Dear Editor,
Asthenozoospermia is a common cause of decreased male fertility, and several factors can inhibit or prevent sperm motility, including ultrastructural defects of the sperm flagellum.1 An ultrastructural defect leading to severe asthenozoospermia is a syndrome called dysplasia of the fibrous sheath (DFS),1 which results in short, thick, and irregular sperm tails; this feature is caused by severe disturbances in the fibrous sheath (FS) and other cytoskeletal components and(or) the dynein arms. DFS is also known as tail stump syndrome2 or short-tailed spermatozoa and is a likely variant of primary ciliary dyskinesia (PCD).3 However, multiple morphological abnormalities of the sperm flagella (MMAF) is a more accurate description for this syndrome.4
No empirical medicinal treatment has been reported to improve the semen parameters of MMAF men, and intracytoplasmic sperm injection (ICSI) is the only way to conceive with a female partner.1 Chemes and Alvarez1 reviewed the ICSI outcomes of 12 cases, the total fertilization rate was 63 ± 16%; 10 pregnancies followed, and 14 babies were delivered.
Six Chinese men (age, 31–34 years) with MMAF were diagnosed between March 2012 and October 2014. All of the participants had suffered from primary infertility for 1–7 years. All patients were subjected to a complete physical examination, and no significant respiratory symptoms were observed. All of the men showed a normal lymphocyte karyotype, and reproductive hormones were within normal ranges. The female partner of case 1 was diagnosed with mosaic Turner syndrome, and the fluorescence in situ hybridization analysis revealed nuc ish (DXZ1 ×1)[1024]//(DXZ1 ×2)[50]//(DXZ1 ×3)[65]. The other female partners had no special reproductive system problems. Case 2 was diagnosed with varicocele and underwent varicocelectomy at another hospital, but his semen parameters failed to improve. All couples signed informed consent for the ICSI procedure, and our hospital Ethics Committee approved this study.
All cases showed semen pH values of 7.2 to 7.8 and severely impaired sperm motility and flagellar morphology. Sperm concentrations were 7.0–24.6 × 106 ml−1. Cases 1–3 had some motile spermatozoa (4.7%–8.5%), whereas cases 4–6 presented with 100% immotility (Table 1). Most spermatozoa (85.0%–99.5%) showed the MMAF phenotype under light microscopy after modified Papanicolaou staining, as combinations of absent, short, thick, coiled, and bent tails, without systematic anomalies on sperm head (Figure 1). A transmission electron microscopy assessment was carried out on cases 1, 4, and 5, and showed poorly assembled flagella.5
Table 1.
Laboratory testing and intracytoplasmic sperm injection outcomes of the six MMAF couples
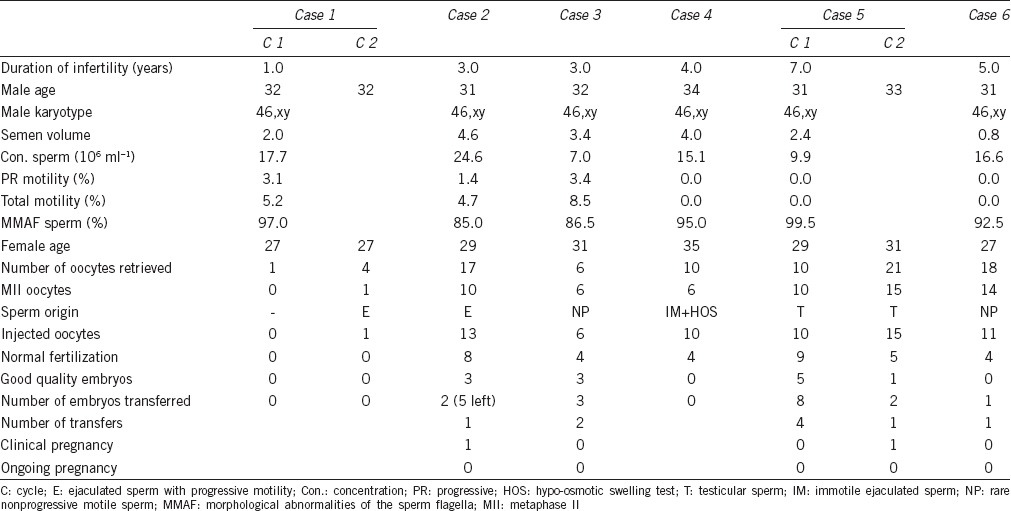
Figure 1.
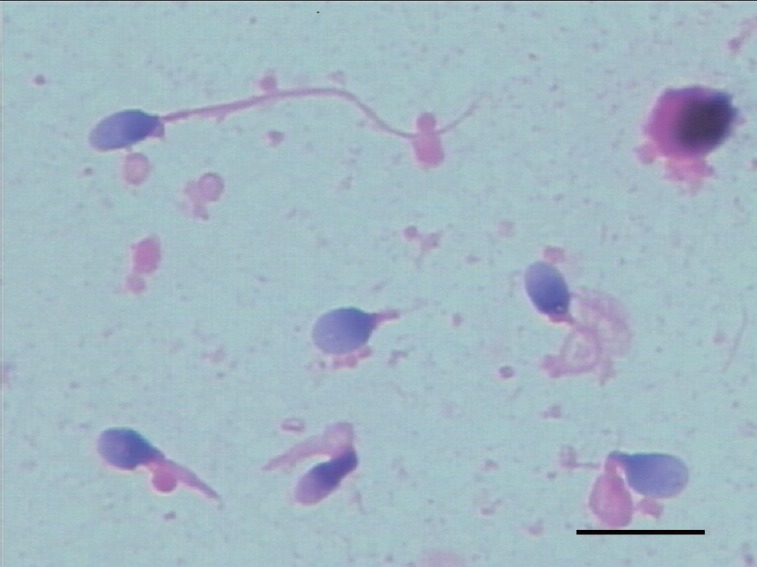
Spermatozoa with multiple morphological abnormalities of the sperm flagella under light microscopy. Scale bar = 10 μm.
The female partners underwent an ovulation induction protocol, according to our routine procedures. Case 1 was induced to ovulate with a microstimulation protocol, who was treated with clomiphene citrate (Codal Synto, Ltd., Limassol, Cyprus), and the others underwent the long protocol, in which follicular development was stimulated with recombinant follicle stimulating hormone (FSH; Merck Serono SA, Aubonne Branch, Switzerland) after pituitary desensitization with a gonadotropin-releasing hormone agonist (Ferring Pharmaceutical Co. Ltd., St-Prex, Switzerland). When the dominant follicle reached a diameter of 18 mm or two follicles were >16 mm, 10 000 IU human chorionic gonadotropin (hCG; Beijing Saisheng Pharmaceutical Co. Ltd., Beijing, China) was administered. Oocytes were retrieved under transvaginal ultrasound guidance 36 h after hCG was administered, and metaphase II oocytes were fertilized 4–6 h after retrieval.
Semen samples were collected by masturbation to confirm the presence of motile sperm on the day oocytes were retrieved. After gradient centrifugation, motile spermatozoa from cases 1 to 3 were injected. Viable spermatozoa were confirmed with the hypo-osmotic swelling (HOS) test and injected in case 4, and fresh testicular spermatozoa of case 5 were injected. The 16 oocytes from case 6 were cryopreserved, and some sluggish and switching type motile spermatozoa from two later ejaculations were injected. The ICSI results are summarized in Table 1.
No embryo was available for case 1 after one oocyte injected in two cycles. Case 2 achieved a 61.5% (8/13) fertilization rate, and seven transferrable embryos were obtained. Although the pregnancy ended in abortion, five embryos remained for subsequent transfers. The nonprogressive motile spermatozoa of case 3 fertilized four of the six oocytes but resulted in a failed pregnancy. For case 4, four fertilized oocytes (4/10) produced no transferable embryos. The testicular spermatozoa obtained from case 5 were mostly tailless and 100% immotile. The first attempt achieved a 90.0% (9/10) fertilization rate, but no pregnancies occurred after eight embryos were transferred. A clinical pregnancy was confirmed after the second attempt, even though a lower fertilization rate (33.3%) and fewer embryos (two) were observed. However, a fetal heartbeat was not detected by ultrasound, and the villus showed a karyotype of 46,xx,14pstk+. The fertilization rate for case 6 was 36.4% (4/11), and the only transferable embryo failed to implant. In summary, the total fertilization rate of the six cases was 51.5% (34/66), with two pregnancies ending in abortion, and five embryos remaining. Both motile ejaculated and testicular sperm showed the potential for fertilization and embryonic development but the immotile ejaculated sperm did not. However, we had a small number of cases; thus, a definitive conclusion is not possible.
We identified seven reports on 24 males that referred to ICSI outcomes from males with DFS/MMAF.2,3,6,7,8,9,10 The immotile spermatozoa showed a viability of 15%–57%. The fertilization rate in their data varied from 38.9% to 75%, clinical pregnancy occurred in 54.2% (13/24) of the couples, and 11 offspring were delivered. Both ejaculated immotile sperm and testicular sperm achieved fertilization and successful pregnancies in some reports,3,8 but not all.9
Selecting live sperm when no motile sperm is present was crucial for successful ICSI. The HOS test was valuable to identify live sperm but offered limited information in MMAF men with such severely distorted sperm tails. We used the HOS test in case 4; however, judging swollen sperm tails was confusing. Testicular sperm was better than immotile ejaculated sperm and has been recommended for use rather than completely immotile ejaculated sperm.11 In our observation, the viability of MMAF spermatozoa varies from 9% to 80%.5 A randomly selected immotile ejaculated spermatozoon may have a high risk of being dead, which would lead to a disappointing ICSI outcome. McLachlan et al.3 used testicular sperm from a man with MMAF and achieved a fertilization rate of 66.7% as well as a successful delivery.
In conclusion, ICSI technique should be used to fertilize the oocytes in patients with MMAF, although the fertilization rate is variable and depends on the presence of live sperm. In our observations, testicular sperm is an alternative when motile sperm were unavailable.
AUTHOR CONTRIBUTIONS
SMY designed the study, and participated in semen analysis, and drafted the manuscript. XYY participated in the design of the study. YD, HL, WW and JYL carried out the artificial reproduction to the affected couples. DGW participated in the design of the study and revised the article critically for important intellectual content. All authors have read and approved the final manuscript.
COMPETING FINANCIAL INTERESTS
All authors declare no competing financial interests.
ACKNOWLEDGMENTS
This study was funded by the Suzhou Youth Project of Science and Education for Medicine (KJXW2013025) from the Suzhou Municipal Bureau of Health.
REFERENCES
- 1.Chemes HE, Alvarez SC. Tales of the tail and sperm head aches: changing concepts on the prognostic significance of sperm pathologies affecting the head, neck and tail. Asian J Androl. 2012;14:14–23. doi: 10.1038/aja.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stalf T, Sanchez R, Kohn FM, Schalles U, Kleinstein J, et al. Pregnancy and birth after intracytoplasmic sperm injection with spermatozoa from a patient with tail stump syndrome. Hum Reprod. 1995;10:2112–4. doi: 10.1093/oxfordjournals.humrep.a136244. [DOI] [PubMed] [Google Scholar]
- 3.McLachlan RI, Ishikawa T, Osianlis T, Robinson P, Merriner DJ, et al. Normal live birth after testicular sperm extraction and intracytoplasmic sperm injection in variant primary ciliary dyskinesia with completely immotile sperm and structurally abnormal sperm tails. Fertil Steril. 2012;97:313–8. doi: 10.1016/j.fertnstert.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Ben KM, Coutton C, Zouari R, Karaouzene T, Rendu J, et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet. 2014;94:95–104. doi: 10.1016/j.ajhg.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang SM, Li HB, Wang JX, Shi YC, Cheng HB, et al. Morphological characteristics and initial genetic study of multiple morphological anomalies of the flagella in China. Asian J Androl. 2015;17:513–5. doi: 10.4103/1008-682X.146100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olmedo SB, Nodar F, Chillik C, Chemes HE. Successful intracytoplasmic sperm injection with spermatozoa from a patient with dysplasia of the fibrous sheath and chronic respiratory disease. Hum Reprod. 1997;12:1497–9. doi: 10.1093/humrep/12.7.1497. [DOI] [PubMed] [Google Scholar]
- 7.Favero R, Rizzo F, Baccetti B, Piomboni P. Embryo development, pregnancy and twin delivery after microinjection of ‘stump’ spermatozoa. Andrologia. 1999;31:335–8. doi: 10.1046/j.1439-0272.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- 8.Olmedo SB, Rawe VY, Nodar FN, Galaverna GD, Acosta AA, et al. Pregnancies established through intracytoplasmic sperm injection (ICSI) using spermatozoa with dysplasia of fibrous sheath. Asian J Androl. 2000;2:125–30. [PubMed] [Google Scholar]
- 9.Ravel C, Chantot-Bastaraud S, Siffroi JP, Escalier D, Antoine JM, et al. Tail stump syndrome associated with chromosomal translocation in two brothers attempting intracytoplasmic sperm injection. Fertil Steril. 2006;86:719.e1–7. doi: 10.1016/j.fertnstert.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell V, Rives N, Albert M, Peers MC, Selva J, et al. Outcome of ICSI with ejaculated spermatozoa in a series of men with distinct ultrastructural flagellar abnormalities. Hum Reprod. 2006;21:2065–74. doi: 10.1093/humrep/del130. [DOI] [PubMed] [Google Scholar]
- 11.Shulman A, Feldman B, Madgar I, Levron J, Mashiach S, et al. In-vitro fertilization treatment for severe male factor: the fertilization potential of immotile spermatozoa obtained by testicular extraction. Hum Reprod. 1999;14:749–52. doi: 10.1093/humrep/14.3.749. [DOI] [PubMed] [Google Scholar]


