Abstract
Ten percent of deaths worldwide are due to trauma, and it is the third most common cause of death in the United States. Despite a profound upregulation in procoagulant mechanisms, one-quarter of trauma patients present with laboratory-based evidence of trauma-induced coagulopathy (TIC), which is associated with poorer outcomes including increased mortality. The most common causes of death after trauma are hemorrhage and traumatic brain injury (TBI). The management of TIC has significant implications in both because many hemorrhagic deaths could be preventable, and TIC is associated with progression of intracranial injury after TBI. This review covers the most recent evidence and advances in our understanding of TIC, including the role of platelet dysfunction, endothelial activation, and fibrinolysis. Trauma induces a plethora of biochemical and physiologic changes, and despite numerous studies reporting differences in coagulation parameters between trauma patients and uninjured controls, it is unclear whether some of these differences may be “normal” after trauma. Comparisons between trauma patients with differing outcomes and use of animal studies have shed some light on this issue, but much of the data continue to be correlative with causative links lacking. In particular, there are little data linking the laboratory-based abnormalities with true clinically evident coagulopathic bleeding. For these reasons, TIC continues to be a significant diagnostic and therapeutic challenge.
Introduction
Injury is the third leading cause of death in the United States and is the leading cause of death in those aged 44 years or younger.1 Ten percent of deaths worldwide are due to injury,2 greater than that of HIV, tuberculosis, and malaria combined.3 In contrast to other causes of trauma death such as traumatic brain injury (TBI), sepsis, and multiple organ failure, exsanguination occurs rapidly (median 2-3 hours after presentation) and accounts for roughly half of trauma deaths.4,5
Despite a profound upregulation in procoagulant mechanisms and increased thrombin-generating potential after injury,6 at least one-quarter of civilian trauma patients7 and one-third of military trauma patients8 will present with a laboratory-defined coagulopathy. In 1969, Simmons et al were the first to report a relationship between shock and prolonged prothrombin time (PT) and partial thromboplastin time (PTT) in combat trauma patients during the Vietnam War.9 Since that time, research efforts have focused on a distinct posttraumatic coagulopathy independent from classic sequelae of iatrogenic resuscitation injury such as hemodilution and hypothermia. This trauma-induced coagulopathy (TIC) is associated with increased transfusion requirements, risk of complications, and mortality.7,8 The majority of posttraumatic bleeding is noncoagulopathic: bleeding from arteries and veins which can be controlled with compression, embolization, or suture repair/ligation. Conversely, coagulopathic bleeding is unusual and represents a failure to form hemostatic clots even at the level of the capillary bed, resulting in diffuse bleeding involving uninjured sites and is extremely difficult to stop with mechanical interventions. However, the relationship between the laboratory-based abnormalities on which current research efforts are focused and clinically evident coagulopathic bleeding is unclear. Although progress is being made on understanding the mechanisms of TIC (the focus of this review), it continues to be a significant diagnostic and therapeutic challenge.
Mechanisms of trauma-induced coagulopathy
Activated protein C
Activated protein C (APC) has been postulated as a major driver of TIC. Circulating protein C is activated by binding to endothelial protein C receptor in the presence of the thrombin-thrombomodulin complex and protein S. APC is anticoagulant (inactivating factors Va and VIIIa), profibrinolytic (inhibiting plasminogen activator inhibitor [PAI-1]), and cytoprotective (activating anti-inflammatory and antiapoptotic cell signaling pathways).
Early studies by Brohi et al found significant associations between systemic hypoperfusion, thrombomodulin activity, prolonged PT and PTT, fibrinolytic activity, and decreased levels of protein C.10,11 Although APC activity was not measured directly, the authors postulated that hypoperfusion upregulated thrombomodulin activity, which led to increased activation of protein C and subsequently increased coagulopathy and fibrinolysis. A single-center prospective study of 203 trauma patients by Cohen et al reported that coagulopathy and fibrinolysis were significantly correlated with elevated APC activity, which was only present with concomitant hypoperfusion (base deficit > 6) and severe injury (Injury Severity Score [ISS] > 15).12
These findings were subsequently corroborated by Cohen et al13 utilizing data from the Prospective, Observational, Multicenter, Major Trauma Transfusion (PROMMTT) study, which enrolled 1245 bleeding trauma patients from 10 major trauma centers.4 Analyzing blood samples from a subset of 165 patients who had coagulation factor levels obtained, the investigators found that the combination of increased injury severity and increased hypoperfusion was significantly associated with increased APC activity, prolonged PT and PTT, increased fibrinolysis, and depletion of factors I, II, V, VII, VIII, IX, and X. Significantly, decreases in factor V and VIII levels (those directed cleaved by APC) were most strongly correlated with severe injury and hypoperfusion, suggesting a critical role of APC in mediating TIC.
Although APC has been implicated as a contributor to TIC, the notion that APC is the primary driver of TIC has been challenged. Investigators have shown that isolated blunt TBI14,15 or pulmonary contusion16 are sufficient to cause coagulopathy in the absence of hypoperfusion, although these studies did not measure protein C levels or APC activity. Jansen et al17 and Burggraf et al18 described little correlation in trauma patients between degree of hypoperfusion and activity of multiple clotting factors including factors Va and VIIIa. Campbell et al studied the contribution of purified APC to TIC in vitro and found that physiologic concentrations of APC did not significantly deplete plasma or platelet-derived factor Va, and furthermore did not induce fibrinolysis in the presence or absence of tissue plasminogen activator (tPA), although the authors do acknowledge that there are significant ex vivo limitations to such experiments.19 Finally, Chapman et al found that overwhelming release of tPA, not degradation of PAI-1 (the purported mechanism of APC-mediated fibrinolysis), was the cause of hyperfibrinolysis in severely injured trauma patients.20
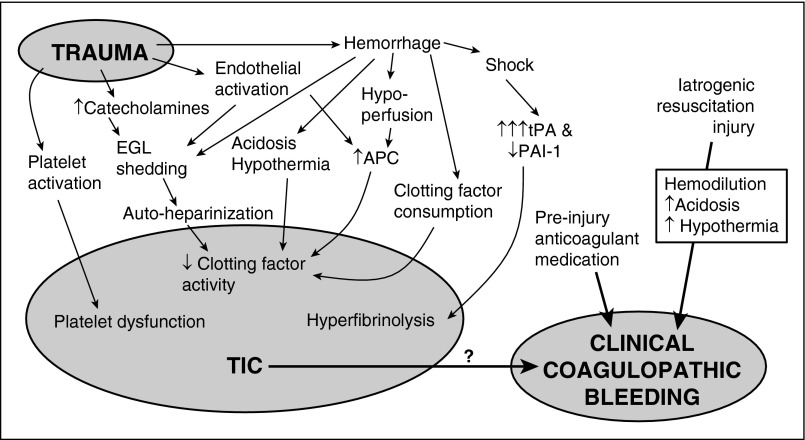
One of the most important observations in the last few years has been the sheer complexity of TIC (Figure 1) which in itself argues against the dominance of any single protein and instead supports the existence of multiple interrelated pathways (including iatrogenic injury) which converge into the TIC phenotype. A principal component analysis of thromboelastograms (TEGs) from 98 severely injured trauma patients identified at least 3 distinct patterns of coagulopathy: “global” coagulopathy with impairment of coagulation factor and platelet activities, hyperfibrinolysis, and a third pattern which may indicate endogenous anticoagulation from APC and endothelial factors.21 As TIC research proceeds, untangling its multiple interrelated pathways will not be straightforward.
Figure 1.
Schematic overview of TIC. Trauma induces a laboratory-evident coagulopathy through a variety of different pathways, which is likely modulated by baseline patient factors such as genetics and comorbidities. Unfortunately, few causative relationships are currently known. TIC is a separate entity from iatrogenic causes of coagulopathy including hemodilution and preinjury anticoagulant therapy. Currently, the literature only identifies TIC based on laboratory abnormalities, and its relationship to true clinical coagulopathic bleeding is unknown.
Role of the endothelium
The quiescent endothelium expresses many anticoagulant molecules on its surface including thrombomodulin, endothelial protein C receptors, and endothelial glycocalyx layer (EGL) components. Several mechanisms may induce activation of endothelial cells after trauma including vasoactive catecholamines, inflammatory mediators such as tumor necrosis factor-α, thrombin, and hypoxia.22 Although endothelial activation entails the upregulation of both procoagulant and anticoagulant mechanisms, the net result is a localized procoagulant milieu. After severe trauma and blood loss, however, the organism is faced with the competing interests of both limiting further blood loss and limiting microvascular thrombosis to maintain end-organ perfusion in a low-flow state. From a teleological perspective, it is possible that increased anticoagulation and fibrinolysis in the blood may serve to counterbalance the increased procoagulant properties of endothelial surfaces.23
As the interface between the endothelium and the blood, the EGL likely plays a key role. The EGL is a matrix of proteoglycans (syndecan-1, hyaluronic acid, heparan sulfate, and chondroitin sulfate) which projects into the lumen of the blood vessel from the endothelial surface.24 Loss of the EGL (“shedding”) can be quantified by a concurrent increase in circulating levels of EGL components, particularly syndecan-1.25 In addition to anticoagulant properties, the intact EGL appears instrumental in maintaining microvascular integrity: EGL injury leads to the extravasation of protein and fluid, leading to edema.26,27 EGL shedding has been detected in a variety of acute and chronic inflammatory states including trauma.28 The exact mechanisms are unclear but may involve several mediators25 including catecholamines.29,30
Of particular interest are 2 anticoagulant EGL components: chondroitin sulfate and heparan sulfate, which increase the efficiency of thrombomodulin31 and antithrombin III,32 respectively. Shedding of these components into the circulation may result in “autoheparinization” and contribute to TIC.33,34 Xu et al investigated the link between catecholamines and coagulopathy in a rat model of trauma and hemorrhage.35 The investigators found that inhibition of catecholamine secretion by chemical sympathectomy significantly reduced markers of endothelial injury (soluble thrombomodulin, syndecan-1) and inflammation (tumor necrosis factor-α, interleukin-6) compared with positive controls. Interestingly, markers for fibrinolysis (tPA, plasmin-antiplasmin [PAP] complex) were also decreased, but PT and PTT were similar.
The interaction of transfused plasma on the EGL and endothelium may partially explain the improved survival associated with plasma-based resuscitation of hemorrhagic shock.4,5,36 The mechanistic basis for this benefit is unclear, but given that plasma contains thousands of unique moieties, it is likely more than simple replacement of volume and clotting factors. For example, in vitro37-39 and animal studies40,41 demonstrate that, compared with crystalloid resuscitation, plasma resuscitation repairs and reverses hemorrhagic shock–induced EGL shedding and vascular permeability, although this finding has yet to be demonstrated in patients. Recently, the relationship between “endotheliopathy,” coagulopathy, and sympathoadrenal activation has also been investigated in 404 severely injured trauma patients: elevated circulating syndecan-1 (EGL shedding), vascular endothelial–cadherin (endothelial tight junction disruption), and epinephrine (sympathoadrenal activation), as well as low platelet count, high ISS, and prehospital packed red blood cell transfusions were independent predictors of hypocoagulability on admission TEG, whereas elevated circulating E-selectin (endothelial activation) and prehospital plasma transfusions predicted a more hypercoagulable TEG at presentation (Sisse R. Ostrowski, Hanne H. Henriksen, Jakob Stensballe, Mikkel Gybel-Brask, J.C.C., Lisa A. Baer, Bryan A. Cotton, J.B.H., C.E.W., and Pär I. Johansson, manuscript submitted May 2016). These data indicate a close relationship between the endothelium, coagulation, and sympathoadrenal axis, although mechanistic data continue to be an area of active investigation. Isolation of the specific moieties by which plasma confers EGL repair would be of tremendous therapeutic potential.
Platelet dysfunction
Activated platelets play a pivotal role in hemostasis, serving as a lipid-rich scaffold which brings all of the necessary proteolytic machinery in close proximity to generate a thrombin burst. Unsurprisingly, a lower platelet count is associated with increased bleeding, progression of intracranial hemorrhage, and mortality.42,43 However, it is clear that platelet dysfunction can occur when the platelet count is well within the reference range, preventing simple reliance on platelet count to guide therapy.44 Viscoelastic tests provide some information on platelet function, but the angle and maximum amplitude/clot firmness variables on TEG and rotational thromboelastometry (ROTEM) are also dependent on fibrinogen levels,45 and these tests are furthermore not effective at detecting inhibition by antiplatelet agents.46 Specialized assays including TEG platelet mapping (TEG-PM) and aggregometry modalities assay platelet function by measuring their response to various agonists. However, diagnostic cutoffs for pathologic platelet dysfunction after trauma have not been well established.
In 2012, Kutcher et al reported a platelet function study using multiple electrode aggregometry of preresuscitation blood samples from 101 trauma patients.44 The investigators found platelet “hypofunction” in response to at least 1 of 4 agonists (adenosine diphosphate [ADP], arachidonic acid [AA] thrombin receptor–activating peptide, and collagen) in 45% of trauma patients on admission and 90% of patients at some point during their intensive care unit stay when compared with healthy controls. Platelet hypofunction on admission was associated with nearly 10-fold increased mortality despite normal platelet counts; inhibition to AA and collagen stimulation predicted mortality. In contrast to this finding, a similar study by Solomon et al found that inhibition along the ADP and thrombin receptor–activating peptide pathways was associated with mortality.47 Although there are clear interspecies differences in coagulation between humans and other mammals, studies utilizing a rat model of isolated TBI15 and a porcine model of concomitant hemorrhage and TBI48 both demonstrated platelet inhibition to ADP stimulation within 15 minutes of injury. Finally, Sirajuddin et al recently reported on the apparent ubiquity of platelet dysfunction in a cohort of 459 minimally injured trauma patients (median ISS = 5).49 Patients in their study had lower platelet inhibition to ADP and AA stimulation (58% and 30%, respectively) than patients in other studies presenting with hemorrhagic shock (97% and 50%, respectively)50 or severe TBI (87% and 38%, respectively),51 but nevertheless had significant platelet inhibition compared with uninjured controls.
How should these data be interpreted? It is possible that platelet dysfunction is one of the earliest and most sensitive indicators of TIC. The specific mechanism is unknown but may involve “platelet exhaustion,” where platelets become activated en masse and are refractory to further stimulation for up to 24 hours afterward.52,53 However, because the minimally injured patients above49 had little evidence of bleeding (2% mortality and minimal transfusion requirements [Babak Sarani, George Washington University, electronic communication, May 17, 2016]) despite inhibition of platelet function on TEG-PM, the contribution of such platelet dysfunction to TIC is ambiguous. A recent study by Stalker et al investigating location-dependent differential platelet activation and organization may shine some light on this question.54 Through an elegant use of confocal intravital imaging in a mouse model of vascular injury, the investigators described a hemostatic plug consisting of a “core” region of tightly packed platelets surrounded by an outer “shell” region of more loosely packed platelets. Whereas platelets in the core region are isolated from the plasma and exposed to high levels of thrombin and collagen, the shell region is exposed to circulating plasma and grows by platelet-ADP interactions. Some degree of inhibition along the ADP pathway may therefore be normal after trauma to counterbalance widespread activation of procoagulant mechanisms; another finding supporting this hypothesis is that platelet inhibition along the ADP pathway increases clot sensitivity to tPA-mediated fibrinolysis.55 Alternatively, platelet assays may inherently select for more dysfunctional platelets because functional platelets were removed from the circulation and incorporated into clots.
Another open question is the role of platelet transfusions. Retrospective studies show an association between platelet transfusions and improved outcomes.56,57 The Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) study randomized 680 bleeding trauma patients to receive high or low ratios of plasma and platelets to packed red blood cells (1:1:1 vs 1:1:2). Although all-cause mortality was no different, the high plasma and platelet ratio (1:1:1) group had reduced risk of exsanguination (9% vs 15%) and improved achievement of clinical hemostasis (86% vs 78%) compared with the 1:1:2 group.5 The study design precluded estimation of the independent effects of plasma and platelet transfusion, however. The hypofunction of stored platelets also remains a concern with regard to platelet transfusion. Ponschab et al demonstrated that the platelet aggregation of in vitro–reconstituted whole blood (1:1:1 ratio) using 5-day-old apheresis platelets was below the lower limit as determined by blood samples from healthy volunteers.58 Although the authors call for monitoring of platelet function after platelet transfusions, our lack of understanding regarding what constitutes clinically pathologic platelet function after trauma makes interpretation of such results difficult. Given the presence of endogenous platelet hypofunction after even minimal trauma, it is possible that circulating factors in trauma patients may further decrease the function of transfused platelets. Further studies investigating the role of platelets in mediating TIC, as well as the role of platelet transfusions, are clearly warranted.
Fibrinogen, fibrin, and fibrinolysis
The cleavage of fibrinogen into fibrin and its polymerization into a fibrin mesh are the final steps of coagulation and are necessary to stabilize the platelet plug. Kornblith et al investigated the longitudinal fibrin and platelet contributions to clot strength by TEG maximum amplitude in 251 trauma patients.45 They found that the fibrin contribution to clot strength (31% at time of presentation, which increased to 44% by 72 hours) is much higher than that of uninjured controls at 20%.59 Lower fibrinogen contribution to MA and lower functional fibrinogen level on TEG were both associated with increased transfusions and mortality. The combination of low clot strength and low functional fibrinogen level was particularly lethal (26% mortality), which may be a target population who may benefit from early aggressive replacement of fibrinogen with fibrinogen concentrate (off-label in United States and parts of Europe) or cryoprecipitate. Interestingly, mortality was not elevated in patients with depressed functional fibrinogen level and normal or elevated clot strength, possibly due to higher thrombin concentration at time of clot formation60 or a compensatory augmentation of platelet function in these patients.
Fibrinolysis, plasmin-mediated degradation of the fibrin mesh, is an essential process which maintains vascular patency at homeostasis. Plasmin is formed by the cleavage of plasminogen by tPAs and urokinase plasminogen activators (uPAs), respectively. tPA is secreted by endothelial cells in response to a variety of stimuli including catecholamines, bradykinin, and thrombin.61 Overactivity of this system, termed hyperfibrinolysis, is associated with lethal hemorrhage after injury.62 Raza et al demonstrated in 303 trauma patients that fibrinolytic activation as detected by elevated PAP levels is common (59% of patients), but hyperfibrinolysis as detected by ROTEM (5%) was not.63 Given that PAP clearance has a half-life of 3 to 6 hours in healthy individuals,64 many patients with elevated PAP levels may not have had ongoing elevation of fibrinolytic activity at the time of ROTEM assay. Increasing PAP levels were nonetheless independently associated with increased mortality, with the combination of elevated PAP level and ROTEM lysis being particularly deadly (40% mortality). Cardenas et al analyzed fibrinolytic activity in 163 trauma patients and found that increasing PAP levels were associated with significantly increased levels of tPA (odds ratio, 4.2; 95% confidence interval [CI], 2.0-8.9) and smaller decreases in PAI-1 (odds ratio, 0.97; 95% CI, 0.96-0.99).65 This was corroborated by Chapman et al, who recently demonstrated that tPA levels were significantly higher and free PAI-1 levels were significantly lower in hyperfibrinolytic vs nonhyperfibrinolytic patients. Total (both free and complexed) PAI-1 levels were similar between the 2 groups, providing further evidence that hyperfibrinolysis was driven by increased tPA and not PAI-1 loss.20
Overall, hyperfibrinolysis appears closely associated with lethal hemorrhagic shock and is relatively independent of injury severity, which was corroborated in an animal model where isolated hemorrhagic shock induced tPA-mediated hyperfibrinolysis whereas isolated tissue injury reduced fibrinolytic activity.66 Because of the poor outcomes associated with hyperfibrinolysis, the Clinical Randomisation of an Antifibrinolytic in Significant Haemorrhage 2 (CRASH-2) trial was performed to analyze the effect of empiric antifibrinolytic treatment.67 The trial randomized over 20 000 trauma patients at risk for hemorrhage to receive placebo or tranexamic acid (TXA), which binds to plasminogen and inhibits its activation by tPA. Importantly, the trial was conducted in mostly low-to-moderate income countries that often lacked mature trauma systems, and no testing for fibrinolysis was conducted. TXA reduced all-cause mortality (relative risk [RR], 0.91; 95% CI, 0.85-0.97) with the greatest benefit seen in decreased death due to bleeding (RR, 0.85; 95% CI, 0.76-0.96). Post hoc exploratory analyses68 demonstrated a time-dependent outcome after TXA use: the largest reduction of exsanguination was seen when TXA was given within 1 hour of injury (RR, 0.68; 95% CI, 0.57-0.82), with a lesser benefit observed when given between 1 and 3 hours (RR, 0.79; 95% CI, 0.64-0.97), whereas exsanguination was increased when TXA was given after 3 hours (RR, 1.44; 95% CI, 1.12-1.84). For patients receiving TXA within 3 hours of injury, the absolute risk reduction for exsanguination was 1.9%.68 Although it is intuitive that a treatment which allegedly stops bleeding is most efficacious when given early, it is paradoxical that it appears to increase bleeding when given late. One explanation may be the differential effect of TXA on tPA- vs uPA-mediated fibrinolysis combined with time-varying expression of these plasminogen activators: although the TXA-induced conformational change on plasminogen reduces tPA-mediated activation, it actually accelerates uPA-mediated activation,69 and Hijazi et al recently demonstrated in a mouse model of isolated TBI that cerebrospinal fluid (CSF) and brain parenchyma levels of tPA peaked within 3 hours, whereas uPA levels peaked at 8 hours: after tPA levels had already decreased.70 Although this finding plausibly explains the time-dependent effect of TXA on bleeding in CRASH-2, confirmation is required in human studies.
Interestingly, recent data has demonstrated that the converse of hyperfibrinolysis, termed “fibrinolytic shutdown,” is also associated with increased mortality.71 In a multicenter prospective observational study which categorized 2540 trauma patients into 3 fibrinolytic groups based on degree of clot lysis at 30 minutes on TEG (LY30), hyperfibrinolysis was the least common phenotype (18% of study population) but was associated with the highest mortality (34%), whereas fibrinolytic shutdown was the most common phenotype (46%) and was associated with increased mortality (22%), compared with patients with fibrinolysis not in either extreme (“physiologic fibrinolysis”: 14% mortality).72 Hemorrhage was the leading cause of death in hyperfibrinolytic patients, whereas organ failure was the leading cause of death in shutdown patients, although 15% of shutdown patients still succumbed to hemorrhage. A fundamental mechanistic question is whether fibrinolysis shutdown is a consequence of fibrin mesh architecture, a deficiency of the fibrinolytic machinery, or both. The “shutdown” phenomenon has also added a new dimension of controversy regarding TXA use: whereas some believe it should be reserved for patients who demonstrate hyperfibrinolysis on viscoelastic assays and avoided in those with shutdown,73 others argue that early TXA use could potentially benefit any patient at risk for hemorrhage.74 We agree that randomized control trials75-77 to definitively analyze its risks, benefits, and mechanisms of action in mature trauma systems are worthwhile.
Coagulopathy after brain injury: similarities and differences
The incidence of TIC after isolated TBI is comparable to that of other trauma.78,79 Similar patterns of platelet dysfunction were observed between these 2 populations as well: inhibition to ADP and AA stimulation on TEG-PM was detected in patients presenting with isolated TBI15,51 or hemorrhage,50 with inhibition to ADP significantly associated with increasing TBI severity or hemorrhagic shock. Additionally, animal models of TBI with and without hemorrhagic shock demonstrated platelet inhibition to these stimuli within 15 minutes of injury.15,48 However, isolated TBI likely induces coagulopathy through different mechanisms owing to: (1) a lack of significant hemorrhage, hypoperfusion, and shock, giving APC a much lesser role; (2) high levels of tissue factor in brain parenchyma,80 which could be released into the circulation after injury; and (3) “novel” interactions between plasma proteins and brain tissue which is normally prevented by an intact blood-brain barrier.
Microparticles (MPs), small 0.1- to 1-µm phospholipid vesicles released from the cell membrane as a consequence of cell death or certain stimuli, may play a large role. MPs carry different membrane proteins specific to their cell of origin, which can be identified by flow cytometry.81 Several studies have identified an increase in circulating MP levels after many types of trauma82-86 including TBI.87,88 A small French study of 16 patients with isolated severe TBI found elevated plasma and CSF levels of MPs mainly of platelet and endothelial cell origin.88 Sustained high levels of MPs in the CSF 10 days postinjury were associated with poor outcome, possibly indicating persistent injury to the blood-brain barrier. This is in contrast to many other studies of polytrauma patients where high circulating levels of MPs were associated with improved outcomes.82,83,85 A recent study by Tian et al investigated the role of MPs from neurons and glial cells, brain-derived MPs (BDMPs), in the pathogenesis of TIC after TBI.89 Isolated TBI in mice induced a reversible hypercoagulability dependent on the presence of circulating BDMPs, which are highly procoagulant due to pronounced levels of tissue factor and phosphatidylserine. Rapid infusion of BDMPs into uninjured mice resulted in a hypercoagulable state which converted to a consumptive coagulopathy with sustained infusion. Interestingly, BDMPs activated human platelets in vitro, but did not induce platelet aggregation. Given that animal models of TBI detected platelet inhibition to ADP and AA stimulation 15 minutes after injury,15,48 exposure of platelets to BDMPs may be inducing “platelet exhaustion” but requires further studies for confirmation.
Recently, Hijazi et al performed an elegant study which implicated primary fibrinolysis in post-TBI coagulopathy in a mouse model.70 As expected, uPA and tPA knockout mice had reduced intracranial hemorrhage (ICH) after TBI compared with wild type, whereas PAI-1 knockout mice had worse ICH. As mentioned earlier, CSF and brain parenchyma levels of tPA peaked several hours before uPA levels; in this context, early TXA reduced ICH, whereas late TXA exacerbated it. However, a tPA variant which inhibited both tPA and uPA decreased ICH, even in an animal which was anticoagulated with warfarin prior to injury. The authors concluded that fibrinolysis, initiated first by tPA and then maintained by uPA, was the primary driver of delayed ICH progression in a mouse model of isolated TBI. This provides a mechanistic basis for the time-dependent differential effect of TXA observed in the CRASH-2 trial,90 as well as pointing to a profibrinolytic mechanism, and not an anticoagulant one, as the primary driver of continued ICH after TBI. Randomized controlled trials investigating the early use of TXA in severe TBI patients are currently under way.91,92
Conclusion
As investigation of TIC proceeds, its complexity seems to deepen. A major challenge is that trauma produces a plethora of biochemical and physiologic changes, and despite many studies showing differences in laboratory parameters between trauma patients and healthy controls, it remains unclear whether certain “abnormalities” may in fact be “normal” after trauma. Comparisons between trauma patients with differing outcomes (survival vs mortality, for example) combined with mechanistic data from animal models have shed some light on this question, but the majority of findings are correlative with currently little causative data, leading to disagreement and controversy over how best to manage these patients. More high-level data from randomized controlled trials combined with mechanistic studies are needed to overcome the diagnostic and therapeutic challenge posed by TIC.
Acknowledgment
R.C. was supported by a T32 fellowship (grant no. 5T32GM008792) from the National Institutes of Health, National Institute of General Medical Sciences.
Authorship
Contribution: R.C. performed the literature review, and drafted the manuscript and figure; J.C.C. assisted with the literature review and preparation of manuscript and figure; and C.E.W. and J.B.H. reviewed and edited the manuscript and figure.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ronald Chang, University of Texas Health Science Center, 6410 Fannin St, Suite 1100, Houston, TX 77030; e-mail: ronald.chang@uth.tmc.edu.
References
- 1.National Vital Statistics System, National Center for Health Statistics, Centers for Disease Control and Prevention. 10 Leading Causes of Death by Age Group, United States—2013. Atlanta, GA: Office of Statistics and Programing, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/injury/images/lc-charts/leading_causes_of_death_by_age_group_2013-a.gif. Accessed January 11, 2016.
- 2.Norton R, Kobusingye O. Injuries. N Engl J Med. 2013;368(18):1723–1730. doi: 10.1056/NEJMra1109343. [DOI] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2013;381(9867):628]. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holcomb JB, del Junco DJ, Fox EE, et al. PROMMTT Study Group. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holcomb JB, Tilley BC, Baraniuk S, et al. PROPPR Study Group. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardenas JC, Rahbar E, Pommerening MJ, et al. Measuring thrombin generation as a tool for predicting hemostatic potential and transfusion requirements following trauma. J Trauma Acute Care Surg. 2014;77(6):839–845. doi: 10.1097/TA.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 7.Brohi K, Singh J, Heron M, Coats T. Acute traumatic coagulopathy. J Trauma. 2003;54(6):1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 8.Niles SE, McLaughlin DF, Perkins JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008;64(6):1459–1465. doi: 10.1097/TA.0b013e318174e8bc. [DOI] [PubMed] [Google Scholar]
- 9.Simmons RL, Collins JA, Heisterkamp CA, III, Mills DE, Andren R, Phillips LL. Coaguliation disorders in combat casualties. I: acute changes after wounding. Ann Surg. 1969;169(4):455–462. doi: 10.1097/00000658-196904000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet J-F. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245(5):812–818. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brohi K, Cohen MJ, Ganter MT, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma. 2008;64(5):1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 12.Cohen MJ, Call M, Nelson M, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. 2012;255(2):379–385. doi: 10.1097/SLA.0b013e318235d9e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MJ, Kutcher M, Redick B, et al. PROMMTT Study Group. Clinical and mechanistic drivers of acute traumatic coagulopathy. J Trauma Acute Care Surg. 2013;75(1 suppl 1):S40–S47. doi: 10.1097/TA.0b013e31828fa43d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lustenberger T, Talving P, Kobayashi L, et al. Early coagulopathy after isolated severe traumatic brain injury: relationship with hypoperfusion challenged. J Trauma. 2010;69(6):1410–1414. doi: 10.1097/TA.0b013e3181cdae81. [DOI] [PubMed] [Google Scholar]
- 15.Castellino FJ, Chapman MP, Donahue DL, et al. Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. J Trauma Acute Care Surg. 2014;76(5):1169–1176. doi: 10.1097/TA.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright AP, Wade CE, Camp E, et al. Pulmonary contusion on admission chest x-ray is associated with coagulopathy and mortality in trauma patients. J Emerg Med Trauma Surg Care. 2015;2(2):011.
- 17.Jansen JO, Scarpelini S, Pinto R, Tien HC, Callum J, Rizoli SB. Hypoperfusion in severely injured trauma patients is associated with reduced coagulation factor activity. J Trauma. 2011;71(5 suppl 1):S435–S440. doi: 10.1097/TA.0b013e318232e5cb. [DOI] [PubMed] [Google Scholar]
- 18.Burggraf M, Payas A, Kauther MD, Schoeneberg C, Lendemans S. Evaluation of clotting factor activities early after severe multiple trauma and their correlation with coagulation tests and clinical data. World J Emerg Surg. 2015;10:43. doi: 10.1186/s13017-015-0038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell JE, Meledeo MA, Cap AP. Comparative response of platelet fV and plasma fV to activated protein C and relevance to a model of acute traumatic coagulopathy. PLoS One. 2014;9(6):e99181. doi: 10.1371/journal.pone.0099181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman MP, Moore EE, Moore HB, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg. 2016;80(1):16–25. doi: 10.1097/TA.0000000000000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin TL, Moore EE, Moore HB, et al. A principal component analysis of postinjury viscoelastic assays: clotting factor depletion versus fibrinolysis. Surgery. 2014;156(3):570–577. doi: 10.1016/j.surg.2014.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hinsbergh VW. Endothelium--role in regulation of coagulation and inflammation. Semin Immunopathol. 2012;34(1):93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johansson PI, Ostrowski SR. Acute coagulopathy of trauma: balancing progressive catecholamine induced endothelial activation and damage by fluid phase anticoagulation. Med Hypotheses. 2010;75(6):564–567. doi: 10.1016/j.mehy.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 24.Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108(3):384–394. doi: 10.1093/bja/aer515. [DOI] [PubMed] [Google Scholar]
- 25.Schött U, Solomon C, Fries D, Bentzer P. The endothelial glycocalyx and its disruption, protection and regeneration: a narrative review. Scand J Trauma Resusc Emerg Med. 2016;24(1):48. doi: 10.1186/s13049-016-0239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol. 2004;557(Pt 3):889–907. doi: 10.1113/jphysiol.2003.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levick JR. Revision of the Starling principle: new views of tissue fluid balance. J Physiol. 2004;557(Pt 3):704. doi: 10.1113/jphysiol.2004.066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahbar E, Cardenas JC, Baimukanova G, et al. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med. 2015;13:117. doi: 10.1186/s12967-015-0481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makhmudov RM, Mamedov YaD, Dolgov VV, Repin VS. Catecholamine-mediated injury to endothelium in rabbit perfused aorta: a quantitative analysis by scanning electron microscopy. Cor Vasa. 1985;27(6):456–463. [PubMed] [Google Scholar]
- 30.Dolgov VV, Makhmudov RM, Bondarenko MF, Repin VS. Deleterious action of adrenaline on the endothelial lining of the vessels [in Russian]. Arkh Patol. 1984;46(10):31–36. [PubMed] [Google Scholar]
- 31.Sadler JE, Lentz SR, Sheehan JP, Tsiang M, Wu Q. Structure-function relationships of the thrombin-thrombomodulin interaction. Haemostasis. 1993;23(suppl 1):183–193. doi: 10.1159/000216927. [DOI] [PubMed] [Google Scholar]
- 32.Shworak NW, Kobayashi T, de Agostini A, Smits NC. Anticoagulant heparan sulfate to not clot--or not? Prog Mol Biol Transl Sci. 2010;93:153–178. doi: 10.1016/S1877-1173(10)93008-1. [DOI] [PubMed] [Google Scholar]
- 33.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012;73(1):60–66. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 34.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Yu W-K, Lin Z-L, et al. Chemical sympathectomy attenuates inflammation, glycocalyx shedding and coagulation disorders in rats with acute traumatic coagulopathy. Blood Coagul Fibrinolysis. 2015;26(2):152–160. doi: 10.1097/MBC.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 36.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 37.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wataha K, Menge T, Deng X, et al. Spray-dried plasma and fresh frozen plasma modulate permeability and inflammation in vitro in vascular endothelial cells. Transfusion. 2013;53(suppl 1):80S–90S. doi: 10.1111/trf.12040. [DOI] [PubMed] [Google Scholar]
- 39.Potter DR, Baimukanova G, Keating SM, et al. Fresh frozen plasma and spray-dried plasma mitigate pulmonary vascular permeability and inflammation in hemorrhagic shock. J Trauma Acute Care Surg. 2015;78(6 suppl 1):S7–S17. doi: 10.1097/TA.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 40.Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng Z, Pati S, Potter D, et al. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock. 2013;40(3):195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown LM, Call MS, Margaret Knudson M, et al. Trauma Outcomes Group. A normal platelet count may not be enough: the impact of admission platelet count on mortality and transfusion in severely injured trauma patients. J Trauma. 2011;71(2 suppl 3):S337–S342. doi: 10.1097/TA.0b013e318227f67c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnüriger B, Inaba K, Abdelsayed GA, et al. The impact of platelets on the progression of traumatic intracranial hemorrhage. J Trauma. 2010;68(4):881–885. doi: 10.1097/TA.0b013e3181d3cc58. [DOI] [PubMed] [Google Scholar]
- 44.Kutcher ME, Redick BJ, McCreery RC, et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 2012;73(1):13–19. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kornblith LZ, Kutcher ME, Redick BJ, Calfee CS, Vilardi RF, Cohen MJ. Fibrinogen and platelet contributions to clot formation: implications for trauma resuscitation and thromboprophylaxis. J Trauma Acute Care Surg. 2014;76(2):255–263. doi: 10.1097/TA.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swallow RA, Agarwala RA, Dawkins KD, Curzen NP. Thromboelastography: potential bedside tool to assess the effects of antiplatelet therapy? Platelets. 2006;17(6):385–392. doi: 10.1080/09537100600757521. [DOI] [PubMed] [Google Scholar]
- 47.Solomon C, Traintinger S, Ziegler B, et al. Platelet function following trauma. A multiple electrode aggregometry study. Thromb Haemost. 2011;106(2):322–330. doi: 10.1160/TH11-03-0175. [DOI] [PubMed] [Google Scholar]
- 48.Sillesen M, Johansson PI, Rasmussen LS, et al. Platelet activation and dysfunction in a large-animal model of traumatic brain injury and hemorrhage. J Trauma Acute Care Surg. 2013;74(5):1252–1259. doi: 10.1097/TA.0b013e31828c7a6b. [DOI] [PubMed] [Google Scholar]
- 49.Sirajuddin S, Valdez C, DePalma L, et al. Inhibition of platelet function is common following even minor injury [published online ahead of print March 28, 2016]. J Trauma Acute Care Surg. [DOI] [PubMed]
- 50.Wohlauer MV, Moore EE, Thomas S, et al. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J Am Coll Surg. 2012;214(5):739–746. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davis PK, Musunuru H, Walsh M, et al. Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit Care. 2013;18(2):201–208. doi: 10.1007/s12028-012-9745-6. [DOI] [PubMed] [Google Scholar]
- 52.Pareti FI, Capitanio A, Mannucci L, Ponticelli C, Mannucci PM. Acquired dysfunction due to the circulation of “exhausted” platelets. Am J Med. 1980;69(2):235–240. doi: 10.1016/0002-9343(80)90383-6. [DOI] [PubMed] [Google Scholar]
- 53.Jacoby RC, Owings JT, Holmes J, Battistella FD, Gosselin RC, Paglieroni TG. Platelet activation and function after trauma. J Trauma. 2001;51(4):639–647. doi: 10.1097/00005373-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Stalker TJ, Traxler EA, Wu J, et al. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013;121(10):1875–1885. doi: 10.1182/blood-2012-09-457739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore HB, Moore EE, Chapman MP, et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J Thromb Haemost. 2015;13(10):1878–1887. doi: 10.1111/jth.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 57.Gunter OL, Jr, Au BK, Isbell JM, Mowery NT, Young PP, Cotton BA. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J Trauma. 2008;65(3):527–534. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 58.Ponschab M, Schlimp CJ, Zipperle J, et al. Platelet function in reconstituted whole blood variants: An observational study over 5 days of storage time. J Trauma Acute Care Surg. 2015;79(5):797–804. doi: 10.1097/TA.0000000000000852. [DOI] [PubMed] [Google Scholar]
- 59.Shen L, Lorand L. Contribution of fibrin stabilization to clot strength. Supplementation of factor XIII-deficient plasma with the purified zymogen. J Clin Invest. 1983;71(5):1336–1341. doi: 10.1172/JCI110885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolberg AS, Campbell RA. Thrombin generation, fibrin clot formation and hemostasis. Transfus Apheresis Sci. 2008;38(1):15–23. doi: 10.1016/j.transci.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129(3):307–321. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 62.Cotton BA, Harvin JA, Kostousouv V, et al. Hyperfibrinolysis at admission is an uncommon but highly lethal event associated with shock and prehospital fluid administration. J Trauma Acute Care Surg. 2012;73(2):365–370. doi: 10.1097/TA.0b013e31825c1234. [DOI] [PubMed] [Google Scholar]
- 63.Raza I, Davenport R, Rourke C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost. 2013;11(2):307–314. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 64.Chandler WL, Alessi MC, Aillaud MF, Vague P, Juhan-Vague I. Formation, inhibition and clearance of plasmin in vivo. Haemostasis. 2000;30(4):204–218. doi: 10.1159/000054136. [DOI] [PubMed] [Google Scholar]
- 65.Cardenas JC, Matijevic N, Baer LA, Holcomb JB, Cotton BA, Wade CE. Elevated tissue plasminogen activator and reduced plasminogen activator inhibitor promote hyperfibrinolysis in trauma patients. Shock. 2014;41(6):514–521. doi: 10.1097/SHK.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 66.Moore HB, Moore EE, Lawson PJ, et al. Fibrinolysis shutdown phenotype masks changes in rodent coagulation in tissue injury versus hemorrhagic shock. Surgery. 2015;158(2):386–392. doi: 10.1016/j.surg.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shakur H, Roberts I, et al. CRASH-2 trial collaborators. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010. 376(9734):23-32. [DOI] [PubMed]
- 68.Roberts I, Shakur H, et al. CRASH-2 collaborators. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011. 377(9771):1096-1101. [DOI] [PubMed]
- 69.Markus G, Priore RL, Wissler FC. The binding of tranexamic acid to native (Glu) and modified (Lys) human plasminogen and its effect on conformation. J Biol Chem. 1979;254(4):1211–1216. [PubMed] [Google Scholar]
- 70.Hijazi N, Abu Fanne R, Abramovitch R, et al. Endogenous plasminogen activators mediate progressive intracerebral hemorrhage after traumatic brain injury in mice. Blood. 2015;125(16):2558–2567. doi: 10.1182/blood-2014-08-588442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77(6):811–817. doi: 10.1097/TA.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moore HB, Moore EE, Liras IN, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2540 severely injured patients. J Am Coll Surg. 2016;222(4):347–355. doi: 10.1016/j.jamcollsurg.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore EE, Moore HB, Gonzalez E, Sauaia A, Banerjee A, Silliman CC. Rationale for the selective administration of tranexamic acid to inhibit fibrinolysis in the severely injured patient. Transfusion. 2016;56(suppl 2):S110–S114. doi: 10.1111/trf.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roberts I. Fibrinolytic shutdown: fascinating theory but randomized controlled trial data are needed. Transfusion. 2016;56(suppl 2):S115–S118. doi: 10.1111/trf.13490. [DOI] [PubMed] [Google Scholar]
- 75.Mitra B, Mazur S, Cameron PA, et al. PATCH-Trauma Study Investigators. Tranexamic acid for trauma: filling the ‘GAP’ in evidence. Emerg Med Australas. 2014;26(2):194–197. doi: 10.1111/1742-6723.12172. [DOI] [PubMed] [Google Scholar]
- 76.Brown JB, Neal MD, Guyette FX, et al. Design of the Study of Tranexamic Acid during Air Medical Prehospital Transport (STAAMP) Trial: addressing the knowledge gaps. Prehosp Emerg Care. 2015;19(1):79–86. doi: 10.3109/10903127.2014.936635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Washington University School of Medicine. Tranexamic Acid Mechanisms and Pharmacokinetics in Traumatic Injury (TAMPITI). NLM identifier: NCT02535949. Available at: https://clinicaltrials.gov/ct2/show/study/NCT02535949. Accessed May 23, 2016.
- 78.Wafaisade A, Lefering R, Tjardes T, et al. Trauma Registry of DGU. Acute coagulopathy in isolated blunt traumatic brain injury. Neurocrit Care. 2010;12(2):211–219. doi: 10.1007/s12028-009-9281-1. [DOI] [PubMed] [Google Scholar]
- 79.Epstein DS, Mitra B, O’Reilly G, Rosenfeld JV, Cameron PA. Acute traumatic coagulopathy in the setting of isolated traumatic brain injury: a systematic review and meta-analysis. Injury. 2014;45(5):819–824. doi: 10.1016/j.injury.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 80.Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg. 2009;108(5):1447–1452. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mooberry MJ, Key NS. Microparticle analysis in disorders of hemostasis and thrombosis. Cytometry A. 2016;89(2):111–122. doi: 10.1002/cyto.a.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Windeløv NA, Johansson PI, Sørensen AM, et al. Low level of procoagulant platelet microparticles is associated with impaired coagulation and transfusion requirements in trauma patients. J Trauma Acute Care Surg. 2014;77(5):692–700. doi: 10.1097/TA.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 83.Matijevic N, Wang YW, Wade CE, et al. PROMMTT Study Group. Cellular microparticle and thrombogram phenotypes in the Prospective Observational Multicenter Major Trauma Transfusion (PROMMTT) study: correlation with coagulopathy. Thromb Res. 2014;134(3):652–658. doi: 10.1016/j.thromres.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park MS, Owen BA, Ballinger BA, et al. Quantification of hypercoagulable state after blunt trauma: microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery. 2012;151(6):831–836. doi: 10.1016/j.surg.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Curry N, Raja A, Beavis J, Stanworth S, Harrison P. Levels of procoagulant microvesicles are elevated after traumatic injury and platelet microvesicles are negatively correlated with mortality. J Extracell Vesicles. 2014;3:25625. doi: 10.3402/jev.v3.25625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park MS, Xue A, Spears GM, et al. Thrombin generation and procoagulant microparticle profiles after acute trauma: a prospective cohort study. J Trauma Acute Care Surg. 2015;79(5):726–731. doi: 10.1097/TA.0000000000000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nekludov M, Mobarrez F, Gryth D, Bellander BM, Wallen H. Formation of microparticles in the injured brain of patients with severe isolated traumatic brain injury. J Neurotrauma. 2014;31(23):1927–1933. doi: 10.1089/neu.2013.3168. [DOI] [PubMed] [Google Scholar]
- 88.Morel N, Morel O, Petit L, et al. Generation of procoagulant microparticles in cerebrospinal fluid and peripheral blood after traumatic brain injury. J Trauma. 2008;64(3):698–704. doi: 10.1097/TA.0b013e31816493ad. [DOI] [PubMed] [Google Scholar]
- 89.Tian Y, Salsbery B, Wang M, et al. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood. 2015;125(13):2151–2159. doi: 10.1182/blood-2014-09-598805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Medcalf RL. The traumatic side of fibrinolysis. Blood. 2015;125(16):2457–2458. doi: 10.1182/blood-2015-02-629808. [DOI] [PubMed] [Google Scholar]
- 91.Dewan Y, Komolafe EO, Mejía-Mantilla JH, et al. CRASH-3 - tranexamic acid for the treatment of significant traumatic brain injury: study protocol for an international randomized, double-blind, placebo-controlled trial. Trials. 2012. 13:87. [DOI] [PMC free article] [PubMed]
- 92.Resuscitation Outcomes Consortium. Prehospital Tranexamic Acid Use for Traumatic Brain Injury (TXA). NLM identifier: NCT01990768. Available at: https://clinicaltrials.gov/ct2/show/study/NCT01990768. Accessed May 18, 2016.