Abstract
We conducted a community-based cross-sectional study to evaluate the role of genetics in determining the individual difference in total testosterone and sex hormone-binding globulin levels. Study participants comprised 730 Korean men consisting of 142 pairs of monozygotic twins, 191 pairs of siblings, and 259 father-offspring pairs from 270 families who participated in the Healthy Twin study. Serum concentration of total testosterone and sex hormone-binding globulin were measured by chemiluminescence immunoassay, and free testosterone and bioavailable testosterone were calculated using Vermeulen's method. Quantitative genetic analysis based on a variance decomposition model showed that the heritability of total testosterone, free testosterone, bioavailable testosterone, and sex hormone-binding globulin were 0.56, 0.45, 0.44, and 0.69, respectively after accounting for age and body mass index. Proportions of variance explained by age and body mass index varied across different traits, from 8% for total testosterone to 31% for sex hormone-binding globulin. Bivariate analysis showed a high degree of additive genetic correlation (ρG = 0.67) and a moderate degree of individual-specific environmental correlation (ρE = 0.42) between total testosterone and sex hormone-binding globulin. The findings confirmed the important role of genetics in determining the individually different levels of testosterone and sex hormone-binding globulin during adulthood in Korean men as found in non-Asian populations, which may suggest that common biologic control for determining testosterone level directly or indirectly through binding protein are largely shared among different populations.
Keywords: genetics, Korean, sex hormone-binding globulin, testosterone, twin study
INTRODUCTION
Testosterone, a male sex hormone primarily secreted from the testes, has been suggested to be associated with the aging process, sexual function, and various health conditions, including cardiovascular, endocrine, and bone health in men.1 Testosterone levels vary between individuals even in the same ethnic population.2,3,4 Evidence from twin studies and family studies indicates that genetics contributes to the inter-individual variation in testosterone levels,5,6 in addition to age and a range of health conditions.7,8 However, most previous studies included only the Caucasians and the reported heritability (h2) of testosterone levels varies across studies, ranging from very mild (h2 =0.2) to very strong (h2 =0.7) for total testosterone (TT)5,9,10,11,12 and from 0.19 to 0.60 for free testosterone.10,11,12,13 In addition, the hitherto reported levels of testosterone heritability have been obtained mostly from twin-only studies or nontwin family studies. Although studies involving twins can discriminate genetic influences from environmental influences, the estimated heritability from a twin-only study tends to be inflated, because it includes dominant genetic effects in addition to additive genetic effects which are the main interest.14 Inclusion of more diverse family relationship such as father-offspring pairs who share same additive genetics but do not share dominant genetics at all would provide much better chance of testing dominant genetic component as well as shared environmental components.
The clinical action of testosterone largely depends on the level of free or bioavailable testosterone rather than TT, because 50% to 60% of TT is bound to sex hormone-binding globulin (SHBG). SHBG also exhibits wide inter-individual variation, and diverse factors contribute to the regulation of SHBG, which makes the study of testosterone more complicated.15,16 Therefore, it seems necessary to know the influence of genetics on SHBG, which confounds the understanding of how SHBG controls testosterone levels and its effects on health.10,13
We conducted this study in 730 Korean men from extended families composed of twins and their brothers and fathers to investigate the contributions of genetic factors to TT, functional testosterone, and SHBG and to understand the relationship between testosterone and SHBG.
MATERIALS AND METHODS
Study subjects
Study subjects included 730 Korean men consisting of 142 pairs of monozygotic (MZ) twins, 191 pairs of siblings, and 259 father-offspring pairs from 270 families who participated in the Healthy Twin study between April 2005 and April 2010. Dizygotic twins were included in the sibling group, because the level of genetic resemblance between dizygotic twins is similar to that between nontwin brothers, and the number of DZ twins was too small (33 pairs) to be analyzed as a separate group. Details of the study design and protocols have been previously published.17 In brief, the Healthy Twin study is a community-based cohort study ongoing since 2005, which has recruited Korean adult twins and their first-degree adult family members without ascertainment of their health status, through a nationwide advertisement and a mailing to some members of the Korean twin-family register whose contact information was available. During the first and second wave surveys between April 2005 and April 2010, a total of 742 men with at least one more male family member underwent measurement of blood levels of testosterone and SHBG. Among these, 12 men with a cancer history or a testosterone treatment were not included. All participants provided written informed consent. The Institutional Review Board of each participating center approved the study protocol.
Study variables
Blood samples were drawn in the morning (around 10 a.m.) after overnight fasting. Serum was immediately separated and stored at −70°C. We measured TT and SHBG immediately after thawing the frozen serum by electrochemiluminescence immunoassay using commercial ADVIA Centaur XP (Siemens, Erlange, Germany) for TT and a MODULAR ANALYTICS E170 analyzer (Roche, Basel, Switzerland) for SHBG. The minimum concentration measurable at the laboratory was 0.35 nmol L−1 for TT and SHBG. Inter-assay coefficients of variation were <7.6% for TT and <2% for SHBG. Albumin levels in fresh sera were measured by colorimetry using an ADVIA 1650 chemistry analyzer (Siemens). Free testosterone (cFT) and bioavailable testosterone (cBAT) levels were calculated using Vermeulen's method.18
Body mass index (BMI) was calculated using measured weight and height (kg m−2). Information on health habits was obtained using a self-administered questionnaire.
We ascertained the zygosity of twin pairs using 16 short tandem repeat markers including one sex-determining marker for 67% of twin pairs and a self-administered zygosity questionnaire for the remaining 33%. Ascertainment of zygosity using the questionnaire was 94.3% accurate compared to ascertainment by the short tandem repeat marker.19
Statistics
The characteristics of offspring and fathers were compared by t-test and Chi-square test and intraclass correlation coefficients (ICCs) between various intra-familial pairs were estimated using linear mixed regression analysis using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
Quantitative genetic analysis was conducted using Sequential Oligogenic Linkage Analysis Routines (SOLAR) version 2.0 (Southwest Foundation for Biomedical Research, San Antonio, TX, USA).20 To eliminate the influence of skewness and residual kurtosis, inverse normal transformation was performed for TT, SHBG, cFT, and cBAT before they were entered into the analytic models.
A variance component approach was used to partition the total phenotypic variation (бp2) of each trait into its additive genetic component (бa2) and unmeasured environmental component (бe2).21,22 In addition, unmeasured shared environmental components within a family (бc2) were tested and added to the model if adding бc2 effect significantly increased overall model fitting in terms of likelihood compared with models without бc2 (i.e., increase in 2ln(L)>2, L: likelihood). The key assumption of this model is that the effects of environmental factors are common to the members of a family and that the three factors, бa2, бc2, and бe2, have independent and additive effects on the trait variance with the total residual variance being the sum of the additive and individual-specific variance components (бp2= бa2+ бc2+ бe2). Heritability represents the proportion of residual variance attributed to additive genetic factors (бa2/бp2). By using the strength of twin and family design, we tried to estimate narrow-sense heritability, instead of the broad-sense heritability that includes both dominant genetic effects and additive genetic effects as usually estimated from twin-only studies.
We also estimated the additive genetic correlations between TT and SHBG by performing a bivariate variance component-based genetic analysis to ascertain evidence of a common genetic regulation of the traits.20 Through the bivariate variance-component analysis, the phenotypic correlations between two traits are partitioned into genetic (ρG) and environmental (ρE) correlations. Thus, this provides insight as to whether or not the correlation between two or more phenotypes of an individual is codetermined by shared genes and environment.
RESULTS
Table 1 shows the characteristics of the study subjects. Compared with their fathers, offspring had higher levels of cFT and cBAT and lower SHBG levels. Current alcohol drinking and smoking were more prevalent, and participation in an adequate level of physical exercise was less prevalent among offspring than among their fathers (Table 1).
Table 1.
Characteristics of study participants
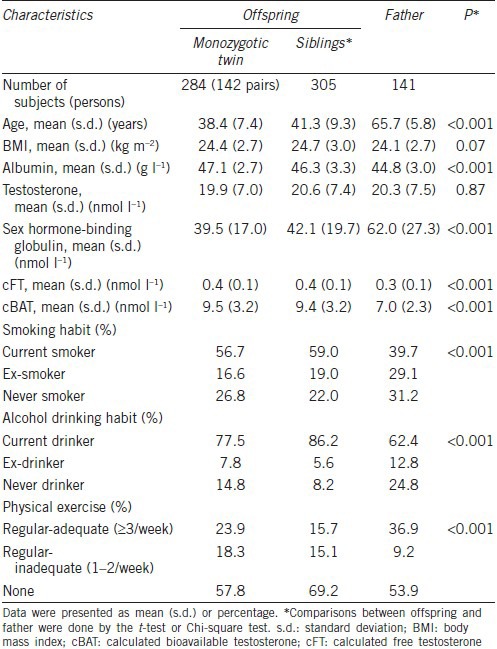
Table 2 shows the age-adjusted intraclass correlation coefficients (ICCs) between various intra-familial pairs and heritability estimates for each trait. The ICC between MZ twin pairs was moderately high for all traits (between 0.51 and 0.61), and was around twice as high as the ICC estimates for sibling pairs and father-offspring pairs. However, the ICC estimates for sibling pairs were similar to those for father-offspring pairs.
Table 2.
Intraclass correlation coefficients and heritability of testosterone and sex hormone-binding globulin
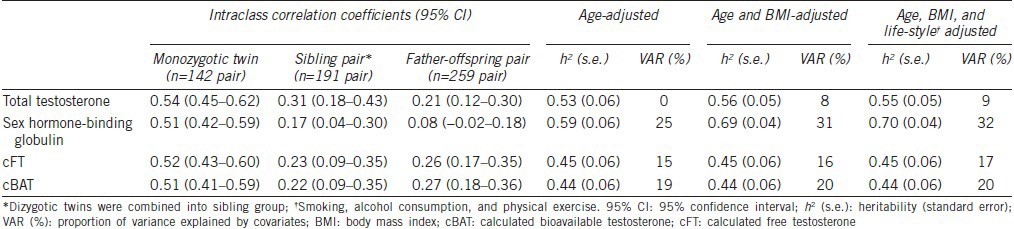
Age-adjusted heritability was moderately high for all traits (between 0.44 and 0.59) with the highest estimate being that of SHBG. When BMI was additionally adjusted, the heritability of SHBG was increased by 10%. Proportions of variance explained by covariates varied across different traits, from 8% for TT to 31% for SHBG. When we further adjusted for health behaviors such as smoking, alcohol, and regular physical activity, the estimated heritability was almost unchanged.
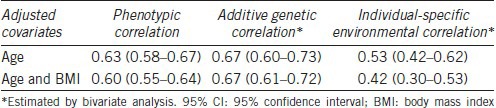
Table 3 shows the cross-trait correlations between SHBG and TT. There was a moderately high (P < 0.01) positive phenotypic correlation between TT and SHBG. When the phenotypic correlation between SHBG and TT was decomposed, both additive genetic and unique environmental correlations were statistically significant (P < 0.05). These finding did not change materially when BMI was adjusted in addition to age.
Table 3.
Cross-trait correlation (95% CI) between sex hormone-binding globulin and total testosterone
DISCUSSION
Although studies including ours have consistently suggested an important genetic influence on individual differences in TT and SHBG levels at adulthood, the reported heritability estimates range widely. The difference in adjusted covariates, the extent of involved intra-familial relationships, ethnicity, or the age range of studied participants may explain the variability in reported heritability.
Classical twin studies have reported heritability estimates of 22%–59% in males,5,11,12,23 which are slightly lower than the heritability of 40%–73% found in family-based studies.9,10,13,24 Heritability estimates in study involving both twins and their families may be lower compared to twin-only study or family-only study by avoiding overestimation of heritability through the comparisons of phenotypic correlations between various familial relationships.14 However, the heritability estimates of TT and SHBG in our twin and family study were similar to those in twin-only or family-based studies.
Most previous studies were conducted in Caucasian populations5,10,11,12,13,25 with only three studies including Mexican-Americans15,23 or African-Americans.9,23 In our study, the heritability estimates for testosterone and SHBG were similar to or higher than those found in Caucasians. However, it is unclear whether the genetic role is stronger in Asian men due to the lack of other Asian studies.
Only a few studies have accounted for covariates.10,13,15 Adiposity has been found to be inversely associated with TT and SHBG.13,26 We found that heritability estimates of SHBG and TT increased substantially after adjusting for BMI while the study by Bogaert et al.13 found that heritability estimate of TT decreased and that of SHBG increased after adjusting for BMI. Although we could not elucidate the reason for the different findings between our study and the study by Bogaert et al.,13 we think that the difference supports the importance of BMI adjustment, especially when comparisons are made between populations with different BMI distributions.
Considering that the variance explained by covariates other than age and BMI was only about 1% in our study, it seems less likely that the differences in adjustment for lifestyle factors between studies have contributed to a lot to the variation of heritability estimates for TT and SHBG between studies.
Previous studies have reported wide range of heritability estimates for TT and SHBG by the age range of study participants with very low heritability at birth27 and higher heritability in adolescents23 and adults.5,13 These findings seem to suggest that inter-individual variability of TT is determined mainly by environmental factors in early infancy27,28 while the influence of genetic factors becomes more evident in older age.10
Supporting the role of genetic factors in determining individual differences in TT and SHBG levels in men, genome-wide association studies (GWAS) and meta-analysis revealed several SNPs associated with TT levels6,29,30 and SHBG levels6,30,31 at several genomic regions. However, most studies were conducted in the Caucasians, and many of the identified SNPs in the Caucasian studies6,29 were not repeatedly identified in Chinese study.30 Therefore, studies are needed to identify more pleiotropic loci, especially in Asians and other non-Caucasian populations. In addition, clinical relevance of genetically determined individual differences in the levels of testosterone and SHBG should be further evaluated, considering that the SNP on the SHBG gene that seems to determine testosterone level was not associated with the risk of cardiovascular and metabolic diseases associated with low testosterone level in a study.32
Polymorphisms in the genes encoding the enzymes involved in TT biosynthesis and metabolism may also contribute to inter-individual variability of testosterone.33
Although controversial findings existed,34,35 androgen receptor CAG repeat polymorphism has been found to be involved in determining inter-individual variability of circulating testosterone and SHBG level, probably through negative feedback mechanism.36,37,38 Ackerman et al.39 also demonstrated interethnic differences in the allele frequencies of the AR (CAG)n polymorphism, such as the longest repeat length in Thai population followed by Hispanic, Caucasian, and Afro-Caribbean. Although further studies are needed to confirm, the interethnic differences in the AR (CAG)n polymorphism may explain the interethnic difference in testosterone level at least in part.
The genetic and environmental correlations between TT and SHBG found in our study were very similar to those found in a Belgian study (ρG = 0.67 and ρE = 0.42).13 Although they differed slightly from those found in the Framingham Heart Study (ρG = 0.87 and ρE = 0.25),10 these findings strongly suggest that TT and SHBG levels are codetermined by shared genes. Travison et al.10 reported that the genetic correlations between TT and SHBG (ρG = 0.87) were much higher than the correlation between free T and SHBG (ρG = 0.24),10 which suggests that genetic factors determining concentration of SHBG rather than those determining binding capacity of SHBG are likely to codetermine TT levels.
In accordance with this finding, several SNPs (rs12150660, rs727428, and rs2075230) at the SHBG locus were identified to be associated with variation in serum TT as well as SHBG.6,30,40 In addition, a genetic mutation in exon 8 (Asp327Asn) and a (TAAAA)(n)-repeat in the promoter region of the SHBG gene were suggested to contribute to genetically-determined inter-individual variation in TT through variation in SHBG concentration.41 Given that the system regulating sex steroid hormone levels is very complicated and SHBG is under influence of various endogenous hormones such as leptin, IGF-1 system, and insulin,25,42,43 understanding the molecular basis is essential in the search for genes underlying the association between TT and SHBG.
Our study has some limitations. First, we measured TT and SHBG levels just once and thus could not consider intra-individual variability. Second, we could not use gold standard method for measuring TT and free testosterone. Although the accepted gold standard method for free testosterone measurement is equilibrium dialysis and that for TT is tandem mass spectrometry, we could not use those methods because they are costly and time-consuming processes.18 Furthermore, we used calculated free testosterone because free T measurement using an easier and cheaper method (i.e., radioimmunoassay method) has been criticized as inaccurate.
CONCLUSION
We confirmed that genetics plays an important role in determining the inter-individually different levels of testosterone and SHBG during adulthood in Korean men. This finding is consistent with the findings from the studies in non-Asian populations, which suggests that common biologic control for determining testosterone level directly or indirectly through binding protein are largely shared among different populations.
AUTHOR CONTRIBUTION
JS participated in its design, performed the statistical analysis, and drafted the manuscript. YMS conceived of the study, participated in its design, carried out the survey, interpreted the data, and revised the manuscript. Both authors read and approved the final manuscript.
COMPETING INTERESTS
The authors have no conflicts of interest to disclose.
ACKNOWLEDGMENTS
This research was supported by the Samsung Biomedical Research Institute grant (GL1B32111) and by a grant from the Korea Health Technology R and D Project through the Korea Health Industry Development Institute (KHIDI, funding HI14C0064). The funding sources had no role in conducting this study.
REFERENCES
- 1.Jones TH. Advances in the Management of Testosterone Deficiency. London: Karger; 2009. [DOI] [PubMed] [Google Scholar]
- 2.Mazur A. The age-testosterone relationship in black, white, and Mexican-American men, and reasons for ethnic differences. Aging Male. 2009;12:66–76. doi: 10.1080/13685530903071802. [DOI] [PubMed] [Google Scholar]
- 3.Jakobsson J, Ekstrom L, Inotsume N, Garle M, Lorentzon M, et al. Large differences in testosterone excretion in Korean and Swedish men are strongly associated with a UDP-glucuronosyl transferase 2B17 polymorphism. J Clin Endocrinol Metab. 2006;91:687–93. doi: 10.1210/jc.2005-1643. [DOI] [PubMed] [Google Scholar]
- 4.Heald AH, Ivison F, Anderson SG, Cruickshank K, Laing I, et al. Significant ethnic variation in total and free testosterone concentration. Clin Endocrinol. 2003;58:262–6. doi: 10.1046/j.1365-2265.2003.01653.x. [DOI] [PubMed] [Google Scholar]
- 5.Ring HZ, Lessov CN, Reed T, Marcus R, Holloway L, et al. Heritability of plasma sex hormones and hormone binding globulin in adult male twins. J Clin Endocrinol Metab. 2005;90:3653–8. doi: 10.1210/jc.2004-1025. [DOI] [PubMed] [Google Scholar]
- 6.Ohlsson C, Wallaschofski H, Lunetta KL, Stolk L, Perry JR, et al. Genetic determinants of serum testosterone concentrations in men. PLoS Genet. 2011;7:e1002313. doi: 10.1371/journal.pgen.1002313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. 2007;92:549–55. doi: 10.1210/jc.2006-1859. [DOI] [PubMed] [Google Scholar]
- 8.Lapauw B, Goemaere S, Zmierczak H, Van Pottelbergh I, Mahmoud A, et al. The decline of serum testosterone levels in community-dwelling men over 70 years of age: descriptive data and predictors of longitudinal changes. Eur J Endocrinol. 2008;159:459–68. doi: 10.1530/EJE-07-0873. [DOI] [PubMed] [Google Scholar]
- 9.Hong Y, Gagnon J, Rice T, Perusse L, Leon AS, et al. Familial resemblance for free androgens and androgen glucuronides in sedentary black and white individuals: the HERITAGE Family Study. Health, risk factors, exercise training and genetics. J Endocrinol. 2001;170:485–92. doi: 10.1677/joe.0.1700485. [DOI] [PubMed] [Google Scholar]
- 10.Travison TG, Zhuang WV, Lunetta KL, Karasik D, Bhasin S, et al. The heritability of circulating testosterone, oestradiol, oestrone and sex hormone binding globulin concentrations in men: the Framingham Heart Study. Clin Endocrinol. 2014;80:277–82. doi: 10.1111/cen.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meikle AW, Bishop DT, Stringham JD, West DW. Quantitating genetic and nongenetic factors that determine plasma sex steroid variation in normal male twins. Metabolism. 1986;35:1090–5. doi: 10.1016/0026-0495(86)90020-x. [DOI] [PubMed] [Google Scholar]
- 12.Bishop DT, Meikle AW, Slattery ML, Stringham JD, Ford MH, et al. The effect of nutritional factors on sex hormone levels in male twins. Genet Epidemiol. 1988;5:43–59. doi: 10.1002/gepi.1370050105. [DOI] [PubMed] [Google Scholar]
- 13.Bogaert V, Taes Y, Konings P, Van Steen K, De Bacquer D, et al. Heritability of blood concentrations of sex-steroids in relation to body composition in young adult male siblings. Clin Endocrinol. 2008;69:129–35. doi: 10.1111/j.1365-2265.2008.03173.x. [DOI] [PubMed] [Google Scholar]
- 14.Tenesa A, Haley CS. The heritability of human disease: estimation, uses and abuses. Nat Rev Genet. 2013;14:139–49. doi: 10.1038/nrg3377. [DOI] [PubMed] [Google Scholar]
- 15.Jaquish CE, Blangero J, Haffner SM, Stern MP, MacCluer JW. Quantitative genetics of serum sex hormone-binding globulin levels in participants in the San Antonio Family Heart Study. Metabolism. 1997;46:988–91. doi: 10.1016/s0026-0495(97)90266-3. [DOI] [PubMed] [Google Scholar]
- 16.Ahrentsen OD, Jensen HK, Johnsen SG. Sex-hormone-binding globulin deficiency. Lancet. 1982;2:377. doi: 10.1016/s0140-6736(82)90560-8. [DOI] [PubMed] [Google Scholar]
- 17.Gombojav B, Song YM, Lee K, Yang S, Kho M, et al. The Healthy Twin Study, Korea updates: resources for omics and genome epidemiology studies. Twin Res Hum Genet. 2013;16:241–5. doi: 10.1017/thg.2012.130. [DOI] [PubMed] [Google Scholar]
- 18.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–72. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 19.Song YM, Lee D, Lee MK, Lee K, Lee HJ, et al. Validity of the zygosity questionnaire and characteristics of zygosity-misdiagnosed twin pairs in the Healthy Twin Study of Korea. Twin Res Hum Genet. 2010;13:223–30. doi: 10.1375/twin.13.3.223. [DOI] [PubMed] [Google Scholar]
- 20.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allison DB, Neale MC, Zannolli R, Schork NJ, Amos CI, et al. Testing the robustness of the likelihood-ratio test in a variance-component quantitative-trait loci-mapping procedure. Am J Hum Genet. 1999;65:531–44. doi: 10.1086/302487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JT, Blangero J. Comparison of variance components and sibpair-based approaches to quantitative trait linkage analysis in unselected samples. Genet Epidemiol. 1999;16:113–34. doi: 10.1002/(SICI)1098-2272(1999)16:2<113::AID-GEPI1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Harden KP, Kretsch N, Tackett JL, Tucker-Drob EM. Genetic and environmental influences on testosterone in adolescents: evidence for sex differences. Dev Psychobiol. 2014;56:1278–89. doi: 10.1002/dev.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meikle AW, Stanish WM, Taylor N, Edwards CQ, Bishop CT. Familial effects on plasma sex-steroid content in man: testosterone, estradiol and sex-hormone-binding globulin. Metabolism. 1982;31:6–9. [PubMed] [Google Scholar]
- 25.Kuijper EA, Lambalk CB, Boomsma DI, van der Sluis S, Blankenstein MA, et al. Heritability of reproductive hormones in adult male twins. Hum Reprod. 2007;22:2153–9. doi: 10.1093/humrep/dem145. [DOI] [PubMed] [Google Scholar]
- 26.Gates MA, Mekary RA, Chiu GR, Ding EL, Wittert GA, et al. Sex steroid hormone levels and body composition in men. J Clin Endocrinol Metab. 2013;98:2442–50. doi: 10.1210/jc.2012-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakai LM, Baker LA, Jacklin CN, Shulman I. Sex steroids at birth: genetic and environmental variation and covariation. Dev Psychobiol. 1991;24:559–70. doi: 10.1002/dev.420240804. [DOI] [PubMed] [Google Scholar]
- 28.Caramaschi D, Booij L, Petitclerc A, Boivin M, Tremblay RE. Genetic and environmental contributions to saliva testosterone levels in male and female infant twins. Psychoneuroendocrinology. 2012;37:1954–9. doi: 10.1016/j.psyneuen.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Jin G, Sun J, Kim ST, Feng J, Wang Z, et al. Genome-wide association study identifies a new locus JMJD1C at 10q21 that may influence serum androgen levels in men. Hum Mol Genet. 2012;21:5222–8. doi: 10.1093/hmg/dds361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Tao S, Gao Y, Zhang J, Hu Y, et al. Genome-wide association study of sex hormones, gonadotropins and sex hormone-binding protein in Chinese men. J Med Genet. 2013;50:794–801. doi: 10.1136/jmedgenet-2013-101705. [DOI] [PubMed] [Google Scholar]
- 31.Coviello AD, Haring R, Wellons M, Vaidya D, Lehtimaki T, et al. A genome-wide association meta-analysis of circulating sex hormone-binding globulin reveals multiple loci implicated in sex steroid hormone regulation. PLoS Genet. 2012;8:e1002805. doi: 10.1371/journal.pgen.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svartberg J, Schirmer H, Wilsgaard T, Mathiesen EB, Njolstad I, et al. Single-nucleotide polymorphism, rs1799941 in the sex hormone-binding globulin (SHBG) gene, related to both serum testosterone and SHBG levels and the risk of myocardial infarction, type 2 diabetes, cancer and mortality in men: the Tromso Study. Andrology. 2014;2:212–8. doi: 10.1111/j.2047-2927.2013.00174.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Li Q, Wang T, Yang G, Wang Y, et al. A common polymorphism in the human aromatase gene alters the risk for polycystic ovary syndrome and modifies aromatase activity in vitro. Mol Hum Reprod. 2011;17:386–91. doi: 10.1093/molehr/gar007. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen TL, Hagen C, Wraae K, Bathum L, Larsen R, et al. The impact of the CAG repeat polymorphism of the androgen receptor gene on muscle and adipose tissues in 20-29-year-old Danish men: Odense Androgen Study. Eur J Endocrinol. 2010;162:795–804. doi: 10.1530/EJE-09-0763. [DOI] [PubMed] [Google Scholar]
- 35.Lapauw B, Goemaere S, Crabbe P, Kaufman JM, Ruige JB. Is the effect of testosterone on body composition modulated by the androgen receptor gene CAG repeat polymorphism in elderly men? Eur J Endocrinol. 2007;156:395–401. doi: 10.1530/EJE-06-0607. [DOI] [PubMed] [Google Scholar]
- 36.Haring R, Ernst F, Schurmann C, Homuth G, Volker U, et al. The androgen receptor CAG repeat polymorphism as a risk factor of low serum testosterone and its cardiometabolic effects in men. Int J Androl. 2012;35:511–20. doi: 10.1111/j.1365-2605.2011.01220.x. [DOI] [PubMed] [Google Scholar]
- 37.Mouritsen A, Hagen CP, Sorensen K, Aksglaede L, Mieritz MG, et al. Androgen receptor CAG repeat length is associated with body fat and serum SHBG in boys: a prospective cohort study. J Clin Endocrinol Metab. 2013;98:E605–9. doi: 10.1210/jc.2012-3778. [DOI] [PubMed] [Google Scholar]
- 38.De Naeyer H, Bogaert V, De Spaey A, Roef G, Vandewalle S, et al. Genetic variations in the androgen receptor are associated with steroid concentrations and anthropometrics but not with muscle mass in healthy young men. PLoS One. 2014;9:e86235. doi: 10.1371/journal.pone.0086235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ackerman CM, Lowe LP, Lee H, Hayes MG, Dyer AR, et al. Ethnic variation in allele distribution of the androgen receptor (AR) (CAG) n repeat. J Androl. 2012;33:210–5. doi: 10.2164/jandrol.111.013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prescott J, Thompson DJ, Kraft P, Chanock SJ, Audley T, et al. Genome-wide association study of circulating estradiol, testosterone, and sex hormone-binding globulin in postmenopausal women. PLoS One. 2012;7:e37815. doi: 10.1371/journal.pone.0037815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanbillemont G, Bogaert V, De Bacquer D, Lapauw B, Goemaere S, et al. Polymorphisms of the SHBG gene contribute to the interindividual variation of sex steroid hormone blood levels in young, middle-aged and elderly men. Clin Endocrinol. 2009;70:303–10. doi: 10.1111/j.1365-2265.2008.03365.x. [DOI] [PubMed] [Google Scholar]
- 42.Gomez JM, Maravall FJ, Gomez N, Navarro MA, Soler J. Determinants of sex hormone-binding globulin concentrations in a cross-sectional study of healthy men randomly selected. J Nutr Health Aging. 2007;11:60–4. [PubMed] [Google Scholar]
- 43.Danielson KK, Drum ML, Lipton RB. Sex hormone-binding globulin and testosterone in individuals with childhood diabetes. Diabetes Care. 2008;31:1207–13. doi: 10.2337/dc07-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]


