Abstract
IMPORTANCE
An increasing percentage of births are conceived with assisted reproductive technology (ART) and other infertility treatment. Despite findings that such treatments may be associated with diminished gestation and birth size, scarce data exist regarding infertility treatments and children’s development in the United States.
OBJECTIVE
To assess the use and type of infertility treatment in relation to children’s development through age 36 months.
DESIGN, SETTING, AND PARTICIPANTS
Prospective cohort study (conducted 2008–2014) that sampled based on infertility treatment and plurality. Included in the study were infants born between 2008 and 2010 in New York state (excluding New York City) whose parents completed developmental screening instruments through 36 months of age. A total of 4824 mothers (97%of 4989) completed 1 or more developmental screening instruments for 5841 children, including 1830 conceived with infertility treatment and 2074 twins.
EXPOSURES
Maternal self-report of any infertility treatment was further categorized into ART and ovulation induction/intrauterine insemination. Assisted reproductive technology use was previously validated by linkage with the Society for Assisted Reproductive Technology–Clinical Outcome Reporting System.
MAIN OUTCOMES AND MEASURES
Five developmental domains (fine motor, gross motor, communication, personal-social functioning, and problem-solving ability), as measured by the parental completion of the Ages and Stages Questionnaires at 4, 8, 12, 18, 24, 30, and 36 months of age. Generalized linear mixed modeling techniques estimated adjusted odds ratios (aORs) and 95%CIs for use and type of infertility treatment in relation to failing a developmental domain. Data were stratified by plurality and weighted for the sampling scheme.
RESULTS
There were 1422 mothers (29.5%; mean [SD], age, 34.1 [5.2] years) who underwent infertility treatment. Infertility treatment was not associated with risk of their children failing any developmental domain (aOR, 1.33; 95%CI, 0.94–1.89). Assisted reproductive technology was associated with increased risk for failing any developmental domain but only when singletons and twins were evaluated together (aOR, 1.81; 95%CI, 1.21–2.72). Adjustment for birth weight further attenuated this estimate (aOR, 1.26; 95%CI, 0.82–1.93). After stratifying by plurality, type of treatment also was not significantly associated with failing any developmental domain for ovulation induction/intrauterine insemination (aOR, 1.00; 95%CI, 0.57–1.77 for singletons and aOR, 1.30; 95%CI, 0.76–2.21 for twins) or ART (aOR, 1.38; 95%CI, 0.78–2.43 for singletons and aOR, 1.58; 95%CI, 0.94–2.65 for twins).
CONCLUSIONS AND RELEVANCE
After considering plurality, children’s development through age 3 years was similar irrespective of infertility treatment or specific type. To our knowledge, these findings are among the first to focus on non-ART treatments in the United States.
Infertility treatment, including assisted reproductive technology (ART), has helped many people become parents.1,2 In 2011, 1.5%of US births were conceived with ART.1 Conceptions by ovulation induction were estimated to be much higher, accounting for 3% to 7% of US births.3,4
Concern about the neurodevelopment of children conceived with such modalities is longstanding, given the potential for developmental “programming” at any stage of treatment including in vitro culture5 and intracytoplasmic sperm injection (ICSI).6 Conception by fertility drugs may also be relevant owing to ovarian stimulation possibly impacting endometrium receptivity and placentation.7 Observations that children conceived by infertility treatment are born earlier and with lower birth weight add to concerns.8,9
Many studies, as reviewed elsewhere,10–12 have investigated neurodevelopmental outcomes with ART but the evidence remains equivocal and largely based on a few follow-up studies conducted outside of the United States. In Sweden, a small significant association with 18% higher risk for intellectual disabilities was found, driven by procedures with ICSI,6 whereas in Denmark, no association was observed.13 Other studies investigating cognitive or neuromotor function have largely found no difference.11 One linkage study from the United States observed an increased risk for autism associated with ART,14 which seemed largely due to socioeconomic differences in surveillance,15 and no significant associations were found in large registry studies from Scandinavia.6,13,16
Studies investigating non-ART infertility treatments and children’s development remain rare. One study found a small increased risk in any mental disorders for children conceived with ovulation induction (OI), with or without intrauterine insemination (IUI), relative to children conceived without any treatment using registry data from Denmark.13 These results contradict earlier studies from Finland,17 the United States,18 and the United Kingdom that found no differences with respect to children conceived by OI/IUI.19
In response to critical data gaps, we designed the Upstate KIDS Study to specifically assess the association between the mode of conception and children’s development through age 3 years. Because ART technique differs between the United States and abroad (eg, including the number of embryos transferred or use of assisted hatching),20 our findings are particularly important for communicating with US couples seeking infertility treatment.
Methods
Study Design and Population
The Upstate KIDS Study recruited infants born to women delivering in New York state (excluding New York City) between 2008 and 2010.21 All infants whose birth certificates indicated infertility treatment use comprised the exposure cohort and their parents were invited to participate. All multiples were also invited to participate. Singletons not conceived with infertility treatment were recruited at a 3:1 ratio to the exposure cohort frequency matched on the state’s perinatal region of birth. In total, 5034 mothers (27%of 18 479 approached) of 6171 children enrolled in the study, with higher response (32%) among exposed mothers.21 We have described recruitment and follow-up procedures, as well as demonstrated the validity of this sampling framework relative to birth certificate information and the absence of major differences in baseline characteristics by participation status.21 The New York State Department of Health and the University at Albany (State University of New York) institutional review boards approved the study and served as the institutional review boards designated by the National Institutes of Health under a reliance agreement. All parents provided written informed consent.
Infertility Treatment Exposure
At 4 months post partum, mothers selected all medical services or medications used to become pregnant on the questionnaire. Two subcategories of exposure were defined: (1) ART use consisted of in vitro fertilization (with or without ICSI), assisted hatching, frozen embryo transfer, zygote intrafallopian transfer, gamete intrafallopian transfer, and/or donor eggs or embryos and (2) OI via oral or injectable medications with or without IUI.21 Maternal report was linked with the Society for Assisted Reproductive Technology–Clinical Outcome Reporting System (SART-CORS), a database of ART outcomes reported from member US clinics to the Centers for Disease Control and Prevention. The sensitivity and specificity of maternal report compared with SART-CORS were high (93% and 99%, respectively).22 Given the absence of a registry for verifying OI/IUI and geographic limitation of the SART linkage, we used maternal report for all infertility treatment information and relied on birth certificate data only when missing (n = 147; 3%).
Developmental Assessment
The Ages and Stages Questionnaire (ASQ) is a validated developmental screening instrument recommended for early identification of developmental delays.23–25 The ASQ encourages parents to perform activities with their children to accurately respond to questions capturing 5 developmental domains (ie, fine motor, gross motor, communication, personal-social functioning, and problem-solving ability). Parents completed the ASQ at 4, 6, 8, 12, 18, 24, 30, and 36 months of age, corrected for gestational age.26,27 We implemented the ASQ–second edition27 for screening at ages 4 to 12 months and the third edition26 from ages 18 months onwards as ASQ-3 became available in 2009.
Each item was scored as recommended26,27 (“yes” = 10 points, “sometimes” = 5 points, and “not yet” = 0 points) and summed for each domain (0–60 points). Domain-specific fails were defined as scores 2 SDs below the mean for normative data.26,27 Study personnel followed up with parents when the child failed any of the 5 domains or parental concern was noted. Trained specialists implemented an age-appropriate follow-up ASQ for the domain(s) that failed or discussed concerns. We defined age at fail as the time of the initial fail. When follow-up was incomplete after an initial fail, the child remained as failing that ASQ. Screening instruments were considered valid only if completed in the specified age windows.26,27 For children who failed any ASQ domain, a referral was made to the New York State Early Intervention Program (EIP), which provides free developmental services as needed after clinical evaluation.28
Additional Developmental Data
We linked EIP data to assess whether the proportions of children referred for developmental evaluation differed by infertility treatment exposure. Records were matched on identifiers (ie, birth dates and names). The Early Intervention Program provided aggregate tables for proportions of Upstate KIDS children referred for evaluation stratified by plurality and infertility treatment status. Individual-level data from EIP required parental consent, which was solicited from those whose child failed a previous ASQ and were received for 478 children.
Questionnaires accompanying the ASQ at 30 and 36 months also asked mothers to report whether their children received any developmental services. The positive and negative predictive values of reported use of developmental services compared against EIP linkage were high for the 478 infants (86.2% and 95.0%, respectively).
Last, a subgroup of 314 singletons (8%) and 132 twin pairs (12%) underwent clinical diagnostic examinations at 3 to 4 years of age. For this assessment, children who failed the ASQ screening (at 30 or 36 months) and the Modified Checklist for Autism in Toddlers29 (at 18 months) were invited along with a random sample of children with no history of fails. Based on this evaluation, clinicians provided diagnostic codes for each child. Developmental disability was defined as having 1 or more of these diagnoses: autism spectrum disorder (ASD); attention-deficit/hyperactivity disorder; language, learning, and speech disorders; cognitive deficits; cerebral palsy; and/or sensory impairments.
Covariates
Covariate information came from vital records (ie, maternal and paternal ages, insurance status, plurality, previous live birth, prepregnancy body mass index [BMI, calculated as weight in kilograms divided by height in meters squared], birth weight; and gestational age) or by baseline maternal report (ie, paternal BMI, marital status, race/ethnicity, education, and smoking and alcohol use during pregnancy).
Statistical Methods
Sociodemographic characteristics relative to infertility treatment were compared using χ2 and t tests. Infant characteristics are shown for all singletons and a randomly selected twin of each sibling pair. Triplets and quadruplets from 45 families were excluded owing to small numbers (n = 134 children).
We first evaluated the prospective associations between infertility treatment exposure (yes/no) and failing any ASQ domain and then by each of the 5 domains. Treatment type was further assessed separating ART and OI/IUI. Children conceived without infertility treatment were the reference group in all analyses. We used generalized linear mixed-effects models with a logit link to estimate the associations between infant treatment and failing the ASQ. These models provided adjusted odds ratios (aORs) and 95% CIs. All models included maternal-level random intercepts and nested infant-level random intercepts to account for the correlation owing to repeated measures and clustering of infants within mothers.30 In models based only on singletons or primary cohort where only 1 infant was included from each mother, an infant-level random intercept was included to account for repeated measures of infants. Sampling weights were applied to account for the study’s design of oversampling infants conceived with infertility treatment and twins.21 Weights were derived using New York state birth certificate data on infertility treatment, plurality, and region of birth for all infants born during the period of recruitment. Time was modeled assuming a nonlinear trajectory of the odds of failing. The estimation was based on weighted likelihood function, which was computed using an adaptive quadrature and the standard errors were calculated based on sandwich-type variance estimation. Our longitudinal methods accounted for children’s varying developmental stages and varying failures over the course of follow-up, allowing flexibility of children to fail at any point in time but not necessarily subsequent follow-up screenings.
A priori factors known to be associated with development (such as socioeconomic status or smoking)31,32 and associated with infertility treatment were adjusted for including maternal age, race/ethnicity, education, insurance, married/living as married, previous live birth, and smoking during pregnancy. Birth weight was included in a separate model to avoid potential overadjustment bias.33 Missing data on marital status (n = 206 infants) and prior live birth (n = 43) were completed using multiple imputations after creating 10 data sets based on a Bernoulli distribution, with probabilities dependent on their observed distributions by infertility treatment type. For variables where few (n <10) were missing (such as smoking or insurance), the missing were imputed using the most frequent response.
Because the ASQ is a screening rather than diagnostic tool, we also evaluated cross-sectional associations between infertility treatment and maternal reported use of developmental services reported at 30 and/or 36 months of age, using logistic regression and adjusting for similar covariates and weighted for the sampling framework. Analyses accounted for missing questionnaire data by inverse probability weighting.34 To construct the weights for missing data, a regression model was run to determine the probability of missing data at 30 and 36 months (yes/no) by infertility treatment exposure and plurality. Sampling weights were multiplied to missing weights to further take into account missing data. Total weights were trimmed at the 95th percentile. For twins, generalized estimating equations were used to account for the correlation between twin pairs. Last, for the children with diagnostic information at 3 to 4 years, χ2 tests of the frequencies were made with respect to developmental diagnoses among singletons (n = 314) and 1 twin of each pair (n = 132). All analyses were performed using SAS version 9.3 (SAS Institute Inc).
Results
In total, 3402 mothers (of 4011 children) unexposed and 1422 mothers (of 1830 children) exposed to infertility treatment were included in our analysis. Six to 10 percent of children failed at least 1 of the ASQ developmental domains at each screen (eTable in the Supplement). Domain-specific fails were fewer, ranging from 2% to 5% per screen. Longitudinal follow-up decreased overtime in the families. However, almost all children had at least 1 valid ASQ screen between 4 and 36 months. Mothers (n = 4824; 97% of 4989) who returned the ASQ were more frequently white (81% vs 65%) compared with mothers who never returned one (n = 165; 3%) but did not otherwise differ.
Table 1 shows the differences in characteristics by use of any infertility treatment. Differences between the specific treatment types were also observed, with older parental ages; more twin births; higher education and paternal BMI; and lower maternal prepregnancy BMI, birth weight, and gestational age in the ART group compared with the OI/IUI group.
Table 1.
Baseline Characteristics by Infertility Treatment Status in the Upstate KIDS Study (2008–2010)a
| Characteristic | No. (%) | |||
|---|---|---|---|---|
| Infertility Treatment | OI/IUI | ART | ||
| None | Any | |||
| No. (%) | 3402 (70.5) | 1422 (29.5) | 742 (15.4) | 679 (14.1) |
| Age, mean (SD), yb,c | ||||
| Maternal | 28.9 (5.7) | 34.1 (5.2) | 32.5 (4.6) | 35.8 (5.2) |
| Paternal | 31.7 (6.6) | 36.4 (6.3) | 34.8 (5.8) | 38.1 (6.3) |
| Maternal race/ethnicityb | ||||
| Non-Hispanic white | 2654 (78.0) | 1237 (87.0) | 664 (89.5) | 573 (84.4) |
| Non-Hispanic black | 195 (5.7) | 37 (2.6) | 16 (2.1) | 20 (3.0) |
| Non-Hispanic Asian | 82 (2.4) | 45 (3.2) | 16 (2.2) | 29 (4.2) |
| Hispanic | 248 (7.3) | 34 (2.4) | 15 (2.0) | 19 (2.8) |
| Mixed race or ethnicity/other | 223 (6.6) | 69 (4.8) | 31 (4.2) | 38 (5.6) |
| Maternal educationb,c | ||||
| <High school | 278 (8.2) | 13 (1.0) | 11 (1.5) | 2 (0.3) |
| High school or GED equivalent | 555 (16.3) | 67 (4.7) | 36 (4.9) | 31 (4.6) |
| Some college | 1157 (34.0) | 302 (21.2) | 183 (24.7) | 119 (17.5) |
| College | 666 (19.6) | 401 (28.2) | 201 (27.0) | 199 (29.3) |
| Advanced degree | 746 (21.9) | 639 (44.9) | 311 (41.9) | 328 (48.3) |
| Private insuranceb,c | 2270 (66.8) | 1349 (94.9) | 695 (93.8) | 654 (96.3) |
| Married/living as married | 2770 (85.3) | 1302 (95.3) | 680 (95.0) | 622 (95.7) |
| Any alcohol during pregnancyb | 436 (12.8) | 150 (10.6) | 74 (10.0) | 76 (11.2) |
| Smoked during pregnancyb | 624 (18.4) | 55 (3.9) | 32 (4.3) | 23 (3.4) |
| BMI, mean (SD) | ||||
| Prepregnancyc | 27.0 (6.8) | 27.3 (6.9) | 28.3 (7.5) | 26.1 (5.9) |
| Paternalb,c | 27.9 (5.4) | 28.9 (5.4) | 29.3 (5.7) | 28.4 (5.0) |
| Previous live birthb | 2035 (60.2) | 576 (40.9) | 300 (40.5) | 275 (41.1) |
| Pluralityb,c | ||||
| Singleton | 2767 (81.3) | 1000 (70.3) | 549 (74.0) | 450 (66.3) |
| Twin | 635 (18.7) | 422 (29.7) | 193 (26.0) | 229 (33.7) |
| Birth weight, mean (SD), gb,c,d | 3218 (672) | 3084 (728) | 3133 (730) | 3030 (723) |
| Gestational age, mean (SD), wkb,c,d | 38.2 (2.4) | 37.6 (2.7) | 37.8 (2.7) | 37.4 (2.7) |
| Age at last follow-up, mean (SD), mob,d | 23.3 (13.3) | 26.6 (12.4) | 26.3 (12.5) | 26.9 (12.2) |
Abbreviations: ART, assisted reproductive technology; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GED, General Educational Development; OI/IUI, ovulation induction/intrauterine insemination.
Missing data: specific infertility treatment type (n = 1), paternal age (n = 336), insurance (n = 4), married/living as married (n = 209), alcohol (n = 2), smoking (n = 2), maternal BMI (n = 11), paternal BMI (n = 538), and prior live birth (n = 37).
P < .05 for comparisons between no infertility treatment and any treatment (first 2 columns).
P < .05 for comparisons between OI/IUI and ART (last 2 columns).
For infant-level data (ie, birth weight, gestational age, age at last follow-up, and ever using services), descriptive statistics were derived from all singletons and 1 randomly selected twin of each pair.
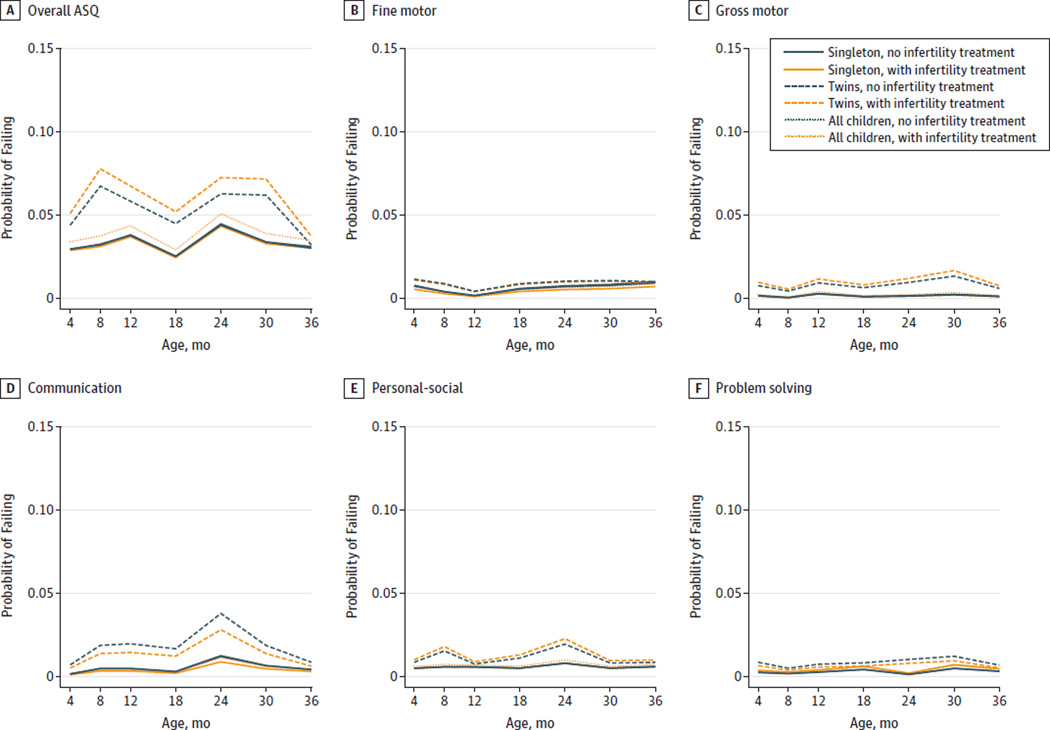
The unadjusted longitudinal trajectories of the ASQ failures were similar, irrespective of infertility treatment among all children and after stratifying by plurality (Figure). Adjustment for covariates strengthened associations but they did not reach statistical significance (aOR, 1.33; 95% CI, 0.94–1.89; Table 2). Assisted reproductive technology was associated with an increased risk for failing any developmental domain (aOR, 1.81; 95% CI, 1.21–2.72) and, specifically, the personal-social (aOR, 2.03; 95% CI, 1.15–3.59) and problem-solving (aOR, 2.33; 95% CI, 1.15–4.74) domains. However, given the higher probability of failing the ASQ among twins and the higher proportion of twins in the ART group (34% vs 19%) than the unexposed group, results were further stratified by plurality. No statistically significant differences were observed for any of the 5 domains and even when further adjusting for birth weight among singletons or twins.
Figure. Probabilities of Failing the Ages and Stages Questionnaire (ASQ) From 4 to 36 Months by Infertility Treatment Status and Plurality.
The probabilities of failing any ASQ domain and of the specific domains are shown. The longitudinal crude probabilities of failure by infertility treatment status for all children and stratified by plurality were estimated by generalized linear mixed-effects models using ASQ data from 4 to 36 months in the Upstate KIDS Study.
Table 2.
Development Delays According to ASQ Screening From 4 to 36 Months by Infertility Treatment Status in the Upstate KIDS Study (2008–2010)a
| Adjusted Odds Ratio (95% CI) | ||||||
|---|---|---|---|---|---|---|
| All | Singletons | Twins | ||||
| Variable | Model 1b | Model 2c | Model 1b | Model 2c | Model 1b | Model 2c |
| Any Treatment | ||||||
| No. | 5840 | 5840 | 3766 | 3766 | 2074 | 2074 |
| Any fail | 1.33 (0.94–1.89) |
1.17 (0.82–1.68) |
1.10 (0.71–1.69) |
1.15 (0.69–1.91) |
1.42 (0.91–2.20) |
1.28 (0.82–2.01) |
| Fine motor | 1.05 (0.65–1.70) |
1.14 (0.65–1.99) |
0.78 (0.40–1.52) |
1.53 (0.79–2.97) |
1.28 (0.72–2.28) |
1.24 (0.60–2.59) |
| Gross motor | 1.63 (0.60–4.40) |
1.36 (0.79–2.34) |
1.11 (0.58–2.14) |
1.56 (0.78–3.13) |
1.62 (0.89–2.96) |
1.18 (0.64–2.19) |
| Communication | 1.01 (0.58–1.75) |
0.97 (0.53–1.76) |
0.83 (0.41–1.67) |
1.07 (0.49–2.33) |
1.04 (0.54–2.01) |
1.06 (0.63–1.77) |
| Personal-social | 1.45 (0.84–2.52) |
1.27 (0.73–2.21) |
1.16 (0.62–2.16) |
1.36 (0.65–2.84) |
1.47 (0.78–2.79) |
1.39 (0.70–2.75) |
| Problem solving | 1.87 (0.53–6.54) |
1.68 (0.88–3.22) |
1.71 (0.86–3.40) |
1.29 (0.53–3.12) |
1.01 (0.52–1.96) |
0.92 (0.41–2.10) |
| OI/IUI | ||||||
| No. | 4938 | 4938 | 3316 | 3316 | 1622 | 1622 |
| Any fail | 1.16 (0.72–1.86) |
1.10 (0.68–1.77) |
1.00 (0.57–1.77) |
1.05 (0.54–2.02) |
1.30 (0.76–2.21) |
1.14 (0.66–1.97) |
| Fine motor | 0.98 (0.49–1.96) |
1.09 (0.50–2.38) |
0.84 (0.35–2.01) |
1.38 (0.57–3.34) |
0.98 (0.48–1.98) |
0.83 (0.33–2.06) |
| Gross motor | 1.02 (0.49–2.09) |
1.23 (0.64–2.35) |
0.72 (0.30–1.77) |
1.35 (0.61–2.99) |
1.48 (0.69–3.15) |
1.18 (0.55–2.55) |
| Communication | 0.89 (0.42–1.87) |
0.95 (0.43–2.09) |
0.76 (0.31–1.88) |
0.97 (0.38–2.44) |
0.98 (0.51–1.89) |
0.88 (0.45–1.74) |
| Personal-social | 1.16 (0.60–2.25) |
1.08 (0.54–2.19) |
1.01 (0.45–2.27) |
1.22 (0.51–2.94) |
1.14 (0.57–2.28) |
0.90 (0.46–1.74) |
| Problem solving | 1.57 (0.73–3.37) |
1.22 (0.50–3.00) |
1.65 (0.70–3.90) |
1.31 (0.46–3.72) |
0.71 (0.33–1.54) |
0.64 (0.25–1.64) |
| ART | ||||||
| No. | 4913 | 4913 | 3217 | 3217 | 1696 | 1696 |
| Any fail | 1.81 (1.21–2.72) |
1.26 (0.82–1.93) |
1.38 (0.78–2.43) |
1.31 (0.66–2.61) |
1.58 (0.94–2.65) |
1.30 (0.77–2.22) |
| Fine motor | 1.36 (0.56–3.30) |
1.35 (0.28–6.52) |
0.69 (0.38–1.22) |
1.79 (0.94–3.44) |
1.58 (0.81–3.10) |
1.55 (0.66–3.64) |
| Gross motor | 2.87 (0.98–8.41) |
1.53 (0.69–3.38) |
2.23 (0.91–5.45) |
2.01 (0.64–6.26) |
1.68 (0.83–3.42) |
1.13 (0.54–2.39) |
| Communication | 1.26 (0.64–2.49) |
1.02 (0.50–2.09) |
0.82 (0.30–2.29) |
1.20 (0.40–3.64) |
1.12 (0.54–2.32) |
1.08 (0.52–2.23) |
| Personal-social | 2.03 (1.15–3.59) |
1.46 (0.73–2.92) |
1.43 (0.61–3.35) |
1.59 (0.51–4.95) |
1.64 (0.67–4.00) |
1.28 (0.62–2.61) |
| Problem solving | 2.33 (1.15–4.74) |
1.40 (0.56–3.52) |
2.11 (0.80–5.58) |
1.25 (0.26–5.95) |
1.27 (0.58–2.81) |
1.24 (0.44–3.51) |
Abbreviations: ART, assisted reproductive technology; ASQ, Ages and Stages Questionnaire; OI/IUI, ovulation induction/intrauterine insemination.
Reference group consists of children who were not conceived by any infertility treatment (n = 4011 children including 2767 singletons and 1244 twins).
Model 1 adjusted for maternal age, race/ethnicity, education, insurance, married/living as married, prior live birth, and smoking during pregnancy.
Model 2 adjusts for covariates listed in model 1 plus birth weight.
When we linked our cohort with the state’s EIP data, we observed no significant differences in the percentages of children referred for evaluation by infertility treatment status. That is, among 3904 singletons, 21.2% of children conceived with treatment and 20.7% of children not conceived with treatment were referred. Among 1084 nonrelated twins, 40.8% of children conceived with treatment and 38.6% of children not conceived with treatment were referred. In addition, no differences were found in maternal report of children’s use of developmental services (eg, aOR, 1.26; 95% CI,0.69–2.30 for any treatment) (Table 3).
Table 3.
Use of Developmental Services From Maternal Report at 30 and/or 36 Months by Infertility Treatment Exposure in the Upstate KIDS Studya
| Variable | Adjusted Odds Ratios (95% CI) | ||
|---|---|---|---|
| Allb | Singletons | Twins | |
| Unadjusted, No./No.c | 492/2826 | 361/2300 | 131/526 |
| Treatment | 1.15 (0.64–2.05) | 1.02 (0.50–2.08) | 0.82 (0.23–2.88) |
| OI/IUI | 1.00 (0.42–2.39) | 0.87 (0.30–2.48) | 0.82 (0.14–4.67) |
| ART | 1.28 (0.59–2.78) | 1.19 (0.46–3.10) | 0.82 (0.18–3.69) |
| Model 1, No./No.c,d | 488/2790 | 360/2276 | 128/514 |
| Treatment | 1.26 (0.69–2.30) | 1.13 (0.54–2.33) | 1.03 (0.24–4.35) |
| OI/IUI | 1.11 (0.46–2.67) | 0.95 (0.33–2.72) | 1.02 (0.16–6.60) |
| ART | 1.42 (0.64–3.17) | 1.33 (0.50–3.56) | 1.04 (0.19–5.78) |
Abbreviations: ART, assisted reproductive technology; OI/IUI, ovulation induction/intrauterine insemination.
Reference group includes children who were not conceived by any infertility treatment.
Includes all singletons and a random twin of the pair.
Number of children reporting use of services/number of children not reporting use.
Model 1 adjusted for maternal age, race/ethnicity, education, insurance, married/living as married, prior live birth, and smoking during pregnancy.
Among children who completed diagnostic evaluations at 3 to 4 years, 73 (16%) had a developmental disability that included 1 or multiple of the following: language-, learning-, or speech-related disorders (n = 49), ASD (n = 17), attention-deficit/hyperactivity disorder and its subtypes (n = 16), cognitive deficits (n = 6), cerebral palsy (n = 2), and sensory impairments (n = 2). Frequency of any disability did not differ by infertility treatment exposure; 18% of children without treatment (n = 266) and 13% of children conceived by infertility treatment (n = 180) had a diagnosis (P = .15). Similarly, further stratifying by type of treatment showed no differences: 16% among children conceived with OI/IUI (n = 89) having a diagnosis and 11% of children conceived with ART (n = 91) (P = .25).
Discussion
In the first US birth cohort designed specifically to assess infertility treatment and children’s development through 3 years of age, we observed that ART was associated with increased risk for developmental delays but associations were primarily owing to the higher twinning rate associated with ART and were not statistically significant after stratifying by plurality. We also observed no difference in parental reports of children using developmental services or referral for EIP evaluation relative to infertility treatment. Moreover, the prevalence of clinically diagnosed developmental disabilities at 3 to 4 years of age was not associated with infertility treatment in a subsample of children undergoing clinical evaluations. These findings suggest that infertility treatment is not associated with developmental delays through 3 years of age, irrespective of treatment type, after accounting for plurality.
Studies on children conceived by infertility treatment in the United States remain scarce and have predominantly focused on the risk for ASD, mostly finding no association.12,15,18,35 In particular, several analyses based on linkages between the National ART Surveillance System and developmental services among Californian children (born 1997–2006) have been conducted to evaluate autism risk in relation to ART.14,15,20 Although a higher risk was found in association with ART,14 socioeconomic factors related to surveillance may largely explain this finding.15 However, further analyses by the same authors found that ICSI was associated with an increased risk for autism in the first 5 years of life (adjusted hazard ratio, approximately 1.65 among singletons and multiples) compared with in vitro fertilization.20 According to our SART-CORS linkage, more than 70% of ART children were conceived with ICSI and fewer with in vitro fertilization, prohibiting further comparisons of the techniques. Our cohort was systematically followed up through age 3 years and will continue through age 8 years, allowing for a more complete assessment of development including disabilities that may not manifest until later ages.
Our findings of the OI/IUI group suggest that these non-ART treatments are also not likely to lead to large short-term differences in a child’s development (ie, up to 3 years of age). Other studies have also found no statistically significant differences in developmental disorders,17 cognitive development,19 or autism.12,18 A registry study from Denmark suggested a small increased risk for mental disorders associated with OI/IUI.13 Specifically, risks were observed for ASD (hazard ratio, 1.20; 95% CI, 1.05–1.37) but not intellectual disabilities (1.02; 95% CI, 0.81–1.28).13 Although our study could not evaluate autism given the follow-up through 3 years, the ASQ was designed to screen for developmental delays associated with intellectual disabilities or ASD.36 However, given our relatively smaller sample size in comparison with the study from Denmark (approximately 33 000 exposed), we were unable to rule out weak associations with certainty, although some of the ASQ domains were found to have associations in the 1.20 range for OI/IUI.
Of note, the ASQ was designed as a screening not diagnostic instrument, and its concurrent validity in comparison with other clinically administered developmental assessment tools varies.23,26,27,37,38 For the Upstate KIDS Study, cutoffs at 2 SDs below the average were used to maximize specificity and minimize overreferral that might result in undue parental distress. A small Iranian study using the ASQ at 60 months similarly reported no differences in the failure of domains among 61 singlet on term infants conceived by ART compared with 61 infants spontaneously conceived after adjusting for education and birth weight.39
Our findings are strengthened by the multiple methods used to evaluate childhood development (which is important given that children can develop in an uneven manner), the demonstrated validity of maternal-reported ART treatment data compared with clinical data from SART-CORS,22 and our ability to link children with the state’s EIP. Still, our study had limitations including attrition over time, particularly for the unexposed cohort and twins, and missing data stemming from ASQs being completed by parents outside of established age ranges as observed in a previous study.28 However, we accounted for missing data via generalized linear mixed-effects modeling40 and by inverse probability weighting for missing responses.34 The sample sizes of the 2 specific treatment groups, particularly among twins, were under powered to detect small associations that could not be ruled out by our analysis. The low initial enrollment rate and its difference by exposure status (32% among exposed vs 26% among nonexposed) may limit generalizability.
Conclusions
We found no evidence suggesting that children’s development through age 3 years—using a standardized parental rating instrument and augmented by registry linkage for developmental services—is associated with any type of infertility treatment in comparison with children conceived without such treatments after accounting for plurality. The elevated probability of delay associated with multiple births remains a risk to be weighed given the higher twinning rate after use of ART. Continued follow-up of children conceived by infertility treatment is needed to ensure the absence of later-onset conditions.
Supplementary Material
At a Glance.
The Upstate KIDS Study, which recruited mothers and their children from New York state (excluding New York City) between 2008 and 2010, was designed to assess the association between the mode of conception and children’s development through age 3 years.
Use of any infertility treatment was not associated with risk for failing any developmental domain as assessed by the Ages and Stages Questionnaire.
Specific treatment using ovulation induction with or without intrauterine insemination was not associated with failing any developmental domain.
Conception by assisted reproductive technology was associated with failing any domain, but not significantly, after accounting for plurality.
No difference in parental reports of children using developmental services or referral for Early Intervention Program evaluation was found relative to infertility treatment or the specific types.
Acknowledgments
Funding/Support: This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts HHSN275201200005C and HHSN267200700019C).
Role of the Funder/Sponsor: The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: We thank all of the Upstate KIDS Study participants and staff for their important contributions, as well as Donna M. Noyes, PhD, Kirsten Siegenthaler, PhD, MSPH, and Daniel Kellis from the New York State Department of Health, Bureau of Early Intervention who performed the New York State Early Intervention Program linkage and provided aggregated data frequencies. None received compensation for their contribution.
Footnotes
Author Contributions: Drs Yeung and Xie had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bell, Kus, Buck Louis.
Acquisition, analysis, or interpretation of data: Yeung, Sundaram, Bell, Druschel, Ghassabian, Bello, Xie, Buck Louis.
Drafting of the manuscript: Yeung, Buck Louis.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Yeung, Sundaram, Bell, Xie.
Obtained funding: Druschel, Buck Louis.
Administrative, technical, or material support: Yeung, Bell, Kus, Ghassabian, Buck Louis.
Study supervision: Yeung.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Barfield WD Centers for Disease Control and Prevention (CDC) Assisted reproductive technology surveillance: United States, 2011. MMWR Surveill Summ. 2014;63(10):1–28. [PubMed] [Google Scholar]
- 2.Kupka MS, Ferraretti AP, de Mouzon J, et al. European IVF-Monitoring Consortium; for the European Society of Human Reproduction and Embryology. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE. Hum Reprod. 2014;29(10):2099–2113. doi: 10.1093/humrep/deu175. [DOI] [PubMed] [Google Scholar]
- 3.Schieve LA, Devine O, Boyle CA, Petrini JR, Warner L. Estimation of the contribution of non-assisted reproductive technology ovulation stimulation fertility treatments to US singleton and multiple births. Am J Epidemiol. 2009;170(11):1396–1407. doi: 10.1093/aje/kwp281. [DOI] [PubMed] [Google Scholar]
- 4.Duwe KN, Reefhuis J, Honein MA, Schieve LA, Rasmussen SA. Epidemiology of fertility treatment use among US women with liveborn infants, 1997–2004. J Womens Health (Larchmt) 2010;19(3):407–416. doi: 10.1089/jwh.2009.1499. [DOI] [PubMed] [Google Scholar]
- 5.Nelissen EC, Van Montfoort AP, Coonen E, et al. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod. 2012;27(7):1966–1976. doi: 10.1093/humrep/des145. [DOI] [PubMed] [Google Scholar]
- 6.Sandin S, Nygren KG, Iliadou A, Hultman CM, Reichenberg A. Autism and mental retardation among offspring born after in vitro fertilization. JAMA. 2013;310(1):75–84. doi: 10.1001/jama.2013.7222. [DOI] [PubMed] [Google Scholar]
- 7.Devroey P, Bourgain C, Macklon NS, Fauser BC. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15(2):84–90. doi: 10.1016/j.tem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Ombelet W, Martens G, De Sutter P, et al. Perinatal outcome of 12,021 singleton and 3108 twin births after non-IVF-assisted reproduction: a cohort study. Hum Reprod. 2006;21(4):1025–1032. doi: 10.1093/humrep/dei419. [DOI] [PubMed] [Google Scholar]
- 9.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A Knowledge Synthesis Group. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146(2):138–148. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 10.Hediger ML, Bell EM, Druschel CM, Buck Louis GM. Assisted reproductive technologies and children's neurodevelopmental outcomes. Fertil Steril. 2013;99(2):311–317. doi: 10.1016/j.fertnstert.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart R, Norman RJ. The longer-term health outcomes for children born as a result of IVF treatment, part II: mental health and development outcomes. Hum Reprod Update. 2013;19(3):244–250. doi: 10.1093/humupd/dmt002. [DOI] [PubMed] [Google Scholar]
- 12.Lyall K, Baker A, Hertz-Picciotto I, Walker CK. Infertility and its treatments in association with autism spectrum disorders: a review and results from the CHARGE Study. Int J Environ Res Public Health. 2013;10(8):3715–3734. doi: 10.3390/ijerph10083715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bay B, Mortensen EL, Hvidtjorn D, Kesmodel US. Fertility treatment and risk of childhood and adolescent mental disorders: register based cohort study. BMJ. 2013;347:f3978. doi: 10.1136/bmj.f3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fountain C, Zhang Y, Kissin DM, et al. Association between assisted reproductive technology conception and autism in California, 1997–2007. Am J Public Health. 2015;105(5):963–971. doi: 10.2105/AJPH.2014.302383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schieve LA, Fountain C, Boulet SL, et al. Does autism diagnosis age or symptom severity differ among children according to whether assisted reproductive technology was used to achieve pregnancy? J Autism Dev Disord. 2015;45(9):2991–3003. doi: 10.1007/s10803-015-2462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehti V, Brown AS, Gissler M, Rihko M, Suominen A, Sourander A. Autism spectrum disorders in IVF children: a national case-control study in Finland. Hum Reprod. 2013;28(3):812–818. doi: 10.1093/humrep/des430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klemetti R, Sevón T, Gissler M, Hemminki E. Health of children born as a result of in vitro fertilization. Pediatrics. 2006;118(5):1819–1827. doi: 10.1542/peds.2006-0735. [DOI] [PubMed] [Google Scholar]
- 18.Lyall K, Pauls DL, Spiegelman D, Santangelo SL, Ascherio A. Fertility therapies, infertility and autism spectrum disorders in the Nurses' Health Study II. Paediatr Perinat Epidemiol. 2012;26(4):361–372. doi: 10.1111/j.1365-3016.2012.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carson C, Kelly Y, Kurinczuk JJ, Sacker A, Redshaw M, Quigley MA. Effect of pregnancy planning and fertility treatment on cognitive outcomes in children at ages 3 and 5: longitudinal cohort study. BMJ. 2011;343:d4473. doi: 10.1136/bmj.d4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kissin DM, Zhang Y, Boulet SL, et al. Association of assisted reproductive technology (ART) treatment and parental infertility diagnosis with autism in ART-conceived children. Hum Reprod. 2015;30(2):454–465. doi: 10.1093/humrep/deu338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buck Louis GM, Hediger ML, Bell EM, et al. Methodology for establishing a population-based birth cohort focusing on couple fertility and children’s development: the Upstate KIDS Study. Paediatr Perinat Epidemiol. 2014;28(3):191–202. doi: 10.1111/ppe.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buck Louis GM, Druschel C, Bell E, et al. Use of assisted reproductive technology treatment as reported by mothers in comparison with registry data: the Upstate KIDS Study. Fertil Steril. 2015;103(6):1461–1468. doi: 10.1016/j.fertnstert.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gollenberg AL, Lynch CD, Jackson LW, McGuinness BM, Msall ME. Concurrent validity of the parent-completed Ages and Stages Questionnaires, 2nd Ed with the Bayley Scales of Infant Development II in a low-risk sample. Child Care Health Dev. 2010;36(4):485–490. doi: 10.1111/j.1365-2214.2009.01041.x. [DOI] [PubMed] [Google Scholar]
- 24.Schonhaut L, Armijo I, Schönstedt M, Alvarez J, Cordero M. Validity of the ages and stages questionnaires in term and preterm infants. Pediatrics. 2013;131(5):e1468–e1474. doi: 10.1542/peds.2012-3313. [DOI] [PubMed] [Google Scholar]
- 25.Guevara JP, Gerdes M, Localio R, et al. Effectiveness of developmental screening in an urban setting. Pediatrics. 2013;131(1):30–37. doi: 10.1542/peds.2012-0765. [DOI] [PubMed] [Google Scholar]
- 26.Squires J, Bricker D. Ages & Stages Questionnaires, Third Edition (ASQ-3) Baltimore, MD: Brookes Publishing; 2009. [Google Scholar]
- 27.Squires J, Potter L, Bricker D. The ASQ User’s Guide for the Ages & Stages Questionnaires: A Parent-Completed, Child-Monitoring System. Baltimore, MD: Paul H. Brookes Publishing Co; 1999. [Google Scholar]
- 28.Education of the Handicapped Act Amendments of 1986. Pub L No.99-457, 100 Stat 1145. [Google Scholar]
- 29.Robins DL, Fein D, Barton ML. The Modified Checklist for Autism in Toddlers (M-CHAT) Storrs, CT: Self-published; 1999. [Google Scholar]
- 30.Almgren M, Schlinzig T, Gomez-Cabrero D, et al. Cesarean delivery and hematopoietic stem cell epigenetics in the newborn infant: implications for future health? Am J Obstet Gynecol. 2014;211(5):502.e1–502.e8. doi: 10.1016/j.ajog.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Potijk MR, Kerstjens JM, Bos AF, Reijneveld SA, de Winter AF. Developmental delay in moderately preterm-born children with low socioeconomic status: risks multiply. J Pediatr. 2013;163(5):1289–1295. doi: 10.1016/j.jpeds.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Julvez J, Ribas-Fitó N, Torrent M, Forns M, Garcia-Esteban R, Sunyer J. Maternal smoking habits and cognitive development of children at age 4 years in a population-based birth cohort. Int J Epidemiol. 2007;36(4):825–832. doi: 10.1093/ije/dym107. [DOI] [PubMed] [Google Scholar]
- 33.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 35.Ackerman S, Wenegrat J, Rettew D, Althoff R, Bernier R. No increase in autism-associated genetic events in children conceived by assisted reproduction. Fertil Steril. 2014;102(2):388–393. doi: 10.1016/j.fertnstert.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardy S, Haisley L, Manning C, Fein D. Can screening with the Ages and Stages Questionnaire detect autism? J Dev Behav Pediatr. 2015;36(7):536–543. doi: 10.1097/DBP.0000000000000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steenis LJ, Verhoeven M, Hessen DJ, van Baar AL. Parental and professional assessment of early child development: the ASQ-3 and the Bayley-III-NL. Early Hum Dev. 2015;91(3):217–225. doi: 10.1016/j.earlhumdev.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Simard MN, Luu TM, Gosselin J. Concurrent validity of ages and stages questionnaires in preterm infants. Pediatrics. 2012;130(1):e108–e114. doi: 10.1542/peds.2011-3532. [DOI] [PubMed] [Google Scholar]
- 39.Fallah R, Akhavan Karbasi S, Galalian MT, Dehghani-Firouzabadi R. Comparison of developmental status of 5-year-old singleton children born through assisted and natural conceptions. Iran J Reprod Med. 2013;11(5):365–370. [PMC free article] [PubMed] [Google Scholar]
- 40.Molenberghs GV. Models for Discrete Longitudinal Data. New York, NY: Springer Science+Business Media Inc; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.