1002/j.1460-2075.1987.tb04737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. E., Coleclough C., Perry R. P. Functional significance and evolutionary development of the 5'-terminal regions of immunoglobulin variable-region genes. Cell. 1982 Jun;29(2):681–689. doi: 10.1016/0092-8674(82)90184-2. [DOI] [PubMed] [Google Scholar]
- Kendall A. G., Tashian R. E. Erythrocyte carbonic anhydrase I: inherited deficiency in humans. Science. 1977 Jul 29;197(4302):471–472. doi: 10.1126/science.406674. [DOI] [PubMed] [Google Scholar]
- Kozak M. Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol Cell Biol. 1989 Nov;9(11):5073–5080. doi: 10.1128/mcb.9.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987 Oct;7(10):3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Evaluation of the fidelity of initiation of translation in reticulocyte lysates from commercial sources. Nucleic Acids Res. 1990 May 11;18(9):2828–2828. doi: 10.1093/nar/18.9.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eucaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nucleic Acids Res. 1984 May 11;12(9):3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Lowe N., Edwards Y. H., Edwards M., Butterworth P. H. Physical mapping of the human carbonic anhydrase gene cluster on chromosome 8. Genomics. 1991 Aug;10(4):882–888. doi: 10.1016/0888-7543(91)90176-f. [DOI] [PubMed] [Google Scholar]
- Peabody D. S., Berg P. Termination-reinitiation occurs in the translation of mammalian cell mRNAs. Mol Cell Biol. 1986 Jul;6(7):2695–2703. doi: 10.1128/mcb.6.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C. A., Piatigorsky J. Preferential conservation of the globular domains of the beta A3/A1-crystallin polypeptide of the chicken eye lens. Gene. 1986;45(2):139–147. doi: 10.1016/0378-1119(86)90248-9. [DOI] [PubMed] [Google Scholar]
- Tashian R. E., Goodman M., Headings V. E., Ward R. H., DeSimone J. Genetic variation and evolution in the red cell carbonic anhydrase isozymes of macaque monkeys. Biochem Genet. 1971 Apr;5(2):183–200. doi: 10.1007/BF00485644. [DOI] [PubMed] [Google Scholar]
- Tashian R. E., Kendall A. G., Carter N. D. Inherited variants of human red cell carbonic anhydrases. Hemoglobin. 1980;4(5-6):635–651. doi: 10.3109/03630268008997733. [DOI] [PubMed] [Google Scholar]
- Tashian R. E., Venta P. J., Nicewander P. H., Hewett-Emmett D. Evolution, structure, and expression of the carbonic anhydrase multigene family. Prog Clin Biol Res. 1990;344:159–175. [PubMed] [Google Scholar]