Summary
The engulfment of apoptotic cells by phagocytes, a process referred to as efferocytosis, is essential for maintenance of normal tissue homeostasis and a prerequisite for the resolution of inflammation. Neutrophils are the predominant circulating white blood cell in humans, and contain an arsenal of toxic substances that kill and degrade microbes. Neutrophils are short lived and spontaneously die by apoptosis. This review will highlight how the engulfment of apoptotic neutrophils by human phagocytes occurs, how heterogeneity of phagocyte populations influences efferocytosis signaling, and downstream consequences of efferocytosis. The efferocytosis of apoptotic neutrophils by macrophages promotes anti-inflammatory signaling, prevents neutrophil lysis, and dampens immune responses. Given the immunomodulatory properties of efferocytosis, understanding pathways that regulate and enhance efferocytosis could be harnessed to combat infection and chronic inflammatory conditions.
Keywords: Efferocytosis, neutrophil, macrophages, apoptosis, inflammation, infection
Introduction
The ability of macrophages to remove injured host cells was described in the late nineteenth century by Elie Metchnikoff, the father of phagocyte biology. He described the homeostatic removal of dead cells and referred to the process as physiologic inflammation. deCathelineau and Henson later coined the term efferocytosis, meaning ‘to carry to the grave’, to refer to the phagocytosis of apoptotic cells (1). Professional and non-professional phagocytes are involved in the recognition and elimination of apoptotic cells from circulation. Neutrophils are the predominant circulating white blood cell in humans and play a critical role in host defense against bacterial and fungal infections. Neutrophils have an inherently short lifespan, and only live for approximately 24 hours (2). Older neutrophils undergo constitutive, spontaneous apoptosis and are cleared by efferocytosis. It is estimated that 109 neutrophils/kg of body weight are turned over every day in the absence of inflammation (3).
The transfer of autologous radiolabeled neutrophils into patients with inflammation results in the trafficking of these cells from the blood to abscesses, lung, spleen, liver, and bone marrow. Retention of the radiolabelled cells in the spleen, liver, and bone marrow after the disappearance from the blood suggests these organs are the sites of neutrophil destruction (4, 5). In vitro, the appearance of apoptotic features in neutrophils, including nuclear condensation and DNA-laddering, coincides with their removal by macrophages, a subset of phagocytes (6). In some instances, removal by macrophages precedes the appearance of gross apoptotic features (7). These data indicate that efferocytosis of neutrophils occurs in vivo and involves recognition and engulfment by phagocytes.
The removal of apoptotic neutrophils from circulation regulates granulopoiesis (8), prevents secondary lysis and spillage of noxious neutrophil substances (9, 10). Moreover, apoptotic neutrophils trigger an anti-inflammatory and pro-resolving response in macrophages, monocytes, and dendritic cells (11-13). Thus, it can be surmised that efferocytosis of apoptotic neutrophils is a fundamental, innate process required for tissue homeostasis and immunity, and its dysregulation can lead to unwanted excessive inflammation, autoimmunity, and exacerbation of infection.
Human Phagocytes
The complexity and heterogeneity of human phagocytes complicates our current understanding of efferocytosis and defining how phagocyte diversity contributes to efferocytosis is essential for untangling the role of specific ligands and receptors. Unlike processes regulated by adaptive immunity, efferocytosis can occur in a non-autologous system and between different species (14). For example, mouse phagocytes can engulf apoptotic human cells and vice versa. A great amount of work has been done identifying the important receptor-ligand interactions and signaling pathways engaged during efferocytosis (15, 16); however, not everything that is known about this process can be applied to efferocytosis of apoptotic neutrophils by human phagocytes. For example, engulfment of apoptotic thymocytes involves CD14, whereas the engulfment of apoptotic neutrophils by macrophages does not (17). In addition, the phagocytosis of apoptotic neutrophils by fibroblasts involves a mannose/fucose specific lectin, whereas engulfment by unstimulated macrophages does not (18, 19). The remarkable plasticity and host-to-host variation of human phagocytes also influences the requirement for certain receptors and confounds existing data, making these cells notoriously difficult to study in the context of efferocytosis. Because the source of apoptotic cells, the species of origin, and the heterogeneity of the phagocyte population can influence the requirement for specific factors (20), the over generalization of this process should be avoided. Therefore, this review will focus primarily on the engulfment of human neutrophils by human macrophages and, to a lesser extent, dendritic cells.
Death of the neutrophil
Neutrophils are the first immune cells recruited to the site of injury or infection. Antimicrobial killing by neutrophils relies on the generation of toxic chemicals and delivery of pre-synthesized destructive enzymes from granules to phagosomes. While these degradative enzymes contribute to a beneficial antimicrobial response inside phagosomes, they can contribute to tissue damage if released extracellularly. Teleologically, efferocytosis of apoptotic neutrophils serves to prevent neutrophil lysis and subsequent release of cytotoxic and inflammatory components.
The true first step in efferocytosis is the induction of apoptosis of the target cell, therefore understanding how and when neutrophils die is crucial. Cellular apoptosis is characterized by phosphatidylserine (PS) externalization, DNA fragmentation, and membrane blebbing, resulting in phagocytosis by neighboring cells. Neutrophils undergo constitutive apoptosis after approximately 24 hours in circulation, although the exact timing is a subject of debate (21). Neutrophil apoptosis is usually initiated by one of two signaling cascades: the intrinsic or extrinsic apoptotic pathways. The intrinsic pathway relies on the loss of mitochondrial membrane integrity caused by the imbalance of pro- and anti- apoptotic factors in the cell and subsequent release of cytochrome c from the mitochondria. Cytochrome c facilitates caspase-9-dependent assembly of the Apaf-1-containing apoptosome and eventual activation of executioner caspase-3. The extrinsic pathway responds to extracellular signaling through death receptors to drive caspase-8-dependent activation of caspase-3. Fas-L, TNF-α, and TRAIL are all examples of soluble proteins that mediate extrinsic apoptosis through engagement of their cognate receptors expressed on the surface of neutrophils (22, 23).
Apoptosis of neutrophils can be accelerated, delayed, or derailed during several inflammatory and infectious conditions (reviewed in (2)). For example, phagocytosis of Fc- and complement-opsonized latex beads accelerates the apoptotic program of neutrophils (24). Some viral and bacterial pathogens also accelerate activation of apoptosis, whereas others initiate apoptosis and then abruptly cause lysis (25, 26). Conversely, neutrophil lifespan can be extended by G-CSF, GM-CSF, IL-8, bacterial components, C-reactive protein, serum amyloid A and other pro-inflammatory mediators (27-29). The ability of soluble mediators to extend neutrophil lifespan could ensure that complete bacterial clearance occurs prior to the onset of apoptosis and phagocytosis by macrophages. In contrast, it has been speculated that pathogens that accelerate apoptosis are able gain silent access into macrophages via efferocytosis (30). Despite the ability of some pathogens to exploit apoptosis, it remains an evolutionarily conserved pathways designed to promote normal cell turn over.
Macrophage heterogeneity
The role of phagocytes extends beyond host defense. Phagocytes play an important role during tissue homeostasis as they readily migrate towards and phagocytose senescent cells in circulation. Resident tissue macrophages, infiltrating macrophages, dendritic cells and non-professional phagocytes are all capable of engulfing apoptotic neutrophils (31).
The behavior of phagocytes, or phenotype, depends on specific stimuli derived from the extracellular milieu. In vivo, the number, frequency, and combination of exogenous stimuli that polarize macrophages toward different phenotypes seems almost limitless. Although more expansive naming systems have been proposed (32), this review will use the simplistic nomenclature of M1, M2a, M2b, and M2c to define macrophage subsets where relevant (33). M1 macrophages are generated by stimulation with IFN-γ and LPS, M2a macrophages with IL-4 or IL-13, M2b macrophages by immune complexes in combination with IL-1β or LPS, and M2c macrophages with IL-10, TGF-β or glucocorticoids (33). Because treatment with different exogenous stimuli program the macrophage to express a defined set of genes, the different macrophage subsets are beneficial in specialized contexts. For example, M1 macrophages upregulate the expression of those genes that are important in antimicrobial responses, whereas M2a macrophages upregulate the expression of genes that are important for anti-parasitic responses (34). Unsurprisingly, macrophage subsets also can express different sets of receptors, thereby influencing their phagocytic ability and efficiency. The mechanisms by which macrophage subsets and dendritic cells engulf apoptotic neutrophils, and their role in resolving inflammation, is addressed in the following sections.
Untangling the phagocytic synapse
Changes on the cell membrane of apoptotic cells initiate tethering to and engulfment by phagocytes. These changes to the cell surface include direct modification of lipids and proteins and the ‘indirect’ binding, or bridging, of serum components to the modified surface antigens. The receptor-ligand interactions between apoptotic cells and phagocytes form an engulfment synapse. This section will highlight some of the nuances regarding the discrimination of apoptotic neutrophils by human macrophages
Neutrophils downregulate a number of membrane proteins as they age, including CD16 (FcγRIII), CD31 (PECAM-1), CD32, CD35 (Complement receptor 1), CD43, CD45, CD44, CD47 (IAP), CD50 (ICAM-3), CD55 (Complement regulatory protein DAF), CD62L (L-selectin), CD63, CD66b, CD87 (uPAR), CD88 (C5a receptor), and CD120a (tumor necrosis factor receptor 1), thus significantly changing the apoptotic neutrophil surface (35-40). Some of these proteins, including CD31 and ICAM-3, are also functionally modified (41, 42), whereas other proteins, including ICAM-1, are upregulated on apoptotic neutrophils (41-43). Finally, new antigens are expressed on the surface of apoptotic neutrophils. Apoptotic neutrophils release intracellular proteins, including Annexin-I and calreticulin, which associate with the membrane and act as ligands for efferocytosis (reviewed in (44)). Furthermore, inactivation of an aminophospholipid translocase and activation of a lipid scramblase in apoptotic cells causes phophatidylserine (PS) accumulation on the cell's outer leaflet (45). Many serum components also recognize and bind to surface exposed PS, thereby amplifying the number of ligands present on apoptotic neutrophils. Since healthy neutrophils have no PS, or only transient PS, in the outer-leaflet of the cell membrane, surface detection of PS by labeled Annexin-V and exclusion of vital dyes has become the gold standard for detection of apoptosis.
These newly exposed ligands expressed on or bound to apoptotic neutrophils are recognized by a diverse group of receptors expressed on the surface of professional phagocytes. When studying the first identified efferocytosis receptors, αvβ3 integrin and PS receptor, investigators realized that different macrophage subsets exhibit a differential requirement for these two receptors (14). Given that the species and type of phagocyte and apoptotic cell alter the preference or requirement for different receptor-ligand pairs, the discussion of receptors will be limited to those that have been studied using primary human phagocytes and neutrophils.
Integrins
Unstimulated human macrophages primarily use the αvβ3 integrin (vitronectin receptor, CD61) system to recognize and engulf apoptotic neutrophils (Fig. 1). Savill and colleagues were the first to demonstrate that integrin αvβ3 recognizes the bridging molecule thrombospondin, which in cooperation with CD36, tethers and engulfs the apoptotic neutrophil (46, 47). Cultured macrophages produce thrombospondin and blockade of thrombospondin production or αvβ3 integrin on unstimulated macrophages reduces efferocytosis of neutrophils (17, 47, 48). Ligand redundancy exists for the αvβ3 integrin receptor. Serum-component milk fat globule-epidermal growth factor 8 (MFG-E8, lactadherin) also acts as bridging molecule between PS on apoptotic cells and αvβ3 integrin on phagocytes (49). These data demonstrate that in some instances where macrophages do not supply bridging molecules, serum may play an important role in efferocytosis.
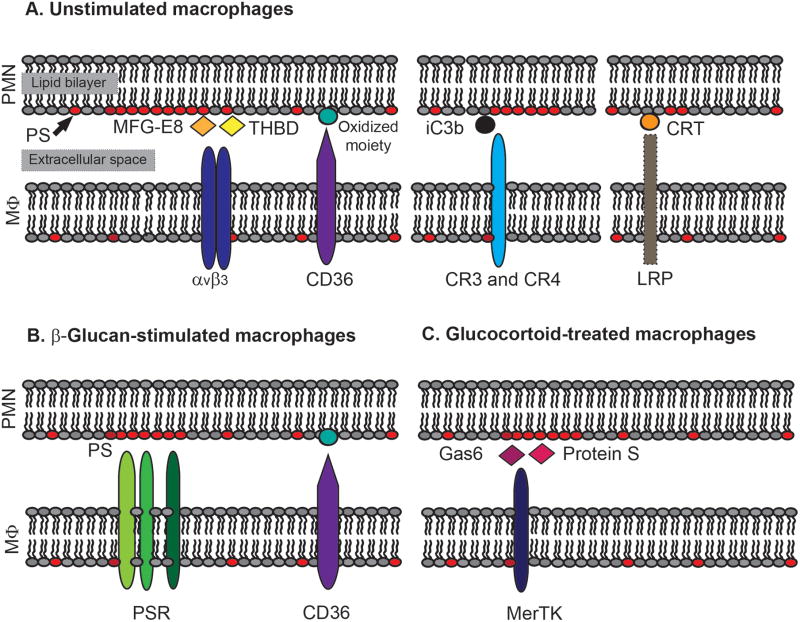
Figure 1. Examples of receptor-ligand interactions during efferocytosis of apoptotic neutrophils.
Unstimulated macrophages (MΦ) primarily use receptors αvβ3 integrin and CD36 to efferocytose apoptotic neutrophils (PMN, A, left). Ligands on apoptotic PMN for αvβ3 integrin include thrombospondin (THBD) and MFG-E8, whereas ligands for CD36 likely include oxidized-LDL-like- or oxidized PS-moieties (A, left). Accessory interactions at the synapse between apoptotic PMN and unstimulated MΦ also include complement receptors CR3 and CR4 recognition of iC3b-opsonized apoptotic PMN (A, middle), and LRP-1 recognition of plasma membrane-associated CRT (A, right). Alternatively, β-glucan-stimulated MΦ recognize PS on apoptotic neutrophils via PSRs (B), and glucocorticoid-treated MΦ enhance efferocytosis through MerTK recognition of PS-bridging molecules Gas6 and Protein S (C).
In contrast to the role of αvβ3 in macrophages, αvβ3 does not play a large role in DC efferocytosis (50); however αvβ5 integrin might serve as an alternative receptor for DC efferocytosis. Integrin αvβ5 also recognizes MFG-E8 and is required for DC engulfment of apoptotic red blood cells, HeLa cells, and apoptotic, mycobacteria-laden neutrophils (50). In addition to αvβ5 integrin, CD36, DC-SIGN, and the TAM family of tyrosine kinases also play important roles in DC efferocytosis (50-52).
Finally, the interaction of apoptotic neutrophils and macrophages may involve β2 integrins, and ligands for β2 integrins are present on apoptotic cells. For example, αLβ2 (lymphocyte function-associated antigen 1, LFA-1) can recognize neutrophil ICAM-3 (intracellular adhesion molecule-3) and αMβ2 (complement receptor 3, CR3) can recognize deposited complement component iC3b (43, 53). In addition, β2 is involved in lipoxin-mediated efferocytosis pathways (54); however other data suggests that the role of β2 integrins in the engulfment of apoptotic cells may be context dependent (43, 55).
PS receptors
Ingestion of apoptotic cells correlates with loss of phospholipid asymmetry and appearance of PS on the outer leaflet of the plasma membrane (17, 56, 57). PS is the best characterized “eat me” signal on the surface of apoptotic cells, and its stereospecific recognition has been shown to function in the interactions leading to engulfment of apoptotic cells. However, unlike many other cell types (14, 56), human macrophages do not inherently favor recognition of apoptotic neutrophils via the PS-PS receptor (PSR) system as demonstrated by studies showing blockade of PS with PS-liposomes does not reduce macrophage efferocytosis of neutrophils (17). Macrophages that are stimulated with β-glucan, on the other hand, down regulate αvβ3 integrin, causing macrophages to switch from the αvβ3 integrin recognition to PSR/PS-dependent recognition and uptake is blocked by PS-liposomes (Fig. 1) (17). Additional studies are required to determine which of the PS receptors (BAI-1, TIM4, Stabilin-1 and -2, or RAGE (58-60) modulate tethering and/or internalization of apoptotic neutrophils by human macrophages.
CD36
CD36 is constitutively used as an efferocytosis receptor (Fig. 1). Blockade of CD36 reduces efferocytosis by human macrophages relying on the PS-receptor or αvβ3 integrin for efferocytosis of apoptotic neutrophils. It is unlikely that CD36 is an additional receptor for PS as inhibition of phagocytosis by anti-CD36 antibodies and PS-liposomes is partially additive (17). Rather, it is suggested that the CD36 ligand is an oxidized-LDL-like or oxidized PS moiety (17, 59, 61). The cooperative nature of CD36 and PS-engagement in human macrophages was examined further by exploiting biotin-avidin binding to label erythrocytes with protein, antibody, or lipid (62). In this study, CD36 promoted tethering, but not engulfment, of erythrocytes, and addition of PS-liposomes promoted internalization (62). These data suggest that some receptors serve a tethering purpose and are constitutively engaged, whereas other receptors engage the cytoskeleton machinery, and their activity may depend on the polarization state of the macrophage.
Complement receptors
These first studies on efferocytosis argued that additional serum components, such as complement factors, were not essential for efferocytosis (18). Conflict in the literature arises because macrophages secrete many of the proteins it uses as bridging molecules. In addition, the role of specific serum factors is dependent on the context. For example, complement-related collectins mediate efferocytosis by alveolar macrophages, which is consistent with the high levels of the collectins surfactants A and D (SP-A and SP-D) in the lung (63). Defects in efferocytosis are associated with several pathological conditions that manifest in the lung (64); therefore, complement directed therapeutics might be extremely promising in this context.
In addition, the clearance of apoptotic cells exhibiting features of secondary necrosis (frequently referred to as late apoptotic cells) seems to preferentially involve complement opsonization. In the latter example CR3 and CR4 mediate efferocytosis of complement-opsonized apoptotic neutrophils (Fig. 1) (53). Furthermore, dying neutrophils downregulate complement regulatory proteins (38), which may facilitate deposition of opsonic complement fragments on the cell membrane (65). Accordingly, serum enhances efferocytosis of neutrophil preparations containing higher percentages of late apoptotic neutrophils (20).
MerTK
Mer tyrosine kinase (MerTK), AXL, and TYRO3 are a family of efferocytosis receptors that recognize the serum proteins and PS-bridging molecules, Gas6 and Protein S (Fig. 1) (66). Mice deficient in MerTK signaling display a defect in the engulfment of apoptotic cells and develop autoantibodies, and mice with a triple deficiency in MerTK, AXL, and TYRO3 develop autoimmune disease (67, 68). The importance of MerTK in efferocytosis of apoptotic neutrophils is evident when macrophages are programmed to an M2c or M2c-like phenotype (69). Treatment with IL-10 and M-CSF, or glucocorticoid steroids, generate M2c macrophages, which preferentially engulf apoptotic cells compared to M1, M2a, and M2b macrophages (70). Following polarization, M2c macrophages upregulate MerTK surface expression, and synthesize Protein S and Gas6 and secrete them into serum (69-71). Accordingly, blockade of MerTK in M2c macrophages restores macrophage efferocytosis to baseline levels (69).
Interestingly antagonizing the nuclear receptor peroxisome proliferator-activated receptor (PPAR)-γ upregulates MerTK and Gas6 but fails to enhance uptake of apoptotic neutrophils (71), suggesting that MerTK expression alone is insufficient. Of interest, conventional M2c macrophages also exhibit rounding and higher levels of Rac activity, which may further augment the MerTK efferocytosis pathway (72). Similar to glucocorticoid steroids and PPAR-γ antagonists, complement component C1q also regulates macrophage expression of MerTK and Gas6 (73), but to determine if C1q plays a role in efferocytosis of apoptotic neutrophils by human macrophages requires further investigation.
LRP1
Lipoprotein receptor-related protein (LRP or CD91) recognizes a diverse set of ligands, including calreticulin (CRT, Fig. 1) (74). CRT normally functions as a chaperone protein in the endoplasmic reticulum (75). During apoptosis, CRT can be detected on the membrane of cells, including neutrophils (40). It is not fully understand how CRT is trafficked from the endoplasmic reticulum to the cell membrane during apoptosis; however, once at the membrane, CRT has the capacity to induce membrane ruffling and macropinocytosis in the murine macrophage cell line J774 macrophages (40). Compared to human monocytes, expression of LRP on resting human macrophages is low, and inhibition of LRP on human macrophages causes a small, but significant, reduction in efferocytosis of human neutrophils (76). It is possible that LRP may play a larger role in efferocytosis by other cell types or different macrophage subtypes; although, activation of macrophages to M1, M2a, M2b, or M2c subtypes fails to upregulate gene expression of LRP in human macrophages (77).
Receptor redundancy and specificity
Blockade of a single receptor has never been shown to completely abolish efferocytosis suggesting that either multiple, redundant efferocytosis pathways exist or individual receptors cooperate with other receptors in a synergistic manner. Efferocytosis receptors, including SR-A, LOX1, CD68, CD300, ATP binding cassette (ABC) A1 and A7, and ligands, including lysophosphatidylserine (lyso-PS), CCN1, C1q, MBL, and Annexin I, may all participate in neutrophil efferocytosis by human macrophages under certain conditions (74, 78-83). For example, previous data suggest a minor, or non-existent role, for lectin-mediated efferocytosis of apoptotic neutrophils (17, 18); however, more recent studies show that galectin-3-mediated enhanced macrophage efferocytosis of neutrophils occurs via a putative lectin-receptor (84).
The data discussed in this section highlight how different macrophage subtypes, and possibly macrophages cultured in different conditions, might generate conflicting results regarding the requirement for particular ligands and receptors; therefore, it is imperative that when studying efferocytosis we carefully characterize both the phagocyte and apoptotic neutrophil in a given experimental system. Overall, we can surmise that the efferocytosis pathways initiated in vivo involves the integration of signals from the microenvironment, tissue, individual cell, and presence or absence of accompanying inflammation.
Preferred methods for measuring efferocytosis in vitro
To continue to evaluate efferocytosis in primary human cells, in vitro assays are essential. Characterization of macrophage subtypes and death modalities in neutrophils are two aspects of improving these experimental systems, but it will also be imperative to distinguish between macrophages with tethered and internalized apoptotic cells.
Multi-color flow cytometry has proved advantageous for measuring efferocytosis. In this method, macrophages are co-cultured with pre-labeled apoptotic neutrophils and following a period of phagocytosis, neutrophils are subsequently counterstained with a separate label or marker. This method allows investigators to distinguish between macrophages with tethered neutrophils, which emit fluorescence for both labels, and macrophages with completely ingested neutrophils, which only emit fluorescence for the first label. This experimental system is good because it is sensitive, high-powered and unbiased; however, some minor caveats exist. Using conventional flow cytometry, it is not possible to determine if a single macrophage contains both engulfed and tethered apoptotic neutrophils. For assessing efferocytosis and infection, it is not impossible to discern if neutrophils laden with microbes or individual neutrophils and microbes are in the same compartment in the macrophage. These considerations often confound data regarding silent entry of pathogens into macrophages.
Microscopy of efferocytosis also has significant merit for the reasons addressed above. In particular, three-dimensional reconstruction from a stack of serial cross sections confocal images of cells can be useful to confirm complete internalization of apoptotic neutrophils and microbe-laden neutrophils by macrophages. Together, these approaches can help identify molecular processes involved in efferocytosis in disease and infection.
Intracellular signaling and digestion
Phagocytosis requires actin cytoskeleton rearrangements. Our understanding regarding cytoskeletal rearrangement during efferocytosis has been primarily examined using cell lines and model organisms amenable to genetic manipulation. The key findings from these studies are reviewed extensively elsewhere (1, 44, 45), however, a few key features are described here. C. elegans nematodes are commonly used to study efferocytosis, because 131 cells of the 1090 total cells in the worm are reproducibly destined to die. Characterization of this model system by Sydney Brenner, H. Robert Horvitz and John Sulston was recognized with a Nobel Prize in physiology and medicine in 2002. Using this model system, several groups identified a collection of signaling molecules that are involved in two pathways for the removal of apoptotic cells: the CED-12, -2, and -5 pathway and the CED-1, -7, and -6 pathway. The mammalian homologs of CED-12, -2, and -5 are ELMO, CrkII, and Dock180, respectively. CED-1 has sequence homology to scavenger receptors and the intracellular portion of LRP, and CED-7 and CED-6 are homologous to ABC-A1 and GULP, respectively. These signaling modules converge to activate CED-10 in C. elegans and the Rho family GTPase, Rac1, in mammals (1, 44, 45).
Phagocytosis is the ingestion of particles greater than 0.5 μm and it can occur through distinct mechanisms. During Fc-mediated phagocytosis by macrophages, pseudopodia containing Fc-receptors extend and bind to sequential ligands on the target forming a tight-fitting phagosome. This process has been termed the zipper-mechanism of phagocytosis and requires Rac1 (85, 86). In C. elegans and mouse macrophage cell line Mf4/4, efferocytosis morphologically resembles the zipper mechanism of phagocytosis with the pseudopods reaching around the target to form a tight fitting phagosome (45, 87).
Alternatively, two pathways of phagocytosis have been described which result in the formation of spacious phagosome: complement-mediated and macropinocytosis. In complement-mediated phagocytosis, complement coated particles interact with their cognate receptor, thereby triggering the sinking of the target into the macrophage (85). Unlike Fc-mediated phagocytosis, this pathway activates Rho family GTPase RhoA. In macropinocytosis, extensive membrane ruffling entraps particles as the membrane folds back onto itself, and like Fc-mediated phagocytosis, macropinocytosis requires Rac1 (1). PS-dependent efferocytosis closely resembles macropinocytosis (64) and PS-liposomes trigger extensive membrane ruffling and bystander engulfment (74). Moreover, PS-dependent efferocytosis by fibroblasts or J774 macrophages is sensitive to the macropinocytosis inhibitor, amiloride (40, 62). Together, these data indicate that multiple pathways are involved in efferocytosis, but much more work needs to be done to discern shared and distinct features of integrin- and complement-mediated macrophage efferocytosis of apoptotic neutrophils and conventional phagocytosis mediated by the same receptors.
Engulfed apoptotic cells are degraded through the process of phagosome maturation (44, 88). Studies comparing the rate of phagosome maturation in human macrophages showed that phagosomes containing apoptotic neutrophils acidify more rapidly than phagosomes containing antibody-opsonized apoptotic neutrophils. The rapid acidification of phagosomes containing apoptotic cells is dependent on Rho GTPase activation of ezrin-radixin-moesin (ERM) proteins, suggesting that (1) phagosome cargo dictates processing efficiency and (2) efferocytosis relies on a balance of Rho and Rac signaling (89).
Enhancing efferocytosis and implications in disease
Impaired efferocytosis has been observed in many human inflammatory and autoimmune diseases, including cystic fibrosis, chronic obstructive pulmonary disease, asthma, idiopathic pulmonary fibrosis, rheumatoid arthritis, systemic lupus erythematous, glomerulonephritis, and atherosclerosis (64). The failure to clear apoptotic cells may exacerbate inflammation (as discussed below), and therapies targeted at enhancing efferocytosis may improve outcomes in these conditions. In proven therapies and novel experimental conditions, enhanced expression of efferocytosis receptor-ligand pairs and activation of signaling moieties contributes to increased efferocytosis.
Glucocorticoid treatment leading to M2c programming is the quintessential example of a therapeutic agent that drives enhanced efferocytosis. Enhanced efferocytosis mediated by glucocorticoids is attributed to (1) enhanced expression of MerTK and ligands Gas 6 and protein S (69), (2) enhanced expression of Annexin I and its receptor ALXR (90), and (3) enhanced activation of Rac1 (72). The upregulation efferocytosis ligands and receptors following exposure to glucocorticoid steroids could also be attributed to peroxisome proliferator-activated receptor (PPAR)-γ activity during the differentiation from monocyte to macrophage (91). Inhibiting activation of PPAR-γ with antagonist GW9962 during differentiation reduces CD36 and AXL expression and reduces basal and glucocorticoid steroid-enhanced efferocytosis of neutrophils by human macrophages (71, 91). Interestingly, the same concentrations of GW9962 enhance MerTK and Gas6 expression by human macrophages, demonstrating that efferocytosis receptors MerTK, AXL, and CD36 are differentially regulated (71, 91). Treating human macrophages with low concentrations (0.1-10 μM) PPAR-γ activating ligand rosiglitazone during monocyte to macrophage maturation, increases CD36 expression, but is insufficient to alter MerTK expression or stimulate efferocytosis of apoptotic neutrophils by human macrophages (71, 91). Although there were no effects with rosiglitazone, it is worth noting here that PPAR-γ activating ligand pioglitazone normalizes the defective efferocytosis in monocytes from patients with chronic granulomatous disease (CGD) (92), and may be a promising therapeutic in this context.
Several therapeutics and experimental manipulations enhance efferocytosis by altering the RhoA-Rac1 balance in cells in favor of Rac activation. These treatments include lovastatin, N-acetylcysteine and CD44-ligation (93-95). Endogenous lipid mediator, Lipoxin-A4, also enhances Rac1 activity in human macrophages and promotes efferocytosis in a CD36, αvβ3, and β2-dependent manner (54, 96). Accordingly, microparticles derived from activated neutrophils that contain pro-resolving lipids and cause macrophages to produce pro-resolving lipids, including lipoxins, enhance efferocytosis of apoptotic neutrophils by GM-CSF-treated macrophages (97, 98). Lyso-PS signaling through G2A also stimulates Rac1 activity and potentiates efferocytosis by murine macrophages and potentially human macrophages as well (83).
Macrolide antibiotics have a variety of immunomodulatory properties (99). Included in their repertoire is the ability of macrolides belonging to the 14-member and 15-member macrolide family, including erythromycin and azithromycin, to enhance efferocytosis of neutrophils by human alveolar macrophages. Enhanced efferocytosis driven by erythromycin is dependent on PS-PRS signaling, but not integrin signaling (100). In addition, azithromycin upregulated expression of mannose receptor (MR) in human alveolar macrophages (101), and MR might play a role in neutrophil efferocytosis. General immunosuppression by steroids and statins could be detrimental in the context of infection, whereas the dual bactericidal and pro-resolving action of macrolides would be more advantageous. Likewise, data supports that macrolide antibiotics are clearly beneficial in the treatment of community-acquired pneumonia (102).
Regulating efferocytosis
Neutrophils can expose PS on their surface in the absence of apoptosis (103, 104). Since neutrophils and other cells (105) can expose PS on their surfaces, yet resist engulfment, then other mechanisms must exist to control this process. The regulatory membrane proteins that prevent efferocytosis are referred to as ‘don't eat me’ signals and protect viable, healthy cells from phagocyte engulfment.
‘Don't eat me’ signals are difficult to study because the output is the absence of efferocytosis, and to date, only a small number of these signals have been fully described. The regulation of ‘don't eat me’ signaling is complex, and evidence suggests that at least three features contribute to turning on and off ‘don't eat me’ signaling, including activity, expression, and location of the ‘don't eat me’ molecules. CD31 and CD47 are the best characterized don't eat me signals that prevent the ingestion of viable leukocytes. In viable neutrophils, CD31 interacts homotypically with CD31 on phagocytes to mediate detachment. In apoptotic cells, CD31 is inactivated and these cells remained attached to the phagocytes and are internalized (42). Little is known about the deactivation of CD31 during apoptosis, but it is worth exploring.
Permissive attachment of inactive CD31 on apoptotic cells and active CD31 on macrophages further augments efferocytosis. Macrophage contact with apoptotic cells causes macrophage membrane depolarization, a process that facilitates firm binding of apoptotic cells, and CD31 homophilic ligation prevents macrophage repolarization through CD31-dependent inhibition of the voltage-gated potassium channel ether-à-go-go-related gene (ERG) (106). In summary, active CD31 promotes detachment of viable cells from phagocytes, whereas inactive CD31 prompts attachment of apoptotic cells to phagocytes, homotypic CD31 ligation in macrophages, and efferocytosis.
Apoptotic cells downregulate CD47 and sequester this molecule away from the phagocytic synapse during efferocytosis. In viable cells, including neutrophils and red blood cells, CD47 interacts with immunoreceptor trypsine-based inhibitory motif (ITIM)-containing protein SIRPα. Previous studies postulate that this interaction causes activation of inhibitory tyrosine phosphatases SHP-1 and SHP-2 to shut down actin-cytoskeleton rearrangement and phagocytosis (40, 107). Consistent with this earlier observation, blockade of either CD47 on the neutrophil or SIRPα on the phagocyte triggers extensive membrane ruffling and ingestion of viable cells (40).
CD300a (108), plasminogen activator inhibitor-1 (PAI-1) (109), and the urokinase receptor (uPAR) (110) have been identified as ‘don't eat me’ receptors using either cell lines or animal models. In addition, soluble factors including vitronectin (111), TNF-α (112, 113), HMGB1 (114-116), prototypic tissue pentraxin PTX3 (117), extracellular histones (118), and PGE2-mediated elevation of intracellular cAMP (119) inhibit efferocytosis pathways, and understanding these inhibitory signaling pathways may be of therapeutic value.
Production of anti-inflammatory mediators
Because an enormous number of neutrophils are eliminated every day, any accompanying inflammation would be devastating for an individual. Efferocytosis of neutrophils is considered an anti-inflammatory and pro-resolving event. As such, harnessing the anti-inflammatory and pro-resolving nature of efferocytosis could be targeted therapeutically to combat chronic inflammatory conditions, including graft-verses-host disease, Crohn's disease, scleroderma, and atopic dermatitis (120). The anti-inflammatory nature of neutrophil efferocytosis is discussed here.
The first studies investigating whether apoptotic neutrophils trigger cytokine production by macrophages show that efferocytosis is a silent process, resulting in no production of pro-inflammatory cytokines (121, 122). Subsequent studies showed that efferocytosis is not merely a silent process, but actually immunosupressive. Engulfment of unopsonized, UV-irradiated, apoptotic neutrophils by macrophages actively inhibits LPS- and zymosan- dependent production of IL-1β, GM-CSF, TNF-α, and IL-10. In parallel, 18-hour coculture of apoptotic neutrophils with macrophages stimulates the production of anti-inflammatory mediators, TGF-β and PGE2, but not IL-10 (12). We observed that macrophages produced increased amounts of TGF-β and IL-10 when co-cultured with apoptotic neutrophils for 18 hours in serum-containing media (unpublished data); however, macrophages given a shorter, one hour exposure to apoptotic neutrophils (in which more than 50% of the macrophages ingest apoptotic neutrophils) do not produce elevated IL-10 after an additional 12 hours in culture in serum-free media (26). These data suggest that the production of anti-inflammatory cytokines relies on either the continual presence of apoptotic neutrophils or serum components.
Immunosuppression caused by apoptotic neutrophils is receptor mediated. Using monocytes, which differ from macrophages in that they are unable to engulf apoptotic cells at least in vitro (123), allowed investigators to show that contact between dying cells and monocytes is sufficient to induce immunosuppression (11, 124). Monocytes cultured with apoptotic neutrophils suppress proinflammatory cytokine production following LPS stimulation and increased production of TGF-β and IL-10 (12, 124). Interestingly, Fc-mediated phagocytosis of antibody-opsonized neutrophils by macrophages or monocytes fails to inhibit LPS-mediated pro-inflammatory cytokine production (12, 124), whereas CD36 ligation on monocytes is sufficient to induce the anti-inflammatory effect on LPS-activated monocytes (11). It is also plausible that complement fragment iC3b on apoptotic cells promotes anti-inflammatory cytokine production, because iC3b recognition by monocytes promotes IL-10 production and dampens IL-12 production (125, 126).
The study of resolving inflammation has helped to identify a novel class of lipid mediators called specialized pro-resolving mediators (SPM). SPM, including lipoxins, resolvins, protectins and maresins, have potent anti-inflammatory properties (127). The Serhan group has elegantly demonstrated that efferocytosis of apoptotic neutrophils by macrophages leads to increased production of SPM including, Resolvin D1 (RvD1), RvD2, and lipoxin B4. Additionally, PGE2 production accompanies SPM production, and together these soluble mediators play a critical role in resolving inflammation (97). Blockade of soluble mediators, including TGFβ, PAF, or PGE2 restored LPS-mediated cytokine production demonstrating that these anti-inflammatory mediators have a paracrine effect of blocking proinflammatory cytokine production by macrophages (12).
Prevention of secondary necrosis and necrosis
Neutrophils are full of noxious substances, including proteolytic enzymes and danger-associated molecular patterns that propagate inflammation and cause host tissue damage. Unfortunately, when efferocytosis is delayed necrotic corpses can accumulate. Many groups have compared how apoptotic neutrophils and necrotic neutrophils stimulate macrophages. Lysed neutrophils cause macrophages to produce the pro-inflammatory cytokine, TNF-α, as well as IL-10, whereas apoptotic neutrophils or necrotic T cells do not (128). The proinflammatory effects of lysed neutrophils are dependent on neutrophil elastase (128). In mice, coculture with necrotic neutrophils causes up regulation of costimulatory molecule CD40 on murine bone marrow-derived macrophages (BMDM) and enhance T cell responses, whereas colculture with apoptotic neutrophils has no effect (129), suggesting that necrotic debris has adjuvant like properties, whereas apoptotic cells do not. Finally, imaging studies conducted in laser-injured mice revealed that the cell death of one neutrophil amplifies chemotaxis of surrounding cells (130).
Although the clinical picture supports the notion that defects in the clearance of apoptotic neutrophils contributes to tissue damage and unwanted inflammation, there is also evidence that in certain contexts late apoptotic and necrotic neutrophils stimulate resolution. In the set of experiments analyzing the proinflammatory effects of necrotic neutrophils, it was noted that late apoptotic neutrophils did not stimulate macrophage pro-inflammatory cytokine production, and it is thought that caspases might inactivate inflammatory substances (131). Also, under serum-free conditions human necrotic neutrophils inhibit LPS-mediated cytokine production. Alpha-defensins are anti-microbial, cationic peptides expressed by human, but not mouse, neutrophils. Alpha-defensins are inactivated in serum; however, in the absence of serum, alpha-defensins released from dying neutrophils or liberated from necrotic neutrophils by freeze-thaw have potent anti-inflammatory activity (132). Understanding how necrotic neutrophils stimulate or fail to stimulate inflammation in different contexts is an important and clinically relevant area of study.
Insights into neutrophil efferocytosis in small rodent models
Much of the work investigating the process of eliminating neutrophils in vivo relies on studies involving animal models. Although little to no intravital imaging of efferocytosis currently exists in animal models, neutrophil efferocytosis by phagocytes has been demonstrated in vivo using a variety of other analytical techniques, including radiolabeling, and confirms this process occurs in the lung, spleen, liver, and bone marrow (4, 7, 133, 134). Consistent with the trafficking and destruction of apoptotic neutrophils in the liver, blocking Kuppfer cell phagocytosis in rats causes systemic neutrophilia and a particular increase in neutrophils in the lung and spleen (7). Clearance in the bone marrow is especially interesting, since intact and viable cells appear to migrate back to bone marrow for destruction (5). The CXCR4/SDP-1α signaling axis mediates trafficking into the bone marrow (135). Aging neutrophils upregulate CXCR4 (136), and while still Annexin-V-negative, older neutrophils can migrate towards the CXCR4 ligand, SDF-1a/CXCL12. When injected into mice, the CXCR4-high neutrophils home to a hematopoietic compartment of the bone marrow, where SDF-1a is constitutively expressed (135).
In vivo evidence of efferocytosis has also emerged from studies investigating the production of leukocytes from the bone marrow, or granulopoiesis. Several studies have suggested that efferocytosis in tissues is a prerequisite for turning off neutrophil granulopoesis. Interestingly, mice deficient in factors required for neutrophil extravasation (i.e. β2 integrin/CD18 and P-selectin) into tissue exhibit neutrophilia; therefore, Ley and colleagues speculate that turning off neutrophil granulopoiesis requires extravasation into tissues (8). The engulfment of apoptotic neutrophils dampens IL-23 production, and IL-23-dependent IL-17 production by T cells. Because IL-17 is diminished, IL-17-dependent, GCSF-driven neutrophil granulopoiesis is therefore reduced. In support of these data, transfer of normal neutrophils into CD18-/- mice restores IL-23 levels, reduces IL-17, and normalizes neutrophil numbers in the blood (8, 137).
Specialized subsets of efferocytic macrophages are detected during resolving inflammation in mice. During the resolution of murine peritonitis a CD11b-low population of macrophages emerges. Gene expression in the CD11b-low population diverges from the M1 and M2 framework because classic macrophage phenotype markers, arginase-1 and iNOS, are not found in the CD11b-low population. Regardless, CD11b-low macrophages engulf higher numbers of apoptotic neutrophils, and produce less pro-inflammatory cytokines but more TGF-β in response to TLR-ligands. Moreover, CD11b-low cells are less phagocytic than their CD11b-high counterparts and are more likely to migrate to the lymph nodes (138). The observed decrease in phagocyte appetite could be triggered by PGE2 production and elevation of intracellular cAMP (119) or alterations in the Rho-Rac1 balance. Looking at the effects of efferocytosis on alveolar macrophage antibacterial function, Medeiros et al. demonstrated that signaling leading to production of PGE2 and PGE2 signaling through the EP2 receptor (but not TGF-β signaling) activates cAMP. Likewise, activation of cAMP causes a reduction in the ability of rat alveolar macrophages to engulf Fc-osponized targets and reduces the ability of alveolar macrophages to kill Streptococcus pneumoniae (139). These data highlight the plasticity of macrophages in vivo and how extracellular stimuli influence their effector functions.
Finally, although neutrophil reverse transmigration has been reported to occur in zebrafish, mice, and humans, only an extremely small percentage of human neutrophils (0.25% in healthy patients) have this capacity (140). Therefore, disappearance of neutrophils from sites of inflammation is most likely mediated by efferocytosis in the inflamed tissues.
Regulation of dendritic cell function
DC reside in tissues as an immature cell type and subsequently mature into professional antigen presenting cells capable of stimulating a T cell response. Phagosome maturation in DC is slow and less efficient than macrophages, favoring antigen presentation to T cells (141). Expression of costimulatory molecules on DC is also a prerequisite for maturation and T cell stimulation, whereas lack of costimulatory molecules contributes to T cell tolerance (142). DC are one of the cell types capable of recognizing and engulfing apoptotic neutrophils, thus understanding how DCs promote tolerance to self-antigen rather than immunity is a subject of significant interest.
Typical of DC, the phagosomes of DC containing either apoptotic cells or antibody-opsonized apoptotic cells mature at a slower rate than in macrophages (89), indicating that phagosome maturation in DC is different than macrophages in which apoptotic cargo acidify more rapidly than antibody-opsonized cells.
In line with their other immunomodulatory properties of apoptotic cells, engulfment of either apoptotic or necrotic neutrophils fails to induce expression of important costimulatory molecules on immature DC (143). Even in the response to pro-inflammatory molecules TNF-α or LPS, DC cocultured with apoptotic neutrophils failed to mature to the same extent as DC treated with TNF-α or LPS alone (89, 143). In contrast, necrotic tumor cells have been shown to induce DC maturation, whereas necrotic neutrophils, apoptotic neutrophils and apoptotic tumor cell lines tend to be suppressive (144). It is currently unknown how necrotic neutrophils differ from necrotic tumor cells in their inability to induce DC maturation.
Despite strong evidence that apoptotic cells prevent DC maturation, there is evidence that neutrophil efferocytosis is important for DC cross-priming of CD8+ T cells (145). Cross-priming occurs when extracellular antigen gains access to the cytosol and is then loaded into MHC class I molecules and presented to cytotoxic CD8+ T cell. Neutrophils carrying tumor antigen, Escherichia coli, or Mycobacterium tuberculosis are able induce cross priming in DC as evidenced by lymphocyte proliferation (146-148).
The exception are Leishmania major-laden neutrophils, which fail to cross-prime DC as described above. This parasite enters the skin via the bite of an infected sand fly, where it immediately triggers neutrophil infiltration. Neutrophils fail to control parasite burden and directly promote infection (149). Likewise, neutropenia has been associated with a better immune response, suggesting that neutrophils may be a double-edged sword in Leishmania infection. To study how neutrophil effector functions and lifespan could impair immunity to Leishmania, Ribeiro-Gomes and colleagues infected mice with Leishmania major and isolated neutrophils 12 hours following inoculation. They demonstrated that cultured DC are capable of capturing sorted uninfected and parasite-laden neutrophils, albeit at an extremely low rate (∼1.2% phagocytosis). Furthermore, DC associated with parasite-laden neutrophils are less capable of CD8+ T cells cross-priming compared to uninfected neutrophils from the same mouse. In contrast, DC that capture either uninfected or Toxoplama gondii-laden neutrophils are able to support CD8+ T cell cross-priming to the same extent indicating that inhibition of cross-priming is unique to Leishmania (52). Overall, these data demonstrate that efferocytosis is important for cross-priming during infection, and is targeted by at least one pathogen.
Macrophage efferocytosis and infection
An exciting recent hypothesis is that neutrophils containing viable bacteria can hijack efferocytosis to gain silent entry into phagocytes. Many examples of this phenomenon exist, as certain parasites and bacteria have been shown to survive neutrophil-mediated killing, modulate apoptosis, and be engulfed by phagocytes; in some instances, efferocytosis of neutrophils containing viable organisms results in a detrimental consequences for the host. Yet in other instances, efferocytosis has been shown to be beneficial in promoting a host response or efferocytosis simply does not happen at all.
Chlamydia pneumoniae, a Gram-negative, obligate intracellular bacterium, Yersinia pestis, a Gram-negative, facultative intracellular bacterium, and Leishmania major, an obligate intracellular protozoan, are engulfed by human neutrophils, survive within the phagosome, and modulate apoptosis (149-152). Neutrophils containing these viable microorganisms are subsequently engulfed by human macrophages (149-152). Entry via the neutrophil appears to enhance intracellular survival in macrophages; however, it is unclear if this is due to (1) cloaked entry via efferocytosis (2) upregulation of bacterial/parasitic pro-survival factors while in the neutrophil or (3) unchecked proliferation in the neutrophil and therefore higher initial bacterial or parasitic burden delivered to macrophages or other mechanisms. Despite the higher number of microorganisms present, macrophages cocultured with microbe-laden neutrophils produced less proinflammatory cytokines and more anti-inflammatory cytokines, including TGF-β and IL-1RA (150-152). These data suggest that pathogens can exploit efferocytosis to gain entry into a host cell. Nevertheless, the relevance of this pathway to pathogen dissemination and overall manipulation of the inflammatory response remains somewhat controversial and merits further study.
In contrast to the pathogens described above, efferocytosis is important for the control of Mycobacteria tuberculosis. Typically, M. tuberculosis prevents macrophage phagosome-lysosome fusion and replicates in the phagosomal niche. Efferocytosis of mycobacteria-containing apoptotic macrophages results in phagosome-lysosome fusion and pathogen elimination (153). Although neutrophil products contribute to anti-mycobacterial responses (154), M. tuberculosis delays neutrophil apoptosis and impairs T cell responses (155), suggesting that the ability to avoid efferocytosis contributes to M. tuberculosis pathogenicity. Future studies are required to confirm this hypothesis; however, it is still plausible that neutrophil efferocytosis contributes to antibacterial responses through expedited phagosome maturation, acquisition of anti-microbial neutrophil products, or through DC-cross priming as discussed above.
Finally, our lab has shown that community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA), prevents macrophage efferocytosis of CA-MRSA-laden neutrophils. CA-MRSA initially induces a program of accelerated apoptosis in neutrophils following phagocytosis; however, the neutrophil abruptly lyses without caspase activation (26, 156). While CA-MRSA-neutrophils are still intact, they bind to macrophages, but resist internalization. CD47 is upregulated on CA-MRSA laden neutrophils, but blockade of CD47 alone is not sufficient to induce efferocytosis. The inability of macrophages to engulf CA-MRSA-laden neutrophils likely perpetuates inflammation in two complementary ways: (1) by preventing macrophage anti-inflammatory cytokine production and (2) by culminating in neutrophil lysis and release of host and bacterial constituents that can activate macrophages and contribute to tissue damage (26, 157, 158). Especially in cases of antibiotic resistance, there is an urgent need for novel targets and therapeutics to treat S. aureus and other infections. Elucidating how individual pathogens manipulate efferocytosis may provide important insights into targets for antimicrobial therapies.
Conclusions
Efferocytosis of human neutrophils by phagocytes is critical for normal tissue homeostasis and resolution of inflammation. Outstanding questions remain in several areas, including the role of macrophage heterogeneity in neutrophil efferocytosis, and how specific pathogens modulate neutrophil survival and efferocytosis. Given the anti-inflammatory nature of efferocytosis, understanding the molecular players that initiate recognition and engulfment and downstream signaling events may lead to the development of novel therapeutics targeted at curbing unwanted inflammation.
Acknowledgments
I would like to thank Maya Amjadi, Lauren Kinkead, and Drs. Mark Wacker and Lee-Ann Allen for careful review of the manuscript. This work was generously supported by the Nauseef laboratory and funding from Horizon Pharma and the National Institute of Health grants AI70958 and AI044642 (WMN). The Nauseef lab is also supported by a Merit Review award and use of facilities at the Iowa City Department of Veterans Affairs Medical Center, Iowa City, IA 52246
References
- 1.deCathelineau AM, Henson PM. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays in biochemistry. 2003;39:105–117. doi: 10.1042/bse0390105. [DOI] [PubMed] [Google Scholar]
- 2.McCracken JM, Allen LA. Regulation of human neutrophil apoptosis and lifespan in health and disease. Journal of cell death. 2014;7:15–23. doi: 10.4137/JCD.S11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Athens JW, et al. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. The Journal of clinical investigation. 1961;40:989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thakur ML, Coleman RE, Welch MJ. Indium-111-labeled leukocytes for the localization of abscesses: preparation, analysis, tissue distribution, and comparison with gallium-67 citrate in dogs. The Journal of laboratory and clinical medicine. 1977;89:217–228. [PubMed] [Google Scholar]
- 5.Saverymuttu SH, Peters AM, Keshavarzian A, Reavy HJ, Lavender JP. The kinetics of 111indium distribution following injection of 111indium labelled autologous granulocytes in man. British journal of haematology. 1985;61:675–685. doi: 10.1111/j.1365-2141.1985.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 6.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. The Journal of clinical investigation. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi J, Gilbert GE, Kokubo Y, Ohashi T. Role of the liver in regulating numbers of circulating neutrophils. Blood. 2001;98:1226–1230. doi: 10.1182/blood.v98.4.1226. [DOI] [PubMed] [Google Scholar]
- 8.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 10.Weiss SJ. Tissue destruction by neutrophils. The New England journal of medicine. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 11.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 12.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. The Journal of clinical investigation. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korns D, Frasch SC, Fernandez-Boyanapalli R, Henson PM, Bratton DL. Modulation of macrophage efferocytosis in inflammation. Frontiers in immunology. 2011;2:57. doi: 10.3389/fimmu.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadok VA, et al. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. Journal of immunology. 1992;149:4029–4035. [PubMed] [Google Scholar]
- 15.Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–550. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nature reviews Immunology. 2007;7:964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 17.Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) Journal of immunology. 1998;161:6250–6257. [PubMed] [Google Scholar]
- 18.Savill JS, Henson PM, Haslett C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel “charge-sensitive” recognition mechanism. The Journal of clinical investigation. 1989;84:1518–1527. doi: 10.1172/JCI114328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall SE, Savill JS, Henson PM, Haslett C. Apoptotic neutrophils are phagocytosed by fibroblasts with participation of the fibroblast vitronectin receptor and involvement of a mannose/fucose-specific lectin. Journal of immunology. 1994;153:3218–3227. [PubMed] [Google Scholar]
- 20.Dransfield I, Rossi AG, Brown SB, Hart SP. Neutrophils: dead or effete? Cell surface phenotype and implications for phagocytic clearance. Cell death and differentiation. 2005;12:1363–1367. doi: 10.1038/sj.cdd.4401695. [DOI] [PubMed] [Google Scholar]
- 21.Tak T, Tesselaar K, Pillay J, Borghans JA, Koenderman L. What's your age again? Determination of human neutrophil half-lives revisited. Journal of leukocyte biology. 2013;94:595–601. doi: 10.1189/jlb.1112571. [DOI] [PubMed] [Google Scholar]
- 22.Gabelloni ML, Trevani AS, Sabatte J, Geffner J. Mechanisms regulating neutrophil survival and cell death. Seminars in immunopathology. 2013;35:423–437. doi: 10.1007/s00281-013-0364-x. [DOI] [PubMed] [Google Scholar]
- 23.Geering B, Simon HU. Peculiarities of cell death mechanisms in neutrophils. Cell death and differentiation. 2011;18:1457–1469. doi: 10.1038/cdd.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi SD, Voyich JM, Buhl CL, Stahl RM, DeLeo FR. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fate is regulated at the level of gene expression. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6901–6906. doi: 10.1073/pnas.092148299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi SD, et al. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10948–10953. doi: 10.1073/pnas.1833375100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, Nauseef WM. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. Journal of immunology. 2014;192:4709–4717. doi: 10.4049/jimmunol.1302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. Journal of leukocyte biology. 1993;54:283–288. [PubMed] [Google Scholar]
- 28.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 29.Simon HU. Neutrophil apoptosis pathways and their modifications in inflammation. Immunological reviews. 2003;193:101–110. doi: 10.1034/j.1600-065x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- 30.Thwaites GE, Gant V. Are bloodstream leukocytes Trojan Horses for the metastasis of Staphylococcus aureus? Nature reviews. Microbiology. 2011;9:215–222. doi: 10.1038/nrmicro2508. [DOI] [PubMed] [Google Scholar]
- 31.Hart SP, Dransfield I, Rossi AG. Phagocytosis of apoptotic cells. Methods. 2008;44:280–285. doi: 10.1016/j.ymeth.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Murray PJ, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Frontiers in bioscience : a journal and virtual library. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 34.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews Immunology. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 35.Dransfield I, Buckle AM, Savill JS, McDowall A, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. Journal of immunology. 1994;153:1254–1263. [PubMed] [Google Scholar]
- 36.Dransfield I, Stocks SC, Haslett C. Regulation of cell adhesion molecule expression and function associated with neutrophil apoptosis. Blood. 1995;85:3264–3273. [PubMed] [Google Scholar]
- 37.Homburg CH, de Haas M, von dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roos D. Human neutrophils lose their surface Fc gamma RIII and acquire Annexin V binding sites during apoptosis in vitro. Blood. 1995;85:532–540. [PubMed] [Google Scholar]
- 38.Jones J, Morgan BP. Apoptosis is associated with reduced expression of complement regulatory molecules, adhesion molecules and other receptors on polymorphonuclear leucocytes: functional relevance and role in inflammation. Immunology. 1995;86:651–660. [PMC free article] [PubMed] [Google Scholar]
- 39.Hart SP, Ross JA, Ross K, Haslett C, Dransfield I. Molecular characterization of the surface of apoptotic neutrophils: implications for functional downregulation and recognition by phagocytes. Cell death and differentiation. 2000;7:493–503. doi: 10.1038/sj.cdd.4400680. [DOI] [PubMed] [Google Scholar]
- 40.Gardai SJ, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–334. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 41.Moffatt OD, Devitt A, Bell ED, Simmons DL, Gregory CD. Macrophage recognition of ICAM-3 on apoptotic leukocytes. Journal of immunology. 1999;162:6800–6810. [PubMed] [Google Scholar]
- 42.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 43.Kristof E, Zahuczky G, Katona K, Doro Z, Nagy E, Fesus L. Novel role of ICAM3 and LFA-1 in the clearance of apoptotic neutrophils by human macrophages. Apoptosis: an international journal on programmed cell death. 2013;18:1235–1251. doi: 10.1007/s10495-013-0873-z. [DOI] [PubMed] [Google Scholar]
- 44.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harbor perspectives in biology. 2013;5:a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nature reviews. Immunology. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 46.Savill J, Hogg N, Haslett C. Macrophage vitronectin receptor, CD36, and thrombospondin cooperate in recognition of neutrophils undergoing programmed cell death. Chest. 1991;99:6S–7S. doi: 10.1378/chest.99.3_supplement.6s-a. [DOI] [PubMed] [Google Scholar]
- 47.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. The Journal of clinical investigation. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 49.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 50.Albert ML, et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. The Journal of experimental medicine. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Majai G, et al. PPARgamma modulated inflammatory response of human dendritic cell subsets to engulfed apoptotic neutrophils. Journal of leukocyte biology. 2010;88:981–991. doi: 10.1189/jlb.0310144. [DOI] [PubMed] [Google Scholar]
- 52.Ribeiro-Gomes FL, et al. Apoptotic cell clearance of Leishmania major-infected neutrophils by dendritic cells inhibits CD8(+) T-cell priming in vitro by Mer tyrosine kinase-dependent signaling. Cell death & disease. 2015;6:e2018. doi: 10.1038/cddis.2015.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. The Journal of experimental medicine. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. Journal of immunology. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 55.Ren Y, et al. Nonphlogistic clearance of late apoptotic neutrophils by macrophages: efficient phagocytosis independent of beta 2 integrins. Journal of immunology. 2001;166:4743–4750. doi: 10.4049/jimmunol.166.7.4743. [DOI] [PubMed] [Google Scholar]
- 56.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. Journal of immunology. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 57.Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. The Journal of biological chemistry. 2001;276:1071–1077. doi: 10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- 58.Armstrong A, Ravichandran KS. Phosphatidylserine receptors: what is the new RAGE? EMBO reports. 2011;12:287–288. doi: 10.1038/embor.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. The Journal of experimental medicine. 2010;207:1807–1817. doi: 10.1084/jem.20101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bratton DL, Henson PM. Apoptotic cell recognition: will the real phosphatidylserine receptor(s) please stand up? Current biology: CB. 2008;18:R76–79. doi: 10.1016/j.cub.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 61.Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. The Journal of experimental medicine. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoffmann PR, et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. The Journal of cell biology. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vandivier RW, et al. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. Journal of immunology. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- 64.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 65.Gaipl US, et al. Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell death and differentiation. 2001;8:327–334. doi: 10.1038/sj.cdd.4400826. [DOI] [PubMed] [Google Scholar]
- 66.Tsou WI, et al. Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. The Journal of biological chemistry. 2014;289:25750–25763. doi: 10.1074/jbc.M114.569020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scott RS, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 68.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 69.McColl A, et al. Glucocorticoids induce protein S-dependent phagocytosis of apoptotic neutrophils by human macrophages. Journal of immunology. 2009;183:2167–2175. doi: 10.4049/jimmunol.0803503. [DOI] [PubMed] [Google Scholar]
- 70.Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. Journal of immunology. 2012;189:3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zizzo G, Cohen PL. The PPAR-gamma antagonist GW9662 elicits differentiation of M2c-like cells and upregulation of the MerTK/Gas6 axis: a key role for PPAR-gamma in human macrophage polarization. Journal of inflammation. 2015;12:36. doi: 10.1186/s12950-015-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giles KM, Ross K, Rossi AG, Hotchin NA, Haslett C, Dransfield I. Glucocorticoid augmentation of macrophage capacity for phagocytosis of apoptotic cells is associated with reduced p130Cas expression, loss of paxillin/pyk2 phosphorylation, and high levels of active Rac. Journal of immunology. 2001;167:976–986. doi: 10.4049/jimmunol.167.2.976. [DOI] [PubMed] [Google Scholar]
- 73.Galvan MD, Foreman DB, Zeng E, Tan JC, Bohlson SS. Complement component C1q regulates macrophage expression of Mer tyrosine kinase to promote clearance of apoptotic cells. Journal of immunology. 2012;188:3716–3723. doi: 10.4049/jimmunol.1102920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ogden CA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. The Journal of experimental medicine. 2001;194:781–795. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nauseef WM, McCormick SJ, Clark RA. Calreticulin functions as a molecular chaperone in the biosynthesis of myeloperoxidase. The Journal of biological chemistry. 1995;270:4741–4747. doi: 10.1074/jbc.270.9.4741. [DOI] [PubMed] [Google Scholar]
- 76.Gabillet J, et al. Proteinase 3, the autoantigen in granulomatosis with polyangiitis, associates with calreticulin on apoptotic neutrophils, impairs macrophage phagocytosis, and promotes inflammation. Journal of immunology. 2012;189:2574–2583. doi: 10.4049/jimmunol.1200600. [DOI] [PubMed] [Google Scholar]
- 77.Sudan B, Wacker MA, Wilson ME, Graff JWA. Systematic Approach to Identify Markers of Distinctly Activated Human Macrophages. Frontiers in immunology. 2015;6:253. doi: 10.3389/fimmu.2015.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nature reviews Molecular cell biology. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 79.Jun JI, Kim KH, Lau LF. The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nature communications. 2015;6:7386. doi: 10.1038/ncomms8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murakami Y, Tian L, Voss OH, Margulies DH, Krzewski K, Coligan JE. CD300b regulates the phagocytosis of apoptotic cells via phosphatidylserine recognition. Cell death and differentiation. 2014;21:1746–1757. doi: 10.1038/cdd.2014.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Penberthy KK, Ravichandran KS. Apoptotic cell recognition receptors and scavenger receptors. Immunological reviews. 2016;269:44–59. doi: 10.1111/imr.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scannell M, et al. Annexin-1 and peptide derivatives are released by apoptotic cells and stimulate phagocytosis of apoptotic neutrophils by macrophages. Journal of immunology. 2007;178:4595–4605. doi: 10.4049/jimmunol.178.7.4595. [DOI] [PubMed] [Google Scholar]
- 83.Frasch SC, et al. NADPH oxidase-dependent generation of lysophosphatidylserine enhances clearance of activated and dying neutrophils via G2A. The Journal of biological chemistry. 2008;283:33736–33749. doi: 10.1074/jbc.M807047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Karlsson A, et al. Galectin-3 functions as an opsonin and enhances the macrophage clearance of apoptotic neutrophils. Glycobiology. 2009;19:16–20. doi: 10.1093/glycob/cwn104. [DOI] [PubMed] [Google Scholar]
- 85.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annual review of immunology. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 86.Allen LA, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. The Journal of experimental medicine. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Krysko DV, et al. Macrophages use different internalization mechanisms to clear apoptotic and necrotic cells. Cell death and differentiation. 2006;13:2011–2022. doi: 10.1038/sj.cdd.4401900. [DOI] [PubMed] [Google Scholar]
- 88.Lu N, Zhou Z. Membrane trafficking and phagosome maturation during the clearance of apoptotic cells. International review of cell and molecular biology. 2012;293:269–309. doi: 10.1016/B978-0-12-394304-0.00013-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Erwig LP, McPhilips KA, Wynes MW, Ivetic A, Ridley AJ, Henson PM. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12825–12830. doi: 10.1073/pnas.0605331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maderna P, Yona S, Perretti M, Godson C. Modulation of phagocytosis of apoptotic neutrophils by supernatant from dexamethasone-treated macrophages and annexin-derived peptide Ac(2-26) Journal of immunology. 2005;174:3727–3733. doi: 10.4049/jimmunol.174.6.3727. [DOI] [PubMed] [Google Scholar]
- 91.Majai G, Sarang Z, Csomos K, Zahuczky G, Fesus L. PPARgamma-dependent regulation of human macrophages in phagocytosis of apoptotic cells. European journal of immunology. 2007;37:1343–1354. doi: 10.1002/eji.200636398. [DOI] [PubMed] [Google Scholar]
- 92.Fernandez-Boyanapalli RF, et al. Impaired efferocytosis in human chronic granulomatous disease is reversed by pioglitazone treatment. The Journal of allergy and clinical immunology. 2015;136:1399–1401. e1391–1393. doi: 10.1016/j.jaci.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morimoto K, et al. Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. Journal of immunology. 2006;176:7657–7665. doi: 10.4049/jimmunol.176.12.7657. [DOI] [PubMed] [Google Scholar]
- 94.Moon C, Lee YJ, Park HJ, Chong YH, Kang JL. N-acetylcysteine inhibits RhoA and promotes apoptotic cell clearance during intense lung inflammation. American journal of respiratory and critical care medicine. 2010;181:374–387. doi: 10.1164/rccm.200907-1061OC. [DOI] [PubMed] [Google Scholar]
- 95.Hart SP, Rossi AG, Haslett C, Dransfield I. Characterization of the effects of cross-linking of macrophage CD44 associated with increased phagocytosis of apoptotic PMN. PloS one. 2012;7:e33142. doi: 10.1371/journal.pone.0033142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reville K, Crean JK, Vivers S, Dransfield I, Godson C. Lipoxin A4 redistributes myosin IIA and Cdc42 in macrophages: implications for phagocytosis of apoptotic leukocytes. Journal of immunology. 2006;176:1878–1888. doi: 10.4049/jimmunol.176.3.1878. [DOI] [PubMed] [Google Scholar]
- 97.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dalli J, et al. Heterogeneity in neutrophil microparticles reveals distinct proteome and functional properties. Molecular & cellular proteomics: MCP. 2013;12:2205–2219. doi: 10.1074/mcp.M113.028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shinkai M, Henke MO, Rubin BK. Macrolide antibiotics as immunomodulatory medications: proposed mechanisms of action. Pharmacology & therapeutics. 2008;117:393–405. doi: 10.1016/j.pharmthera.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 100.Yamaryo T, Oishi K, Yoshimine H, Tsuchihashi Y, Matsushima K, Nagatake T. Fourteen-member macrolides promote the phosphatidylserine receptor-dependent phagocytosis of apoptotic neutrophils by alveolar macrophages. Antimicrobial agents and chemotherapy. 2003;47:48–53. doi: 10.1128/AAC.47.1.48-53.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hodge S, et al. Azithromycin improves macrophage phagocytic function and expression of mannose receptor in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2008;178:139–148. doi: 10.1164/rccm.200711-1666OC. [DOI] [PubMed] [Google Scholar]
- 102.Wunderink RG, Mandell L. Adjunctive therapy in community-acquired pneumonia. Seminars in respiratory and critical care medicine. 2012;33:311–318. doi: 10.1055/s-0032-1315643. [DOI] [PubMed] [Google Scholar]
- 103.Kuijpers TW, et al. Neutrophils in Barth syndrome (BTHS) avidly bind annexin-V in the absence of apoptosis. Blood. 2004;103:3915–3923. doi: 10.1182/blood-2003-11-3940. [DOI] [PubMed] [Google Scholar]
- 104.Frasch SC, Henson PM, Nagaosa K, Fessler MB, Borregaard N, Bratton DL. Phospholipid flip-flop and phospholipid scramblase 1 (PLSCR1) co-localize to uropod rafts in formylated Met-Leu-Phe-stimulated neutrophils. The Journal of biological chemistry. 2004;279:17625–17633. doi: 10.1074/jbc.M313414200. [DOI] [PubMed] [Google Scholar]
- 105.Appelt U, Sheriff A, Gaipl US, Kalden JR, Voll RE, Herrmann M. Viable, apoptotic and necrotic monocytes expose phosphatidylserine: cooperative binding of the ligand Annexin V to dying but not viable cells and implications for PS-dependent clearance. Cell death and differentiation. 2005;12:194–196. doi: 10.1038/sj.cdd.4401527. [DOI] [PubMed] [Google Scholar]
- 106.Vernon-Wilson EF, et al. CD31 delays phagocyte membrane repolarization to promote efficient binding of apoptotic cells. Journal of leukocyte biology. 2007;82:1278–1288. doi: 10.1189/jlb.0507283. [DOI] [PubMed] [Google Scholar]
- 107.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 108.Simhadri VR, Andersen JF, Calvo E, Choi SC, Coligan JE, Borrego F. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood. 2012;119:2799–2809. doi: 10.1182/blood-2011-08-372425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park YJ, et al. PAI-1 inhibits neutrophil efferocytosis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:11784–11789. doi: 10.1073/pnas.0801394105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park YJ, Liu G, Tsuruta Y, Lorne E, Abraham E. Participation of the urokinase receptor in neutrophil efferocytosis. Blood. 2009;114:860–870. doi: 10.1182/blood-2008-12-193524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bae HB, et al. Vitronectin inhibits efferocytosis through interactions with apoptotic cells as well as with macrophages. Journal of immunology. 2013;190:2273–2281. doi: 10.4049/jimmunol.1200625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McPhillips K, et al. TNF-alpha inhibits macrophage clearance of apoptotic cells via cytosolic phospholipase A2 and oxidant-dependent mechanisms. Journal of immunology. 2007;178:8117–8126. doi: 10.4049/jimmunol.178.12.8117. [DOI] [PubMed] [Google Scholar]
- 113.Michlewska S, Dransfield I, Megson IL, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is critically regulated by the opposing actions of pro-inflammatory and anti-inflammatory agents: key role for TNF-alpha. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:844–854. doi: 10.1096/fj.08-121228. [DOI] [PubMed] [Google Scholar]
- 114.Banerjee S, de Freitas A, Friggeri A, Zmijewski JW, Liu G, Abraham E. Intracellular HMGB1 negatively regulates efferocytosis. Journal of immunology. 2011;187:4686–4694. doi: 10.4049/jimmunol.1101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Friggeri A, Yang Y, Banerjee S, Park YJ, Liu G, Abraham E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. American journal of physiology Cell physiology. 2010;299:C1267–1276. doi: 10.1152/ajpcell.00152.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu G, et al. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. Journal of immunology. 2008;181:4240–4246. doi: 10.4049/jimmunol.181.6.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Rossum AP, et al. The prototypic tissue pentraxin PTX3, in contrast to the short pentraxin serum amyloid P, inhibits phagocytosis of late apoptotic neutrophils by macrophages. Arthritis and rheumatism. 2004;50:2667–2674. doi: 10.1002/art.20370. [DOI] [PubMed] [Google Scholar]
- 118.Friggeri A, et al. Extracellular histones inhibit efferocytosis. Molecular medicine. 2012;18:825–833. doi: 10.2119/molmed.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rossi AG, McCutcheon JC, Roy N, Chilvers ER, Haslett C, Dransfield I. Regulation of macrophage phagocytosis of apoptotic cells by cAMP. Journal of immunology. 1998;160:3562–3568. [PubMed] [Google Scholar]
- 120.Knobler R, et al. Guidelines on the use of extracorporeal photopheresis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2014;28 Suppl 1:1–37. doi: 10.1111/jdv.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meagher LC, Savill JS, Baker A, Fuller RW, Haslett C. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2. Journal of leukocyte biology. 1992;52:269–273. [PubMed] [Google Scholar]
- 122.Stern M, Savill J, Haslett C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by alpha v beta 3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. The American journal of pathology. 1996;149:911–921. [PMC free article] [PubMed] [Google Scholar]
- 123.Newman SL, Henson JE, Henson PM. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. The Journal of experimental medicine. 1982;156:430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Byrne A, Reen DJ. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. Journal of immunology. 2002;168:1968–1977. doi: 10.4049/jimmunol.168.4.1968. [DOI] [PubMed] [Google Scholar]
- 125.Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. The Journal of experimental medicine. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yoshida Y, et al. Monocyte induction of IL-10 and down-regulation of IL-12 by iC3b deposited in ultraviolet-exposed human skin. Journal of immunology. 1998;161:5873–5879. [PubMed] [Google Scholar]
- 127.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Seminars in immunology. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fadok VA, Bratton DL, Guthrie L, Henson PM. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. Journal of immunology. 2001;166:6847–6854. doi: 10.4049/jimmunol.166.11.6847. [DOI] [PubMed] [Google Scholar]
- 129.Barker RN, Erwig LP, Hill KS, Devine A, Pearce WP, Rees AJ. Antigen presentation by macrophages is enhanced by the uptake of necrotic, but not apoptotic, cells. Clinical and experimental immunology. 2002;127:220–225. doi: 10.1046/j.1365-2249.2002.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lammermann T, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ren Y, Savill J. Apoptosis: the importance of being eaten. Cell death and differentiation. 1998;5:563–568. doi: 10.1038/sj.cdd.4400407. [DOI] [PubMed] [Google Scholar]
- 132.Miles K, et al. Dying and necrotic neutrophils are anti-inflammatory secondary to the release of alpha-defensins. Journal of immunology. 2009;183:2122–2132. doi: 10.4049/jimmunol.0804187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lovas K, Knudsen E, Iversen PO, Benestad HB. Sequestration patterns of transfused rat neutrophilic granulocytes under normal and inflammatory conditions. European journal of haematology. 1996;56:221–229. doi: 10.1111/j.1600-0609.1996.tb01933.x. [DOI] [PubMed] [Google Scholar]
- 134.Suratt BT, Young SK, Lieber J, Nick JA, Henson PM, Worthen GS. Neutrophil maturation and activation determine anatomic site of clearance from circulation. American journal of physiology Lung cellular and molecular physiology. 2001;281:L913–921. doi: 10.1152/ajplung.2001.281.4.L913. [DOI] [PubMed] [Google Scholar]
- 135.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- 136.Nagase H, et al. Cytokine-mediated regulation of CXCR4 expression in human neutrophils. Journal of leukocyte biology. 2002;71:711–717. [PubMed] [Google Scholar]
- 137.von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. Journal of immunology. 2008;181:5183–5188. doi: 10.4049/jimmunol.181.8.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A. Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. European journal of immunology. 2011;41:366–379. doi: 10.1002/eji.201040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. The Journal of experimental medicine. 2009;206:61–68. doi: 10.1084/jem.20082058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Buckley CD, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. Journal of leukocyte biology. 2006;79:303–311. doi: 10.1189/jlb.0905496. [DOI] [PubMed] [Google Scholar]
- 141.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunological reviews. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 142.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. The Journal of experimental medicine. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Clayton AR, Prue RL, Harper L, Drayson MT, Savage CO. Dendritic cell uptake of human apoptotic and necrotic neutrophils inhibits CD40, CD80, and CD86 expression and reduces allogeneic T cell responses: relevance to systemic vasculitis. Arthritis and rheumatism. 2003;48:2362–2374. doi: 10.1002/art.11130. [DOI] [PubMed] [Google Scholar]
- 144.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. The Journal of experimental medicine. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boudaly S. Activation of dendritic cells by polymorphonuclear neutrophils. Frontiers in bioscience. 2009;14:1589–1595. doi: 10.2741/3326. [DOI] [PubMed] [Google Scholar]
- 146.Tomihara K, et al. Antigen-specific immunity and cross-priming by epithelial ovarian carcinoma-induced CD11b(+)Gr-1(+) cells. Journal of immunology. 2010;184:6151–6160. doi: 10.4049/jimmunol.0903519. [DOI] [PubMed] [Google Scholar]
- 147.Aleman M, et al. Spontaneous or Mycobacterium tuberculosis-induced apoptotic neutrophils exert opposite effects on the dendritic cell-mediated immune response. European journal of immunology. 2007;37:1524–1537. doi: 10.1002/eji.200636771. [DOI] [PubMed] [Google Scholar]
- 148.Alfaro C, et al. Dendritic cells take up and present antigens from viable and apoptotic polymorphonuclear leukocytes. PloS one. 2011;6:e29300. doi: 10.1371/journal.pone.0029300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peters NC, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Zandbergen G, et al. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. Journal of immunology. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 151.Rupp J, et al. Chlamydia pneumoniae hides inside apoptotic neutrophils to silently infect and propagate in macrophages. PloS one. 2009;4:e6020. doi: 10.1371/journal.pone.0006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Spinner JL, et al. Yersinia pestis survival and replication within human neutrophil phagosomes and uptake of infected neutrophils by macrophages. Journal of leukocyte biology. 2014;95:389–398. doi: 10.1189/jlb.1112551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martin CJ, Peters KN, Behar SM. Macrophages clean up: efferocytosis and microbial control. Current opinion in microbiology. 2014;17:17–23. doi: 10.1016/j.mib.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Silva MT, Silva MN, Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microbial pathogenesis. 1989;6:369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- 155.Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell host & microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kobayashi SD, et al. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. Journal of innate immunity. 2010;2:560–575. doi: 10.1159/000317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zheng L, He M, Long M, Blomgran R, Stendahl O. Pathogen-induced apoptotic neutrophils express heat shock proteins and elicit activation of human macrophages. Journal of immunology. 2004;173:6319–6326. doi: 10.4049/jimmunol.173.10.6319. [DOI] [PubMed] [Google Scholar]
- 158.Holzinger D, et al. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. Journal of leukocyte biology. 2012;92:1069–1081. doi: 10.1189/jlb.0112014. [DOI] [PMC free article] [PubMed] [Google Scholar]