Abstract
CTLA-4 is a critical inhibitory “checkpoint” molecule of immune activation. Several recent reports have described patients with immune dysregulation and lymphoproliferative disease resulting from 2 different genetic diseases that directly or indirectly cause CTLA-4 deficiency. Numerous articles have also been published describing CTLA-4 blockade in cancer immunotherapy and its side effects, which are ultimately the consequence of treatment-induced CTLA-4 deficiency. Here, we review these 2 diseases and CTLA-4 blockade therapy, emphasizing the crucial role of CTLA-4 in immune checkpoint regulation.
Introduction
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is a critical and potent inhibitor of T-cell proliferation that serves as a “checkpoint” of immune responses.1 The essential role of CTLA-4 in lymphocyte homeostasis and tolerance is illustrated by Ctla4 knockout mice, which develop rapidly fatal destructive multiorgan lymphocytic infiltration.2,3 CTLA4 recessive disease has not yet been reported in humans. However, disease because of partial loss of CTLA-4 was recently described.4,5 “CTLA-4 haploinsufficiency with autoimmune infiltration” (CHAI) patients have heterozygous loss-of-function mutations in CTLA-4 and develop lymphocytic infiltration of multiple nonlymphoid organs similar to Ctla4 knockout mice. Additionally, prior reports have described patients clinically resembling CHAI disease, but with recessive inheritance. These patients harbor biallelic mutations in the “lipopolysaccharide-responsive vesicle trafficking, beach- and anchor-containing” (LRBA) gene rather than in CTLA4.6-14 However, we discovered that LRBA deficiency causes a secondary loss of CTLA-4 by affecting the internal trafficking of this protein, thus elucidating the underlying molecular link between these 2 similar diseases.12 We call this recessive disease “LRBA deficiency with autoantibodies, regulatory T (Treg) cell defects, autoimmune infiltration, and enteropathy” (LATAIE), which emphasizes the most prominent features of this disorder. Here, we review CHAI and LATAIE disease, emphasizing their shared mechanism of autoimmunity, and discuss potential treatment strategies. We also discuss CTLA-4 blockade in cancer immunotherapy, which may induce clinical symptoms of CHAI and LATAIE disease.
Clinical manifestations
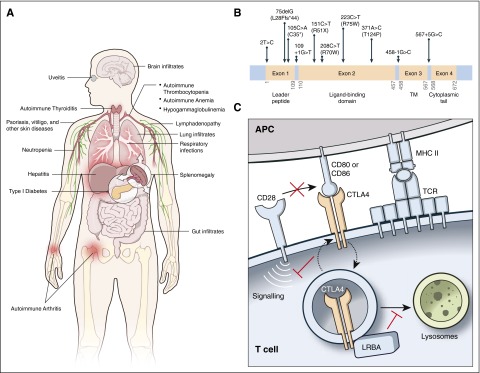
Patients with CHAI and LATAIE present with autoantibody-mediated cytopenias, lymphadenopathy/splenomegaly, hypogammaglobulinemia, organ-specific autoimmunity, and lymphocytic infiltration of nonlymphoid organs. Although features of CHAI and LATAIE are similar, a notable difference is the typically earlier age of onset with LATAIE, where disease onset is often apparent in preschool-age children, whereas CHAI presents in older children or young adults. The autoantibody-mediated cytopenias (ie, autoimmune hemolytic anemia, autoimmune thrombocytopenia, and neutropenia), lymphadenopathy, and splenomegaly resemble the autoimmune lymphoproliferative syndrome (ALPS) in many patients.15 Table 1 and Figure 1A summarize the common clinical features of CHAI and LATAIE disease and their frequencies in published reports. The clinical phenotypes for LATAIE patients have been comprehensively reviewed, and their frequencies are comparable to that which we report here.17,18 Notably, frequencies of different clinical features and organ involvement vary greatly between different clinical cohorts. Patients with hypogammaglobulinemia, often diagnosed as common variable immune deficiency, have increased infections, especially of the respiratory tract. The frequency of patients with interstitial lung disease may potentially be higher for LATAIE disease in which patients are frequently described with chronic lung disease and/or digital clubbing, but interstitial lung disease was not always documented.6,7,16 Furthermore, disease penetrance can vary. Penetrance for CHAI disease is incomplete, with an estimated 40% of CTLA4 mutation-positive family members appearing clinically healthy.5 By contrast, LATAIE disease shows nearly complete penetrance, with only 1 clinically healthy LRBA mutation-positive individual reported.11 Despite near universal penetrance, the severity of LATAIE disease varies substantially, even within individual kindreds.
Table 1.
Characteristic features of CHAI and LATAIE disease
| Patient | Mutation | HGG | AIHA | ITP | LAD | Splenomegaly | ILD | Enteropathy | Brain lesions | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| CHAI | ||||||||||
| P1 | p.R51* | + | + | + | 4 | |||||
| P2 | p.R51* | + | + | + | + | + | 4 | |||
| P3 | p.L28Ffs*44 | + | + | + | + | + | + | + | 4 | |
| P4 | Splice site, c.458-1G>C | + | + | + | + | + | 4 | |||
| P5 | Splice site, c.567+5G>C | + | + | + | + | + | + | 4 | ||
| P6 | Splice site, c.567+5G>C | + | + | + | + | + | + | 4 | ||
| P7 | p.C35* | + | + | + | 5 | |||||
| P8 | p.C35* | + | + | + | 5 | |||||
| P9 | p.C35* | + | + | + | + | 5 | ||||
| P10 | p.C35* | + | + | + | + | 5 | ||||
| P11 | p.C35* | + | + | + | + | + | + | 5 | ||
| P12 | Splice site, c.110+1G>T | ND | ND | + | 5 | |||||
| P13 | Splice site, c.110+1G>T | ND | ND | ND | ND | + | 5 | |||
| P14 | Splice site, c.110+1G>T | + | + | 5 | ||||||
| P15 | Splice site, c.110+1G>T | + | + | 5 | ||||||
| P16 | p.R70W | + | + | + | + | + | + | 5 | ||
| P17 | p.R70W | + | ND | 5 | ||||||
| P18 | p.T124P | + | + | + | + | + | 5 | |||
| P19 | p.R75W | + | + | + | + | + | + | + | 5 | |
| P20 | Start codon, c.2T>C | + | + | + | + | 5 | ||||
| P21 | p.L28Sfs*40 | + | + | 16 | ||||||
| LATAIE | ||||||||||
| P1 | p.I2657S | + | + | + | + | 6 | ||||
| P2 | p.I2657S | + | + | 6 | ||||||
| P3 | p.R1683* | + | + | + | + | + | + | + | 6 | |
| P4 | p.E59* | + | + | + | + | + | 6 | |||
| P5 | Exon 1-2 deletion | + | + | 6 | ||||||
| P6 | p.E2219Dfs*3 | + | + | + | 7 | |||||
| P7 | p.E2219Dfs*3 | + | 7 | |||||||
| P8 | p.E2219Dfs*3 | + | + | 7 | ||||||
| P9 | p.E2219Dfs*3 | + | + | + | + | 7 | ||||
| P10 | p.E2219Dfs*3 | + | + | 7 | ||||||
| P11 | Exons 1-30 deletion | + | + | + | + | + | + | 8 | ||
| P12 | p.T2388Pfs*7 | + | + | + | + | 10 | ||||
| P13 | p.T2388Pfs*7 | + | + | + | 10 | |||||
| P14 | p.Q678* | + | + | 9 | ||||||
| P15 | p.C289Cfs*3 | + | + | + | + | + | 9 | |||
| P16 | p.C289Cfs*3 | + | + | + | + | 9 | ||||
| P17 | p.I2657S | + | + | + | + | + | 11 | |||
| P18 | p.S2713fs | + | + | + | + | 11 | ||||
| P19 | p.S2713fs | + | + | + | + | 11 | ||||
| P20 | p.R1445Q | + | + | + | + | + | + | + | 12 | |
| P21 | p.R1445Q | + | + | + | + | + | 12 | |||
| P22 | p.K175*; T1587fs | + | + | + | + | + | + | 12 | ||
| P23 | p.R1445* | + | + | + | + | + | 12 | |||
| P24 | p.S1233F; Q2506* | + | + | + | + | 12 | ||||
| P25 | p.V2249F; L1834P | + | + | + | + | + | + | + | 12 | |
| P26 | p.R655* | + | + | + | + | + | + | + | 12 | |
| P27 | p.D248G; c.8502-1G>C | + | + | + | 12 | |||||
| P28 | p.D1329fs | + | 12 | |||||||
| Frequency (%) | ||||||||||
| In CHAI | 75 | 38 | 43 | 40 | 53 | 63 | 81 | 20 | ||
| In LATAIE | 57 | 54 | 64 | 50 | 57 | 43 | 71 | 11 | ||
CTLA4 mutations in CHAI patients are heterozygous. LRBA mutations in LATAIE patients are homozygous or compound heterozygous.
AIHA, autoimmune hemolytic anemia; HGG, hypogammaglobulinemia; ILD, interstitial lung disease; ITP, immune thrombocytopenia; LAD, lymphadenopathy; ND, not determined; P, patient.
Figure 1.
CHAI and LATAIE disease phenotype and mechanism. (A) Clinical features of CHAI and LATAIE disease. (B) Schematic of the CTLA4 exons showing the mutations in CHAI patients. TM, transmembrane domain. A schematic displaying LRBA mutations causing LATAIE can be found in Lo et al,12 Alkhairy et al,17 and Gámez-Díaz et al.18 (C) Model depicting the function of CTLA-4 and its regulation by LRBA.
Despite varying clinical manifestations, most CHAI and LATAIE patients have lymphocytic overactivation and infiltration of at least 1 nonlymphoid organ, usually the intestine, lungs, or brain. For both diseases, intestinal involvement, leading to enteropathy, is most common. The lungs are the second most frequently infiltrated organ, and infiltrates in the brain are less common. In our experience, lymphocytic infiltrates in the brain seem to cause disease mainly by edema and compression rather than direct tissue destruction as seen in multiple sclerosis.
Importantly, there are some LATAIE patients that present only with ALPS-like disease and no other autoimmune manifestations or lymphocytic infiltration.11 These mutations may retain residual LRBA function and/or protein expression leading to a milder phenotype.
In addition to the ALPS-like disorder and lymphocytic infiltration of gut, lungs, and/or brain, some CHAI and LATAIE patients have other autoimmune manifestations or infiltration of other organs (ie, kidney, liver, or bone marrow) less frequently. Autoimmune disorders reported include type 1 diabetes, autoimmune thyroiditis, arthritis, psoriasis or other skin disorders, uveitis, vitiligo, and myasthenia gravis. These additional autoimmune disorders appear to be more common in LATAIE than CHAI disease.
Genetic mutation spectrum
CHAI and LATAIE disease are caused by loss-of-function mutations in CTLA4 and LRBA, respectively. CTLA-4 is a single-pass type I transmembrane cell surface receptor of 223 amino acids (∼25 kDa). The majority of CTLA4 reported mutations causing CHAI resulted in loss of protein expression and include insertions/deletions (frameshift), nonsense, missense, splicing mutations, and 1 substitution mutation disrupting the start codon (Figure 1B).4,5,16 Two mutations led to alternative splicing, which removed the transmembrane domain resulting in soluble CTLA-4 rather than the more functional membrane-bound form (Figure 1B).4 Two missense mutations appeared to interfere with ligand binding.5 LRBA is an enormous protein (2863 amino acids, ∼319 kDa) with multiple lipid and protein interaction domains. Biallelic LRBA mutations in LATAIE disease include frameshift (insertions/deletions), nonsense, missense, splice site mutations, and large multiexon deletions.6-14,18 All mutations decrease LRBA protein levels, and missense mutations that permit residual LRBA protein expression cause milder clinical disease.12 Thus, the common mechanism shared by these 2 disorders is that the mutations, whether directly in the CTLA4 gene or in its regulator LRBA, lead to deficient CTLA-4 surface expression or function.
Pathogenesis
CTLA-4 is a crucial T-cell inhibitory receptor. It restrains immune responses by negative signaling or competing with its homolog CD28, the principal T-cell costimulatory molecule, critical for inducing maximal T-cell proliferation.19 CD28 and CTLA-4 compete for the same ligands, CD80 and CD86, on the surface of antigen-presenting cells (Figure 1C). Moreover, CTLA-4 binds CD80 and CD86 with significantly higher affinity and avidity than CD28 and outcompetes CD28 for its ligands. CTLA-4 can also strip these ligands from antigen-presenting cells by transendocytosis, via which the ligands are internalized and degraded in the T cell.20-22 CD28 is expressed constitutively on all naïve and most resting T cells; however, CTLA-4 is expressed only after activation in conventional T cells while it is constitutively expressed on Tregs.23-25 Thus, Tregs are the chief mediator of CTLA-4 inhibitory function. Most CTLA-4 is stored in recycling endosomes, which cycle to the cell surface following T-cell activation.26,27 This allows CTLA-4 to spatially and temporally control T-cell responses. The multiorgan lymphocytic infiltrative disease in Ctla4 knockout mice and CTLA-4 haploinsufficient (CHAI) patients illustrates the importance of CTLA-4 in restraining T-cell responses. In mice with complete germ-line deficiency of CTLA-4, unrestrained T-cell expansion causes death at 4 weeks old,2,3 whereas the loss of CTLA-4 selectively on Treg cells leads to fatal lymphoproliferation by ∼7 weeks of age.28 In CHAI patients, compromising a single allele can predispose them to severe infiltrative T lymphoproliferative disease, with the onset of disease varying among patients.4,5 Thus, CTLA-4 performs vital quantitative regulation of T lymphocyte expansion.
LATAIE patients have lymphocytic infiltrative disease resembling that of CHAI patients, and we now understand why.12 LRBA is a member of a gene family involved in vesicle trafficking,29,30 and we found that LRBA regulates CTLA-4 turnover in endosomes (Figure 1C).12 LRBA helps maintain an intracellular vesicular pool of CTLA-4 for immediate mobilization to the cell surface as needed.12 Loss of LRBA leads to the rapid shuttling of CTLA-4 vesicles to lysosomes for degradation. We found that inhibiting lysosomal degradation with the drug chloroquine or other inhibitors of the lysosome rescued CTLA-4 loss in vitro.12 Interestingly, CTLA-4 degradation because of defective LRBA in LATAIE patients can lead to lower total overall levels of CTLA-4 than seen in CHAI, which may explain why disease is more penetrant in LATAIE than in CHAI patients.
In both CHAI and LATAIE disease, hypogammaglobulinemia and low circulating B-cell numbers are observed. However, because optimal humoral immunity depends on CD28 signaling and T-cell help, CTLA-4 loss might have been expected to augment antibody levels and B-cell numbers as seen in knockout mouse models.2,31-33 Instead, patient B-cell numbers and antibody levels progressively decline over time. These findings appear to be explained, at least in part, by a corresponding increase in CD21lo B cells, which have previously been described as “exhausted,” characterized by a state of functional unresponsiveness following persistent activation.4,34,35 Thus, the heightened T-cell help and chronic B-cell stimulation because of CTLA-4 loss ultimately compromises B-cell function. Although B-cell abnormalities in LATAIE patients resemble that found in CHAI patients, indicating CTLA-4 loss as the culprit, LRBA is expressed in B cells and other non-CTLA-4–expressing cell types and, therefore, may also have yet undiscovered CTLA-4–independent cellular effects.
Therapeutic considerations
Since both CHAI and LATAIE disease result from a functional loss of CTLA-4, drugs targeting the CD28/CTLA-4 pathway or Tregs, which constitutively express CTLA-4, could be effective treatments for either disease. Abatacept, a CTLA-4–immunoglobulin fusion drug, which may act as a pharmacologic “replacement” of CTLA-4, has achieved substantial and lasting improvement of interstitial lung disease in LATAIE patients.12 Abatacept has also been reported to mitigate autoimmune symptoms in a CHAI patient.36 CTLA-4 is critical for the proper immunosuppressive function of Tregs, which preserve immune homeostasis and tolerance.28 Sirolimus, a widely used mechanistic target of rapamycin inhibitor, suppresses conventional T-cell responses but allows Tregs to preferentially expand and maintain their suppressive function because they resist its inhibitory effects.37-40 Accordingly, sirolimus has been reported to alleviate autoimmune symptoms in some CHAI patients.4 In our unpublished experience, sirolimus has also been observed to benefit some LATAIE patients, though not all. A variety of other immunosuppressive drugs have been used for treating CHAI and LATAIE with varying results, and those for LATAIE patients have been briefly reviewed by Gámez-Díaz et al.18
Chloroquine, a lysosomal inhibitor drug, was found to augment CTLA-4 levels in vitro in Treg or activated T cells, especially for LRBA-deficient cells.12 Therefore, chloroquine or hydroxychloroquine, a more widely used derivative, warrant further study of their utility as a therapy for CTLA-4–deficiency diseases. Although chloroquine/hydroxychloroquine are most widely used as antimalarials, they have also shown efficacy in treating rheumatoid arthritis and lupus.41-43 It would be interesting to investigate whether the latter may be attributable to CTLA-4.
Another therapeutic strategy for CHAI and LATAIE patients has been hematopoietic stem cell transplantation (HSCT). At least 8 CHAI and 8 LATAIE patients have undergone HSCTs.10-13,18,44,45 Eleven of the transplants were successful, although 1 died 2.5 years post-HSCT because of diabetic ketoacidosis. Three other transplants resulted in death shortly after transplant, and 2 outcomes have yet to be reported. Four of the CHAI patients experienced graft-versus-host disease with 1 being fatal. Thus, aggressive graft-versus-host disease prophylaxis may be recommended for future patients. Overall, HSCT may be an effective treatment option, but because of its mortality risk and the variability of patient phenotypes, it should perhaps be reserved for more severe cases.
CTLA-4 blockade in cancer immunotherapy
Immune checkpoint therapy has shown considerable promise in enhancing antitumor responses that improve clinical outcome for cancer patients.1 However, the severe clinical phenotypes of CHAI and LATAIE patients underscore the importance of these negative regulatory molecules in preventing autoimmunity, lymphoproliferation, and unnecessary tissue damage in humans. Ipilimumab, a monoclonal antibody directed against CTLA-4, was recently approved by the US Food and Drug Administration in 2011 for the treatment of metastatic melanoma. This approval was the result of multiple late-phase clinical trials demonstrating that ipilimumab significantly increased the mean and long-term survival of patients with advanced melanoma.46,47 Notably, approximately two-thirds of patients treated with ipilimumab experienced immune-related adverse events associated with CTLA-4 blockade. Similar to individuals with genetic CTLA-4 deficiency, these immunotoxicities often affected the gastrointestinal tract with approximately one-third of ipilimumab-treated patients having diarrhea or colitis. CTLA-4 blockade in melanoma patients also commonly caused skin-related disorders associated with increased lymphocytic infiltration and melanocyte apoptosis, which differs from those afflicted with CHAI and LATAIE disease.46,48 These side effects were reported to be successfully managed with systemic corticosteroids and/or anti–tumor necrosis factor α antibody (for diarrhea or colitis).46 Depending on the length of CTLA-4 blockade treatment, other organ-specific pathologies or autoantibody-mediated disorders observed in CHAI and LATAIE disease may emerge. In the investigations thus far, the survival benefits for advanced-stage cancer patients outweigh the risks because the immune-related adverse effects have generally not been life-threatening.
Conclusion
Although the immune dysregulation disorders CHAI and LATAIE are caused by mutations in 2 different genes, they share a common underlying etiology, the functional loss of the critical immune regulatory molecule CTLA-4. Thus, they share similar clinical phenotypes, and the same treatment strategies may be effective for either disease. CTLA-4 blockade in cancer immunotherapy can result in adverse events that resemble the autoimmune phenomena of CHAI and LATAIE disease. Overall, these 2 genetic diseases and the autoimmune adverse events of CTLA-4 blockade therapy illustrate the crucial role of CTLA-4 in suppressing autoimmunity and lymphoproliferation in humans.
Acknowledgments
The authors thank Juan Ravell, Yu Zhang, Tom Fleisher, Steve Holland, and V. Koneti Rao for valuable advice and assistance, as well as Ryan Kissinger for his talented assistance in generating the figure illustrations.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Authorship
Contribution: B.L., J.M.F., H.C.S., G.U., M.B.J., and M.J.L. wrote, reviewed, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael J. Lenardo, Laboratory of Immunology, 10 Center Dr, Building 10, Room 11D14, Bethesda, MD 20892; e-mail: lenardo@nih.gov; and Bernice Lo, Division of Translational Medicine, Sidra Medical and Research Center, P.O. Box 26999, Doha, Qatar; e-mail: blo@sidra.org.
References
- 1.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 2.Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 3.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 4.Kuehn HS, Ouyang W, Lo B, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345(6204):1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubert D, Bode C, Kenefeck R, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20(12):1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Herrera G, Tampella G, Pan-Hammarström Q, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90(6):986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alangari A, Alsultan A, Adly N, et al. LPS-responsive beige-like anchor (LRBA) gene mutation in a family with inflammatory bowel disease and combined immunodeficiency. J Allergy Clin Immunol. 2012;130(2):481–488. doi: 10.1016/j.jaci.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burns SO, Zenner HL, Plagnol V, et al. LRBA gene deletion in a patient presenting with autoimmunity without hypogammaglobulinemia. J Allergy Clin Immunol. 2012;130(6):1428–1432. doi: 10.1016/j.jaci.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charbonnier LM, Janssen E, Chou J, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol. 2015;135(1):217–227. doi: 10.1016/j.jaci.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidel MG, Hirschmugl T, Gamez-Diaz L, et al. Long-term remission after allogeneic hematopoietic stem cell transplantation in LPS-responsive beige-like anchor (LRBA) deficiency. J Allergy Clin Immunol. 2015;135(5):1384–1390. doi: 10.1016/j.jaci.2014.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Revel-Vilk S, Fischer U, Keller B, et al. Autoimmune lymphoproliferative syndrome-like disease in patients with LRBA mutation. Clin Immunol. 2015;159(1):84–92. doi: 10.1016/j.clim.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Lo B, Zhang K, Lu W, et al. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349(6246):436–440. doi: 10.1126/science.aaa1663. [DOI] [PubMed] [Google Scholar]
- 13.Sari S, Dogu F, Hwa V, et al. A successful HSCT in a girl with novel LRBA mutation with refractory celiac disease. J Clin Immunol. 2016;36(1):8–11. doi: 10.1007/s10875-015-0220-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiner F, Plamper M, Dueker G, et al. Infancy-onset T1DM, short stature, and severe immunodysregulation in two siblings with a homozygous LRBA mutation. J Clin Endocrinol Metab. 2016;101(3):898–904. doi: 10.1210/jc.2015-3382. [DOI] [PubMed] [Google Scholar]
- 15.Price S, Shaw PA, Seitz A, et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123(13):1989–1999. doi: 10.1182/blood-2013-10-535393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayakawa S, Okada S, Tsumura M, et al. A patient with CTLA-4 haploinsufficiency presenting gastric cancer. J Clin Immunol. 2016;36(1):28–32. doi: 10.1007/s10875-015-0221-x. [DOI] [PubMed] [Google Scholar]
- 17.Alkhairy OK, Abolhassani H, Rezaei N, et al. Spectrum of phenotypes associated with mutations in LRBA. J Clin Immunol. 2016;36(1):33–45. doi: 10.1007/s10875-015-0224-7. [DOI] [PubMed] [Google Scholar]
- 18.Gámez-Díaz L, August D, Stepensky P, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. 2016;137(1):223–230. doi: 10.1016/j.jaci.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. 2011;32(9):428–433. doi: 10.1016/j.it.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 20.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11(12):852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 21.Collins AV, Brodie DW, Gilbert RJ, et al. The interaction properties of costimulatory molecules revisited. Immunity. 2002;17(2):201–210. doi: 10.1016/s1074-7613(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 22.Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332(6029):600–603. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149(2):380–388. [PubMed] [Google Scholar]
- 24.Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4(6):535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 25.Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mead KI, Zheng Y, Manzotti CN, et al. Exocytosis of CTLA-4 is dependent on phospholipase D and ADP ribosylation factor-1 and stimulated during activation of regulatory T cells. J Immunol. 2005;174(8):4803–4811. doi: 10.4049/jimmunol.174.8.4803. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi OS, Kaur S, Hou TZ, et al. Constitutive clathrin-mediated endocytosis of CTLA-4 persists during T cell activation. J Biol Chem. 2012;287(12):9429–9440. doi: 10.1074/jbc.M111.304329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wing K, Onishi Y, Prieto-Martin P, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 29.Wang JW, Howson J, Haller E, Kerr WG. Identification of a novel lipopolysaccharide-inducible gene with key features of both A kinase anchor proteins and chs1/beige proteins. J Immunol. 2001;166(7):4586–4595. doi: 10.4049/jimmunol.166.7.4586. [DOI] [PubMed] [Google Scholar]
- 30.de Souza N, Vallier LG, Fares H, Greenwald I. SEL-2, the C. elegans neurobeachin/LRBA homolog, is a negative regulator of lin-12/Notch activity and affects endosomal traffic in polarized epithelial cells. Development. 2007;134(4):691–702. doi: 10.1242/dev.02767. [DOI] [PubMed] [Google Scholar]
- 31.Wang CJ, Heuts F, Ovcinnikovs V, et al. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci USA. 2015;112(2):524–529. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wing JB, Ise W, Kurosaki T, Sakaguchi S. Regulatory T cells control antigen-specific expansion of Tfh cell number and humoral immune responses via the coreceptor CTLA-4. Immunity. 2014;41(6):1013–1025. doi: 10.1016/j.immuni.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Sage PT, Paterson AM, Lovitch SB, Sharpe AH. The coinhibitory receptor CTLA-4 controls B cell responses by modulating T follicular helper, T follicular regulatory, and T regulatory cells. Immunity. 2014;41(6):1026–1039. doi: 10.1016/j.immuni.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205(8):1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isnardi I, Ng YS, Menard L, et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010;115(24):5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee S, Moon JS, Lee CR, et al. Abatacept alleviates severe autoimmune symptoms in a patient carrying a de novo variant in CTLA-4. J Allergy Clin Immunol. 2016;137(1):327–330. doi: 10.1016/j.jaci.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 37.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177(12):8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 38.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 39.Hippen KL, Merkel SC, Schirm DK, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3(83):83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111(1):453–462. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The HERA Study Group. A randomized trial of hydroxychloroquine in early rheumatoid arthritis: the HERA Study. Am J Med. 1995;98(2):156–168. doi: 10.1016/s0002-9343(99)80399-4. [DOI] [PubMed] [Google Scholar]
- 42.Clegg DO, Dietz F, Duffy J, et al. Safety and efficacy of hydroxychloroquine as maintenance therapy for rheumatoid arthritis after combination therapy with methotrexate and hydroxychloroquine. J Rheumatol. 1997;24(10):1896–1902. [PubMed] [Google Scholar]
- 43.Costedoat-Chalumeau N, Dunogué B, Morel N, Le Guern V, Guettrot-Imbert G. Hydroxychloroquine: a multifaceted treatment in lupus. Presse Med. 2014;43(6, pt 2):e167–e180. doi: 10.1016/j.lpm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Tesi B, Priftakis P, Lindgren F, et al. Successful hematopoietic stem cell transplantation in a patient with LPS-responsive beige-like anchor (LRBA) gene mutation. J Clin Immunol. 2016;36(5):480–489. doi: 10.1007/s10875-016-0289-y. [DOI] [PubMed] [Google Scholar]
- 45.Slatter MA, Engelhardt KR, Burroughs LM, et al. Hematopoietic stem cell transplantation for CTLA4 deficiency [published online ahead of print April 18, 2016]. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2016.01.045. doi:10.1016/j.jaci.2016.01.045. [DOI] [PubMed] [Google Scholar]
- 46.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 48.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]