Abstract
Methylation modification is an important cellular mechanism of gene expression regulation. Dimethylarginine dimethylaminohydrolase-2 (DDAH-2) protein is a pivotal molecular for endothelium function. To explore the effects of 5-aza-deoxycytidine (5-aza), a demethylation agent, in hyperhomocysteinemia (hhcy)-related erectile dysfunction (ED) rats, 5-aza (1 mg kg−1) was administrated to Sprague-Dawley hhcy-rats induced by supplemented methionine chow diet. Erectile function, nitric oxide-cyclic guanosine monophosphate (NO-cGMP) levels, expression of DDAH-2 protein and promoter methylation status of DDAH-2 were studied in the corpora cavernosa. We found that supplemented methionine diet induced a high homocysteine level after 6 weeks of treatment. DDAH-2 protein was down-regulated in the corpora cavernosa while the administration of 5-aza up-regulated DDAH-2 expression and restored erectile function. The methionine-fed rats showed high methylation levels of DDAH-2 promoter region while the group treated with 5-aza demonstrated lower-methylation levels when compared to the methionine-fed group. Besides, the administration of 5-aza improved NO and cGMP levels in methionine-fed rats. Therefore, the methylation mechanism involves in ED pathogenesis, and demethylation offers a potential new strategy for ED treatment.
Keywords: DDAH-2, erectile dysfunction, hyperhomocysteinemia, methylation
INTRODUCTION
Homocysteine (hcy) has gained arising attention for its association with increased risk of myocardial infarction, stroke and venous thromboembolism.1,2 Hyperhomocysteinemia (hhcy), is defined as a pathological condition characterized by elevations of total plasma hcy. Erectile dysfunction (ED), also known as impotence, is a sexual dysfunction characterized by the inability to develop or maintain an erection of the penis during sexual activity.3 ED, especially for vasculogenic ED, is a vascular disorder of cavernosal vascular bed. Several clinical trials4,5,6 and animal experiments7,8 conducted on ED have demonstrated its close correlation with hcy.
The underlying mechanisms with which hcy induced vascular injury focused on: increased oxidative stress,9,10,11 decreased bioavailability of endothelium-derived nitric oxide (NO),12,13,14 promoting endothelial inflammation,15 endoplasmic reticulum stress and the unfolded protein response.16,17,18 Studies both in vitro19,20,21 and in vivo22,23,24 have demonstrated hcy induced endothelial dysfunction. Khan et al. reported hcy had an inhibitory effect on endothelium-dependent relaxation and NO formation in rabbit corpus cavernosum and this effect was augmented by copper and reversed by superoxide dismutase or catalase.8 Demir et al. showed that the presence of elevated levels of hcy (>12.1 μmol l−1) strongly predicted the occurrence of ED6 while Al-Hunayan et al. demonstrated that 1 μmol l−1 increase of plasma hcy concentration was associated with 2.95 times the odds of ED when compared to control subjects.4 Wang concluded in a review that hhcy to be “a novel risk factor of ED” and the measurement of plasma hcy might be “added to the ED diagnostic procedure.”25
However, the underlying mechanisms of hcy-related endothelial dysfunction were not fully understood. Protein dimethylarginine dimethylaminohydrolase (DDAH) acts as a pivotal role of endothelium function. DDAH metabolizes asymmetric dimethylarginine (ADMA), which serves as an endogenous inhibitor of nitric oxide synthase (NOS).26,27 There are two types of DDAH identified: DDAH-1 and DDAH-2, which is associated with neuronal NOS and endothelial NOS, respectively.28 On the other hand, recent studies found that DDAH-2 promoter methylation was associated with endothelial dysfunction.29 DNA methylation modification is an important cellular mechanism of gene expression regulation. Based on all the previous findings, in this study, we investigated the hypothesis that DDAH-2 promoter hypermethylation associated with ED pathogenesis and we observed the possible effect of 5-aza-deoxycytidine (5-aza), a demethylation agent, for the treatment of ED in a rat model of hhcy.
MATERIALS AND METHODS
Animal groups and treatment protocol
This study complied with the guidelines of animal experiments of Nanjing University, and was approved by the Laboratory Animal Users Committee at Drum Tower Hospital, Nanjing, China. Forty male 12-week-old Sprague-Dawley rats weighing 220-280 g were purchased from Shanghai Slac Laboratory Animal Co., Ltd., (Shanghai, China). Twelve rats fed a standard diet were regarded as a control group (Con). Twenty-eight rats were fed a standard diet and L-methionine supplemented diet (2 g kg−1 body weight). These rats were randomly divided into a methionine group (Met) (n = 14) that were administered with intraperitoneally injection of normal saline and an experimental group that were administered with intraperitoneally injection of 5-aza (Sigma-Aldrich, USA) at 1 mg kg−1 (Met + 5-aza) (n = 14) at day 0 and every 3 days of the treatment procedure. After the treatment for 6 weeks, blood sample was collected from the ear vein. Plasma hcy levels, evaluated with enzymatic cycling assay (Boster, Wuhan, China) and body weights were measured at day 0 and prior to euthanasia.
In vivo assessment of erectile function and tissue procurement
After 6 weeks, all rats underwent the measurement of erectile function through the stimulation of cavernous nerve. The nerve stimulation was conducted at a frequency of 20 Hz with a pulse width of 5 ms. Stimulations were performed at 5 V for 60 s with resting periods of 5 min between subsequent stimulations. The mean arterial pressure (MAP) and intracavernosal pressure (ICP) were measured as described previously.30 At the end of the experiment, all animals were sacrificed. The penis was excised, and the cavernous tissues were dissected from skin and divided into two fragments: one fragment was for target proteins and mRNA and NO detection, and the remaining part was then frozen in liquid nitrogen and then stored at −80°C for future use.
Measurement of plasma ADMA levels
Plasma ADMA levels were measured using high-performance liquid chromatography (HPLC) as described previously in detail.31,32
Measurement of NO
The NO and cyclic guanosine monophosphate (cGMP) concentration in corpus cavernosum were evaluated with spectrophotometry method, using a commercial kit (Boster, Wuhan, China), following the protocols provided by the manufacturer.
DDAH-2 protein level
DDAH-2 protein expression of the three groups was detected by Western blot. The penile tissue strips were homogenized in a buffer containing 50 mM Tris-HCl PH 7.2, 0.1 M NaCl, 5 mM EDTA, 0.5% Triton X-100 and protease inhibitor. After homogenization, samples were centrifuged at 12 000 g for 15 min at 4°C. The supernatants were collected for protein concentration measurement using bicinchoninic acid protein assay (Keygen, China). Equal amounts of proteins (50 μg) were loaded and run on 10% sodium dodecyl sulfate-polyacrylamide gels and then electrotransferred to nitrocellulose membranes, which were incubated with primary antibody against DDAH-2 (Abcam Inc., China; 1:1000) or β-actin (Abcam Inc., China; 1:1000) at room temperature for 4 h. This was subsequently followed by addition of secondary antibodies (Maibio Inc., China; 1:5000). Detection was performed by chemiluminescence (Boster, Wuhan, China) with exposure to X-ray film.
DDAH-2 mRNA expression
DDAH-2 mRNA levels were analyzed using real-time PCR methods. Total RNA was extracted from the penile strips using Trizol regent (Invitrogen, China) according to the manufacturer's protocol. 1 μg RNA was reversely transcribed into cDNA respectively. The total quantitative real-time PCR volume is 25 μl containing SYBR Green PCR mix (Takara, Japan) and oligonucleotide primers. Primer sequences and PCR reaction conditions were shown in Table 1. The thermal cycling conditions for the real-time PCR was 95°C for 30 s (denaturation), followed by 95°C for 5 s, 60°C for 30 s (40 cycles). Relative expression levels for DDAH-2 were normalized to the expression of housekeeping gene β-actin by the 2−ΔΔCT method: ΔCT = (CTDDAH-2 − CTβ-actin); −ΔΔCT = ΔCT (Met group or Met+5-aza group) − ΔCT (Con group); RQ (Relative Quantitation) = 2−ΔΔCT, RQ (Con group) = 1.
Table 1.
Primer sequences, annealing temperature (Ta) and product size

Detection of DDAH-2 promoter methylation levels
Genomic DNA was extracted from blood samples using TIANamp Blood DNA kit (Tiangen, China) and fresh frozen cavernosum tissues using a DNA isolation kit (Qiagen AB, Solna, Sweden), as per the manufacturer's instructions. Bisulfite modification was undertaken with 500 ng samples of DNA using DNA Methylation Kit™ (Zymo Research, HiSS Diagnostics, CA, USA), as per the manufacturer's instructions. The samples were then eluted in 15 μl of elution buffer. The pyrosequencing assay was used to assess methylation status of the DDAH-2 promoter (Chr 20-NC_005119.4 (5049265-5050613)). This assay detected the level of methylation in a region (Chr 20-NC_005119.4 (5049522-5049598)) of the DDAH-2 gene (Chr 20-NC_005119.4 (5049265-5052573)) (Figure 1). PCR and pyrosequencing primers were designed using PyroMark Assay Design 2.0 (Qiagen, Germany). The PCR volume was 20 μl, and incorporated 0.5 μM forward and reverse primers, 10 μl ZymoTaq™ Premix (Zymo Research, HiSS Diagnostics, CA, USA) and 2 μl bisulfite-modified DNA. The forward and reverse primer sequences were 5’- AGTTTGGGGGTGTTGTATATG-3’ and 5’- biotin-CCACTCCAAACTTCAAAACTCTAT-3’, respectively. PCR testing was carried out at 95°C for 10 min, followed by 40 cycles of 95°C for 30 s, 58°C for 30s, and 72°C for 1 min, with a final extension of 72°C for 10 min. PCR product quality was confirmed on 1% agarose gels with ethidium bromide staining. Pyrosequencing was subsequently carried out on the PyroMark™ Q24 pyrosequencer (Qiagen, Germany), with the sequencing primer 5’- GGGGTGTTGTATATGAG-3. The degree of methylation of all six CpG sites was automatically analyzed by the PyroMark™ Q24 software. Briefly, methylation frequency of CpG site was measured in accordance with the ratio of light units between the sum of methylated alleles and the unmethylated alleles.
Figure 1.
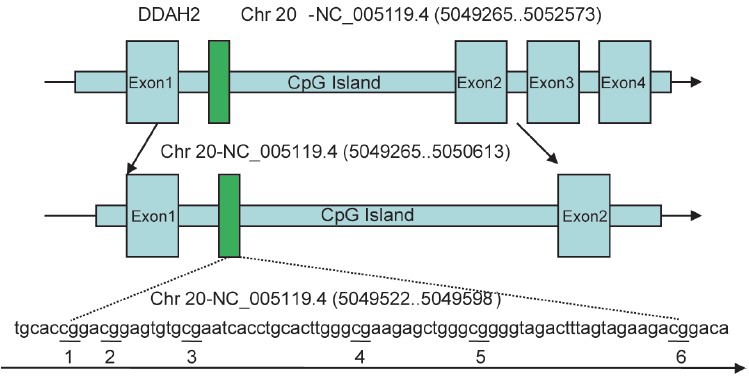
The tested position of DDAH-2 promoter regions.
Statistical analysis
All data were expressed as mean ± standard error. Differences among the groups were assessed using one-way analysis of variance for multiple comparisons, followed by post hoc comparisons using least significant difference test. P < 0.05 was regarded significant.
RESULTS
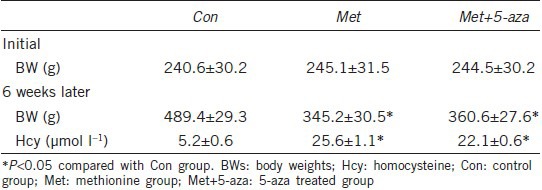
The body weights and plasma hcy levels
The body weights and plasma hcy levels of the experimental rats were shown in Table 2. The body weights of Met group were lower than controls after 6 weeks (P < 0.05). The hcy levels of Met group were statistically higher compared to Con group (P < 0.05). However, 5-aza did not change these values.
Table 2.
BWs and plasma hcy levels
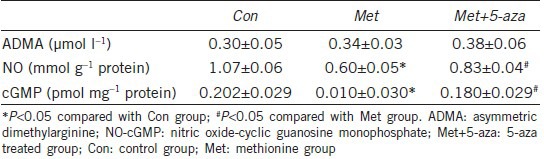
Plasma ADMA and cavernosum NO-cGMP levels
As shown in Table 3, decreased cavernosum NO and cGMP levels were observed in Met group compared to the Con group (P < 0.05). Six weeks of 5-aza administration increased NO and cGMP levels significantly compared with methionine-fed rats (P < 0.05). However, comparisons of ADMA yielded nonsignificant results (P > 0.05).
Table 3.
Plasma ADMA and cavernosum NO-cGMP levels
Erectile function of the three groups
The ICP/MAP ratio was presented in Figure 2. The mean ICP/MAP ratio was decreased in Met group compared to Con group (P < 0.01). However, after 5-aza administration, the mean ICP/MAP ratio increased as compared with Met group (P < 0.05).
Figure 2.
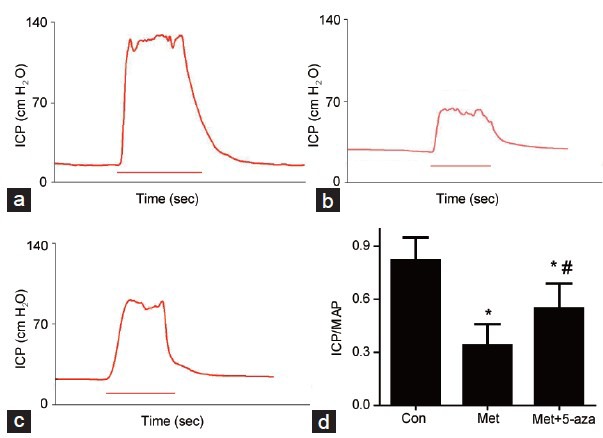
Erectile function evaluation. Intracavernous pressure (ICP) and peak intracavernous pressure/mean systemic arterial pressure (ICP/MAP). (a) Control group. (b) Methionine group. (c) Methionine and 5-aza treated group. (d) Statistical chart of ICP/MAP ratio. The ICP/MAP values in the Met group decreased. 5-aza treatment could partially reverse this reduction, but not to the normal level. *P < 0.05 versus control group; #P < 0.05 versus Met group.
Protein and mRNA levels of DDAH-2 in cavernous tissue
As indicated in Figure 3, the expression of DDAH-2 protein and mRNA decreased significantly in the Met group when compared to Con group (P < 0.01). However, after 5-aza treatment, the expression of DDAH-2 protein and mRNA improved when compared to the Met group (P < 0.05).
Figure 3.
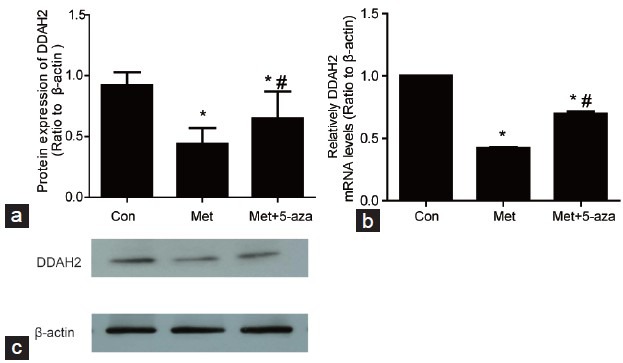
The protein and mRNA levels of DDAH-2 in cavernosum tissues. (a) DDAH-2 protein decreased in Met group compared with Con and increased in Met+5-aza group compared with Met group. (b) The DDAH-2 mRNA decreased in Met group compared with Con group while increased in Met+5-aza group in comparison with Met group. (c) Western blot results of DDAH-2 protein and β-actin. *P < 0.05 versus Con group; #P < 0.05 versus Met group; Con: control group; Met: methionine group; Met+5-aza: 5-aza treated group.
Methylation levels of DDAH-2 promoter region in blood and cavernous tissue
The representative pyrosequencing graphic of the three groups within cavernous tissues were shown in Figure 4a–4c. The mean methylation level of the six detected CpG dinucleotide elevated in Met group compared to the Con group within the blood (P < 0.05) and cavernosum tissues (P < 0.05). However, the methylation level in Met+5-aza group was lower than Met group within the blood (P < 0.05) and cavernous tissues (P < 0.05) (Figure 4d).
Figure 4.
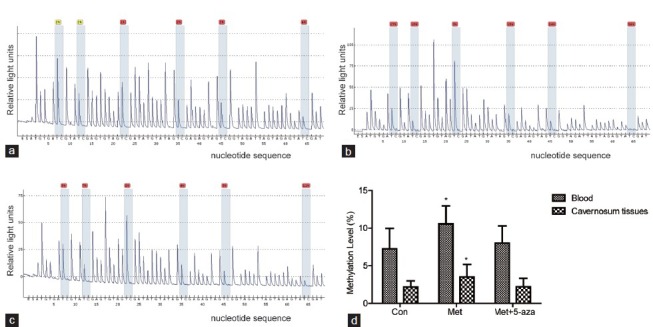
The methylation levels of DDAH-2 promoter region in blood and cavernosum tissues. (a) Pyrosequencing graphic results of Con group in cavernosum tissues. (b) Pyrosequencing graphic results of Met group in cavernosum tissues. (c) Pyrosequencing graphic results of Met+5-aza group in cavernosum tissues. (d) DDAH-2 promoter methylation levels increased in Met group compared to Con group and Met+5-aza group. *P < 0.05 versus Con and Met+5-aza group. Con: control group; Met: methionine group; Met+5-aza: 5-aza treated group.
DISCUSSION
Recent studies have confirmed a positive link between hcy and endothelial dysfunction. However, existing mechanisms, including increased oxidative stress, decreased bioavailability of endothelium-derived NO and promoting endothelial inflammation were not exclusively enough for the explanation of hcy-related endothelial dysfunction. For example, it failed to explain that cysteine, which has a similar molecular structure with hcy, was not regarded as a risk factor for endothelial dysfunction.
ADMA is an endogenous inhibitor of NO synthase, and serves as a possible mediator of hhcy and endothelial dysfunction.33,34 The major pathway of ADMA clearance is DDAH, a key enzyme responsible for ADMA elimination. Liu et al. found that declined DDAH-2 activity was associated with an accumulation of ADMA and decreased NO production in endothelial cells.35 Additionally, Rodionov et al. concluded DDAH-1 overexpression was a protective manner from hhcy-induced alternations in cerebral arteriolar structure and vascular muscle function, and this protective effect was independent from the elevation of serum ADMA.36 DDAH is recognized as a protective factor of endothelial function and plays an important role of endothelium function.
Some studies have suggested elevation of ADMA is an inner mechanism of endothelial dysfunction in hhcy.34,37,38 In the present study, the plasma ADMA levels were of no significance among the three groups. These results were consistent with previous observational studies39,40 that hhcy was not always accompanied with ADMA elevation. A possible explanation is that there is a ADMA-independent pathway of DDAH-mediated endothelium protection. For instance, this protective effect is mediated by DDAH-1-dependent phosphorylation of neurofibromin 1, a molecular that regulate vascular muscle proliferation.41,42 Additionally, Pope et al. concluded that DDAH-mediated endothelium function “is largely ADMA-dependent and latter ADMA-independent.”43 On the other hand, since ADMA is taken up by endothelium, ADMA could accumulate and metabolize by DDAH intracellularly.44
Hcy is a metabolic byproduct forming during the methionine cycle.45,46 Methionine is converted to S-adenosylmethionine (SAM) by the catalysis of methionine adenosyltransferase (MAT). SAM acts as a methyl group donor in the DNA methylation reaction. S-adenosyl-homocysteine (SAH) is formed after SAM donates its methyl group in methylation reactions. SAH is hydrolyzed to hcy by S-adenosylhomocysteine hydrolase (SAHH). Hcy is catabolized through several pathways. Hcy is converted to methionine in remethylation pathway by the action of methylenetetrahy-drofolate reductase (MTHFR) and alternatively turned into cystathionine by cystathione-β synthase (CBS) in the transsulfuration pathway. Hcy involves in the DNA methylation process and may be an intrinsic reflect of DNA methylation modification. DNA methylation serves as an important part of epigenetics and is an important manner of gene expression regulation.
Epigenetics is the study of inherited changes in phenotype or gene expression caused by mechanisms other than changes in the underlying DNA sequence.47 Epigenetics include three distinct, but highly interrelated mechanisms: DNA methylation, histone density and posttranslational modifications and RNA-based mechanisms. DNA methylation is an important part of epigenetics and manner of gene expression regulation.48 DNA methylation is a process that involves the transfer of a methyl-group from a methyl-donor, like SAM, to cytosine in a CpG dinucleotide using DNA methyltransferases, which is 5’-m5CpG-3’. The human genome consists of approximately 28 000 000 CpGs, and only about 7% of all CpGs are within CpG islands.49 CpG islands refer to the regions about 200–500 bp within human genome with relatively high content of CpG dinucleotide. Interestingly, CpG islands are usually associated with gene promoters. About 70% genes promoter regions within human genome have CpG islands.50 CpG dinucleotide are usually to be normally methylated in the transcriptional regions of human genes but tend to be unmethylated in the promoter regions alternatively, which serves as a pretranscriptional regulation of gene expression.51 The CpG island hypermethylation is thought to be associated with transcriptional repression. The possible mechanisms underneath are CpG island hypermethylation attenuating the binding of transcriptional factors to DNA and recruiting the methyl-CpG binding proteins (MBDs), which in turn establishes a transcriptional inactive chromatin environment.52
Recent researches demonstrated that the methylation changes of DDAH-2 gene were associated with hhcy-induced endothelial dysfunction.46 Niu et al. concluded in a clinical study that hypermethylation of DDAH-2 promoter contributed to the impaired function of endothelial progenitor cells in coronary artery disease patients.29 Jia et al. found hcy induced apoptosis of endothelial cells via elevating the methylation level of DDAH-2 promoter regions in cultured human umbilical vein endothelial cells.53 Similar results were further confirmed in some other studies in vivo.46,54 These observations were connected with each other and possibly provided a novel mechanism (DDAH-2 promoter methylation) of hcy-related endothelium impairments.
In the present study, the CpG islands were predicted in the promoter region of DDAH-2 using an online program (http://www.urogene.org/cgi-bin/methprimer). A specific CpG-rich sequence in the promoter region (Chr 20-NC_005119.4 (5049522-5049598)) with six CpG dinucleotide sites included were selected. Tomikawa et al. previously demonstrated that methylation in this region associated with the silencing of DDAH-2 expression.55 These results showed a similar trend with the current experiments.
In the present study, the results indicated a lower-methylation patterns in Con group and Met+5-aza (a demethylation agent) group, as compared with Met group. Otherwise, we detected DDAH-2 protein expression and mRNA levels. The results showed that DDAH-2 protein and mRNA expression were markedly attenuated in the Met group, but elevated in demethylation treatment group and showed a consistent trend with the methylation patterns of DDAH-2 promoter regions. Hence, we successfully demonstrated that down-regulation of DDAH-2 protein in parallel with DDAH-2 promoter region hypermethylation resulted in impaired endothelial function and ED subsequently.
However, there were several limitations of the present study that should take into consideration. First, we did not examine vascular functional studies using endothelium-dependent or endothelium-independent agent in rats cavernosal strips treated with 5-aza. Jones et al. reported an impaired cavernosal relaxation response to carbachol (an endothelium-dependent agent) in isolated hhcy rabbit corpus cavernosum. However, when stimulated with sodium nitroprusside, an endothelium-independent or using nonadrenergic noncholinergic (NANC)-mediated electrical-field stimulation, this relaxation response was unaffected.7 Shukla et al. showed that carbachol-induced relaxation and NANC-mediated electrical-field stimulation-induced relaxation were significantly attenuated in cavernosal strips of diabetes mellitus rabbit. However, this reduced response was reversed by in rats administrated folic acid, which decreased hcy levels.33 The main neurotransmitter released by NANC neurons and endothelium of corpus cavernosum is NO, which is a key molecular that mediates penile erection. In this study, in 5-aza treated rats, elevated DDAH-2 protein level and NO content in cavernous tissues compared with the methionine-fed rats were observed, which possibly showed an elevated response to endothelium-dependent agent or NANC-mediated relaxation in 5-aza treated rats cavernosal strips. This hypothesis, however, is necessary and needs further investigation. Second, DDAH-2 is associated with endothelial NOS while DDAH-1 neuronal NOS and hcy is widely regarded as a risk factor for cardiovascular diseases. Therefore, in the current experiment, the methylation level, protein expression, and mRNA level of DDAH-2 were evaluated, while DDAH-1 remains unknown. Dayal et al. demonstrated both in vitro and in vivo, hhcy caused decreased DDAH-2 mRNA level in the liver.56 Furthermore, Imamura et al. reported that DDAH-1 protein was down-regulated in rabbit cavernous tissue following cigarette smoke extract treatment.57 These results indicated a possibility of DDAH-1-related role in the pathogenesis of hhcy ED. Additionally, using the online program (http://www.urogene.org/cgi-bin/methprimer), CpG islands (Chr 20-NC_005101.4 (251634306-251634769)) were also identified in the promoter region of DDAH-1. This offers a new direction for future studies and enriched the theory of hcy-related ED and clinically beneficial subsequently. Third, in this study, we did not detect the protein levels of eNOS and nNOS in correspondence with DDAH subtypes or eNOS and nNOS activities in rats cavernosal tissues. NO production depends on NOS activity and NOS protein expression. Although studies demonstrated that hcy could inhibit eNOS activity and NO production in vivo46 or promote uncoupling eNOS,58 a condition that eNOS produces superoxide rather than NO, further studies are warranted to illustrate the possible mechanisms of hcy-induced endothelial dysfunction and protective effect of demethylation treatment.
CONCLUSION
For the first time, we provided the evidence that methylation mechanisms involved in ED pathogenesis and demethylation therapy with 5-aza restored erectile function in a rat model of hhcy. This offers new insight into the pathogenesis of ED and thus provides novel therapeutic methods.
AUTHOR CONTRIBUTIONS
ZZ and LLZ carried out the molecular genetic studies, participated in the sequence alignment and drafted the manuscript. HSJ carried out the immunoassays. ZZ and HC participated in the sequence alignment. YC participated in the design of the study and performed the statistical analysis. YTD conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The present study was supported by the National Natural Scientific Foundation of China Grants (No. 81170563).
REFERENCES
- 1.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325:1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288:2015–22. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 3.Solomon H, Man JW, Wierzbicki AS, Jackson G. Relation of erectile dysfunction to angiographic coronary artery disease. Am J Cardiol. 2003;91:230–1. doi: 10.1016/s0002-9149(02)03113-2. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hunayan A, Thalib L, Kehinde EO, Asfar S. Hyperhomocysteinemia is a risk factor for erectile dysfunction in men with adult-onset diabetes mellitus. Urology. 2008;71:897–900. doi: 10.1016/j.urology.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Demir T, Comlekci A, Demir O, Gulcu A, Caliskan S, et al. A possible new risk factor in diabetic patients with erectile dysfunction: homocysteinemia. J Diabetes Complications. 2008;22:395–9. doi: 10.1016/j.jdiacomp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Demir T, Comlekci A, Demir O, Gulcu A, Calypkan S, et al. Hyperhomocysteinemia: a novel risk factor for erectile dysfunction. Metabolism: clin Exp. 2006;55:1564–8. doi: 10.1016/j.metabol.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Jones RW, Jeremy JY, Koupparis A, Persad R, Shukla N. Cavernosal dysfunction in a rabbit model of hyperhomocysteinaemia. BJU Int. 2005;95:125–30. doi: 10.1111/j.1464-410X.2004.05263.x. [DOI] [PubMed] [Google Scholar]
- 8.Khan MA, Thompson CS, Emsley AM, Mumtaz FH, Mikhailidis DP, et al. The interaction of homocysteine and copper markedly inhibits the relaxation of rabbit corpus cavernosum: new risk factors for angiopathic erectile dysfunction? BJU Int. 1999;84:720–4. doi: 10.1046/j.1464-410x.1999.00253.x. [DOI] [PubMed] [Google Scholar]
- 9.Topal G, Brunet A, Millanvoye E, Boucher JL, Rendu F, et al. Homocysteine induces oxidative stress by uncoupling of NO synthase activity through reduction of tetrahydrobiopterin. Free Radic Biol Med. 2004;36:1532–41. doi: 10.1016/j.freeradbiomed.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Au-Yeung KK, Woo CW, Sung FL, Yip JC, Siow YL, et al. Hyperhomocysteinemia activates nuclear factor-kappaB in endothelial cells via oxidative stress. Circ Res. 2004;94:28–36. doi: 10.1161/01.RES.0000108264.67601.2C. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman M. Hypothesis: hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses. 2011;77:1088–93. doi: 10.1016/j.mehy.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Upchurch GR, Jr, Welch GN, Fabian AJ, Freedman JE, Johnson JL, et al. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem. 1997;272:17012–7. doi: 10.1074/jbc.272.27.17012. [DOI] [PubMed] [Google Scholar]
- 13.Stamler JS, Osborne JA, Jaraki O, Rabbani LE, Mullins M, et al. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest. 1993;91:308–18. doi: 10.1172/JCI116187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faraci FM. Hyperhomocysteinemia: a million ways to lose control. Arterioscler Thromb Vasc Biol. 2003;23:371–3. doi: 10.1161/01.ATV.0000063607.56590.7F. [DOI] [PubMed] [Google Scholar]
- 15.Papatheodorou L, Weiss N. Vascular oxidant stress and inflammation in hyperhomocysteinemia. Antioxid Redox Signal. 2007;9:1941–58. doi: 10.1089/ars.2007.1750. [DOI] [PubMed] [Google Scholar]
- 16.Outinen PA, Sood SK, Pfeifer SI, Pamidi S, Podor TJ, et al. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood. 1999;94:959–67. [PubMed] [Google Scholar]
- 17.Outinen PA, Sood SK, Liaw PC, Sarge KD, Maeda N, et al. Characterization of the stress-inducing effects of homocysteine. Biochem J. 1998;332(Pt 1):213–21. doi: 10.1042/bj3320213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokame K, Agarwala KL, Kato H, Miyata T. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J Biol Chem. 2000;275:32846–53. doi: 10.1074/jbc.M002063200. [DOI] [PubMed] [Google Scholar]
- 19.Emsley AM, Jeremy JY, Gomes GN, Angelini GD, Plane F. Investigation of the inhibitory effects of homocysteine and copper on nitric oxide-mediated relaxation of rat isolated aorta. Br J Pharmacol. 1999;126:1034–40. doi: 10.1038/sj.bjp.0702374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang D, Kredan MB, Moat SJ, Hussain SA, Powell CA, et al. Homocysteine-induced inhibition of endothelium-dependent relaxation in rabbit aorta: role for superoxide anions. Arterioscler Thromb Vasc Biol. 2000;20:422–7. doi: 10.1161/01.atv.20.2.422. [DOI] [PubMed] [Google Scholar]
- 21.Ungvari Z, Pacher P, Rischak K, Szollar L, Koller A. Dysfunction of nitric oxide mediation in isolated rat arterioles with methionine diet-induced hyperhomocysteinemia. Arterioscler Thromb Vasc Biol. 1999;19:1899–904. doi: 10.1161/01.atv.19.8.1899. [DOI] [PubMed] [Google Scholar]
- 22.Lentz SR, Sobey CG, Piegors DJ, Bhopatkar MY, Faraci FM, et al. Vascular dysfunction in monkeys with diet-induced hyperhomocyst(e)inemia. J Clin Invest. 1996;98:24–9. doi: 10.1172/JCI118771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tawakol A, Omland T, Gerhard M, Wu JT, Creager MA. Hyperhomocyst(e)inemia is associated with impaired endothelium-dependent vasodilation in humans. Circulation. 1997;95:1119–21. doi: 10.1161/01.cir.95.5.1119. [DOI] [PubMed] [Google Scholar]
- 24.Woo KS, Chook P, Lolin YI, Cheung AS, Chan LT, et al. Hyperhomocyst(e)inemia is a risk factor for arterial endothelial dysfunction in humans. Circulation. 1997;96:2542–4. doi: 10.1161/01.cir.96.8.2542. [DOI] [PubMed] [Google Scholar]
- 25.Wang CH, Huang YF. [Hyperhomocysteinemia and erectile dysfunction: an update] Zhonghua Nan Ke Xue=Natl J Androl. 2011;17:1019–22. [PubMed] [Google Scholar]
- 26.Pope AJ, Karuppiah K, Cardounel AJ. Role of the PRMT-DDAH-ADMA axis in the regulation of endothelial nitric oxide production. Pharmacol Res: official J Ital Pharmacol Soc. 2009;60:461–5. doi: 10.1016/j.phrs.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boger RH. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med. 2006;38:126–36. doi: 10.1080/07853890500472151. [DOI] [PubMed] [Google Scholar]
- 28.Beltowski J, Kedra A. Asymmetric dimethylarginine (ADMA) as a target for pharmacotherapy. Pharmacol Rep. 2006;58:159–78. [PubMed] [Google Scholar]
- 29.Niu PP, Cao Y, Gong T, Guo JH, Zhang BK, et al. Hypermethylation of DDAH2 promoter contributes to the dysfunction of endothelial progenitor cells in coronary artery disease patients. J Transl Med. 2014;12:170. doi: 10.1186/1479-5876-12-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu W, Wan Z, Qiu XF, Chen Y, Dai YT. Resveratrol, an activator of SIRT1, restores erectile function in streptozotocin-induced diabetic rats. Asian J Androl. 2013;15:646–51. doi: 10.1038/aja.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boger RH, Lentz SR, Bode-Boger SM, Knapp HR, Haynes WG. Elevation of asymmetrical dimethylarginine may mediate endothelial dysfunction during experimental hyperhomocyst(e)inaemia in humans. Clin Sci (Lond Engl 1979) 2001;100:161–7. [PubMed] [Google Scholar]
- 32.Esse R, Florindo C, Imbard A, Rocha MS, de Vriese AS, et al. Global protein and histone arginine methylation are affected in a tissue-specific manner in a rat model of diet-induced hyperhomocysteinemia. Biochim Biophys Acta. 2013;1832:1708–14. doi: 10.1016/j.bbadis.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Shukla N, Hotston M, Persad R, Angelini GD, Jeremy JY. The administration of folic acid improves erectile function and reduces intracavernosal oxidative stress in the diabetic rabbit. BJU Int. 2009;103:98–103. doi: 10.1111/j.1464-410X.2008.07911.x. [DOI] [PubMed] [Google Scholar]
- 34.Stuhlinger MC, Stanger O. Asymmetric dimethyl-L-arginine (ADMA): a possible link between homocyst(e)ine and endothelial dysfunction. Curr Drug Metab. 2005;6:3–14. doi: 10.2174/1389200052997393. [DOI] [PubMed] [Google Scholar]
- 35.Liu LH, Guo Z, Feng M, Wu ZZ, He ZM, et al. Protection of DDAH2 overexpression against homocysteine-induced impairments of DDAH/ADMA/NOS/NO pathway in endothelial cells. Cell Physiol Biochem: int J Exp Cell Physiol Biochem Pharmacol. 2012;30:1413–22. doi: 10.1159/000343329. [DOI] [PubMed] [Google Scholar]
- 36.Rodionov RN, Dayoub H, Lynch CM, Wilson KM, Stevens JW, et al. Overexpression of dimethylarginine dimethylaminohydrolase protects against cerebral vascular effects of hyperhomocysteinemia. Circ Res. 2010;106:551–8. doi: 10.1161/CIRCRESAHA.109.200360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuhlinger MC, Oka RK, Graf EE, Schmolzer I, Upson BM, et al. Endothelial dysfunction induced by hyperhomocyst(e)inemia: role of asymmetric dimethylarginine. Circulation. 2003;108:933–8. doi: 10.1161/01.CIR.0000085067.55901.89. [DOI] [PubMed] [Google Scholar]
- 38.Dayal S, Lentz SR. ADMA and hyperhomocysteinemia. Vasc Med (Lond Engl) 2005;10(Suppl 1):S27–33. doi: 10.1191/1358863x05vm599oa. [DOI] [PubMed] [Google Scholar]
- 39.Antoniades C, Tousoulis D, Marinou K, Vasiliadou C, Tentolouris C, et al. Asymmetrical dimethylarginine regulates endothelial function in methionine-induced but not in chronic homocystinemia in humans: effect of oxidative stress and proinflammatory cytokines. Am J Clin Nutr. 2006;84:781–8. doi: 10.1093/ajcn/84.4.781. [DOI] [PubMed] [Google Scholar]
- 40.Korandji C, Zeller M, Guilland JC, Vergely C, Sicard P, et al. Asymmetric dimethylarginine (ADMA) and hyperhomocysteinemia in patients with acute myocardial infarction. Clin Biochem. 2007;40:66–72. doi: 10.1016/j.clinbiochem.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Tokuo H, Yunoue S, Feng L, Kimoto M, Tsuji H, et al. Phosphorylation of neurofibromin by cAMP-dependent protein kinase is regulated via a cellular association of N(G), N(G)-dimethylarginine dimethylaminohydrolase. FEBS Lett. 2001;494:48–53. doi: 10.1016/s0014-5793(01)02309-2. [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Ismat FA, Wang T, Yang J, Epstein JA. NF1 regulates a Ras-dependent vascular smooth muscle proliferative injury response. Circulation. 2007;116:2148–56. doi: 10.1161/CIRCULATIONAHA.107.707752. [DOI] [PubMed] [Google Scholar]
- 43.Pope AJ, Karrupiah K, Kearns PN, Xia Y, Cardounel AJ. Role of dimethylarginine dimethylaminohydrolases in the regulation of endothelial nitric oxide production. J Biol Chem. 2009;284:35338–47. doi: 10.1074/jbc.M109.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, et al. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282:879–87. doi: 10.1074/jbc.M603606200. [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Ingrid S, Ding YG, Liu Y, Qi JG, et al. Imbalance of endogenous homocysteine and hydrogen sulfide metabolic pathway in essential hypertensive children. Chin Med J. 2007;120:389–93. [PubMed] [Google Scholar]
- 46.Zhang JG, Liu JX, Li ZH, Wang LZ, Jiang YD, et al. Dysfunction of endothelial NO system originated from homocysteine-induced aberrant methylation pattern in promoter region of DDAH2 gene. Chin Med J. 2007;120:2132–7. [PubMed] [Google Scholar]
- 47.Wolffe AP, Matzke MA. Epigenetics: regulation through repression. Science (N Y) 1999;286:481–6. doi: 10.1126/science.286.5439.481. [DOI] [PubMed] [Google Scholar]
- 48.Zilberman D. The human promoter methylome. Nat Genet. 2007;39:442–3. doi: 10.1038/ng0407-442. [DOI] [PubMed] [Google Scholar]
- 49.Rollins RA, Haghighi F, Edwards JR, Das R, Zhang MQ, et al. Large-scale structure of genomic methylation patterns. Genome Res. 2006;16:157–63. doi: 10.1101/gr.4362006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saxonov S, Berg P, Brutlag DL. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc Natl Acad Sci U S A. 2006;103:1412–7. doi: 10.1073/pnas.0510310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ioshikhes IP, Zhang MQ. Large-scale human promoter mapping using CpG islands. Nat Genet. 2000;26:61–3. doi: 10.1038/79189. [DOI] [PubMed] [Google Scholar]
- 52.Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet. 2007;16:R50–9. doi: 10.1093/hmg/ddm018. [DOI] [PubMed] [Google Scholar]
- 53.Jia SJ, Lai YQ, Zhao M, Gong T, Zhang BK. Homocysteine-induced hypermethylation of DDAH2 promoter contributes to apoptosis of endothelial cells. Pharmazie. 2013;68:282–6. [PubMed] [Google Scholar]
- 54.Zhang BK, Lai YQ, Niu PP, Zhao M, Jia SJ. Epigallocatechin-3-gallate inhibits homocysteine-induced apoptosis of endothelial cells by demethylation of the DDAH2 gene. Planta Med. 2013;79:1715–9. doi: 10.1055/s-0033-1351017. [DOI] [PubMed] [Google Scholar]
- 55.Tomikawa J, Fukatsu K, Tanaka S, Shiota K. DNA methylation-dependent epigenetic regulation of dimethylarginine dimethylaminohydrolase 2 gene in trophoblast cell lineage. J Biol Chem. 2006;281:12163–9. doi: 10.1074/jbc.M513782200. [DOI] [PubMed] [Google Scholar]
- 56.Dayal S, Rodionov RN, Arning E, Bottiglieri T, Kimoto M, et al. Tissue-specific downregulation of dimethylarginine dimethylaminohydrolase in hyperhomocysteinemia. Am J Physiol Heart Circ Physiol. 2008;295:H816–25. doi: 10.1152/ajpheart.01348.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imamura M, Waseda Y, Marinova GV, Ishibashi T, Obayashi S, et al. Alterations of NOS, arginase, and DDAH protein expression in rabbit cavernous tissue after administration of cigarette smoke extract. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2081–9. doi: 10.1152/ajpregu.00406.2007. [DOI] [PubMed] [Google Scholar]
- 58.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]


