Abstract
To systematically evaluate the prognostic value of lymphovascular invasion (LVI) in radical prostatectomy (RP) by a meta-analysis based on the published literature. To identify relevant studies, PubMed, Cochrane Library, and Web of Science database were searched from 1966 to May 2014. Finally, 25 studies (9503 patients) were included. LVI was found in 12.2% (1156/9503) of the RP specimens. LVI was found to be correlated with higher pathological tumor stages (greater than pT3 stage) (risk ratio [RR] 1.90, 95% confidence interval [CI] 1.73–2.08, P < 0.00001), higher Gleason scores (greater than GS = 7) (RR 1.30, 95% CI 1.23–1.38, P < 0.00001), positive pathological node (pN) status (RR 5.67, 95% CI 3.14–10.24, P < 0.00001), extracapsular extension (RR 1.72, 95% CI 1.46–2.02, P < 0.00001), and seminal vesicle involvement (RR 3.36, 95% CI 2.41–4.70, P < 0.00001). The pooled hazard ratio (HR) was statistically significant for Biochemical Recurrence-Free (BCR-free) probability (HR 2.05, 95% CI 1.64–2.56; Z = 6.30, P < 0.00001). Sensitivity analysis showed that the pooled HR and 95% CI were not significantly altered by the omission of any single study. Begg's Funnel plots showed no significant publication bias (P = 0.112). In conclusion, LVI exhibited a detrimental effect on the BCR-Free probability and clinicopathological features in RP specimens, and may prove to be an independent prognostic factor of BCR.
Keywords: lymphovascular invasion, meta-analysis, prognosis, prostate cancer, radical prostatectomy
INTRODUCTION
Prostate cancer (PCa) is the second most common cancer and the sixth leading cause of cancer-related death in Caucasian men, and there were estimated 238 590 new PCa cases and 29 720 deaths from PCa in the United States in 2014.1 With advances in the minimally invasive technologies, radical prostatectomy (RP) as the standard treatment has made great progress in improving perioperative outcomes. Nevertheless, early biochemical recurrence (BCR) occurred in approximately 20% patients undergoing RP,2,3 in whom the 5-year metastasis rate was as high as 30%–44%.4 Thus, it is imperative for clinicians to identify risk factors of post-RP BCR, and provide advisable indexes for adjuvant therapies including external beam radiotherapy (EBRT), intensity-modulated radiotherapy, and androgen deprivation therapy.
To date, although some potential biomarkers including Lymphovascular Invasion (LVI) have been added to the pathological reports of PCa patients who underwent prostatectomy, their impact on prognosis such as BCR has not been sufficiently evaluated.5 LVI has been documented as a poor prognostic factor in many solid tumors.6,7 Some authors have demonstrated an association between the presence of LVI in prostatectomy specimens and BCR. Although the College of American Pathologists (CAP) suggested that LVI should be reported in the routine examination of RP specimens in the 2010 consensus statement, there is a lack of convincing evidence to support its prognostic value.8 Therefore, we conducted a systematic review of current publications to assess the prognostic value of LVI in BCR, and a meta-analysis was performed for the extracted data that could be merged.
MATERIALS AND METHODS
Literature search
We search Electronic databases including PubMed, Web of Science and the Cochrane Library for published studies that analyzed the prognostic value of LVI in PCa up to May 31, 2014. The following Medical Subject Headings terms and free texts were used: “lymphovascular,” “microvascular,” “vascular,” “vessel,” “invasion,” “prostate,” “prostatic,” “cancer,” “carcinoma,” “neoplasm,” “tumor,” and “mass.” The searching strategies and results are shown in Table 1. In addition, a full manual search from the reference list of each identified article was performed.
Table 1.
Searching strategies and results

Study selection
We defined the inclusion and exclusion criteria at the initiation of the search. Studies were included when they met the following criteria: (1) studies that included definitive diagnosis of PCa; (2) studies that assessed LVI in RP specimens involving lymphatic or vascular invasion for which no attempt was made to differentiate them; (3) studies that chose RP as the only treatment; (4) studies that investigated the relationship between LVI and patient pathological outcomes or the correlation between LVI with preoperative prostate specific antigen (PSA) and pathological parameters; and (5) studies that offered a hazard ratio (HR) and 95% confidence interval (CI) directly or rendered the data that could be used to calculate HR and 95% CI. The exclusion criteria were: (1) review articles, letters to the editor, commentaries, or case reports; (2) studies that duplicated patient populations that had been reported in previous publications; and (3) studies on PCa cell lines or animal models. The whole process was monitored by two reviewers (YH and HH) independently. Discrepancies between the reviewers were resolved by a consensus meeting with three senior investigators (YG, YH, and XGC) who made the final decision regarding inclusion or exclusion of the study.
Data extraction
The following specified data were gathered from each eligible study: (1) main characteristics including the author, country, publication year, institution, recruitment period, study design, pathology stain method, definition of LVI, definition of BCR, the number of patients, median age at operation, the number of pelvic lymph node dissection (PLND), neoadjuvant (neo), androgen deprivation therapy (ADT), external beam radiotherapy (EBRT), and median follow-up time (Supplementary Table 1 (47.1KB, pdf) ); (2) Tumor-Node-Metastasis (TNM) stage characteristics, Gleason score, and correlation between LVI and preoperative PSA and pathological parameters (Supplementary Table 2 (53.5KB, pdf) ); (3) HR of LVI in univariate or multivariate Cox analyses, Co-factors, and the conclusion of each study concerning whether LVI was an independent predictor (Supplementary Table 3 (35.7KB, pdf) ).
Main characteristics of the eligible studies
TNM stage characteristics and correlations between LVI and preoperative PSA and pathological parameters
Estimation of the HR
Statistical analysis
The primary objective of this review was to determine differences in survival outcomes between patients with negative LVI and positive LVI. HR and 95% CI were collected from each study if they were not directly reported, and the HR was estimated according to the method reported by Tierney et al.9 The overall pooled HR was estimated by calculating the weighted average of the log-HRs and their 95% CI from each study. An observed HR >1 implied a poor survival outcome for patients with positive LVI. The impact of LVI on the outcome was considered as an independent predictor if the 95% CI did not overlap with 1 and P < 0.05. Subgroup analysis was performed to check whether the pooled HR was influenced by the region and number of patients, pathologic N stage, median follow-up, analysis results, definition of BCR, staining method, and staging system. In order to assess the stability of the combined HR, sensitivity analysis was performed by removing one study. The heterogeneity of the combined HR was evaluated using the Chi-square (χ2 test) and inconsistency (I2 test). Meta-analysis used the fixed-effect model,10 when P ≥ 0.1 and I2 ≤50%, which indicated a moderate heterogeneity between studies,11 whereas when P < 0.1 or I2 >50%, which indicated large heterogeneity,11 the random-effect model was applied.12 In addition, publication bias was evaluated by Egger's linear regression and Begg's rank correlation.
The secondary objective of this review was to study the relationship between the pathological parameters of PCA and LVI. The data of pathological stage were divided as low-stage (pT2) group and high stage (pT3-4) group. Gleason scores were categorized as low Gleason score (GS <7) and high Gleason score (GS ≥7). The RR of the high stage or high Gleason score along with the corresponding 95% CI was calculated by meta-analysis. In addition, the extracapsular extension (ECE), seminal vesicle involvement (SVI), and pathological node (pN) were directly divided as positive and negative. RR and CI of positive components were analyzed. Stata (Version 12.0; Stata Corp, College station, TX, USA) was used for all statistical analyses.
RESULTS
A total of 25 studies13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 were selected for the systematic review and meta-analysis (Figure 1). With regard to the primary objective, survival outcomes with negative LVI and positive LVI were evaluated. Some studies revealed that LVI was an independent predictor in cancer-specific survival (CSS),13,20 distant metastasis (DM),13,22 progression-free survival (PFS),29 overall survival (OS),13 and these details are shown in Supplementary Table 3 (35.7KB, pdf) , however, the data for CSS, DM, PFS, OS were not available in any study. Nevertheless, 21 studies provided the BCR data, and the meta-analysis showed that positive LVI was correlated with poorer BCR in RP patients (HR = 2.05, 95% CI, 1.64-2.56, P < 0.00001) (Figure 2). Test of Cochrane Q (χ2 = 47.39, P = 0.001) and inconsistency test (I2 =57.8%) could not exclude a significant heterogeneity. Given the large heterogeneity between the studies, subgroup analysis was performed, and the results are shown in Supplementary Table 4 (36.4KB, pdf) . In sensitivity analysis, one-way sensitivity analysis was carried out to exclude a single study and calculated the pooled HR for remaining studies, and omission of each study did not have a significant impact on the merged value of HR. Allowing for publication bias, Begg's funnel plot was performed, and no significant publication bias was detected between these studies regarding HR of BCR with P = 0.112. In addition, Egger's test (P = 0.207) demonstrated a similar result (Figure 3).
Figure 1.
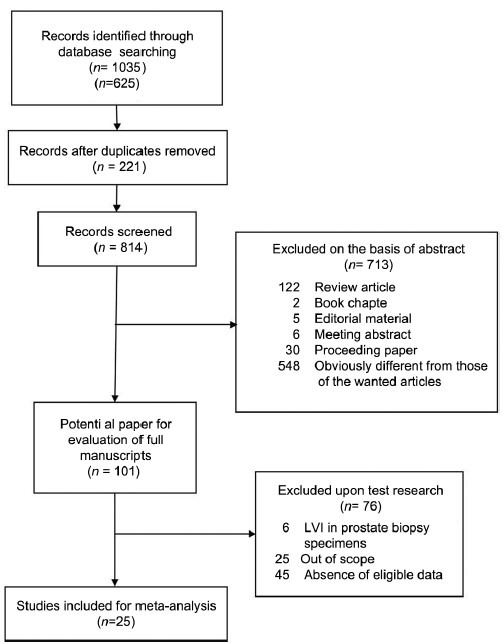
Flow chart of study selection.
Figure 2.
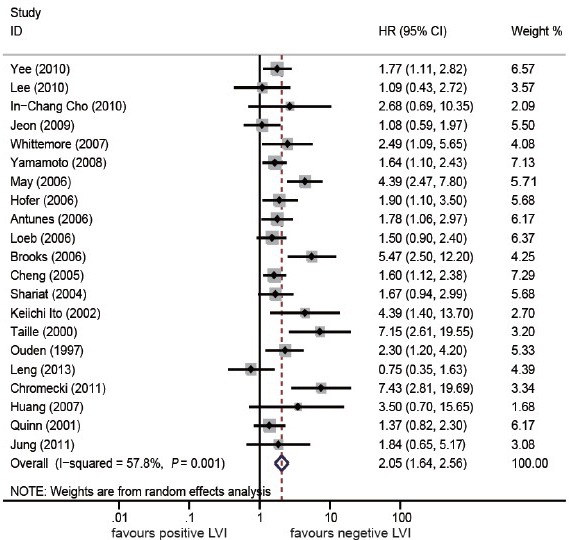
Forest plots of hazard ratios with the random-effects model for lymphovascular invasion in patients with prostate cancer (biochemical recurrence-free probability).
Figure 3.
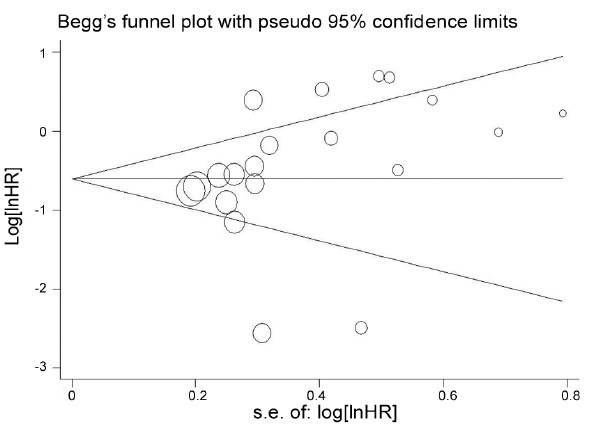
Begg's Funnel plots for publication bias test. Assessment of potential publication bias in studies of lymphovascular invasion in patients with prostate cancer (biochemical recurrence-free probability).
Subgroup analysis of biochemical recurrence-free survival
The secondary objective was to assess the relationship between LVI and higher pathological tumor stages (> pT3 stage), higher Gleason score (>GS = 7), positive pN, ECE and SVI. Ten studies provided data on the number of higher pT stage in the positive LVI groups and negative LVI groups, and the pooled RR was 1.90 (95% CI, 1.73–2.08; Z = 13.45, P < 0.00001) with a moderate heterogeneity (P = 0.054 for heterogeneity; I2 =46.1%) (Figure 4a). Similarly, the data of other pathological parameters were extracted from eligible studies, and we found that LVI was significantly correlated with higher GS (pooled RR, 1.30; 95% CI, 1.23–1.39; Z = 8.55, P < 0.00001) with a moderate heterogeneity (P = 0.019 for heterogeneity; I2 =47.1%) (Figure 4b), positive pN status (pooled RR, 5.67; 95% CI, 3.14–10.24; Z = 5.74, P < 0.00001) with a large heterogeneity (P < 0.00001 for heterogeneity test; I2 =72.8%) (Figure 4c), ECE (pooled RR, 1.72; 95% CI, 1.46–2.02; Z = 6.50, P < 0.00001) with a large heterogeneity (P < 0.00001 for heterogeneity test; I2 =73.6%) (Figure 4d) and SVI (pooled RR, 3.36; 95% CI, 2.41–4.70; Z = 7.11, P < 0.00001) (Figure 4e) despite a large heterogeneity among studies (P < 0.00001 for heterogeneity test; I2 =81.9%).
Figure 4.
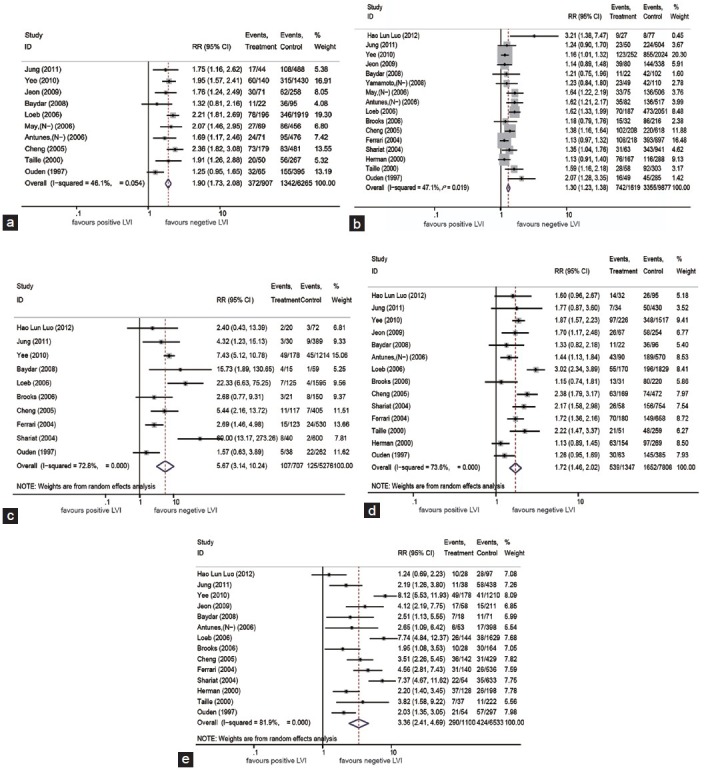
Forest plots of RRs for the Association of LVI with (a) higher pathological tumor stages (>pT3 stage); (b) higher Gleason score (>GS = 7); (c) pathological node (pN); (d) extracapsular extension (ECE); (e) seminal vesicle involvement (SVI). RR: risk ratio.
DISCUSSION
Lymphovascular invasion is defined as the presence of a tumor within an endothelial-lined space,8 which most probably links with the hematogenous spread of tumor cells. Tumor cells first infiltrate into lymphatic and/or vascular vessels, and then disseminate,38,39 which is a much more common phenomenon in malignant tumors including PCA.40 In addition, LVI is a significant prognostic factor in bladder, upper urinary tract urothelial and lung cancers, which has been confirmed in several systematic review studies.41,42,43 As regards to liver and testicular tumors, LVI has been added to the TNM staging system, in terms of improved tumor staging.44,45 Although the prognostic value of LVI in PCA patients after RP has been appraised by a number of studies, the results remain controversial.
The results obtained in our meta-analysis are in line with those in a previous System Review by Ng et al.46 In addition, our study presented a series of advancements in comparison with the previous studies. First, we included more eligible studies with large sample sizes. The Ng's search time was ended in 2009. However, we added 8 extra studies including 2825 patients from 2009 to 2014, thus providing more exact evaluation on the effect and enabling more authentic subgroup analyses. Second, although the same result was obtained in Ng's study reporting a significant relationship between LVI and BCR in RP, we found that the pooled result of LVI had a large heterogeneity (I2 =57.8%) by meta-analysis, and so we conducted a subgroup analysis. Meanwhile, the sensitivity analysis of our study revealed that the omission of each study did not have a significant impact on the merged value of HR. In contrast, Ng et al.46 only assessed the quality of publications and no other analysis on the reliability of the result was done.
In our subgroup analyses of the region, sample size, pN status, follow-up time, negative/positive result of LVI, PSA level definition of BCR and staining method, we found a significant correlation between LVI and poor BCR. Notably, in large sample groups with the number of patients larger than 500, the pooled HR was 1.58 (1.28–1.95). In the short-term follow-up group with the follow-up duration <24 months, we also found that LVI could serve as a predictor in early BCR and be used in Nomogram for predicting BCR.47 Although only one study34 revealed that the addition of LVI only marginally improved the predictive accuracy (from 0.880 to 0.884). In addition, LVI was correlated with higher pT stages, higher GS, positive pN status, ECE, and SVI, indicating that the presence of LVI in PCa may predict the higher risk of progression with poor BCR, PFS, CSS, DM, and OS, and some previous studies13,20,22,29 may support this possibility though we do not have available data to further analysis.
There are some limitations in our meta-analysis. The first is the problem of heterogeneity due to relevant baseline patient characteristics of each study. Although we took into account the heterogeneity in our meta-analysis using the random-effects model, the conclusion drawn in this study should be considered prudently. Second, as some of the studies were unable to provide data available to calculate HRs of BCR, we could not merge their results, although publication bias evaluation of BCR showed no significant difference and sensitivity analysis confirmed the prognostic value of LVI. In addition, as only few included studies covered survival outcomes such as PFS, CSS, DM, and OS, we were unable to perform a meta-analysis for the lack of data available to calculate HR and 95% CI directly or indirectly. Finally, most studies were retrospective, and only two studies included in our meta-analysis were prospective. Therefore, more prospective multicenter trials are required to confirm the conclusion.
In addition to these study limitation, it is usually difficult to completely exclude subjective bias among pathologists in clinical practice.8 Knowing that the surrounding stromal tissue can mimic vascular invasion that cannot be easily be recognized, experts have reached agreement that the report of LVI is only in unequivocal cases.27 With regard to staining method, hematoxylin and eosin (HE) is the most commonly used examination for LVI. However, some included studies incorporated immunohistochemical analysis, and this added measure may increase the detection rate of LVI.26 But as there are still controversies over the use of immunohistochemical analysis, it is not used routinely in clinical practice. What's more, in most studies, tumor cells invasion in lymphatic vessels and vascular vessels were combined as LVI and no effort was made to distinguish between them. One reason for this is the difficulty that there is lack of reproducibility when using routine light microscopy, and previous studies have not fully evaluated the clinical values to assess the survival outcomes of prostate cancer in terms of distinguishing vascular invasion from lymphatic invasion.
CONCLUSION
Our meta-analysis indicates that LVI has a detrimental effect on the BCR-Free probability, and clinicopathological features in RP specimens and, therefore, could be considered as an independent prognostic factor of BCR. It could also be used to predict BCR patients who need further adjuvant therapies.
AUTHOR CONTRIBUTIONS
Y Huang reviewed articles, analyzed data, and drafted the manuscript; HH and XWP reviewed articles, analyzed data, and revised the manuscript critically; HH participated in as the third reviewer and drafting the manuscript; JC and Y Hong participated in data analyzing and revised the manuscript; JQY and LL participated in its design and helped to draft the manuscript; DFX supervised the project and revised manuscript; XGC and YG conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China for Youths (No. 81001136 and 81202020); the National Natural Science Foundation of China (No. 30973006, 81170637); Shanghai Committee of Science and Technology General Program for Medicine (No. 11JC1402302); the Key Project of Science and Innovation Foundation of Shanghai Ministry of Education (14zz084); and the Military Fund for Health Care (13BJZ29).
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Porter CR, Kodama K, Gibbons RP, Correa R, Jr, Chun FK, et al. 25-year prostate cancer control and survival outcomes: a 40-year radical prostatectomy single institution series. J Urol. 2006;176:569–74. doi: 10.1016/j.juro.2006.03.094. [DOI] [PubMed] [Google Scholar]
- 3.Menon M, Bhandari M, Gupta N, Lane Z, Peabody JO, et al. Biochemical recurrence following robot-assisted radical prostatectomy: analysis of 1384 patients with a median 5-year follow-up. Eur Urol. 2010;58:838–46. doi: 10.1016/j.eururo.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65:124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 6.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL, et al. Inclusion of vasculature-related variables in the Dukes staging system of colon cancer. Clin Cancer Res. 2005;11(24 Pt 1):8653–60. doi: 10.1158/1078-0432.CCR-05-1464. [DOI] [PubMed] [Google Scholar]
- 7.Lotan Y, Gupta A, Shariat SF, Palapattu GS, Vazina A, et al. Lymphovascular invasion is independently associated with overall survival, cause-specific survival, and local and distant recurrence in patients with negative lymph nodes at radical cystectomy. J Clin Oncol. 2005;23:6533–9. doi: 10.1200/JCO.2005.05.516. [DOI] [PubMed] [Google Scholar]
- 8.Magi-Galluzzi C, Evans AJ, Delahunt B, Epstein JI, Griffiths DF, et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 3: extraprostatic extension, lymphovascular invasion and locally advanced disease. Mod Pathol. 2011;24:26–38. doi: 10.1038/modpathol.2010.158. [DOI] [PubMed] [Google Scholar]
- 9.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.van den Ouden D, Kranse R, Hop WC, van der Kwast TH, Schröder FH. Microvascular invasion in prostate cancer: prognostic significance in patients treated by radical prostatectomy for clinically localized carcinoma. Urol Int. 1998;60:17–24. doi: 10.1159/000030197. [DOI] [PubMed] [Google Scholar]
- 14.de la Taille A, Rubin MA, Buttyan R, Olsson CA, Bagiella E, et al. Is microvascular invasion on radical prostatectomy specimens a useful predictor of PSA recurrence for prostate cancer patients? Eur Urol. 2000;38:79–84. doi: 10.1159/000020256. [DOI] [PubMed] [Google Scholar]
- 15.Herman CM, Wilcox GE, Kattan MW, Scardino PT, Wheeler TM. Lymphovascular invasion as a predictor of disease progression in prostate cancer. Am J Surg Pathol. 2000;24:859–63. doi: 10.1097/00000478-200006000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Quinn DI, Henshall SM, Haynes AM, Brenner PC, Kooner R, et al. Prognostic significance of pathologic features in localized prostate cancer treated with radical prostatectomy: implications for staging systems and predictive models. J Clin Oncol. 2001;19:3692–705. doi: 10.1200/JCO.2001.19.16.3692. [DOI] [PubMed] [Google Scholar]
- 17.Ito K, Nakashima J, Mukai M, Asakura H, Ohigashi T, et al. Prognostic implication of microvascular invasion in biochemical failure in patients treated with radical prostatectomy. Urol Int. 2003;70:297–302. doi: 10.1159/000070139. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari MK, McNeal JE, Malhotra SM, Brooks JD. Vascular invasion predicts recurrence after radical prostatectomy: stratification of risk based on pathologic variables. Urology. 2004;64:749–53. doi: 10.1016/j.urology.2004.04.070. [DOI] [PubMed] [Google Scholar]
- 19.Shariat SF, Khoddami SM, Saboorian H, Koeneman KS, Sagalowsky AI, et al. Lymphovascular invasion is a pathological feature of biologically aggressive disease in patients treated with radical prostatectomy. J Urol. 2004;171:1122–7. doi: 10.1097/01.ju.0000113249.82533.28. [DOI] [PubMed] [Google Scholar]
- 20.Cheng L, Jones TD, Lin H, Eble JN, Zeng G, et al. Lymphovascular invasion is an independent prognostic factor in prostatic adenocarcinoma. J Urol. 2005;174:2181–5. doi: 10.1097/01.ju.0000181215.41607.c3. [DOI] [PubMed] [Google Scholar]
- 21.Antunes AA, Srougi M, Dall’Oglio MF, Crippa A, Paranhos M, et al. Microvascular invasion is an independent prognostic factor in patients with prostate cancer treated with radical prostatectomy. Int Braz J Urol. 2006;32:668–75. doi: 10.1590/s1677-55382006000600007. discussion 675–7. [DOI] [PubMed] [Google Scholar]
- 22.Brooks JP, Albert PS, O’Connell J, McLeod DG, Poggi MM. Lymphovascular invasion in prostate cancer: prognostic significance in patients treated with radiotherapy after radical prostatectomy. Cancer. 2006;106:1521–6. doi: 10.1002/cncr.21774. [DOI] [PubMed] [Google Scholar]
- 23.Hofer MD, Kuefer R, Huang W, Li H, Bismar TA, et al. Prognostic factors in lymph node-positive prostate cancer. Urology. 2006;67:1016–21. doi: 10.1016/j.urology.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 24.Loeb S, Roehl KA, Yu X, Antenor JA, Han M, et al. Lymphovascular invasion in radical prostatectomy specimens: prediction of adverse pathologic features and biochemical progression. Urology. 2006;68:99–103. doi: 10.1016/j.urology.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Huang SP, Huang CY, Wang JS, Liu CC, Pu YS, et al. Prognostic significance of p53 and X-ray repair cross-complementing group 1 polymorphisms on prostate-specific antigen recurrence in prostate cancer post radical prostatectomy. Clin Cancer Res. 2007;13(22 Pt 1):6632–8. doi: 10.1158/1078-0432.CCR-07-1437. [DOI] [PubMed] [Google Scholar]
- 26.May M, Kaufmann O, Hammermann F, Loy V, Siegsmund M. Prognostic impact of lymphovascular invasion in radical prostatectomy specimens. BJU Int. 2007;99:539–44. doi: 10.1111/j.1464-410X.2006.06650.x. [DOI] [PubMed] [Google Scholar]
- 27.Baydar DE, Baseskioglu B, Ozen H, Geyik PO. Prognostic significance of lymphovascular invasion in clinically localized prostate cancer after radical prostatectomy. Scientific World Journal. 2008;8:303–12. doi: 10.1100/tsw.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whittemore DE, Hick EJ, Carter MR, Moul JW, Miranda-Sousa AJ, et al. Significance of tertiary Gleason pattern 5 in Gleason score 7 radical prostatectomy specimens. J Urol. 2008;179:516–22. doi: 10.1016/j.juro.2007.09.085. discussion 522. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto S, Kawakami S, Yonese J, Fujii Y, Ohkubo Y, et al. Lymphovascular invasion is an independent predictor of prostate-specific antigen failure after radical prostatectomy in patients with pT3aN0 prostate cancer. Int J Urol. 2008;15:895–9. doi: 10.1111/j.1442-2042.2008.02140.x. [DOI] [PubMed] [Google Scholar]
- 30.Jeon HG, Bae J, Yi JS, Hwang IS, Lee SE, et al. Perineural invasion is a prognostic factor for biochemical failure after radical prostatectomy. Int J Urol. 2009;16:682–6. doi: 10.1111/j.1442-2042.2009.02331.x. [DOI] [PubMed] [Google Scholar]
- 31.Cho IC, Chung HS, Cho KS, Kim JE, Joung JY, et al. Bcl-2 as a predictive factor for biochemical recurrence after radical prostatectomy: an interim analysis. Cancer Res Treat. 2010;42:157–62. doi: 10.4143/crt.2010.42.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JT, Lee S, Yun CJ, Jeon BJ, Kim JM, et al. Prediction of perineural invasion and its prognostic value in patients with prostate cancer. Korean J Urol. 2010;51:745–51. doi: 10.4111/kju.2010.51.11.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung JH, Lee JW, Arkoncel FR, Cho NH, Yusoff NA, et al. Significance of perineural invasion, lymphovascular invasion, and high-grade prostatic intraepithelial neoplasia in robot-assisted laparoscopic radical prostatectomy. Ann Surg Oncol. 2011;18:3828–32. doi: 10.1245/s10434-011-1790-4. [DOI] [PubMed] [Google Scholar]
- 34.Yee DS, Shariat SF, Lowrance WT, Maschino AC, Savage CJ, et al. Prognostic significance of lymphovascular invasion in radical prostatectomy specimens. BJU Int. 2011;108:502–7. doi: 10.1111/j.1464-410X.2010.09848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chromecki TF, Cha EK, Pummer K, Scherr DS, Tewari AK, et al. Prognostic value of insulin-like growth factor II mRNA binding protein 3 in patients treated with radical prostatectomy. BJU Int. 2012;110:63–8. doi: 10.1111/j.1464-410X.2011.10703.x. [DOI] [PubMed] [Google Scholar]
- 36.Luo HL, Chiang PH, Chen YT, Cheng YT. Lymphovascular invasion is a pathological feature related to aggressive cancer behavior and predicts early recurrence in prostate cancer. Kaohsiung J Med Sci. 2012;28:327–30. doi: 10.1016/j.kjms.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leng YH, Lee WJ, Yang SO, Lee JK, Jung TY, et al. Oncologic outcomes of patients with Gleason score 7 and tertiary Gleason pattern 5 after radical prostatectomy. Korean J Urol. 2013;54:587–92. doi: 10.4111/kju.2013.54.9.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–6. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 39.Alitalo K, Mohla S, Ruoslahti E. Lymphangiogenesis and cancer: meeting report. Cancer Res. 2004;64:9225–9. doi: 10.1158/0008-5472.CAN-04-2475. [DOI] [PubMed] [Google Scholar]
- 40.Alexander-Sefre F, Singh N, Ayhan A, Salveson HB, Wilbanks G, et al. Detection of tumour lymphovascular space invasion using dual cytokeratin and CD31 immunohistochemistry. J Clin Pathol. 2003;56:786–8. doi: 10.1136/jcp.56.10.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ku JH, Byun SS, Jeong H, Kwak C, Kim HH, et al. Lymphovascular invasion as a prognostic factor in the upper urinary tract urothelial carcinoma: a systematic review and meta-analysis. Eur J Cancer. 2013;49:2665–80. doi: 10.1016/j.ejca.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 42.Kim H, Kim M, Kwak C, Kim HH, Ku JH. Prognostic significance of lymphovascular invasion in radical cystectomy on patients with bladder cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e89259. doi: 10.1371/journal.pone.0089259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mollberg NM, Bennette C, Howell E, Backhus L, Devine B, et al. Lymphovascular invasion as a prognostic indicator in stage I non-small cell lung cancer: a systematic review and meta-analysis. Ann Thorac Surg. 2014;97:965–71. doi: 10.1016/j.athoracsur.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527–36. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 45.Albers P, Siener R, Kliesch S, Weissbach L, Krege S, et al. Risk factors for relapse in clinical stage I nonseminomatous testicular germ cell tumors: results of the German Testicular Cancer Study Group Trial. J Clin Oncol. 2003;21:1505–12. doi: 10.1200/JCO.2003.07.169. [DOI] [PubMed] [Google Scholar]
- 46.Ng J, Mahmud A, Bass B, Brundage M. Prognostic significance of lymphovascular invasion in radical prostatectomy specimens. BJU Int. 2012;110:1507–14. doi: 10.1111/j.1464-410X.2012.11115.x. [DOI] [PubMed] [Google Scholar]
- 47.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;17:1499–507. doi: 10.1200/JCO.1999.17.5.1499. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Main characteristics of the eligible studies
TNM stage characteristics and correlations between LVI and preoperative PSA and pathological parameters
Estimation of the HR
Subgroup analysis of biochemical recurrence-free survival


