Abstract
The impact of erectile dysfunction is distressing to both males and their female partners, but less attention has been paid to identify female partners’ preferred treatment and sexual quality of life outcomes. The present analysis explores female partners’ treatment preference for erectile dysfunction in Chinese Men. This was a phase 4, randomized, open-label, multicenter, crossover study in Chinese men with erectile dysfunction who were naïve to phosphodiesterase type 5 inhibitor treatments. Eligible patients were randomized to sequential 20-mg tadalafil/100-mg sildenafil or 100-mg sildenafil/20-mg tadalafil for 8 weeks each. Of 418 patients, female partners of 64 patients agreed to enter the study; of 64 patients who entered the study with female partners, 63 were randomized, and 62 completed the study. Baseline demographics and disease characteristics were comparable between treatment groups. Significantly more couples preferred tadalafil compared with sildenafil overall (75.4% vs 24.6%; P < 0.001), and irrespective of erectile dysfunction severity at baseline (P ≤ 0.005). Significant improvements in sexual quality of life scores were reported at endpoint (Visit 8) in male patients and female partners in both tadalafil and sildenafil treatment groups (P < 0.001). Significantly higher mean changes from baseline were observed for male patients in the tadalafil group compared with the sildenafil group for the erectile function (P = 0.013) and overall satisfaction (P = 0.019) International Index for Erectile Function domains and the spontaneity domain (P < 0.001) of the Psychological and Interpersonal Relationship Scale. No major safety concerns were reported during the study. Though both treatments were effective, safe, and tolerable, more couples preferred tadalafil compared with sildenafil.
Keywords: erectile dysfunction, partners’ preference, sexual quality of life, sildenafil, tadalafil
INTRODUCTION
Erectile dysfunction (ED) is a prevalent sexual health concern in males1 affecting both the physical and psychological health of male patients, as well as the quality of life (QoL) in both partners.2,3,4 Pharmacotherapy with phosphodiesterase type 5 (PDE5) inhibitors is the preferred first-line therapy for men with ED,5,6 as these drugs have been proven to be effective, safe, and well-tolerated.7 The 3 PDE5 inhibitors currently offered in China for the treatment of ED are sildenafil citrate, tadalafil, and vardenafil as needed (pro re nata [PRN]) along with traditional Chinese medicine. Until recently, the majority of patients in China received prescriptions for sildenafil citrate, as it came into the market first; however, with the current availability of additional safe and effective PDE5 inhibitors, patients and their partners have more options when choosing the best treatment for ED. The fact that patients now have a role in choosing their therapeutic regimen has ignited the concept of preference studies, some of which have shown patients4,8,9 and partners8,10 prefer tadalafil over sildenafil in the treatment of ED; however, no preference studies have been conducted in Chinese men with ED or their female partners.
The results of patients’ preference, degree of preference, efficacy, safety, and tolerability of tadalafil and sildenafil treatment in men with ED in China have been reported previously; both tadalafil and sildenafil were shown to be effective and safe treatments for ED in Chinese patients, and more patients preferred tadalafil.11 Previously reported efficacy and safety results for both tadalafil and sildenafil have recently been corroborated (Wenjun B, MD, unpublished data, 2014).
In addition to efficacy and safety, the objective of ED treatment should focus on sexual QoL due to ED's detrimental effects on the sexual life of couples. Hence, patient's and/or partner's sexual life satisfaction is also an important indicator for the success of ED treatment. Even though ED is distressing to both males and their female partners, and has been shown to have a considerable negative impact on female partners’ sexual QoL,12 most of the research on ED has focused on the males’ preferred treatment for ED and evaluated the outcome of sexual QoL in males.2,4 The details pertaining to the outcome of ED treatment on female partners’ sexual health, as well as female partners’ preferred treatment for male partners’ ED, is limited both in China and globally. Therefore, for this analysis, we focused on male patients who entered the study with female partners. We investigated ED treatment preference and degree of preference in the female partners, along with sexual QoL in both male patients and their female partners following treatment.
MATERIALS AND METHODS
This was a phase 4, randomized, open-label, multicenter, crossover study to evaluate whether male Chinese patients with ED prefer 20-mg tadalafil (Eli Lilly and Company, Indianapolis, IN, USA) PRN, orally, or 100-mg sildenafil (Pfizer and Company, New York, NY, USA) PRN, orally, after 8 weeks of treatment with each. This study was conducted in Chinese male patients and their female partners from June 10, 2011 to July 30, 2012 in 15 study centers in China.13
ED participants
This study included male patients at least 18 years of age and <65 years of age with a history of ED of any etiology (psychogenic, organic, or mixed) and any severity (mild, moderate, or severe) for at least 3 months who had a stable female sexual partner and were naïve to PDE5 inhibitor treatments. Participants were required to make at least 4 sexual intercourse attempts with their female study partner during the 4-week run-in period and during the final 4 weeks of each 8-week treatment period and were required not to use any herbal therapy or traditional Chinese medicine for ED during the study.
Female partners were eligible if they were at least 18 years of age and had the same male study partner throughout the duration of the study and were willing to participate in recording responses to efficacy questionnaires, sexual QoL questionnaires, and other required instruments used in the study.
Patients were excluded if they had ED due to other primary sexual disorders or due to untreated endocrine diseases or a history of radical prostatectomy or other pelvic surgery or a history of penile implant or had a clinically relevant penile deformity in the opinion of the investigator. Patients were also excluded if they had a history of symptomatic hepatobiliary disease, chronic stable angina treated with long-acting nitrates, supraventricular arrhythmia, or sudden cardiac arrest despite medical or device therapy. The complete list of inclusion and exclusion criteria has been reported previously.13
Study design
The complete details of the study design has been reported previously.11 Eligible patients were randomly assigned (1:1 ratio) to either sequential 20-mg tadalafil/100-mg sildenafil (IC/S) or 100-mg sildenafil/20-mg tadalafil (S/IC) using a computer-generated random code. Randomized ED patients then received 8 weeks of treatment with tadalafil or sildenafil PRN. After 8 weeks of treatment, followed by a wash-out period of 7 to 10 days, the patients crossed over to the opposite treatment for another 8 weeks. This was followed by an 8-week extension period (Figure 1). This extension phase was included to identify a patient's behavioral indication for treatment preference.
Figure 1.
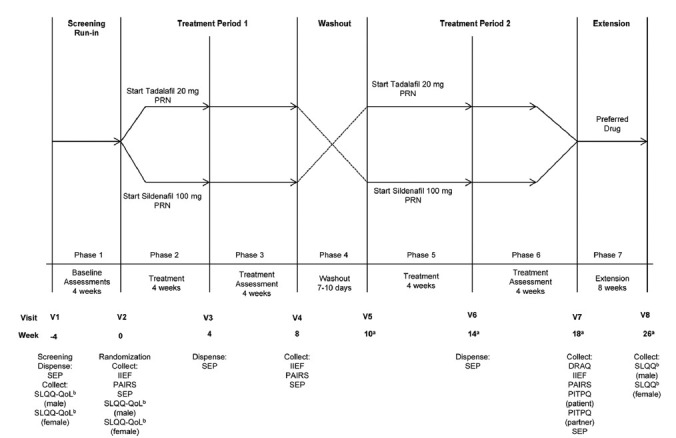
Study design – Randomized patients received 8 weeks of treatment with tadalafil or sildenafil PRN. After 8 weeks of treatment, followed by a wash-out period of 7 to 10 days, the patients crossed over to the opposite treatment for another 8 weeks followed by an 8-week extension period. Abbreviations: DRAQ: drug attribute questionnaire; IIEF: international index of erectile function; PAIRS: psychological and interpersonal relationship scale; PITPQ: phosphodiesterase 5 inhibitor treatment preference questionnaire; PRN: pro re nata (on demand); QoL: quality of life; SEP: sexual encounter profile; SLQQ: sexual life quality questionnaire; THX: treatment satisfaction; V: visit. aWash-out period is 7 to 10 days; up to 10 days have been included in the timeline (i.e., 1.5 weeks). The number of weeks has been rounded up to the nearest integer. bAt screening and baseline, the patient and partner answered the 10-item QoL domain, as the study treatment was not administered. At the end of the extension phase, the complete SLQQ was administered, both QoL and THX domain.
Ethical considerations
The study was conducted in accordance with consensus ethics principles derived from International Ethics Guidelines, International Conference on Harmonization Guideline for Good Clinical Practice E6, and applicable laws and regulations. The study also obtained approval from an Ethics Committee for each subcenter. Written informed consent was obtained from all participants before participation in the study.
Outcome measures
The aim of the present analysis was to determine the treatment preference and degree of treatment preference in female partners using PDE5 Inhibitor Treatment Preference Questionnaire (PITPQ) - partner version and to determine the sexual QoL using the Sexual Life Quality Questionnaire (SLQQ) - Quality of Life (QoL) domain in patients and partners following treatment. The present analysis also evaluated patients’ preferred drug attributes using Drug Attributes Questionnaire (DRAQ), psychosocial and interpersonal outcomes associated with ED and its treatment using Psychological and Interpersonal Relationship Scale (PAIRS), and therapeutic efficacy of ED therapy using International Index of Erectile Dysfunction (IIEF) and Sexual Encounter Profile (SEP) in those patients who had a partner's preferred treatment response reported.
The SLQQ14 is a validated, multidimensional instrument that consists of two domains: sexual QoL (10 questions) and Treatment Satisfaction (THX, 6 questions). The SLQQ-QoL domain compared the patient's and his partner's current sexual experience with their experience prior to the onset of patient's ED. The SLQQ questionnaire was collected at defined time points during the study (Figure 1).
PAIRS is a self-administered 29-item scale containing four domains (Sexual Self-Confidence, Spontaneity, Time Concerns, and Sexual Miscommunication), which are related to the broader psychosocial and interpersonal outcomes associated with ED and its treatment.15 Only three domains are validated: sexual Self-Confidence, Spontaneity, and Time Concerns. The PAIRS score was collected at defined time points during the study (Figure 1).
IIEF is a validated, multidimensional, self-administered questionnaire commonly employed to assess the therapeutic efficacy of ED therapy.16 The questionnaire contains five domains: erectile Function (EF domain, sum of Items 1 through 5 and Item 15), Intercourse Satisfaction (sum of Items 6 through 8), Orgasmic Function (sum of Items 9 and 10), Sexual Desire (sum of Items 11 and 12), and Overall Satisfaction (sum of Items 13 and 14). The IIEF scores were collected at defined time points during the study (Figure 1).
SEP is a self-reporting diary format of 5 questions for which patients record each sexual intercourse attempt. A minimum of 4 attempts are required prior to the collection of SEP at required time points (Figure 1). The baseline and endpoint score for each SEP question is the patient's percentage of “yes” responses to that question during the run-in period and postbaseline period, respectively. The SEP score was collected at defined time points during the study (Figure 1).
DRAQ: patients who answered the PITPQ were asked to answer the DRAQ. DRAQ was administered at the end of the second treatment period (Figure 1).
Statistical analysis
A sample size of 370 patients (185 patients per sequence group) was estimated to achieve 90% power to detect an increased preference for tadalafil over sildenafil citrate of 10% (60% vs 50%) using a two-sided Chi-square test with a significance level of 0.05 assuming 30% of Chinese patients have a missing treatment preference. In this analysis, we discuss the results of the patients whose partners agreed to enter the study.
For all the patients who entered the study with female partners, baseline and efficacy analyses were conducted on an intent-to-treat (ITT) basis. The ITT analysis included all data from randomized subjects and was analyzed according to the treatment assigned in the randomization scheme. The analysis population for the efficacy analyses included all randomized patients who completed both treatment periods (until Visit 7). The safety analysis set consisted of all randomized patients who received at least 1 dose of study drug (either tadalafil or sildenafil) in any of the two treatment periods.
Frequency tables using counts, percentages, and data listings were generated for the categorical efficacy variables (DRAQ, PITPQ Question 1 and 2) by treatment group. Wilson Score method was applied for testing the proportion of patients who choose tadalafil over sildenafil. Whether the treatment sequence had an effect on preference was tested using logistic regression with primary efficacy measure (Question 1 of the PITPQ) as dependent, and sequence group as explanatory.
A contingency table using counts was generated for the consistency between male patients’ responses and their female partners’ responses on the primary efficacy measure (Question 1 of the PITPQ) by treatment group, and Kappa coefficient was calculated.
For patients and their partners, changes from baseline (Visit 2) to endpoint (Visit 8) in the SLQQ-QoL domain score were analyzed using a t-test for least squares (LS) mean changes.
The continuous efficacy variables (all five IIEF domains, three PAIRS domains, and SEP questionnaire) were analyzed using a mixed effect analysis of covariance (ANCOVA) model for crossover designs for the change from baseline to end of each treatment period. The model included treatment, period, sequence, and pooled site as fixed effects, centered baseline value of the efficacy measure (defined as the baseline value for a patient minus the overall baseline mean value) as a covariate, patient within sequence as a random effect, and centered baseline-by-treatment interaction as a fixed effect. Since the wash-out period was planned for more than 7 days (7 to 10 days), no carryover effect was included in the mixed model.
All statistical tests were conducted at a two-sided alpha level of 0.05 unless otherwise stated, and 95% confidence intervals (CIs) for the difference between treatments (tadalafil minus sildenafil citrate) were provided. All statistical analyses were performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC, USA).
RESULTS
Patient disposition and baseline demographics
The overall patient disposition of the study has been reported previously.11 Of 418 patients who entered the study, 64 patients entered the study along with their respective female partners. Of these 64 patients, 63 patients were randomized either to S/IC or IC/S treatment sequences. Of these 63 patients, 32 patients were randomized to IC/S treatment sequence, with 31 patients completing the study and 31 patients were randomized to S/IC treatment sequence with all 31 patients completing the study. One patient randomized to IC/S treatment sequence was discontinued due to withdrawal by subject during treatment period 1. Of these 62 patients, 61 patients responded to Question 1 of the PITPQ. Of these 61 patients, mild, moderate, and severe ED was reported in 8, 17 and 36 patients, respectively.
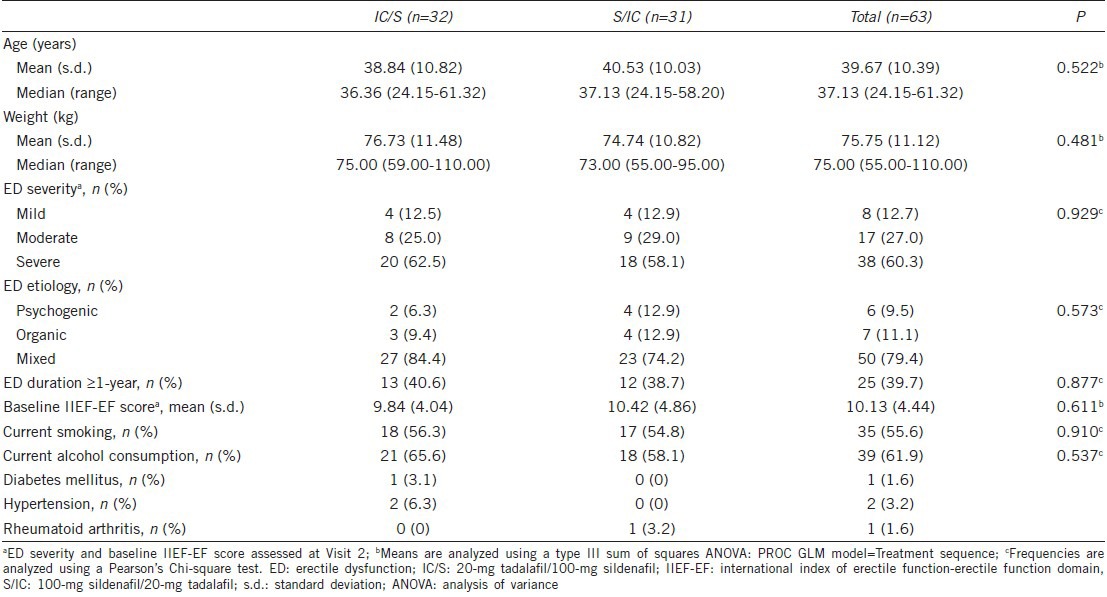
Baseline demographics and other baseline characteristics of the 63 patients who were randomized into the study with partners were comparable. No significant differences were reported between treatment groups (Table 1). The mean (s.d.) age of the patients was 39.67 (10.39) years with a median range of 24.15 to 61.32 years. Most of the patients in the IC/S and S/IC treatment sequence had severe ED (62.5% vs 58.1%, respectively) and mixed etiology (84.4% vs 74.2%, respectively).
Table 1.
Baseline demographics and characteristics
Treatment preference and degree of treatment preference
A total of 61 female partners (30 from IC/S treatment sequence and 31 from S/IC treatment sequence) responded to Question 1 of the PITPQ.
Couples preferred tadalafil compared with sildenafil (75.4% vs 24.6% P < 0.001; Table 2) overall and in both treatment sequences (P < 0.05; Table 2). Treatment sequencing did not show a significant effect on preference of treatment in both female partners and male patients (P = 0.823; Table 2). Table 3, shows that the treatment preferences in male patients and their female partners are consistent overall and in both IC/S and S/IC treatment sequences (Kappa coefficient = 0.607, 0.604, 0.613), respectively. Couples preferred tadalafil compared with sildenafil irrespective of ED severity at baseline (mild ED [75.0% vs 25.0%; P = 0.005]; moderate ED [76.5% vs 23.5%; P < 0.001]; severe ED [75.0% vs 25.0%; P < 0.001]).
Table 2.
Treatment preference and degree of treatment preference using PITPQ by treatment sequence
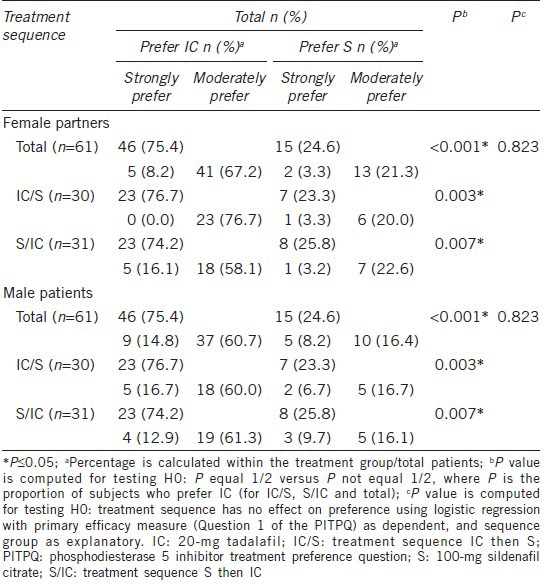
Table 3.
Consistency between couples’ treatment preference and degree of treatment preference using PITPQ by treatment sequence

Of the 46 female partners who preferred tadalafil, 5 female partners strongly preferred tadalafil (all from S/IC treatment sequence) and 41 moderately preferred tadalafil (23 from IC/S and 18 from S/IC treatment sequence). Of the 15 female partners who preferred sildenafil, 2 female partners strongly preferred sildenafil (1 each from IC/S and S/IC treatment sequence) and 13 female partners (6 from IC/S and 7 from S/IC treatment sequence) moderately preferred sildenafil (Table 2).
Of the 46 male patients who preferred tadalafil, 9 male patients (5 from IC/S and 4 from S/IC treatment sequence) strongly preferred tadalafil and 37 male patients (18 from IC/S and 19 from S/IC treatment sequence) moderately preferred tadalafil. Of 15 male patients who preferred sildenafil, 5 male patients (2 from IC/S and 3 from S/IC treatment sequence) strongly preferred tadalafil and 10 male patients (5 from IC/S and 5 from S/IC treatment sequence) moderately preferred sildenafil (Table 2).
More male patients and female partners preferred tadalafil compared with sildenafil (mild ED [6 vs 2 patients; 9.8% vs 3.3%]; moderate ED [13 vs 4 patients; 21.3% vs 6.6%]; severe ED [27 vs 9 patients; 44.3% vs 14.8%]) irrespective of ED severity at baseline.
Sexual life quality questionnaire
The 46 male patients who preferred tadalafil and the 15 male patients who preferred sildenafil answered the SLQQ (QoL and THX). The baseline mean (s.d.) sexual QoL scores for male patients who preferred tadalafil and sildenafil were 14.92 (11.72) and 15.17 (7.56), respectively. The endpoint mean (s.d.) sexual QoL scores were 86.33 (10.42) and 90.50 (5.17), respectively. A significant improvement in QoL (LS mean changes [SE] from baseline to endpoint) was reported in patients who preferred tadalafil (71.41 [1.85 P < 0.001]) and sildenafil (75.33 [3.23 P < 0.001]). The mean (s.d.) THX domain scores at endpoint were 55.58 (16.15) and 65.33 (17.45), respectively, for patients who preferred tadalafil and sildenafil.
The 46 female partners who preferred tadalafil and the 15 female partners who preferred sildenafil answered the complete SLQQ. The baseline and endpoint mean (s.d.) sexual QoL scores for female partners who preferred tadalafil were 23.83 (9.22) and 85.08 (7.80), respectively; while the baseline and endpoint mean (s.d.) sexual QoL scores for female partners who preferred sildenafil were 18.67 (8.64) and 88.92 (5.75), respectively. A significant improvement in QoL (LS mean changes [SE] from baseline to endpoint) was reported in the female partners who preferred tadalafil (61.25 [1.78 P < 0.001]) and sildenafil (70.25 [3.11 P < 0.001]). The mean (s.d.) THX domain scores at endpoint were 50.87 (19.47) and 63.78 (20.03), respectively, for female partners who preferred tadalafil and sildenafil.
International index for erectile function
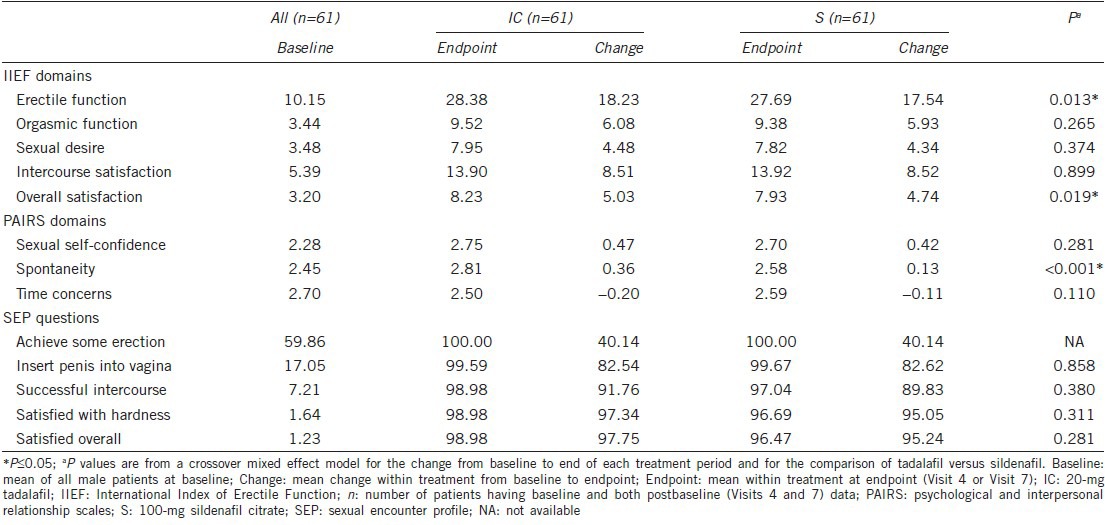
The mean changes from baseline to endpoint were similar for orgasmic function, sexual desire, and intercourse satisfaction IIEF domains between the treatment groups in male patients. However, in male patients, the mean changes from baseline to endpoint were significantly higher in the tadalafil treatment group compared with the sildenafil treatment group for erectile function (18.23 vs 17.54; P = 0.013) and overall satisfaction (5.03 vs 4.74; P = 0.019) IIEF domains (Table 4).
Table 4.
IIEF domains, PAIRS domains, and SEP in male patients
Psychological and interpersonal relationship scale
Mean changes from baseline to endpoint of PAIRS results are shown in Table 4. Changes from baseline in the sexual self-confidence and time concerns domain scores were comparable between the treatment groups. Mean changes from baseline to endpoint in the spontaneity domain score of PAIRS were significantly higher in male patients of the tadalafil group compared with male patients in the sildenafil treatment group (0.36 vs 0.13; P < 0.001).
Sexual encounter profile
Large increases in the mean change from baseline to endpoint were observed for all SEP questions in both the treatment groups; however, no significant differences were observed between the treatment groups (Table 4).
Drug attribute questionnaire
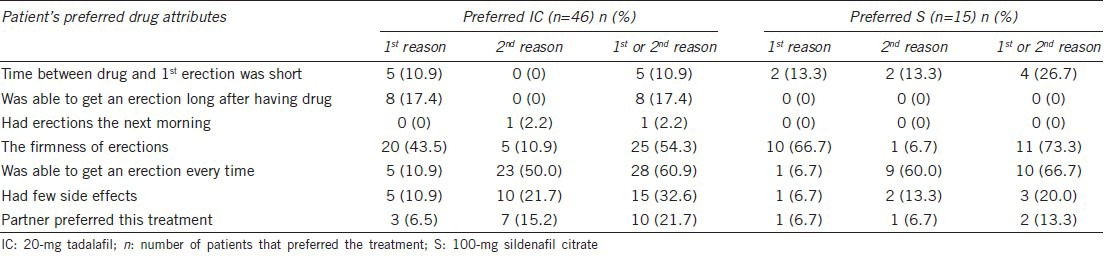
Male patients who had answered PITPQ responded to the DRAQ (Table 5). In both treatment groups, “firmness of erections” (20 [43.5%] for tadalafil and 10 [66.7%] for sildenafil) was identified as the primary reason for treatment preference. The second best (alternate) reason for treatment preference in both groups was identified as “was able to get an erection every time” (23 [50%] for tadalafil and 9 [60%] for sildenafil). Most patients in both treatment groups (28 [60.9%] for tadalafil and 10 [66.7%] for sildenafil) identified “Was able to get an erection every time” as the 1st or 2nd reason for their preference. Of the 46 patients who preferred tadalafil, 10 (21.7%) identified “Partner preferred this treatment” as the 1st or 2nd reason for their preference (Table 5).
Table 5.
Drug attribute questionnaire results in male patients
Safety
The mean (s.d.) dose taken per week by the patients was numerically similar between the treatment groups (6.01 [1.01] vs 5.99 [0.60]). The proportion of patients reporting at least 1 treatment-emergent adverse event (TEAE) was low in both tadalafil and sildenafil treatment groups (0.0% vs 1.6%) during the preextension phase. No deaths or other serious adverse events (SAEs) were reported either during the preextension or the extension phase of the study in both treatment groups. No patient discontinued the study due to an adverse event (AE) during the preextension period or the extension period of the study. A small decrease in mean changes was noticed in heart rate, as well as systolic and diastolic blood pressure; however, no significant differences were observed between the treatment groups.
DISCUSSION
Even though the effects of ED is adverse for both males and their female partners, very limited attention has been paid in evaluating female partners’ preference of treatment and sexual QoL.2,10 However, the partner-orientated approach encourages clinicians to acknowledge women's sexuality with the same importance as male patient sexuality. There is increasing recognition that both partners should be involved in the assessment, diagnosis, patient education, counseling, and choice of treatment for long-term ED treatment to be successful unless the informed patient is unwilling.17
In the open-label, multicenter, randomized crossover study regarding ED patients in China, both tadalafil and sildenafil were found to be effective and safe.11 The present results, in addition to evaluating the female partner's preference of treatment, also demonstrated the outcome of treatment on sexual QoL in both the male patients and their respective female partners.
Treatment preferences were similar in male patients and female partners in the overall study population, as well as in both treatment sequences (IC/S and S/IC). Significantly more couples preferred tadalafil compared with sildenafil irrespective of treatment sequence (75.4% vs 24.6; P < 0.001) and ED severity (P < 0.001 across all baseline severity groups). These results were similar to those that were published previously.10,18 The increased preference for tadalafil compared with sildenafil in female partners may be due to increases in relationship, sexual, and overall satisfaction, as well as less sense of urgency and/or time concerns in male partners, which needs to be confirmed in future studies. The importance of knowing a female partner's preference of male patient's ED treatment will help in the couple's continued long-term use of preferred oral ED medication.
A significant improvement in sexual QoL scores (P < 0.001) was reported at endpoint (Visit 8) in both male patients and female partners in both treatment groups. The increase in sexual QoL and THX scores in couples suggests that treating a male patient's ED may result in a concordant improvement in the female partner's sexual QoL due to female partners’ involvement in assessment and treatment decisions.
The mean increase from baseline in erectile function (18.23 vs 17.54; P = 0.013) and overall satisfaction (5.03 vs 4.47; P = 0.019) of IIEF domains was significantly greater for tadalafil-treated male patients. All the parameters of the SEP questionnaire were greatly improved in both treatment groups. These increases in IIEF and SEP scores indicate an improved erectile function and improved sexual QoL. The increase in improved erectile function and improved sexual QoL also may have had a role in influencing female partners in preferring tadalafil over sildenafil, which needs to be confirmed in future studies.
In this study, significant improvement in the PAIRS spontaneity domain (0.36 vs 0.13; P < 0.001) was observed in male patients who preferred tadalafil treatment, which may be due to its long efficacy duration up to 36 h.19,20 Even though no significant differences were reported between the treatment groups, increased mean changes in sexual self-confidence (0.47 vs 0.42) and decreased mean changes in time concerns (−0.20 vs − 0.11) domains indicated less sense of urgency or less concern about time in relation to sexual activity after treatment with tadalafil or sildenafil. These results support that an effective ED treatment should not only treat the underlying condition, but also improve the psychological well-being of men.20
The first reason listed in the DRAQ for a male patient's preference of treatment was “firmness of erections” (43.5% for tadalafil and 66.7% for sildenafil). The second best (alternate) preference in both treatment groups for choosing respective treatment was provided as “was able to get an erection every time” (50% for tadalafil and 60% for sildenafil). These responses indicate that male patients prefer the medications that allow them to return to an erectile function and partner interaction they experienced prior to having ED. The other notable response chosen by male patients (17.4%) in the tadalafil group was “able to get an erection long after having the drug.” This preference may be due to the long half-life of tadalafil.11,19
Safety results of the trial have been reported previously11 and were similar to safety profiles presented in other tadalafil studies.21 No deaths, SAEs, or discontinuation due to AEs were reported, confirming the safety and tolerability of tadalafil as reported in earlier studies.21
Even though more female partners preferred tadalafil over sildenafil, the sample size was too small to compare the superiority. Another possible study limitation was the open-label design of the study, which reveals the treatment to both patients and partners and could, therefore, interfere with the patient's, as well as the partner's, psychological outcomes.
CONCLUSION
The present results confirm that both partners preferred tadalafil compared with sildenafil irrespective of treatment sequence and ED severity at baseline. Further studies are needed to evaluate why female partners preferred tadalafil, and the influence of female partner's treatment preference on overall sexual satisfaction.
AUTHOR CONTRIBUTIONS
HJL, WPX contributions: drafting and approval of manuscript. CNW contributions: performed the statistical analysis, interpretation, drafting and approval of manuscript. HZL, WJB, and YTD contributed to data collection and intellectual revision.
All authors read, reviewed and approved the final manuscript.
COMPETING INTERESTS
Hong-Jun Li, Wen-Jun Bai, Yu-Tian Dai and Han-Zhong Li declared no competing financial interests.
Wen-Ping Xu and Chia-Ning Wang are employees of Eli Lilly and Company.
ACKNOWLEDGMENTS
This study was supported by Eli Lilly and Company, who was responsible for design and conduct of the study, for monitoring the study, and for verification and analysis of the data. The identifier label for this study is H6D-CR-LVIZ. The authors would like to thank the investigators and their patients who participated in this study. The authors acknowledge Pavan Yenduri and Cindi Wood of inVentiv Health Clinical for manuscript writing and editorial support, respectively.
REFERENCES
- 1.Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–14. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Aurioles E, Kim ED, Rosen RC, Porst H, Burns P, et al. Impact on erectile function and sexual quality of life of couples: a double-blind, randomized, placebo-controlled trial of tadalafil taken once daily. J Sex Med. 2009;6:1314–23. doi: 10.1111/j.1743-6109.2009.01222.x. [DOI] [PubMed] [Google Scholar]
- 3.Wagner G, Fugl-Meyer KS, Fugl-Meyer AR. Impact of erectile dysfunction on quality of life: patient and partner perspectives. Int J Impot Res. 2000;12(Suppl 4):S144–6. doi: 10.1038/sj.ijir.3900594. [DOI] [PubMed] [Google Scholar]
- 4.Brock G, Chan J, Carrier S, Chan M, Salgado L, et al. The treatment of erectile dysfunction study: focus on treatment satisfaction of patients and partners. BJU Int. 2007;99:376–82. doi: 10.1111/j.1464-410X.2006.06586.x. [DOI] [PubMed] [Google Scholar]
- 5.Eardley I, Donatucci C, Corbin J, El-Meliegy A, Hatzimouratidis K, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2010;7(1 PT 2):524–40. doi: 10.1111/j.1743-6109.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 6.Fisher WA, Rosen RC, Mollen M, Brock G, Karlin G, et al. Improving the sexual quality of life of couples affected by erectile dysfunction: a double-blind, randomized, placebo-controlled trial of vardenafil. J Sex Med. 2005;2:699–708. doi: 10.1111/j.1743-6109.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, et al. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998;338:1397–404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Pommerville P, Brock G, Gagnon R, Mehta P, et al. Physician-rated patient preference and patient- and partner-rated preference for tadalafil or sildenafil citrate: results from the Canadian ‘Treatment of Erectile Dysfunction’ observational study. BJU Int. 2006;98:623–9. doi: 10.1111/j.1464-410X.2006.06384.x. [DOI] [PubMed] [Google Scholar]
- 9.Tolrà JR, Campaña JM, Ciutat LF, Miranda EF. Prospective, randomized, open-label, fixed-dose, crossover study to establish preference of patients with erectile dysfunction after taking the three PDE-5 inhibitors. J Sex Med. 2006;3:901–9. doi: 10.1111/j.1743-6109.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- 10.Conaglen HM, Conaglen JV. Investigating women's preference for sildenafil or tadalafil use by their partners with erectile dysfunction: the partners’ preference study. J Sex Med. 2008;5:1198–207. doi: 10.1111/j.1743-6109.2008.00774.x. [DOI] [PubMed] [Google Scholar]
- 11.Bai WJ, Li HJ, Dai YT, He XY, Huang YR, et al. An open-label, multicenter, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in Chinese men naïve to phosphodiesterase 5 inhibitor therapy. Asian J Androl. 2015;17:61–67. doi: 10.4103/1008-682X.143244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher WA, Rosen RC, Eardley I, Sand M, Goldstein I. Sexual experience of female partners of men with erectile dysfunction: the Female Experience of Men's Attitudes to Life Events and Sexuality (FEMALES) study. J Sex Med. 2005;2:675–84. doi: 10.1111/j.1743-6109.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 13.Eli Lilly and Company. A study of tadalafil and sildenafil in men with erectile dysfunction in China. In: ClinicalTrials.gov [Internet]. U.S. National Institutes of Health. NLM Identifier: NCT01352507. [Last cited on 2014 Sep 23]. Available from: http://www.clinicaltrials.gov/show/NCT01352507 .
- 14.Woodward JM, Hass SL, Woodward PJ. Reliability and validity of the sexual life quality questionnaire (SLQQ) Qual Life Res. 2002;11:365–77. doi: 10.1023/a:1015513228469. [DOI] [PubMed] [Google Scholar]
- 15.Swindle RW, Cameron AE, Lockhart DC, Rosen RC. The psychological and interpersonal relationship scales: assessing psychological and relationship outcomes associated with erectile dysfunction and its treatment. Arch Sex Behav. 2004;33:19–30. doi: 10.1023/B:ASEB.0000007459.48511.31. [DOI] [PubMed] [Google Scholar]
- 16.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 17.Dorey G. Partners’ perspective of erectile dysfunction: literature review. Br J Nurs. 2001;10:187–95. doi: 10.12968/bjon.2001.10.3.5382. [DOI] [PubMed] [Google Scholar]
- 18.Eardley I, Mirone V, Montorsi F, Ralph D, Kell P, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naïve to phosphodiesterase 5 inhibitor therapy. BJU Int. 2005;96:1323–32. doi: 10.1111/j.1464-410X.2005.05892.x. [DOI] [PubMed] [Google Scholar]
- 19.Mirone V, Fusco F, Rossi A, Sicuteri R, Montorsi F. Tadalafil and vardenafil vs sildenafil: a review of patient-preference studies. BJU Int. 2009;103:1212–7. doi: 10.1111/j.1464-410X.2008.08267.x. [DOI] [PubMed] [Google Scholar]
- 20.Dean J, Hackett GI, Gentile V, Pirozzi-Farina F, Rosen RC, et al. Psychosocial outcomes and drug attributes affecting treatment choice in men receiving sildenafil citrate and tadalafil for the treatment of erectile dysfunction: results of a multicenter, randomized, open-label, crossover study. J Sex Med. 2006;3:650–61. doi: 10.1111/j.1743-6109.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- 21.Porst H, Kim ED, Casabe AR, Mirone V, Secrest RJ, et al. Efficacy and safety of tadalafil once daily in the treatment of men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia: results of an international randomized, double-blind, placebo-controlled trial. Eur Urol. 2011;60:1105–13. doi: 10.1016/j.eururo.2011.08.005. [DOI] [PubMed] [Google Scholar]


