Summary
Polymorphonuclear neutrophils (PMNs) are innate immune system cells that play an essential role in eradicating invading pathogens. PMN migration to sites of infection/inflammation requires exiting the microcirculation and subsequent crossing of epithelial barriers in mucosa-lined organs such as the lungs and intestines. Although these processes usually occur without significant damage to surrounding host tissues, dysregulated/excessive PMN transmigration and resultant bystander-tissue damage are characteristic of numerous mucosal inflammatory disorders. Mechanisms controlling PMN extravasation have been well characterized, but the molecular details regarding regulation of PMN migration across mucosal epithelia are poorly understood. Given that PMN migration across mucosal epithelia is strongly correlated with disease symptoms in many inflammatory mucosal disorders, enhanced understanding of the mechanisms regulating PMN transepithelial migration should provide insights into clinically relevant tissue-targeted therapies aimed at ameliorating PMN-mediated bystander-tissue damage. This review will highlight current understanding of the molecular interactions between PMNs and mucosal epithelia and the associated functional consequences.
Keywords: Neutrophil, Transepithelial Migration, Mucosal Inflammation
Function of neutrophils during homeostasis and infection/inflammation
Polymorphonuclear neutrophils (PMNs) play a critical role in the innate immune response, representing an essential component of the first line of host defense against invading microbial pathogens. Patients with neutropenia, chronic granulomatous disease (CGD), or leukocyte deficiency syndrome, who are prone to recurrent bacterial and fungal infections, exemplify the critical importance of PMN-mediated phagocytosis and pathogen clearance(1). Similar to other leukocytes, PMNs are derived from pluripotent stem cell progenitors in the bone marrow. Although PMNs have traditionally been considered relatively short-lived effector cells that exit the bone marrow and circulate for about 1.5 hours in mice and 8 hours in humans before undergoing spontaneous apoptosis(2, 3), recent research has indicated that under basal conditions, the half-life of human PMNs can be as long as 5.4 days(4). However, these data remain controversial due to concerns that the presence of contaminating immature bone marrow PMNs in the analyzed samples may have led to overestimation of PMN longevity(5, 6).
Under normal physiologic conditions (i.e., in the absence of inflammation), PMNs are primarily cleared from the circulation in the liver, spleen, and bone marrow(7). Increased C-X-C motif chemokine receptor 4 (CXCR4 CXC) expression in older PMNs directs the cells back to the bone marrow, where they are safely eliminated. Interestingly, it has been suggested that terminal trafficking of aged PMNs into the intestinal tract occurs and that this helps maintain intestinal homeostasis and regulation of commensal microflora(8). It has also been reported that under certain conditions, activated PMNs act as long-lived antigen-presenting cells. For example, activated PMNs isolated from the synovial fluid of rheumatoid arthritis patients express major histocompatibility complex class II (MHC class II), CD80, CD86, and stimulate T cell proliferation(9, 10).
During inflammation, PMNs are activated, resulting in a delay in constitutive PMN apoptosis(11). PMN activation during inflammation can be viewed as a two-step process. First, factors such as bacteria-derived molecules or cytokines/chemokines (including tumor necrosis factor-α [TNF-α], granulocyte-macrophage colony-stimulating factor [GM-CSF], interleukin [IL]-8, and interferon [IFN]-γ(12)) prime quiescent PMNs, which are subsequently mobilized to sites of infection or inflammation, where they encounter additional activating signals that trigger the killing of microbes(13). This delay in apoptosis and increase in PMN lifespan in inflamed tissues indicates that PMNs play a role in the immune response to infection/inflammation that goes beyond their well-described roles in phagocytosis and killing of invading microbes. Indeed, it is now well accepted that once recruited into inflamed tissues, PMNs act as regulators of both the innate and adaptive immune responses by engaging in complex bi-directional interactions with macrophages, natural killer cells, B cells, and T cells(14, 15).
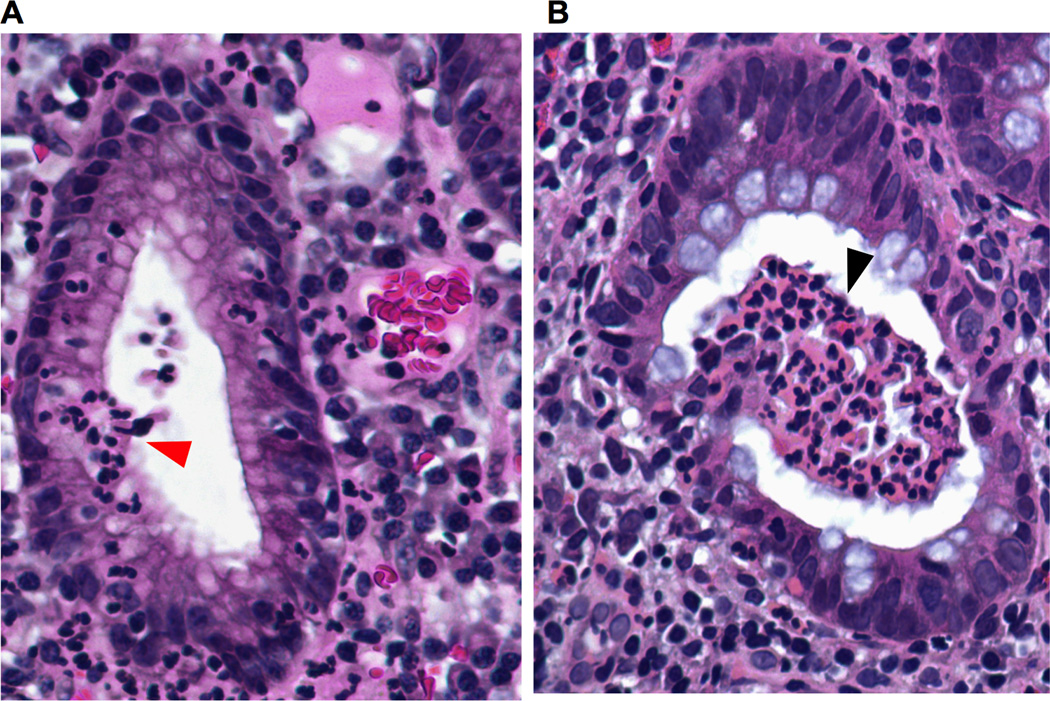
PMNs release a number of cytokines, chemokines, alarmins, proteases, growth factors, and IFNs into the inflammatory milieu, thus playing important roles in eliciting and sustaining inflammation, as well as contributing significantly to the regulation of key immune responses during infection(16–22). The continuous migration of large numbers of cytokine-producing PMNs across the mucosal epithelia can lead to locally high concentrations of cytokines, including (but not limited to) IL-1α, IL-1β, and TNF-α(23), as well as other inflammatory mediators in close proximity to the mucosal epithelium. Although PMNs reportedly produce cytokines in the picogram to nanogram levels in vitro (24, 25), in vivo cytokine production by PMNs has yet to be quantified. However, observations indicating that PMNs are the predominant infiltrating cells during the initial responses to inflammation (outnumbering other leukocytes by several orders of magnitude) strongly suggest that PMNs are an important source of pro-inflammatory mediators at inflamed mucosal epithelial surfaces (Fig. 1A, B).
Fig 1.
Recent evidence has revealed that in addition to their roles in the generation and amplification of inflammatory responses, PMNs also contribute to inflammation resolution and restoration of tissue homeostasis during later stages of the inflammatory response(26). PMN-mediated resolution of inflammation and restoration of tissue homeostasis is facilitated in part through the biosynthesis of growth factors, including vascular endothelial growth factor and lipid mediators such as lipoxins, resolvins, and protectins(27, 28). PMNs also play a role in wound cleaning through the phagocytosis and removal of accumulated cell debris(29). The importance of PMNs during the resolution of inflammation is highlighted by the observation that depletion of PMNs results in exacerbated tissue destruction during colitis in vivo(30). However, despite their importance in inflammation resolution, dysregulated transepithelial migration (TEM) of PMNs and associated bystander-tissue damage is also associated with a range of inflammatory disorders, including inflammatory bowel disease (IBD) and chronic obstructive pulmonary disease (COPD). Therefore, elucidation of the mechanism underlying PMN trafficking into tissues during inflammation/infection represents an important topic for research.
PMN migration across vascular endothelium and interstitium
PMN migration from the microcirculation to the tissues is a complex, multistep process. In the initial step, circulating PMNs exit the vascular endothelium. The sequential nature of PMN transendothelial migration involving the subsequent well-defined steps of tethering, slow rolling, arrest, adhesion, crawling, and paracellular (or transcellular) migration have been extensively characterized and are reviewed elsewhere(31–34). Following transendothelial migration, PMNs must migrate across the basement membrane and pericyte sheath before crossing through the interstitial space(35, 36). The basement membrane (BM) consists of a 300-nm-thick specialized network of extracellular matrix macromolecules that include proteins, glycoproteins, and proteoglycans underlying both endothelial and epithelial cells. The mechanism by which PMNs transmigrate across the BM remains largely uncharacterized, but a role for PMN proteinases in this process has been suggested(37, 38).
Similar to the mechanism of transmigration across the BM, PMN migration through the interstitium remains largely uncharacterized, although the process is reportedly independent of focal adhesions and pericellular proteolysis(39). In addition, interstitial migration of PMNs in response to pro-inflammatory signals, including chemokine (C-C motif) ligand 3 (CCL3) also known as macrophage inflammatory protein 1-alpha (MIP1α), reportedly requires actin polymerization and matrix metalloproteinase (MMP) activation(40). PMNs in the extravascular space reportedly exhibit extremely coordinated chemotaxis behaviors, forming clusters reminiscent of the swarming behaviors of insects. Using a model of sterile skin inflammation, Lammermann et al. demonstrated that local cell death initiates PMN swarming and interstitial recruitment that is dependent on the lipid leukotriene B4 (LTB4), but independent of β2 integrins(41). Although the mechanism of integrin-independent movement of PMNs through the interstitium has yet to be fully characterized, it has been shown that the binding of LTB4 to its G-protein–coupled receptor BLT4 amplifies signaling pathways that lead to F-actin production and changes in PMN polarization that directly guide chemotaxis(42). Integrin-independent migration of PMNs through the interstitium is plausible because this process does not require the crossing of significant tissue barriers (such as continuous endothelial or epithelial linings)(42). Therefore, it follows that the interstitial environment would support stochastic PMN chemotaxis, with migrating cells following soluble cues, such as LTB4, without a need for integrin-mediated adhesion interactions.
PMN transepithelial migration cascade
Following movement through the interstitium, PMNs reach the subepithelial space (in mucosal lined organs such as the lungs and intestine). In the presence of a chemotactic stimulus, PMNs then cross the epithelial barrier before reaching sites of mucosal infection/inflammation. This final migration of PMNs across an epithelial barrier represents a critically important (and generally understudied) step in the immune response, as PMN-mediated epithelial damage and loss of epithelial barrier function are directly correlated with the symptoms of a variety of inflammatory diseases. Indeed, during PMN TEM, unabated activation of PMNs by microbial or host-derived stimuli can result in the release of cytotoxic compounds that damage vicinal epithelial cells. Specifically, both human and animal model studies have shown that PMNs are the primary perpetrators of inflammation-related injury to tissues of the lung(43), urinary tract(44), and intestines(45, 46). Despite the potential tissue damage PMNs can cause, the mechanisms regulating their transepithelial migration remain incompletely understood.
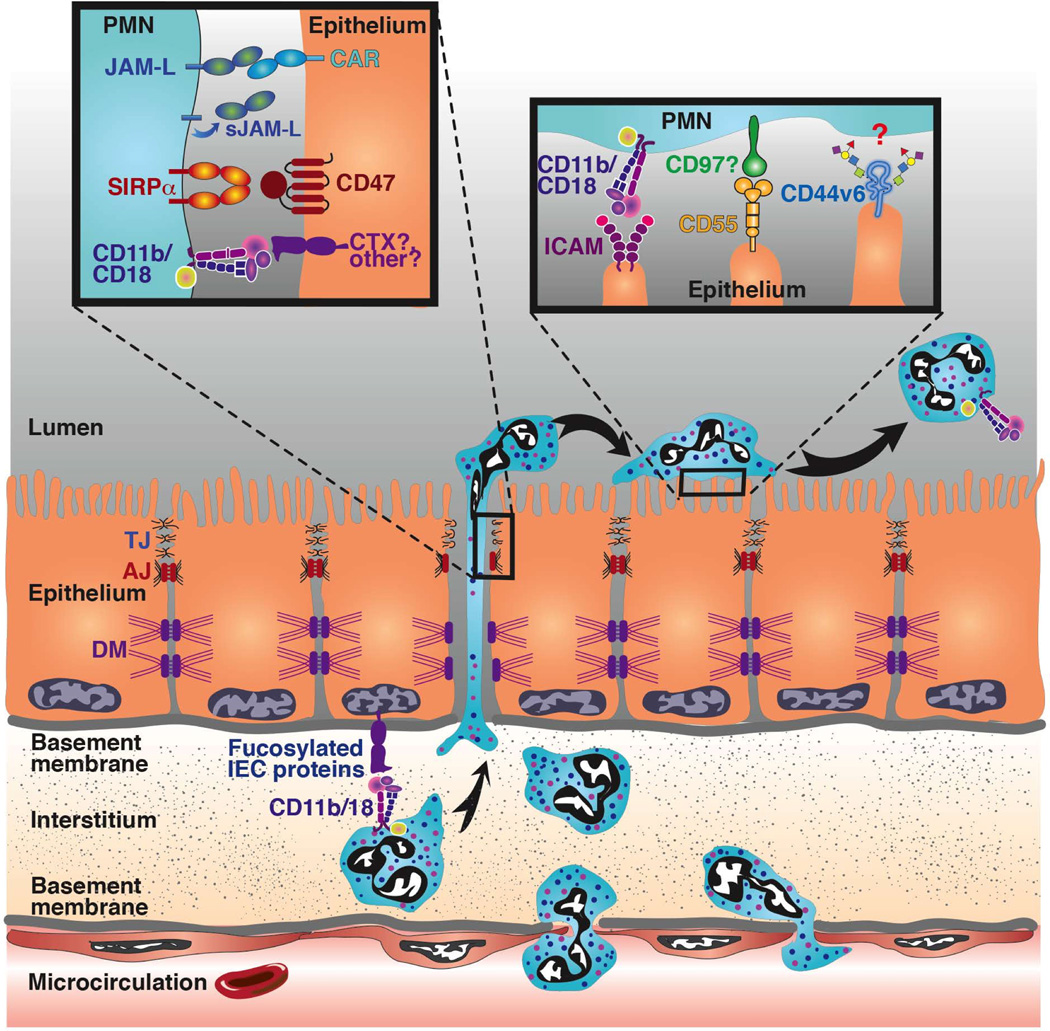
Analogous to PMN transendothelial migration, PMN TEM can also occur in sequential steps that encompass initial adhesion to the epithelium, migration between epithelial cells, and postmigration events at the apical epithelial surface (Fig. 2). However, in contrast to migration from the microcirculation, initial PMN adhesive interactions occur at the basolateral rather than apical epithelial surface. In addition, PMNs arrive at the basolateral epithelium having already crossed the vascular endothelium and migrated through the interstitium, processes that undoubtedly alter the PMN activation state. Another key feature of epithelial PMN trafficking is the considerable length of the intraepithelial space (≥20 µm) that migrating PMNs must traverse to breach the epithelial barrier. Furthermore, this distance is significantly greater than the equivalent space in endothelial cells, which is 2–4 µm at most(47).
Fig 2.
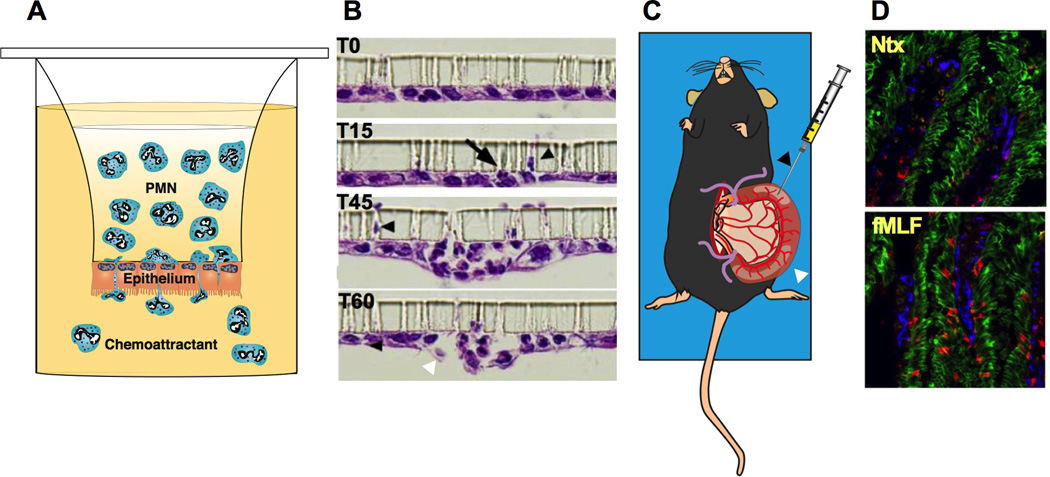
Although much of our knowledge of PMN trafficking across epithelia derives from in vitro studies of PMN migration across layers of cultured epithelial cells, the process has also been studied in vivo(48–50). Systems designed to model the polarity of physiologically relevant basolateral to apical PMN TEM are used to grow epithelial cells in an upside-down/inverted configuration on collagen-coated transwell filters with pore sizes sufficient for PMNs to squeeze through (3–5 µm) (Fig. 3A, B)(51–53). These in vitro PMN migration assay systems paved the way for important investigations of the molecular events and functional biology of PMN TEM in many distinct epithelial cell types(49, 54–58). More recently, in an in vivo model of PMN TEM into the intestinal lumen, a short section of vascularized small intestine was isolated and then injected with various cytokines/chemoattractants that induce translocation of PMNs to the intestinal lumen (Fig. 3C, D)(49, 50). This ‘ileal loop’ model facilitated further investigations of the roles of specific epithelial proteins during PMN TEM, as well as PMN TEM–mediated changes, in the epithelial barrier in vivo(49, 50).
Fig 3.
Interaction of PMNs with basolateral epithelial ligands
PMNs migrating across an epithelial barrier first interact with the basolateral epithelial surface. PMN adhesion to the basolateral epithelium in the lungs, intestines, and urinary tract depends predominantly on CD11b/CD18(52, 59, 60), which is in contrast to PMN endothelial trafficking, where PMNs interact with both CD11a/CD18 and CD11b/CD18(61, 62). However, CD11b/CD18-independent migration of PMNs reportedly occurs under certain circumstances such as at bronchial and alveolar epithelial surfaces. This lung-specific, CD11b/CD18-independent migration might be explained by the particular physiology of the lung, in which PMNs emigrate primarily through capillaries, where the vascular pressure is lower and the PMNs are in closer proximity to the epithelium. However, it has been well documented that anti-CD11b/CD18 antibodies almost completely block PMN trafficking across both the intestinal and airway epithelia, demonstrating that interaction with β2 integrins is a prerequisite for PMN TEM(51–53) under most circumstances. In addition, specific pro-inflammatory stimuli, including TNF-α, histamine, and IFN-γ, increase PMN epithelial adhesive interactions in a CD11b/CD18-dependent fashion(63, 64). Reports that patients with the autosomal recessive disorder leukocyte adhesion deficiency 1 (LAD1), who lack functional CD18, suffer from recurrent mucosal bacterial infections(65) further demonstrates the essential role of CD11b/CD18 in PMN TEM.
CD11b/CD18 is the most promiscuous member of the integrin family, with more than 30 reported ligands(66–91) (Table 1), yet epithelial receptors for this integrin are poorly understood. Classical receptors for CD11b/CD18 include intercellular adhesion molecules 1 and 2(71), complement C3 fragment (67), fibrinogen(92), and several plasma proteins and vascular cell receptors, such as kininogen(88), factor X(66), factor H(72), glycoprotein 1bα(85), urokinase receptor (86), and E-selectin(77). Soluble ligands for CD11b/CD18 have also been reported and include fibronectin(79), neutrophil inhibitory factor(78), laminin(87), collagen(89), zymosan(81), and vitronectin(76). The extensive family of CD11b/CD18 ligands also includes the PMN enzymes elastase(68) and myeloperoxidase(75). Finally, CD11b/CD18 also binds to ligands expressed by several microbial species, including Candida albicans(73), Leishmania promastigotes(82), and Bordetella pertussis(80).
Table 1.
| CDllb/CD18 Ligand | Glycosylation Status |
|---|---|
| Adhesion molecules | |
| ICAM 1 (71) | Glycoprotein |
| ICAM 2 (71) | Glycoprotein |
| ICAM 3 (71) | Glycoprotein |
| Plasma Protein and vascular cell receptors | |
| Complement C3 fragment (iC3b)(67) | Glycoprotein |
| Fibrinogen (91) | Glycoprotein |
| Neutrophil Inhibitory Factor (78) | Glycoprotein |
| Kininogen (88) | Glycoprotein |
| Factor X (66) | Glycoprotein |
| Complement Factor H (72) | Glycoprotein |
| Glycoprotein lb (85) | Glycoprotein |
| Urokinase Receptor (86) | Glycoprotein |
| E Selectin (77) | Glycan-binding protein |
| Extracellular Matrix Proteins | |
| Fibronectin (79) | Glycoprotein |
| Laminin (87) | Glycoprotein |
| Collagen (89) | Not reported |
| Vitronectin (76) | Glycoprotein |
| CYR61/CCN1 (83) | Glycoprotein |
| Microbial Receptors | |
| Candida albicans hyphae (73) | Glycan mediated binding |
| Leishmania promastigotes protein gp63 (82) | Glycoprotein |
| Bordetella pertussis hemagglutinin (80) | Glycoprotein |
| Zymosan (81) | Glycoprotein |
| Enzymes | |
| Elastase (68) | Glycoprotein |
| Myeloperoxidase (75) | Glycoprotein |
| Catalase (69) | Glycoprotein |
| Other | |
| Transferrin (69) | Glycoprotein |
| Casein (69) | Glycoprotein |
| Soybean trypsin inhibitor (69) | Not reported |
| Bovine serum albumin (74) | Not reported |
| Ovalbumin (74) | Glycoprotein |
| Limpet hemocyanin (84) | Glycoprotein |
| Non-protein ligands | |
| Heparin (70) | Glycoprotein |
| β-glucan (90) | Glycoprotein |
| Non-coated plastic (74) | Not Glycosylated |
Intriguingly, despite the wide array of identified CD11b/CD18 ligands, basolaterally expressed, functionally relevant epithelial ligands for CD11b/CD18 that regulate PMN adhesion have yet to be identified. Given the presence of a lectin-like motif between the I domain and C-terminal regions of the extracellular domain (ECD) of CD11b(81, 90), it is tempting to speculate that epithelial cell surface glycans mediate PMN adhesion via binding to CD11b/CD18. In support of this hypothesis, the lectin-like domain of murine CD11b/CD18 was shown to bind to the polysaccharide structure β-glucan, which is present in the cell walls of yeast, bacteria, and fungi(90). Additionally, pretreatment of epithelial cells with various polysaccharides (mannose-6P, glucose-6P, and fucoidin) significantly inhibits PMN TEM(93). The L-fucose–rich polysaccharide fucoidin binds directly to CD11b/CD18 and potently inhibits epithelial adhesion to CD11b/CD18 purified from human PMNs. Adhesion of T84 intestinal epithelial cells (IECs) to PMN CD11b/CD18 is significantly reduced following treatment with p-nitrophenyl-β-D-xylanopyranoside, an inhibitor of proteoglycan biosynthesis. Furthermore, treatment of T84 IECs with fucosidase (but not neuraminidase) significantly reduces adhesion of the IECs to CD11b/CD18, suggesting that fucosylated proteoglycans are the epithelial ligands for PMN CD11b/CD18(94). Taken together, these data suggest that epithelial cell surface proteoglycans decorated with fucose sugar moieties play an important role in CD11b/CD18-mediated PMN adhesion. Although a number of epithelial surface glycoproteins and glycolipids display terminal fucose residues, the exact identity of the CD11b/CD18 ligand remains to be determined.
Another mechanism that could explain the binding between CD11b/CD18 and the epithelial cell surface involves carbohydrate-binding, epithelial membrane–associated proteins such as galectins(95), which can recognize and bind to β-galactose epitopes on PMN CD11b/CD18 molecules. This hypothesis is supported by the fact that by binding to and cross-linking cell surface glycoconjugates, galectins regulate numerous cell processes, including apoptosis, cytokine secretion, cell adhesion, and migration(96). The cell surface–associated galectins 1, 3, 4, and 9 are expressed in human epithelia(97–99). Furthermore, human CD11b/CD18 is extensively decorated with glycoconjugates, including sialyl Lewis X (sLex, Neu5Acα2-3Galβ1-4[Fucα1-3]GlcNAcβ-R) and Lewis X Galβ1-4 (Lex, Fucα1-3)GlcNAcβ-R), which have terminal β-galactose glycans(55, 100, 101). Finally, expression of galectin 3, which promotes inflammatory responses through the activation of naïve and primed PMNs, is altered in the intestinal mucosa of patients with IBD(102).
CD11b/CD18 is the best-characterized PMN ligand known to mediate the adhesion of PMNs to the basolateral surface of the epithelium, with few reports to date of other binding interactions mediating this process. However, PMN interaction with the basolateral epithelial surface results in important functional responses, such as loosening of the epithelial barrier, which facilitates subsequent PMN migration. Specifically in the intestine, contact between PMNs and the basolateral aspect of the epithelium increases myosin light-chain kinase phosphorylation and barrier permeability, independent of redistribution or loss of tight-junction (TJ) proteins(103). Mechanistically, basolaterally adherent PMNs reportedly activate the epithelial protease-activated receptors 1 and 2, which leads to a myosin light chain kinase (MLCK)-dependent increase in intestinal permeability(104).
PMN migration between epithelial cells
Following PMN adherence to the basolateral epithelial surface, the next step in TEM is migration through the lateral paracellular space (Fig. 2). It is well documented that PMN TEM occurs exclusively through a paracellular (as opposed to transcellular) pathway, as was reported for transendothelial migration(105–107). PMNs also reportedly migrate between epithelial cells in clusters (Figs. 1A, 3B)(52, 108). PMNs navigating the paracellular space between epithelial cells encounter a variety of lateral membrane adhesion receptors. One of the cell surface receptors involved in this process is the immunoglobulin superfamily member CD47, which is expressed on both PMNs and the basolateral epithelial surface. Importantly, expression of CD47 on PMNs is up-regulated by pro-inflammatory stimuli, enhanced PMN TEM was shown to correlate with increased epithelial expression of CD47(56), and expression of CD47 is up-regulated in the intestinal mucosa of persons with IBD(109). Furthermore, antibody-mediated inhibition of CD47 or its PMN binding partner, signal regulatory protein α (Sirpα)(110), significantly delays PMN TEM in vitro(56, 111). In addition, a study utilizing a murine model of Escherichia coli–induced peritonitis in CD47-deficient mice demonstrated decreased PMN trafficking, resulting in increased bacterial growth and subsequent mortality(112). Although, the mechanism(s) by which CD47 mediates PMN TEM have yet to be fully elucidated, it is known that PMN CD47 plays a crucial role in this process, as antibody-mediated ligation of PMN-expressed CD47 inhibits migration(56). Mechanistically, PMN-expressed CD47 binding in cis to Sirpα regulates PMN TEM, presumably by signaling through SHP-1 and SHP-2(113). Although PMN-expressed CD47 plays a clearly established role in TEM, PMN TEM across CD47-deficient epithelial cells increases following transfection and induced expression of CD47, suggesting that epithelial CD47 also plays a role in PMN trafficking(56). Taken together, these data suggest that CD47-Sirpα interactions function in controlling the rate of PMN trafficking through tissues via bi-directional signaling events that direct migration and retention of PMNs at sites of infection/inflammation. In addition to its role in PMN trafficking CD47 has been implicated as a marker of self, acting as an important ‘don’t eat me’ signal on a number of cells(114). PMN undergoing apoptosis have decreased expression of CD47(115) and, following phagocytosis of Staphyloccus aureus PMN increase the surface expression of CD47, which results in deceased macrophage mediated PMN efferocytosis(116). However the effect of altered expression of CD47 on PMN and how this might regulate PMN clearance from mucosal tissues and contribute to non-resolving inflammation responses requires further investigation.
Similar to the interactions observed between CD47 and Sirpα, PMN surface proteins interact with intercellular junctional molecules as the cells move between adjoining epithelial cells. The initial migration of PMNs during an acute inflammatory response usually results in a transient decrease in epithelial permeability, followed by barrier restoration and a return to mucosal homeostasis(57, 106, 107). However, uncontrolled or pathologic PMN TEM often results in more persistent disruption of key junctional proteins, leading to serious interruptions in epithelial barrier function, which are associated with the symptoms of a range of inflammatory diseases(117). Therefore, it is important to understand how migrating PMNs interact with epithelial junctional molecules.
PMN Interaction with Desmosomal Proteins
Desmosomes (DMs) represent a formidable barrier to migrating PMNs as they first enter the epithelial paracellular space. DMs are punctate structures that provide mechanical strength to the epithelium via transmembrane complexes formed between desmoglein-2 (Dsg-2) and desmocollin-2, which are anchored to keratin intermediate filaments(118). The importance of DM proteins in regulating cell-cell contacts within the epithelial barrier is well established. Indeed, loss of desmoglein-2, desmocolin-2, and desmoplakin expression in individuals with IBD results in significantly perturbed epithelial barrier function(117). Although direct binding of PMNs to DMs has not been reported, inflammation-induced shedding of the ECD of desmoglein-2 results in impairment of intestinal epithelial barrier function. MMP-9 and a disintegrin and metalloproteinase domain–containing protein 10 (ADAM 10) are sheddases that cleave Dsg-2, resulting in the release of Dsg ECDs from IECs(119). As migrating PMNs secrete both MMP-9(120) and ADAM-10(121), it is tempting to speculate that PMN-mediated shedding of Dsg-2 contributes to epithelial barrier disruption and facilitates the migration of additional immune cells during acute inflammatory responses. This hypothesis is supported by the observation that ligation of Dsg-2 results in activation of PI3K and Erk1/2, leading to the loss of epithelial cell barrier functions(122). Furthermore, cleavage products of Dsg-2 actively regulate cellular processes such as apoptosis and differentiation, thus influencing the delicate balance of epithelial homeostasis(123).
PMN interaction with adherens junction and tight-junction complexes
As PMNs migrate along the paracellular space toward the epithelial lumen, they next encounter the apical junction complex (AJC), which encompasses adherens junctions (AJs) and TJs (Fig. 2). The AJC forms a tight seal between adjacent epithelial cells, creating a selective barrier that separates luminal contents from the surrounding tissue. AJs are formed through homophilic interactions between extracellular epithelial (E)-cadherin and various intracellular proteins, including the catenin family members p120-catenin and β-catenin. Both E-cadherin and catenin family proteins play important roles in the maintenance of epithelial barrier integrity, which is disrupted during PMN TEM. Loss of p120-catenin, for example, results in concomitant loss of E-cadherin and an aberrant PMN-mediated inflammatory response during bacterial colonization(124). PMN TEM across several types of epithelia depletes both E-cadherin and β-catenin from epithelial cell membranes, thus altering barrier integrity. For example, in a model of inflammatory PMN infiltration of the airway epithelium, the ECD of E-cadherin was shown to be cleaved by neutrophil elastase (NE), resulting in the destabilization of AJs(125, 126). Similarly, influx of PMNs across the intestinal epithelium is associated with changes in the localization of E-cadherin and β-catenin, with subsequent AJ disruption(125).
The epithelial adhesion complexes encountered by migrating PMNs localized to the apical-most aspect of the lateral plasma membrane during the later stages of PMN TEM are the TJs. TJs form a tight barrier between the outside world and body tissues, while also preventing the intermixing of apical and basolateral epithelial membrane components. Structurally, TJs are composed of a series of anastomosing intramembranous strands associated with proteins that include members of the claudin family, CTX family proteins including junction adhesion molecules (JAMs), Coxsackie adenovirus receptor (CAR), and CAR-like protein, as well as occludin, tricellulin, and other immunoglobulin family members(127). The binding interactions between migrating PMNs and individual TJ proteins remain poorly characterized, despite the fact that TJ proteins are well positioned to interact with migrating PMNs. However, some binding interactions between PMNs and TJ proteins have been described and will be highlighted below.
The JAM family contains many important TJ proteins that perform tissue-specific functions governed by protein distribution patterns, including regulation of the epithelial barrier(128), endothelial and epithelial cell migration(129), angiogenesis(129), and leukocyte adhesion(130). One member of the JAM family, designated JAM-like molecule (JAML), reportedly exhibits a restricted expression pattern, largely limited to myelomonocytic cells, including PMNs(131). PMN JAML also selectively binds to epithelial TJ-expressed CAR(132). These observations strongly suggest that JAML may function as an adhesion receptor on PMNs used as the cells cross CAR-expressing epithelial TJs (Fig. 2). The demonstration that recombinant ECDs of JAML bind to epithelial CAR and inhibit PMN TEM supports this hypothesis(132). A subsequent study demonstrated that JAML is shed by migrating PMNs and binds to epithelial CAR, initiating signaling events that inhibit barrier recovery and epithelial wound healing in a pro-inflammatory manner(133). Other studies in mice have shown that JAML is also expressed by γδ T cells and interacts with keratinocyte-expressed CAR to promote wound healing in the skin(134). The observed differences in epithelial responses between the gut and skin after JAML-CAR ligation could be because the skin responses were secondary to stimulated growth factor release from T cells downstream of JAML ligation, whereas the intestinal epithelial responses were downstream of epithelial-expressed CAR ligation.
Another member of the JAM family, designated JAM-A, plays a role in regulating PMN migration across the endothelium(135, 136). Interestingly, although JAM-A is abundantly expressed at epithelial TJs, antibody-mediated blockade of JAM-A has no significant effect on PMN TEM(137). The surprising finding that JAM-A does not play a direct role in regulating PMN TEM paved the way for investigations of the function of JAM-A in epithelial cells. It is now well-appreciated that JAM-A regulates epithelial permeability, as knockout animals exhibit ‘leaky’ intestines(138). Also, JAM-A–deficient animals do not get spontaneous colitis, despite having leaky intestines and enhanced bacterial translocation. Other studies revealed that remarkable adaptive immune compensation, which results in elevated tissue and fecal IgA levels, serves to protect JAM-A–deficient mice from developing pathologic colonic inflammation(139).
Another transmembrane TJ protein that has been implicated in regulating PMN TEM is occludin, a tetraspan protein that reportedly modulates transepithelial migration of PMNs, as modifications (mutations) to the N-terminus of occludin lead to enhanced TEM in vitro(140). However, it is unclear whether this enhanced TEM is a direct effect of the protein modification or secondary to mutation-induced alterations in epithelial signaling. In endothelial systems, PMN transmigration reportedly occurs at tricellular junctions regulated by the transmembrane protein tricellulin, which regulates paracellular macromolecule movement(141). Unfortunately, migration of PMNs across epithelial tricellular junctions has not been reported.
Although the roles of specific TJ proteins in regulating PMN trafficking remain incompletely defined, it is clear that migrating PMNs, as well as a cytokine-rich inflammatory milieu, disrupt the TJ structure and thus compromise the epithelial barrier function, which are well-appreciated features of IBD. In particular, migration of PMNs into the intestinal mucosa results in the loss of key TJ proteins, including occludin and zonula occludens (ZO)-1(142), along with disorganization of TJs. Such loss of TJ-associated molecules is not restricted to sites immediately adjacent to transmigrating PMNs but also occurs at sites distant from migrating PMNs. These observations are now explained by numerous studies that have demonstrated potent effects of inflammatory cytokines on epithelial TJs and barrier function. Epithelial cells respond to certain cytokines, such as IFN-ϒ, by selectively internalizing TJ-associated molecules in stimulus-, protein-, and pathway-specific manners that result in compromised barrier function (reviewed elsewhere(143)). As expected, inflammatory conditions that result in barrier compromise initiate a vicious cycle that facilitates further PMN accumulation and the mucosal tissue destruction that is characteristic of disorders including IBD and COPD.
Apical epithelial ligands that interact with migrating PMNs
After navigating the paracellular space between adjacent epithelial cells, PMNs emerge on the apical or luminal membrane (Fig. 2). In the intestines, the microanatomy of the colon (which consists of test-tube shaped crypts that invaginate deeply into the mucosa) facilitates the retention of migrated PMNs at the apical surface, allowing them to interact with epithelial ligands. Furthermore, during colitis, migrated PMNs collect preferentially in the crypt base, which is relatively sterile (compared to the lumen) and provides physical protection from the digestive stream. Recent evidence indicated that post-migrated PMNs on the apical epithelial surface can significantly affect epithelial function. Release of 5’-AMP by PMNs triggers electrogenic chloride secretion, resulting in passive movement of water into the intestinal lumen, a process that forms the molecular basis of secretory diarrhea(144). In addition, during TEM, PMNs trigger the release of E-cadherin fragments at the apical epithelial surface (in an NE-dependent fashion), resulting in a spatially delimited disruption of the AJC(125).
PMN interaction with the apical epithelium represents a critical control point during inflammation, as PMN association with apical epithelial surfaces and accumulation in luminal spaces is closely associated with deleterious symptoms in numerous inflammatory disorders, including acute lung injury (ACI)(145), cystic fibrosis (CF)(146), and IBD(109, 147). Interactions of PMNs with the apical surface are facilitated by inflammation-induced up-regulation of adhesion molecules. For example, intercellular adhesion molecule 1 (ICAM-1) expression is reportedly increased in the epithelium of colonic biopsies from individuals with IBD, as well as in model IEC lines stimulated with pro-inflammatory cytokines. Importantly, ICAM-1 is a ligand for PMN CD11b/CD18 (Table 1) (71), and is expressed predominantly on the apical surface of epithelial cells(148), thus making it an ideal ‘tethering post’ for migrated PMNs at mucosal surfaces. However, a direct role for ICAM-1 during physiologic basolateral to apical PMN TEM has yet to be demonstrated, despite the fact that ICAM-1 binding to PMN CD11b/CD18 facilitates migration in the non-physiologic apical to basolateral direction(148). Clues to the functional significance of apically expressed ICAM-1 have recently been reported. Increased epithelial ICAM-1 expression was shown to enhance the retention of PMNs at the apical epithelial surface(50). In addition, apically localized PMNs exhibit ICAM-1– and CD11b/CD18-dependent increased locomotion. Ligation of ICAM-1 triggers MLCK-dependent actomyosin contractility, resulting in increased epithelial permeability, which in turn facilitates increased recruitment of PMNs into the intestinal lumen. It is easy to imagine that such signaling interactions between ICAM-1 and PMNs(50) would be beneficial for the clearance of invading pathogens during infection. However, such signaling under pathologic conditions could have deleterious effects resulting from increased accumulation of PMNs and associated mucosal injury.
In an analogous fashion to that described above for ICAM-1, increased expression of other apical PMN ligands has also been described in inflamed epithelial tissues. Specifically, epithelial expression of CD55 (decay accelerating factor)(149) increases during inflammation(150, 151). Although epithelial ICAM-1 has been implicated in retention of PMNs at the apical epithelial surface, in contrast, CD55 reportedly mediates rapid detachment of migrated PMNs into the intestinal lumen(58). CD55 was first identified as an inhibitor of complement-mediated cell lysis through interference with C3 and C5 convertase function(152). CD55 (which is highly expressed on the apical aspect of mucosal epithelial cells) also acts as a ligand for pathogenic bacteria, including E. coli(153). Furthermore, CD55 induces downstream epithelial signaling, resulting in NFκB nuclear translocation and activation of mitogen-activated protein kinases (MAPK)-signaling. Although the binding interactions between migrating PMNs and epithelial CD55 have not been fully characterized, it is possible that epithelial-expressed CD55 interacts with its PMN ligand, CD97(154), during PMN trafficking into inflamed intestinal mucosa. In support of this hypothesis, in vivo PMN trafficking to the intestinal mucosa is reportedly reduced when CD97 is inhibited(154).
Role of cell surface glycans in PMN interactions with epithelia
In addition to protein-protein binding interactions regulating PMN trafficking, post-translational glycosylation modifications also control some ligand-receptor recognition interactions during PMN migration from the microcirculation. Glycosylation modifies protein function, both through steric influences and the generation of specific lectin-binding glycan motifs(155, 156). For example, P-selectin glycoprotein ligand 1 is a heavily glycosylated PMN-expressed protein that regulates PMN rolling along the vascular endothelium during inflammatory responses in vivo(157). In addition, Endothelial P- and E-selectin contain binding sites for PMN fucose-containing glycans, including sLex (Neu5Acα2-3Galβ1-4[Fucα1-3]GlcNAcβ-R) and the related glycan Lex (Galβ1-4[Fucα1-3]GlcNAcβ-R)(155, 158, 159). E-Selectin also interacts with specific glycans on the PMN glycoproteins leukosialin and CD44(160, 161). Although selectins are not required for PMN migration across the epithelium (due to the absence of PMN rolling during this process), increasing evidence suggests that other glycan-mediated interactions between PMNs and the epithelium are important for TEM.
Antibodies that specifically recognize the glycan sialyl Lewis A (sLea, Neu5Acα2-3Galβ1-3[Fucα1-4]GlcNAcβ-R) displayed on the epithelial glycoprotein CD44v6 potently inhibit PMN TEM(54). Furthermore, antibody-mediated ligation of sLea prevents shedding of the highly glycosylated ECD of CD44v6 and inhibits detachment of migrated PMNs, resulting in PMN accumulation at the apical epithelial surface. It is tempting to speculate that during PMN TEM, proteolytic enzymes such as MMPs or ADAMs, secreted by migrating PMNs, cleave the CD44v6 ECD, in turn facilitating the detachment of migrating PMNs from the apical epithelial surface into the lumen of the intestine. Okomoto et al. showed that shedding of the highly glycosylated ECD of CD44v6 leads to subsequent presenilin-1/γ-secretase–mediated generation of an intracellular CD44v6 fragment that translocates to the nucleus and promotes transcription through the 12-O-tetradecanoylphorbol-13-acetate–responsive element(162). Therefore, PMN-mediated shedding of the CD44 ECD could represent a key regulatory event for CD44v6 function, with important epithelial signal transduction consequences during inflammation. In addition, although normal epithelium does not express CD44v6/sLea, there is marked expression of both CD44v6 and sLea in inflamed mucosa from individuals with IBD(49). Therefore, sLea may represent a potential therapeutic target to reduce symptoms resulting from uncontrolled PMN influx into the intestines, as well as a potential biomarker for active intestinal inflammatory processes.
In addition to effects downstream of antibody-mediated ligation of epithelial sLea, it was recently reported that targeting of PMN-expressed Lex (Galβ1-4[Fucα1-3]GlcNAcβ-R) also blocks PMN TEM(55). Intriguingly, the Lex-mediated effect on PMN trafficking is specific to PMN epithelial interactions, as ligation of PMN Lex has no effect on PMN migration across endothelial monolayers(55). These observations implicate specific cell surface glycans as key regulators of PMN TEM (and other cellular processes), in part through the generation and masking of Lewis family glycans and other glycan ligands for endogenous lectins, including galectins and selectins. Once secreted from cells (including epithelial cells)(163), the β-galactoside–binding lectin galectin-3 binds to glycoconjugate ligands at the cell surface, where it oligomerizes into a lattice that cross-links cell surface glycoproteins and activates key immune responses, including reactive oxygen species (ROS) production by PMNs, degranulation of mast cells, and chemoattraction of monocytes and macrophages(164–166). In addition, galectin-3 acts as a receptor that mediates PMN adhesion to endothelial cells(167, 168) and laminin(169). In the epithelium, galectin-3 participates in the regulation of epithelial cell apoptosis and proliferation during inflammatory responses(170). Galectin-3 also binds to desmoglein-2 and stabilizes desmosomal cadherins and intercellular adhesion between adjacent IECs(171). Interestingly, immunohistochemical analyses of Streptococcus pneumoniae–infected lung tissue revealed increased association of galectin-3 with the lung epithelial mucosa(167). Therefore, through the cross-linking and activation of glycoproteins, galectin-3 regulates a range of immunomodulatory processes through as of yet poorly defined glycan-mediated mechanisms.
In addition to galectin-3, epithelial cells also express other members of the galectin family, including galectin-1(97) and galectin-9(98). However, in contrast to galectin-3, galectin-1 reportedly exhibits anti-inflammatory activity. Specifically, a study involving a mouse model of paw edema showed that binding of galectin-1 to its glycoside ligand is associated with reduced PMN influx(172), whereas another study demonstrated reduced PMN recruitment in response to IL-8 in an in vitro assay of PMN chemotaxis(173). Beneficial in vivo epithelial-specific effects of galectin-1 have been reported. For example, prophylactic administration of galectin-1 reduces T cell activation, pro-inflammatory cytokine production, and disease progression during 2,4,6-trinitrobenzene sulfonic acid (TNBS) –induced experimental colitis in mice(174). An in vivo model of gram-negative bacterial lung sepsis was used to show that galectin-9−/− mice exhibit less extensive tissue damage and improved lung pathology associated with reduced PMN recruitment(98), thus supporting hypotheses that galectins play important roles during inflammation-induced PMN trafficking.
In addition to galectin-mediated effects on PMN trafficking, other cell surface–expressed glycans also play important roles during leukocyte recruitment. For example, core 2 oligosaccharides generated by the glycosyltransferase core 2-β1,6-N-acetlyglucosaminyltransferase play a role in PMN trafficking to the peritoneum(175). In addition, chemokines such as CXCL8 and MIP-2 bind to (and then slowly dissociate from) glycosaminoglycans in vivo, thus forming spatiotemporal chemokine gradients that facilitate PMN recruitment into inflamed tissues of the lung(176).
Patients with LAD2, who present with neutrophilia, recurrent bacterial infections without pus formation, and chronic gingivitis, exemplify the importance of glycans during PMN trafficking(177). PMNs from these patients do not express the L-selectin–binding ligand sLex and thus fail to migrate into inflamed tissues. Similarly, mice deficient in the specific fucosyltransferases IV and VII exhibit the same deficits in PMN trafficking as selectin-deficient mice(178). Although these defects in sLex production have been linked to impaired PMN trafficking from the microcirculation, specific deficiencies in glycosylation also influence PMN TEM. For example, expression of the mucin-like protein mucosal addressin molecule-1 (MAdCAM-1) is reportedly increased in the mucosal epithelial tissues of individuals with ulcerative colitis(179). Interestingly, increased disease activity in these colitic patients is associated with increased expression of the glycan sulfotransferase GlcNAcGST-1, which preferentially modifies MAdCAM and is thought to contribute to the generation of the glycoepitope sLex(180).
PMN function in inflamed mucosal tissues
During infection by enteropathogenic microbes such as Escherichia coli and Salmonella typhimurium, PMNs undergoing TEM encounter invading microbes present in mucosal tissues and respond to these pathogens with rapid phagocytosis and degranulation(181). PMNs kill invading pathogens that have been engulfed in phagosomes through NADPH oxygenase–dependent mechanisms (e.g., ROS) and release of anti-bacterial proteins such as cathepsins, defensins, lactoferrin, and lysozyme. Similar to recent reports of glycan-mediated effects on PMN TEM, it has been reported that PMN degranulation and phagocytosis can be triggered by engagement of PMN surface glycans. For example, following migration across human intestinal epithelium, PMN surface expression of the Lewis glycan family member Lex increases. Further, targeting of surface Lex glycans (expressed in a terminal position at the ends of glycan chains) on PMNs increases both the rate of phagocytosis and surface expression of CD63 and CD66b, which are markers of primary and specific PMN granules, respectively(55). Thus, glycan-mediated interactions appear to be fundamentally important in PMN trafficking from the vasculature, PMN crossing of epithelial barriers, and augmentation of PMN effector functions at sites of mucosal infection/inflammation.
Dysregulated PMN TEM and tissue damage
PMN-mediated destruction of invading microbes (as described above) is essential for successful immune responses. In addition, during the late stages of acute inflammation, infiltrating PMNs communicate with other inflammatory cells within the mucosal tissues in ways that influence the local microenvironment to promote tissue restitution, wound healing, and homeostasis. However, during persistent inflammatory responses, ongoing pro-inflammatory signaling results in persistent influx of activated PMNs and a delay in the restitution response. Migration of large numbers of activated PMNs results in tissue damage due to the formation of epithelial wounds caused by forced separation and injury of epithelial cells(57, 107, 125). These epithelial wounds are thought to be precursors of the large areas of denuded mucosa or ulcerated epithelium typical of inflammatory disorders such as IBD and ALI/acute respiratory distress syndrome (ARDS). In addition to the mechanical damage caused by large numbers of translocating PMNs, soluble mediators from the migrating PMN arsenal have also been implicated in the disruption of the epithelial barrier and exacerbation of mucosal inflammation. Thus, bystander-tissue damage caused by the release of proteases such as elastase, enzymes such as myeloperoxidase, ROS, and other toxic PMN metabolites contributes directly to the pathobiology of many inflammatory diseases, including rheumatoid arthritis, IBD, CF, and COPD(142, 182–184).
One of the most-studied PMN metabolites is the serine proteinase NE, which is stored predominantly in azurophilic or primary PMN granules. Importantly, high levels of NE in bronchoalveolar lavage (BAL) fluid from patients with ALI/ARDS are correlated with disease severity(185). In addition, in vivo administration of NE results in severe lung damage. Furthermore, elastase inhibition is protective against lung damage, as measured by variables such as permeability of the alveolocapillary membrane(186, 187). NE has also been implicated in the pathology of both CF and COPD; significantly elevated NE levels at infectious sites in pneumonia patients have also been reported(188). In addition to pulmonary inflammation, NE has also been implicated in the pathogenesis of IBD. In fact, both fecal and plasma levels of NE reportedly can be used as markers of disease activity in IBD(189, 190). NE levels and activation are increased during colitis in vivo, potentially contributing to disease progression through the loss of epithelial barrier function(191). Similarly, concentrations of NE are increased in the urine of individuals with interstitial cystitis, a condition characterized by chronic inflammation of the submucosal and muscular layers of the bladder(192). It has been suggested that effects of NE involve disruption of junction-associated proteins such as E-cadherin, enabling further loosening of cell-cell contacts by migrating PMNs, in turn triggering further loss of epithelial barrier function and promoting subsequent migration of trailing leukocytes(125).
PMNs in the mucosal tissues also secrete numerous MMPs that degrade most components of the extracellular matrix, which is a major constituent of the subepithelial space. As well as exerting specific effects on epithelial integrity, increased expression of MMPs derived both from PMNs and macrophages has been implicated in the tissue injury associated with ALI/ARDS(193). Consistent with the above observations, MMP inhibition is protective in experimental models of lung injury(194, 195). Increased detection of MMP-3, MMP-9, and MMP-12 in the inflamed intestinal mucosa of individuals with IBD has also been reported(196, 197). Elevated levels of MMP-9 are associated with mucosal tissue damage and fistula formation in individuals with Crohn’s disease(198), and MMP-9 deficiency or inhibition is correlated with improved outcome and reduced tissue damage in animal models of IBD(199, 200). MMPs reportedly degrade important junction-associated molecules, including E-cadherin and occludin, thus contributing to junction disassembly and destabilization of the epithelial barrier(201, 202). Despite these observations, the discrete effects of individual PMN MMPs on targeting specific junctional proteins during PMN trafficking have yet to be fully characterized.
Besides MMPs, activated PMNs also release small, cationic, arginine-rich peptides, including human neutrophil peptides (HNPs)/defensins, which exhibit antimicrobial activity against both gram-positive and gram-negative bacteria (as well as enveloped viruses and fungi) through permeabilization of the cell membrane(203). Aside from antimicrobial activities, HNPs induce permeability changes in mucosal epithelia through both cytotoxic and non-cytotoxic mechanisms. HNP concentrations are elevated in the BAL fluid of individuals with ARDS and CF. Furthermore, in vivo administration of defensins results in direct increases in lung permeability and injury. Consistent with this observation, intraperitoneal administration of human alpha-defensin 1 (HNP1) to mice during dextran sulfate sodium-induced colitis leads to a more severe disease response, with altered cytokine levels, suggesting a pro-inflammatory role for PMN HNP1 during mucosal inflammation in the intestine(204). Thus, it is not surprising that plasma concentrations of HNP1–3 are significantly increased in individuals with IBD, possibly due to increased expression of HNPs by circulating PMNs(205). Collectively, these data suggest that HNPs play a pathogenic role in diseases characterized by PMN-mediated injury to mucosal epithelia.
Similar to what is described for PMN-produced proteinases and HNPs, PMN-derived oxygen metabolites have been implicated in the mucosal damage associated with dysregulated PMN influx. PMN oxidant–mediated tissue injury is a key factor in the pathogenesis of ARDS, and PMN-derived injurious oxidants induce lung injury in animal models of ALI. ROS and oxidant-induced protein or lipid alterations have been implicated in the pathogenesis of IBD(206), in which intestinal mucosal biopsies revealed increased ROS production during inflammation(207). PMN-produced ROS can be particularly damaging to the intestinal epithelium, as the plasma membrane of IECs contains large amounts of polyunsaturated fatty acids that are highly susceptible to damage by oxidizing radicals(208). In support of the hypothesis that PMN-derived ROS cause tissue damage during intestinal inflammation, PMNs from individuals with IBD reportedly generate increased levels of ROS compared with PMNs from healthy donors(209, 210). . In addition to PMN produced ROS, dual oxidase 2 (DUOX2) an epithelial specific NAPDH oxidase that generates H2O2/ROS is highly expressed in gut epithelium and plays an important role in innate host defense against luminal bacteria(211). Further, DUOX2 expression is markedly upregulated in the intestines of individuals with irritable bowel syndrome and in the inflamed mucosa of individuals with ulcerative colitis and Crohn’s disease suggesting that increased expression of epithelial DUOX2 contributes to mucosal dysbiosis(212).
Through complex mechanisms, reactive oxygen metabolites increase epithelial permeability via disruption of TJs and rearrangement of junctional proteins(213, 214) thus playing an important role in the bystander-tissue damage associated with IBD, COPD, and other inflammatory diseases. However, the ability of PMNs from patients with CGD to efficiently migrate across epithelial barriers suggests that oxidant-associated junction disruption is not an absolute requirement for PMN TEM(105). Interestingly IBD resembling Crohn’s disease occurs in about one third of CGD patients(215) and functional genetic variants in NOX2 (not severe enough to result in CGD) have been associated with very early onset IBD(216). Taken together these data suggest that the absence of PMN-derived oxidants changes the mucosal response to inflammation possibly through increased activation of PMN proteases. In support of this hypothesis CGD mice (lacking a complete respiratory burst) develop accentuated TNBS-mediated colitis (associated with increased PMN infiltration and decreased inflammatory hypoxia) compared to TNBS treated control mice(217).
In addition to PMN-produced proteinases and ROS, PMNs also reportedly release metal-chelating proteins that contribute to mucosal tissue damage at sites of infection(218). One such chelating protein, neutrophil gelatinase-associated lipocalin (NGAL), captures and depletes siderophores, thus depleting levels of available iron and reducing bacterial growth during active infection(219). However, NGAL also binds to and activates MMP-9, leading to increased degradation of collagen and increased tissue damage at sites of inflammation(220). Such increased expression of NGAL has been reported in acute and chronic kidney injury(221), COPD(222), and various cancers(223), as well as in the colonic mucosa, urine, and feces of individuals with IBD(224, 225).
Conclusions
Although there has been progress in elucidating the mechanisms that regulate PMN interactions with epithelial cells, the complex nature of the interplay between migrating PMN and mucosal epithelial as well as the myriad of epithelial functions (including barrier function, ion and water flux, gas exchange and nutrient absorption) means that this challenging area remains generally understudied. Further investigation of the pathobiology of PMN TEM is of major importance, as underscored by the strong link between PMN trafficking across epithelial barriers and the pathology of numerous diseases. Understanding the complex nature of PMN interactions with epithelial cells during PMN trafficking will likely pave the way for tissue-targeted pharmaceutical approaches aimed at ameliorating the consequences of dysregulated inflammation in diseases such as COPD and IBD.
Acknowledgments
Research in the author’s laboratories is supported by funding from the NIH (DK61739, DK72564, DK79392), DoD (PR121194P1) and Crohn’s and Colitis Foundation of America. The authors wish to thank Robin Kunkel for assistance in figure preparation. The authors have no conflicts of interests to declare.
References
- 1.Lakshman R, Finn A. Neutrophil disorders and their management. J Clin Pathol. 2001;54:7–19. doi: 10.1136/jcp.54.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dancey JT, Deubelbeiss KA, Harker LA, Finch CA. Neutrophil kinetics in man. J Clin Invest. 1976;58:705–715. doi: 10.1172/JCI108517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillay J, et al. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood. 2010;116:625–627. doi: 10.1182/blood-2010-01-259028. [DOI] [PubMed] [Google Scholar]
- 5.Tofts PS, Chevassut T, Cutajar M, Dowell NG, Peters AM. Doubts concerning the recently reported human neutrophil lifespan of 5.4 days. Blood. 2011;117:6050–6052. doi: 10.1182/blood-2010-10-310532. author reply 6053–6054. [DOI] [PubMed] [Google Scholar]
- 6.Li KW, Turner SM, Emson CL, Hellerstein MK, Dale DC. Deuterium and neutrophil kinetics. Blood. 2011;117:6052–6053. doi: 10.1182/blood-2010-12-322271. author reply 6053–6054. [DOI] [PubMed] [Google Scholar]
- 7.Hong C, et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J Clin Invest. 2012;122:337–347. doi: 10.1172/JCI58393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubes P, Hunter J, Granger DN. Ischemia/reperfusion-induced feline intestinal dysfunction: importance of granulocyte recruitment. Gastroenterology. 1992;103:807–812. doi: 10.1016/0016-5085(92)90010-v. [DOI] [PubMed] [Google Scholar]
- 9.Cross A, Bucknall RC, Cassatella MA, Edwards SW, Moots RJ. Synovial fluid neutrophils transcribe and express class II major histocompatibility complex molecules in rheumatoid arthritis. Arthritis and rheumatism. 2003;48:2796–2806. doi: 10.1002/art.11253. [DOI] [PubMed] [Google Scholar]
- 10.Gosselin EJ, Wardwell K, Rigby WF, Guyre PM. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. Journal of immunology. 1993;151:1482–1490. [PubMed] [Google Scholar]
- 11.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 12.Hallett MB, Lloyds D. Neutrophil priming: the cellular signals that say ‘amber’ but not ‘green’. Immunol Today. 1995;16:264–268. doi: 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- 13.Liles WC, Ledbetter JA, Waltersdorph AW, Klebanoff SJ. Cross-linking of CD18 primes human neutrophils for activation of the respiratory burst in response to specific stimuli: implications for adhesion-dependent physiological responses in neutrophils. Journal of leukocyte biology. 1995;58:690–697. doi: 10.1002/jlb.58.6.690. [DOI] [PubMed] [Google Scholar]
- 14.Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. The Journal of experimental medicine. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim K, et al. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science. 2015;349:aaa4352. doi: 10.1126/science.aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brazil JC, Louis NA, Parkos CA. The role of polymorphonuclear leukocyte trafficking in the perpetuation of inflammation during inflammatory bowel disease. Inflammatory bowel diseases. 2013;19:1556–1565. doi: 10.1097/MIB.0b013e318281f54e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galbraith GM, Steed RB, Sanders JJ, Pandey JP. Tumor necrosis factor alpha production by oral leukocytes: influence of tumor necrosis factor genotype. J Periodontol. 1998;69:428–433. doi: 10.1902/jop.1998.69.4.428. [DOI] [PubMed] [Google Scholar]
- 18.Gainet J, Chollet-Martin S, Brion M, Hakim J, Gougerot-Pocidalo MA, Elbim C. Interleukin-8 production by polymorphonuclear neutrophils in patients with rapidly progressive periodontitis: an amplifying loop of polymorphonuclear neutrophil activation. Lab Invest. 1998;78:755–762. [PubMed] [Google Scholar]
- 19.Sweeney JF, Rosemurgy AS, Wei S, Djeu JY. Intact autocrine activation and cytokine production by PMNs from injured adults with elevated Candida antigen titres. Injury. 1998;29:35–40. doi: 10.1016/s0020-1383(97)00123-x. [DOI] [PubMed] [Google Scholar]
- 20.Jablonska E. Serum levels of tumor necrosis factor alpha and production of this cytokine by polymorphonuclear cells in breast cancer patients. Arch Immunol Ther Exp (Warsz) 1998;46:93–96. [PubMed] [Google Scholar]
- 21.Jablonska E, Pietruska Z. Changes in soluble IL-6 receptor and IL-6 production by polymorphonuclear cells and whole blood cells of breast cancer patients. Arch Immunol Ther Exp (Warsz) 1998;46:25–29. [PubMed] [Google Scholar]
- 22.Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv Immunol. 1999;73:369–509. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 23.Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–556. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- 24.Brandt E, Woerly G, Younes AB, Loiseau S, Capron M. IL-4 production by human polymorphonuclear neutrophils. Journal of leukocyte biology. 2000;68:125–130. [PubMed] [Google Scholar]
- 25.Van Dervort AL, et al. Nitric oxide regulates endotoxin-induced TNF-alpha production by human neutrophils. Journal of immunology. 1994;152:4102–4109. [PubMed] [Google Scholar]
- 26.Zemans RL, et al. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15990–15995. doi: 10.1073/pnas.1110144108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sylvia CJ. The role of neutrophil apoptosis in influencing tissue repair. J Wound Care. 2003;12:13–16. doi: 10.12968/jowc.2003.12.1.26458. [DOI] [PubMed] [Google Scholar]
- 29.Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- 30.Colgan SP. Targeting hypoxia in inflammatory bowel disease. J Investig Med. 2016;64:364–368. doi: 10.1097/JIM.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller WA. Getting leukocytes to the site of inflammation. Vet Pathol. 2013;50:7–22. doi: 10.1177/0300985812469883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews Immunology. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 33.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nature medicine. 2011;17:1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voisin MB, Woodfin A, Nourshargh S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler Thromb Vasc Biol. 2009;29:1193–1199. doi: 10.1161/ATVBAHA.109.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang S, et al. Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS One. 2012;7:e45499. doi: 10.1371/journal.pone.0045499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nature reviews Immunology. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- 38.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends in cell biology. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Nourshargh S, Hordijk PL, Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 40.Lerchenberger M, et al. Matrix metalloproteinases modulate ameboid-like migration of neutrophils through inflamed interstitial tissue. Blood. 2013;122:770–780. doi: 10.1182/blood-2012-12-472944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lammermann T, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lammermann T, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 43.Worthen GS, Haslett C, Rees AJ, Gumbay RS, Henson JE, Henson PM. Neutrophil-mediated pulmonary vascular injury. Synergistic effect of trace amounts of lipopolysaccharide and neutrophil stimuli on vascular permeability and neutrophil sequestration in the lung. Am Rev Respir Dis. 1987;136:19–28. doi: 10.1164/ajrccm/136.1.19. [DOI] [PubMed] [Google Scholar]
- 44.Lau ME, Loughman JA, Hunstad DA. YbcL of uropathogenic Escherichia coli suppresses transepithelial neutrophil migration. Infect Immun. 2012;80:4123–4132. doi: 10.1128/IAI.00801-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saverymuttu SH, Chadwick VS, Hodgson HJF. Granulocyte Migration in Ulcerative-Colitis. European Journal of Clinical Investigation. 1985;15:60–63. doi: 10.1111/j.1365-2362.1985.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 46.Scholmerich J, Schmidt E, Schumichen C, Billmann P, Schmidt H, Gerok W. Scintigraphic assessment of bowel involvement and disease activity in Crohn’s disease using technetium 99m–hexamethyl propylene amine oxine as leukocyte label. Gastroenterology. 1988;95:1287–1293. doi: 10.1016/0016-5085(88)90363-0. [DOI] [PubMed] [Google Scholar]
- 47.Parkos CA. Cell adhesion and migration. I. Neutrophil adhesive interactions with intestinal epithelium. Am J Physiol. 1997;273:G763–G768. doi: 10.1152/ajpgi.1997.273.4.G763. [DOI] [PubMed] [Google Scholar]
- 48.Walker DC, Behzad AR, Chu F. Neutrophil migration through preexisting holes in the basal laminae of alveolar capillaries and epithelium during streptococcal pneumonia. Microvasc Res. 1995;50:397–416. doi: 10.1006/mvre.1995.1067. [DOI] [PubMed] [Google Scholar]
- 49.Brazil JC, et al. alpha3/4 Fucosyltransferase 3-dependent synthesis of Sialyl Lewis A on CD44 variant containing exon 6 mediates polymorphonuclear leukocyte detachment from intestinal epithelium during transepithelial migration. Journal of immunology. 2013;191:4804–4817. doi: 10.4049/jimmunol.1301307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sumagin R, Robin AZ, Nusrat A, Parkos CA. Transmigrated neutrophils in the intestinal lumen engage ICAM-1 to regulate the epithelial barrier and neutrophil recruitment. Mucosal Immunol. 2014;7:905–915. doi: 10.1038/mi.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu L, Mul FP, Lutter R, Roos D, Knol EF. Transmigration of human neutrophils across airway epithelial cell monolayers is preferentially in the physiologic basolateral-to-apical direction. Am J Respir Cell Mol Biol. 1996;15:771–780. doi: 10.1165/ajrcmb.15.6.8969272. [DOI] [PubMed] [Google Scholar]
- 52.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. The Journal of clinical investigation. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kidney JC, Proud D. Neutrophil transmigration across human airway epithelial monolayers: mechanisms and dependence on electrical resistance. Am J Respir Cell Mol Biol. 2000;23:389–395. doi: 10.1165/ajrcmb.23.3.4068. [DOI] [PubMed] [Google Scholar]
- 54.Brazil JC, Lee WY, Kolegraff KN, Nusrat A, Parkos CA, Louis NA. Neutrophil migration across intestinal epithelium: evidence for a role of CD44 in regulating detachment of migrating cells from the luminal surface. Journal of immunology. 2010;185:7026–7036. doi: 10.4049/jimmunol.1001293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brazil JC, Sumagin R, Cummings RD, Louis NA, Parkos CA. Targeting of Neutrophil Lewis X Blocks Transepithelial Migration and Increases Phagocytosis and Degranulation. The American journal of pathology. 2016;186:297–311. doi: 10.1016/j.ajpath.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y, Merlin D, Burst SL, Pochet M, Madara JL, Parkos CA. The role of CD47 in neutrophil transmigration. Increased rate of migration correlates with increased cell surface expression of CD47. The Journal of biological chemistry. 2001;276:40156–40166. doi: 10.1074/jbc.M104138200. [DOI] [PubMed] [Google Scholar]
- 57.Nusrat A, Parkos CA, Liang TW, Carnes DK, Madara JL. Neutrophil migration across model intestinal epithelia: monolayer disruption and subsequent events in epithelial repair. Gastroenterology. 1997;113:1489–1500. doi: 10.1053/gast.1997.v113.pm9352851. [DOI] [PubMed] [Google Scholar]
- 58.Lawrence DW, et al. Antiadhesive role of apical decay-accelerating factor (CD55) in human neutrophil transmigration across mucosal epithelia. The Journal of experimental medicine. 2003;198:999–1010. doi: 10.1084/jem.20030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agace WW, Patarroyo M, Svensson M, Carlemalm E, Svanborg C. Escherichia coli induces transuroepithelial neutrophil migration by an intercellular adhesion molecule-1-dependent mechanism. Infect Immun. 1995;63:4054–4062. doi: 10.1128/iai.63.10.4054-4062.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tosi MF, Hamedani A, Brosovich J, Alpert SE. ICAM-1-independent, CD18-dependent adhesion between neutrophils and human airway epithelial cells exposed in vitro to ozone. Journal of immunology. 1994;152:1935–1942. [PubMed] [Google Scholar]
- 61.Luscinskas FW, Brock AF, Arnaout MA, Gimbrone MA., Jr Endothelial-leukocyte adhesion molecule-1-dependent and leukocyte (CD11/CD18)-dependent mechanisms contribute to polymorphonuclear leukocyte adhesion to cytokine-activated human vascular endothelium. Journal of immunology. 1989;142:2257–2263. [PubMed] [Google Scholar]
- 62.Lo SK, Van Seventer GA, Levin SM, Wright SD. Two leukocyte receptors (CD11a/CD18 and CD11b/CD18) mediate transient adhesion to endothelium by binding to different ligands. Journal of immunology. 1989;143:3325–3329. [PubMed] [Google Scholar]
- 63.Colgan SP, Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across cultured intestinal epithelial monolayers is modulated by epithelial exposure to IFN-gamma in a highly polarized fashion. The Journal of cell biology. 1993;120:785–798. doi: 10.1083/jcb.120.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyata R, Iwabuchi K, Watanabe S, Sato N, Nagaoka I. Short exposure of intestinal epithelial cells to TNF-alpha and histamine induces Mac-1-mediated neutrophil adhesion independent of protein synthesis. Journal of leukocyte biology. 1999;66:437–446. doi: 10.1002/jlb.66.3.437. [DOI] [PubMed] [Google Scholar]
- 65.Hawkins HK, Heffelfinger SC, Anderson DC. Leukocyte adhesion deficiency: clinical and postmortem observations. Pediatr Pathol. 1992;12:119–130. doi: 10.3109/15513819209023288. [DOI] [PubMed] [Google Scholar]
- 66.Altieri DC, Morrissey JH, Edgington TS. Adhesive receptor Mac-1 coordinates the activation of factor X on stimulated cells of monocytic and myeloid differentiation: an alternative initiation of the coagulation protease cascade. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:7462–7466. doi: 10.1073/pnas.85.20.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beller DI, Springer TA, Schreiber RD. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. The Journal of experimental medicine. 1982;156:1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cai TQ, Wright SD. Human leukocyte elastase is an endogenous ligand for the integrin CR3 (CD11b/CD18, Mac-1, alpha M beta 2) and modulates polymorphonuclear leukocyte adhesion. The Journal of experimental medicine. 1996;184:1213–1223. doi: 10.1084/jem.184.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davis GE. The Mac-1 and p150,95 beta 2 integrins bind denatured proteins to mediate leukocyte cell-substrate adhesion. Experimental cell research. 1992;200:242–252. doi: 10.1016/0014-4827(92)90170-d. [DOI] [PubMed] [Google Scholar]
- 70.Diamond MS, Alon R, Parkos CA, Quinn MT, Springer TA. Heparin is an adhesive ligand for the leukocyte integrin Mac-1 (CD11b/CD1) The Journal of cell biology. 1995;130:1473–1482. doi: 10.1083/jcb.130.6.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diamond MS, et al. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18) The Journal of cell biology. 1990;111:3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DiScipio RG, Daffern PJ, Schraufstatter IU, Sriramarao P. Human polymorphonuclear leukocytes adhere to complement factor H through an interaction that involves alphaMbeta2 (CD11b/CD18) Journal of immunology. 1998;160:4057–4066. [PubMed] [Google Scholar]
- 73.Forsyth CB, Mathews HL. Lymphocytes utilize CD11b/CD18 for adhesion to Candida albicans. Cell Immunol. 1996;170:91–100. doi: 10.1006/cimm.1996.0138. [DOI] [PubMed] [Google Scholar]
- 74.Frieser M, Hallmann R, Johansson S, Vestweber D, Goodman SL, Sorokin L. Mouse polymorphonuclear granulocyte binding to extracellular matrix molecules involves beta 1 integrins. European journal of immunology. 1996;26:3127–3136. doi: 10.1002/eji.1830261245. [DOI] [PubMed] [Google Scholar]
- 75.Johansson MW, Patarroyo M, Oberg F, Siegbahn A, Nilsson K. Myeloperoxidase mediates cell adhesion via the alpha M beta 2 integrin (Mac-1, CD11b/CD18) J Cell Sci. 1997;110(Pt 9):1133–1139. doi: 10.1242/jcs.110.9.1133. [DOI] [PubMed] [Google Scholar]
- 76.Kanse SM, Matz RL, Preissner KT, Peter K. Promotion of leukocyte adhesion by a novel interaction between vitronectin and the beta2 integrin Mac-1 (alphaMbeta2, CD11b/CD18) Arterioscler Thromb Vasc Biol. 2004;24:2251–2256. doi: 10.1161/01.ATV.0000146529.68729.8b. [DOI] [PubMed] [Google Scholar]
- 77.Kotovuori P, et al. The vascular E-selectin binds to the leukocyte integrins CD11/CD18. Glycobiology. 1993;3:131–136. doi: 10.1093/glycob/3.2.131. [DOI] [PubMed] [Google Scholar]
- 78.Muchowski PJ, Zhang L, Chang ER, Soule HR, Plow EF, Moyle M. Functional interaction between the integrin antagonist neutrophil inhibitory factor and the I domain of CD11b/CD18. The Journal of biological chemistry. 1994;269:26419–26423. [PubMed] [Google Scholar]
- 79.Nathan C, et al. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. The Journal of cell biology. 1989;109:1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Relman D, Tuomanen E, Falkow S, Golenbock DT, Saukkonen K, Wright SD. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990;61:1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- 81.Ross GD, Cain JA, Lachmann PJ. Membrane complement receptor type three (CR3) has lectin-like properties analogous to bovine conglutinin as functions as a receptor for zymosan and rabbit erythrocytes as well as a receptor for iC3b. Journal of immunology. 1985;134:3307–3315. [PubMed] [Google Scholar]
- 82.Russell DG, Wright SD. Complement receptor type 3 (CR3) binds to an Arg-Gly-Asp-containing region of the major surface glycoprotein, gp63, of Leishmania promastigotes. The Journal of experimental medicine. 1988;168:279–292. doi: 10.1084/jem.168.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schober JM, Lau LF, Ugarova TP, Lam SC. Identification of a novel integrin alphaMbeta2 binding site in CCN1 (CYR61), a matricellular protein expressed in healing wounds and atherosclerotic lesions. The Journal of biological chemistry. 2003;278:25808–25815. doi: 10.1074/jbc.M301534200. [DOI] [PubMed] [Google Scholar]
- 84.Shappell SB, Toman C, Anderson DC, Taylor AA, Entman ML, Smith CW. Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. Journal of immunology. 1990;144:2702–2711. [PubMed] [Google Scholar]
- 85.Simon DI, et al. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) The Journal of experimental medicine. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simon DI, et al. Identification of a urokinase receptor-integrin interaction site. Promiscuous regulator of integrin function. The Journal of biological chemistry. 2000;275:10228–10234. doi: 10.1074/jbc.275.14.10228. [DOI] [PubMed] [Google Scholar]
- 87.Thompson HL, Matsushima K. Human polymorphonuclear leucocytes stimulated by tumour necrosis factor-alpha show increased adherence to extracellular matrix proteins which is mediated via the CD11b/18 complex. Clin Exp Immunol. 1992;90:280–285. doi: 10.1111/j.1365-2249.1992.tb07943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wachtfogel YT, et al. High molecular weight kininogen binds to Mac-1 on neutrophils by its heavy chain (domain 3) and its light chain (domain 5) The Journal of biological chemistry. 1994;269:19307–19312. [PubMed] [Google Scholar]
- 89.Walzog B, Schuppan D, Heimpel C, Hafezi-Moghadam A, Gaehtgens P, Ley K. The leukocyte integrin Mac-1 (CD11b/CD18) contributes to binding of human granulocytes to collagen. Experimental cell research. 1995;218:28–38. doi: 10.1006/excr.1995.1127. [DOI] [PubMed] [Google Scholar]
- 90.Xia Y, Vetvicka V, Yan J, Hanikyrova M, Mayadas T, Ross GD. The beta-glucan-binding lectin site of mouse CR3 (CD11b/CD18) and its function in generating a primed state of the receptor that mediates cytotoxic activation in response to iC3b-opsonized target cells. Journal of immunology. 1999;162:2281–2290. [PubMed] [Google Scholar]
- 91.Kuijper PH, Gallardo Torres HI, Lammers JW, Sixma JJ, Koenderman L, Zwaginga JJ. Platelet and fibrin deposition at the damaged vessel wall: cooperative substrates for neutrophil adhesion under flow conditions. Blood. 1997;89:166–175. [PubMed] [Google Scholar]
- 92.Wright SD, Weitz JI, Huang AJ, Levin SM, Silverstein SC, Loike JD. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:7734–7738. doi: 10.1073/pnas.85.20.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Colgan SP, et al. Receptors involved in carbohydrate binding modulate intestinal epithelial-neutrophil interactions. The Journal of biological chemistry. 1995;270:10531–10539. doi: 10.1074/jbc.270.18.10531. [DOI] [PubMed] [Google Scholar]
- 94.Zen K, Liu Y, Cairo D, Parkos CA. CD11b/CD18-dependent interactions of neutrophils with intestinal epithelium are mediated by fucosylated proteoglycans. Journal of immunology. 2002;169:5270–5278. doi: 10.4049/jimmunol.169.9.5270. [DOI] [PubMed] [Google Scholar]
- 95.Viguier M, Advedissian T, Delacour D, Poirier F, Deshayes F. Galectins in epithelial functions. Tissue Barriers. 2014;2:e29103. doi: 10.4161/tisb.29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rubinstein N, Ilarregui JM, Toscano MA, Rabinovich GA. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens. 2004;64:1–12. doi: 10.1111/j.0001-2815.2004.00278.x. [DOI] [PubMed] [Google Scholar]
- 97.Perillo NL, Uittenbogaart CH, Nguyen JT, Baum LG. Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. The Journal of experimental medicine. 1997;185:1851–1858. doi: 10.1084/jem.185.10.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steichen AL, Simonson TJ, Salmon SL, Metzger DW, Mishra BB, Sharma J. Alarmin function of galectin-9 in murine respiratory tularemia. PLoS One. 2015;10:e0123573. doi: 10.1371/journal.pone.0123573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huflejt ME, Jordan ET, Gitt MA, Barondes SH, Leffler H. Strikingly different localization of galectin-3 and galectin-4 in human colon adenocarcinoma T84 cells. Galectin-4 is localized at sites of cell adhesion. The Journal of biological chemistry. 1997;272:14294–14303. doi: 10.1074/jbc.272.22.14294. [DOI] [PubMed] [Google Scholar]
- 100.Stockl J, et al. Monoclonal antibodies to the carbohydrate structure Lewis(x) stimulate the adhesive activity of leukocyte integrin CD11b/CD18 (CR3, Mac-1, alpha m beta 2) on human granulocytes. Journal of leukocyte biology. 1993;53:541–549. doi: 10.1002/jlb.53.5.541. [DOI] [PubMed] [Google Scholar]
- 101.Zen K, Cui LB, Zhang CY, Liu Y. Critical role of mac-1 sialyl lewis x moieties in regulating neutrophil degranulation and transmigration. J Mol Biol. 2007;374:54–63. doi: 10.1016/j.jmb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 102.Nieminen J, St-Pierre C, Sato S. Galectin-3 interacts with naive and primed neutrophils, inducing innate immune responses. Journal of leukocyte biology. 2005;78:1127–1135. doi: 10.1189/jlb.1204702. [DOI] [PubMed] [Google Scholar]
- 103.Edens HA, et al. Neutrophil transepithelial migration: evidence for sequential, contact-dependent signaling events and enhanced paracellular permeability independent of transjunctional migration. Journal of immunology. 2002;169:476–486. doi: 10.4049/jimmunol.169.1.476. [DOI] [PubMed] [Google Scholar]
- 104.Chin AC, Lee WY, Nusrat A, Vergnolle N, Parkos CA. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and −2 regulates barrier function and transepithelial migration. Journal of immunology. 2008;181:5702–5710. doi: 10.4049/jimmunol.181.8.5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nash S, Stafford J, Madara JL. The selective and superoxide-independent disruption of intestinal epithelial tight junctions during leukocyte transmigration. Lab Invest. 1988;59:531–537. [PubMed] [Google Scholar]
- 106.Milks LC, Brontoli MJ, Cramer EB. Epithelial permeability and the transepithelial migration of human neutrophils. The Journal of cell biology. 1983;96:1241–1247. doi: 10.1083/jcb.96.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nash S, Stafford J, Madara JL. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987;80:1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev. 2003;83:309–336. doi: 10.1152/physrev.00023.2002. [DOI] [PubMed] [Google Scholar]
- 109.Chin AC, Parkos CA. Neutrophil transepithelial migration and epithelial barrier function in IBD: potential targets for inhibiting neutrophil trafficking. Ann N Y Acad Sci. 2006;1072:276–287. doi: 10.1196/annals.1326.018. [DOI] [PubMed] [Google Scholar]
- 110.Jiang P, Lagenaur CF, Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. The Journal of biological chemistry. 1999;274:559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 111.Parkos CA, et al. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. The Journal of cell biology. 1996;132:437–450. doi: 10.1083/jcb.132.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lindberg FP, Bullard DC, Caver TE, Gresham HD, Beaud AL, Brown EJ, et al. Decreased resistance to bacterial infection and granulocyte defects in IAP-deficient mice. Science. 1996;274:795–798. doi: 10.1126/science.274.5288.795. [DOI] [PubMed] [Google Scholar]
- 113.Liu Y, et al. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. The Journal of biological chemistry. 2002;277:10028–10036. doi: 10.1074/jbc.M109720200. [DOI] [PubMed] [Google Scholar]
- 114.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 115.Lawrence DW, King SB, Frazier WA, Koenig JM. Decreased CD47 expression during spontaneous apoptosis targets neutrophils for phagocytosis by monocyte-derived macrophages. Early human development. 2009;85:659–663. doi: 10.1016/j.earlhumdev.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, Nauseef WM. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. Journal of immunology. 2014;192:4709–4717. doi: 10.4049/jimmunol.1302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gassler N, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–G228. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 118.Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- 119.Kamekura R, et al. Inflammation-induced desmoglein-2 ectodomain shedding compromises the mucosal barrier. Molecular biology of the cell. 2015;26:3165–3177. doi: 10.1091/mbc.E15-03-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Keck T, Balcom JHt, Fernandez-del Castillo C, Antoniu BA, Warshaw AL. Matrix metalloproteinase-9 promotes neutrophil migration and alveolar capillary leakage in pancreatitis-associated lung injury in the rat. Gastroenterology. 2002;122:188–201. doi: 10.1053/gast.2002.30348. [DOI] [PubMed] [Google Scholar]
- 121.Pruessmeyer J, et al. Leukocytes require ADAM10 but not ADAM17 for their migration and inflammatory recruitment into the alveolar space. Blood. 2014;123:4077–4088. doi: 10.1182/blood-2013-09-511543. [DOI] [PubMed] [Google Scholar]
- 122.Wang H, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nature medicine. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nava P, et al. Desmoglein-2: a novel regulator of apoptosis in the intestinal epithelium. Molecular biology of the cell. 2007;18:4565–4578. doi: 10.1091/mbc.E07-05-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smalley-Freed WG, et al. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest. 2010;120:1824–1835. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ginzberg HH, et al. Neutrophil-mediated epithelial injury during transmigration: role of elastase. Am J Physiol Gastrointest Liver Physiol. 2001;281:G705–G717. doi: 10.1152/ajpgi.2001.281.3.G705. [DOI] [PubMed] [Google Scholar]
- 126.Evans SM, Blyth DI, Wong T, Sanjar S, West MR. Decreased distribution of lung epithelial junction proteins after intratracheal antigen or lipopolysaccharide challenge: correlation with neutrophil influx and levels of BALF sE-cadherin. Am J Respir Cell Mol Biol. 2002;27:446–454. doi: 10.1165/rcmb.4776. [DOI] [PubMed] [Google Scholar]
- 127.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 128.Mandell KJ, Babbin BA, Nusrat A, Parkos CA. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on beta1 integrins and Rap1 activity. The Journal of biological chemistry. 2005;280:11665–11674. doi: 10.1074/jbc.M412650200. [DOI] [PubMed] [Google Scholar]
- 129.Naik MU, Vuppalanchi D, Naik UP. Essential role of junctional adhesion molecule-1 in basic fibroblast growth factor-induced endothelial cell migration. Arterioscler Thromb Vasc Biol. 2003;23:2165–2171. doi: 10.1161/01.ATV.0000093982.84451.87. [DOI] [PubMed] [Google Scholar]
- 130.Martin-Padura I, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. The Journal of cell biology. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moog-Lutz C, et al. JAML, a novel protein with characteristics of a junctional adhesion molecule, is induced during differentiation of myeloid leukemia cells. Blood. 2003;102:3371–3378. doi: 10.1182/blood-2002-11-3462. [DOI] [PubMed] [Google Scholar]
- 132.Zen K, et al. Neutrophil migration across tight junctions is mediated by adhesive interactions between epithelial coxsackie and adenovirus receptor and a junctional adhesion molecule-like protein on neutrophils. Molecular biology of the cell. 2005;16:2694–2703. doi: 10.1091/mbc.E05-01-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Weber DA, et al. Neutrophil-derived JAML inhibits repair of intestinal epithelial injury during acute inflammation. Mucosal Immunol. 2014;7:1221–1232. doi: 10.1038/mi.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Witherden DA, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. 2010;329:1205–1210. doi: 10.1126/science.1192698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood. 2009;113:6246–6257. doi: 10.1182/blood-2008-11-188375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Severson EA, Jiang L, Ivanov AI, Mandell KJ, Nusrat A, Parkos CA. Cis-dimerization mediates function of junctional adhesion molecule A. Molecular biology of the cell. 2008;19:1862–1872. doi: 10.1091/mbc.E07-09-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y, et al. Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci. 2000;113(Pt 13):2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- 138.Laukoetter MG, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. The Journal of experimental medicine. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Khounlotham M, et al. Compromised intestinal epithelial barrier induces adaptive immune compensation that protects from colitis. Immunity. 2012;37:563–573. doi: 10.1016/j.immuni.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Balda MS, Flores-Maldonado C, Cereijido M, Matter K. Multiple domains of occludin are involved in the regulation of paracellular permeability. Journal of cellular biochemistry. 2000;78:85–96. [PubMed] [Google Scholar]
- 141.Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. The Journal of cell biology. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. The American journal of pathology. 2001;159:2001–2009. doi: 10.1016/S0002-9440(10)63051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. The American journal of pathology. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Strohmeier GR, et al. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest. 1997;99:2588–2601. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax. 2009;64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- 147.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 148.Parkos CA, et al. Expression and polarization of intercellular adhesion molecule-1 on human intestinal epithelia: consequences for CD11b/CD18-mediated interactions with neutrophils. Mol Med. 1996;2:489–505. [PMC free article] [PubMed] [Google Scholar]
- 149.Louis NA, Hamilton KE, Kong T, Colgan SP. HIF-dependent induction of apical CD55 coordinates epithelial clearance of neutrophils. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:950–959. doi: 10.1096/fj.04-3251com. [DOI] [PubMed] [Google Scholar]
- 150.Beck-Schimmer B, et al. Role of alveolar epithelial ICAM-1 in lipopolysaccharide-induced lung inflammation. Eur Respir J. 2002;19:1142–1150. doi: 10.1183/09031936.02.00236602. [DOI] [PubMed] [Google Scholar]
- 151.Kang BH, Crapo JD, Wegner CD, Letts LG, Chang LY. Intercellular adhesion molecule-1 expression on the alveolar epithelium and its modification by hyperoxia. Am J Respir Cell Mol Biol. 1993;9:350–355. doi: 10.1165/ajrcmb/9.4.350. [DOI] [PubMed] [Google Scholar]
- 152.Lublin DM, Atkinson JP. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu Rev Immunol. 1989;7:35–58. doi: 10.1146/annurev.iy.07.040189.000343. [DOI] [PubMed] [Google Scholar]
- 153.Tieng V, et al. Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2977–2982. doi: 10.1073/pnas.032668099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leemans JC, et al. The epidermal growth factor-seven transmembrane (EGF-TM7) receptor CD97 is required for neutrophil migration and host defense. Journal of immunology. 2004;172:1125–1131. doi: 10.4049/jimmunol.172.2.1125. [DOI] [PubMed] [Google Scholar]
- 155.Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunological reviews. 2009;230:97–113. doi: 10.1111/j.1600-065X.2009.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marth JD, Grewal PK. Mammalian glycosylation in immunity. Nature reviews Immunology. 2008;8:874–887. doi: 10.1038/nri2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sperandio M. Selectins and glycosyltransferases in leukocyte rolling in vivo. The FEBS journal. 2006;273:4377–4389. doi: 10.1111/j.1742-4658.2006.05437.x. [DOI] [PubMed] [Google Scholar]
- 158.Beauharnois ME, et al. Affinity and kinetics of sialyl Lewis-X and core-2 based oligosaccharides binding to L- and P-selectin. Biochemistry. 2005;44:9507–9519. doi: 10.1021/bi0507130. [DOI] [PubMed] [Google Scholar]
- 159.Foxall C, et al. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. The Journal of cell biology. 1992;117:895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Katayama Y, Hidalgo A, Chang J, Peired A, Frenette PS. CD44 is a physiological E-selectin ligand on neutrophils. The Journal of experimental medicine. 2005;201:1183–1189. doi: 10.1084/jem.20042014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jones AT, et al. Characterization of the activation-associated isoform of CD43 on murine T lymphocytes. Journal of immunology. 1994;153:3426–3439. [PubMed] [Google Scholar]
- 162.Okamoto I, et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. The Journal of cell biology. 2001;155:755–762. doi: 10.1083/jcb.200108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sato S, Burdett I, Hughes RC. Secretion of the baby hamster kidney 30-kDa galactose-binding lectin from polarized and nonpolarized cells: a pathway independent of the endoplasmic reticulum-Golgi complex. Experimental cell research. 1993;207:8–18. doi: 10.1006/excr.1993.1157. [DOI] [PubMed] [Google Scholar]
- 164.Massa SM, Cooper DN, Leffler H, Barondes SH. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry. 1993;32:260–267. doi: 10.1021/bi00052a033. [DOI] [PubMed] [Google Scholar]
- 165.Liu FT, Hsu DK, Zuberi RI, Kuwabara I, Chi EY, Henderson WR., Jr Expression and function of galectin-3, a beta-galactoside-binding lectin, in human monocytes and macrophages. The American journal of pathology. 1995;147:1016–1028. [PMC free article] [PubMed] [Google Scholar]
- 166.Yamaoka A, Kuwabara I, Frigeri LG, Liu FT. A human lectin, galectin-3 (epsilon bp/Mac-2), stimulates superoxide production by neutrophils. Journal of immunology. 1995;154:3479–3487. [PubMed] [Google Scholar]
- 167.Sato S, Ouellet N, Pelletier I, Simard M, Rancourt A, Bergeron MG. Role of galectin-3 as an adhesion molecule for neutrophil extravasation during streptococcal pneumonia. Journal of immunology. 2002;168:1813–1822. doi: 10.4049/jimmunol.168.4.1813. [DOI] [PubMed] [Google Scholar]
- 168.Kleshchenko YY, Moody TN, Furtak VA, Ochieng J, Lima MF, Villalta F. Human galectin-3 promotes Trypanosoma cruzi adhesion to human coronary artery smooth muscle cells. Infect Immun. 2004;72:6717–6721. doi: 10.1128/IAI.72.11.6717-6721.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kuwabara I, Liu FT. Galectin-3 promotes adhesion of human neutrophils to laminin. Journal of immunology. 1996;156:3939–3944. [PubMed] [Google Scholar]
- 170.Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. Journal of molecular medicine. 1998;76:402–412. doi: 10.1007/s001090050232. [DOI] [PubMed] [Google Scholar]
- 171.Jiang K, et al. Galectin-3 regulates desmoglein-2 and intestinal epithelial intercellular adhesion. The Journal of biological chemistry. 2014;289:10510–10517. doi: 10.1074/jbc.M113.538538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rabinovich GA, Sotomayor CE, Riera CM, Bianco I, Correa SG. Evidence of a role for galectin-1 in acute inflammation. European journal of immunology. 2000;30:1331–1339. doi: 10.1002/(SICI)1521-4141(200005)30:5<1331::AID-IMMU1331>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 173.La M, et al. A novel biological activity for galectin-1: inhibition of leukocyte-endothelial cell interactions in experimental inflammation. The American journal of pathology. 2003;163:1505–1515. doi: 10.1016/s0002-9440(10)63507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Santucci L, et al. Galectin-1 suppresses experimental colitis in mice. Gastroenterology. 2003;124:1381–1394. doi: 10.1016/s0016-5085(03)00267-1. [DOI] [PubMed] [Google Scholar]
- 175.Broide DH, et al. Core 2 oligosaccharides mediate eosinophil and neutrophil peritoneal but not lung recruitment. Am J Physiol Lung Cell Mol Physiol. 2002;282:L259–L266. doi: 10.1152/ajplung.00214.2001. [DOI] [PubMed] [Google Scholar]
- 176.Tanino Y, et al. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. Journal of immunology. 2010;184:2677–2685. doi: 10.4049/jimmunol.0903274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hidalgo A, Ma S, Peired AJ, Weiss LA, Cunningham-Rundles C, Frenette PS. Insights into leukocyte adhesion deficiency type 2 from a novel mutation in the GDP-fucose transporter gene. Blood. 2003;101:1705–1712. doi: 10.1182/blood-2002-09-2840. [DOI] [PubMed] [Google Scholar]
- 178.Weninger W, et al. Specialized contributions by alpha(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity. 2000;12:665–676. doi: 10.1016/s1074-7613(00)80217-4. [DOI] [PubMed] [Google Scholar]
- 179.Briskin MJ, McEvoy LM, Butcher EC. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature. 1993;363:461–464. doi: 10.1038/363461a0. [DOI] [PubMed] [Google Scholar]
- 180.Kobayashi K, Yokoyama K, Sada M, Saigenji K. [Diagnosis of inflammatory bowel disease. 2. X-ray and endoscopic diagnosis] Nihon Naika Gakkai Zasshi. 2009;98:37–43. doi: 10.2169/naika.98.37. [DOI] [PubMed] [Google Scholar]
- 181.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 182.Fattori V, Amaral FA, Verri WA., Jr Neutrophils and arthritis: Role in disease and pharmacological perspectives. Pharmacol Res. 2016 doi: 10.1016/j.phrs.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 183.Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012;5:354–366. doi: 10.1038/mi.2012.24. [DOI] [PubMed] [Google Scholar]
- 184.Hoenderdos K, Condliffe A. The neutrophil in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;48:531–539. doi: 10.1165/rcmb.2012-0492TR. [DOI] [PubMed] [Google Scholar]
- 185.Donnelly SC, et al. Plasma elastase levels and the development of the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:1428–1433. doi: 10.1164/ajrccm.151.5.7735596. [DOI] [PubMed] [Google Scholar]
- 186.Baird BR, Cheronis JC, Sandhaus RA, Berger EM, White CW, Repine JE. O2 metabolites and neutrophil elastase synergistically cause edematous injury in isolated rat lungs. J Appl Physiol (1985) 1986;61:2224–2229. doi: 10.1152/jappl.1986.61.6.2224. [DOI] [PubMed] [Google Scholar]
- 187.Sakamaki F, et al. Effect of a specific neutrophil elastase inhibitor, ONO-5046, on endotoxin-induced acute lung injury. Am J Respir Crit Care Med. 1996;153:391–397. doi: 10.1164/ajrccm.153.1.8542148. [DOI] [PubMed] [Google Scholar]
- 188.Boutten A, et al. Compartmentalized IL-8 and elastase release within the human lung in unilateral pneumonia. Am J Respir Crit Care Med. 1996;153:336–342. doi: 10.1164/ajrccm.153.1.8542140. [DOI] [PubMed] [Google Scholar]
- 189.Gouni-Berthold I, Baumeister B, Wegel E, Berthold HK, Vetter H, Schmidt C. Neutrophil-elastase in chronic inflammatory bowel disease: a marker of disease activity? Hepatogastroenterology. 1999;46:2315–2320. [PubMed] [Google Scholar]
- 190.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. The American journal of gastroenterology. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 191.Tarlton JF, et al. The role of up-regulated serine proteases and matrix metalloproteinases in the pathogenesis of a murine model of colitis. The American journal of pathology. 2000;157:1927–1935. doi: 10.1016/S0002-9440(10)64831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kuromitsu S, Yokota H, Hiramoto M, Morita S, Mita H, Yamada T. Increased concentration of neutrophil elastase in urine from patients with interstitial cystitis. Scand J Urol Nephrol. 2008;42:455–461. doi: 10.1080/00365590802025881. [DOI] [PubMed] [Google Scholar]
- 193.Fligiel SE, et al. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum Pathol. 2006;37:422–430. doi: 10.1016/j.humpath.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 194.Kim KH, et al. Tissue inhibitor of metalloproteinase-1 deficiency amplifies acute lung injury in bleomycin-exposed mice. Am J Respir Cell Mol Biol. 2005;33:271–279. doi: 10.1165/rcmb.2005-0111OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Steinberg J, et al. Metalloproteinase inhibition reduces lung injury and improves survival after cecal ligation and puncture in rats. J Surg Res. 2003;111:185–195. doi: 10.1016/s0022-4804(03)00089-1. [DOI] [PubMed] [Google Scholar]
- 196.Baugh MD, et al. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814–822. doi: 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]
- 197.Shimshoni E, Yablecovitch D, Baram L, Dotan I, Sagi I. ECM remodelling in IBD: innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut. 2015;64:367–372. doi: 10.1136/gutjnl-2014-308048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Makitalo L, Sipponen T, Karkkainen P, Kolho KL, Saarialho-Kere U. Changes in matrix metalloproteinase (MMP) and tissue inhibitors of metalloproteinases (TIMP) expression profile in Crohn’s disease after immunosuppressive treatment correlate with histological score and calprotectin values. Int J Colorectal Dis. 2009;24:1157–1167. doi: 10.1007/s00384-009-0756-5. [DOI] [PubMed] [Google Scholar]
- 199.Castaneda FE, et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005;129:1991–2008. doi: 10.1053/j.gastro.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 200.Medina C, et al. Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G116–G122. doi: 10.1152/ajpheart.00036.2002. [DOI] [PubMed] [Google Scholar]
- 201.Gorodeski GI. Estrogen decrease in tight junctional resistance involves matrix-metalloproteinase-7-mediated remodeling of occludin. Endocrinology. 2007;148:218–231. doi: 10.1210/en.2006-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pflugfelder SC, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. The American journal of pathology. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang H, Porro G, Orzech N, Mullen B, Liu M, Slutsky AS. Neutrophil defensins mediate acute inflammatory response and lung dysfunction in dose-related fashion. Am J Physiol Lung Cell Mol Physiol. 2001;280:L947–L954. doi: 10.1152/ajplung.2001.280.5.L947. [DOI] [PubMed] [Google Scholar]
- 204.Cunliffe RN, Kamal M, Rose FR, James PD, Mahida YR. Expression of antimicrobial neutrophil defensins in epithelial cells of active inflammatory bowel disease mucosa. J Clin Pathol. 2002;55:298–304. doi: 10.1136/jcp.55.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yamaguchi N, et al. Concentrations of alpha- and beta-defensins in plasma of patients with inflammatory bowel disease. Inflamm Res. 2009;58:192–197. doi: 10.1007/s00011-008-8120-8. [DOI] [PubMed] [Google Scholar]
- 206.McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Davies GR, Simmonds NJ, Stevens TR, Grandison A, Blake DR, Rampton DS. Mucosal reactive oxygen metabolite production in duodenal ulcer disease. Gut. 1992;33:1467–1472. doi: 10.1136/gut.33.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Naito Y, Takagi T, Yoshikawa T. Neutrophil-dependent oxidative stress in ulcerative colitis. J Clin Biochem Nutr. 2007;41:18–26. doi: 10.3164/jcbn.2007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Shiratora Y, et al. Oxygen-derived free radical generating capacity of polymorphonuclear cells in patients with ulcerative colitis. Digestion. 1989;44:163–171. doi: 10.1159/000199906. [DOI] [PubMed] [Google Scholar]
- 210.Biagioni C, et al. Redox state and O2*- production in neutrophils of Crohn’s disease patients. Exp Biol Med (Maywood) 2006;231:186–195. doi: 10.1177/153537020623100209. [DOI] [PubMed] [Google Scholar]
- 211.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 212.Grasberger H, et al. Increased Expression of DUOX2 Is an Epithelial Response to Mucosal Dysbiosis Required for Immune Homeostasis in Mouse Intestine. Gastroenterology. 2015;149:1849–1859. doi: 10.1053/j.gastro.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Musch MW, Walsh-Reitz MM, Chang EB. Roles of ZO-1, occludin, and actin in oxidant-induced barrier disruption. Am J Physiol Gastrointest Liver Physiol. 2006;290:G222–G231. doi: 10.1152/ajpgi.00301.2005. [DOI] [PubMed] [Google Scholar]
- 215.Marciano BE, et al. Gastrointestinal involvement in chronic granulomatous disease. Pediatrics. 2004;114:462–468. doi: 10.1542/peds.114.2.462. [DOI] [PubMed] [Google Scholar]
- 216.Hayes P, et al. Defects in NADPH Oxidase Genes and in Very Early Onset Inflammatory Bowel Disease. Cellular and molecular gastroenterology and hepatology. 2015;1:489–502. doi: 10.1016/j.jcmgh.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Campbell EL, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tschesche H, Zolzer V, Triebel S, Bartsch S. The human neutrophil lipocalin supports the allosteric activation of matrix metalloproteinases. Eur J Biochem. 2001;268:1918–1928. doi: 10.1046/j.1432-1327.2001.02066.x. [DOI] [PubMed] [Google Scholar]
- 219.Flo TH, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 220.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. The Journal of biological chemistry. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 221.Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology. 2010;15:419–428. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 222.Eagan TM, et al. Neutrophil gelatinase-associated lipocalin: a biomarker in COPD. Chest. 2010;138:888–895. doi: 10.1378/chest.09-2718. [DOI] [PubMed] [Google Scholar]
- 223.Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nielsen OH, et al. Rectal dialysate and fecal concentrations of neutrophil gelatinase-associated lipocalin, interleukin-8, and tumor necrosis factor-alpha in ulcerative colitis. The American journal of gastroenterology. 1999;94:2923–2928. doi: 10.1111/j.1572-0241.1999.01439.x. [DOI] [PubMed] [Google Scholar]
- 225.Manfredi MA, Zurakowski D, Rufo PA, Walker TR, Fox VL, Moses MA. Increased incidence of urinary matrix metalloproteinases as predictors of disease in pediatric patients with inflammatory bowel disease. Inflammatory bowel diseases. 2008;14:1091–1096. doi: 10.1002/ibd.20419. [DOI] [PubMed] [Google Scholar]