In this issue of ATVB, Chen and coworkers report that a monoclonal antibody whose epitope includes the ADAM17 cleavage site in human platelet glycoprotein Ibα (GPIbα) inhibits GPIbα shedding during platelet storage under blood banking conditions and improves the recovery and survival of platelets in murine platelet transfusion models.
The lifespan of circulating endogenous platelets is determined by a combination of random platelet removal and a non-random aging mechanism after which senescent platelets are removed by the reticuloendothelial (RE) system. At least of a portion of platelet senescence is regulated by an intrinsic apoptotic pathway that acts as an “internal timer” 1, 2. Platelets contain members of the anti-apoptotic Bcl-2 family such as Bcl-xL, as well as the pro-apoptotic proteins Bax and Bak 1, 3. The half life of Bcl-xL in platelets is shorter than Bak 1, 4. Thus, it is likely that as the effect of Bcl-xL declines, a point is reached at which Bak is able to cause platelet apoptosis 2. How apoptosis causes platelet clearance is unknown. Apoptosis causes phosphotidylserine (PS) exposure on cell surfaces, a signal for the phagocytosis of apoptotic cells 5, but it remains to be established whether PS exposure plays a role in the clearance of senescent platelets.
In contrast to endogenous platelets, transfused platelets appear to be cleared because glycans and/or proteins on their surface are perturbed during storage before transfusion. Short-term platelet storage in the cold (i.e., hours) results in GPIb clustering, removal of small amounts of sialic acid from the GPIbα ligand binding domain, removal of newly-exposed galactose residues leading to exposure of N-acetylglucosamine, and platelet clearance via the integrin αMβ2 expressed on hepatic macrophages 6. However, although galactosylation improved the survival of short-term chilled platelets in mice, transfusing galactosylated platelets refrigerated for 48 hours into humans did not extend their circulation time, implying that other cold-induced lesions had occurred 7. Sialic acid removal alone causes the rapid clearance of transfused platelets 6. Moreover, platelet storage for 48 hours in the cold causes desialylation of platelet glycoproteins when the platelets are re-warmed, likely due to cold-induced up-regulation of the sialidase Neu-1 on the platelet surface. Then rapid platelet clearance occurs when the platelets are transfused 6, 8, 9. Sialic acid loss exposes penultimate galactose residues, predominantly on platelet GPIbα, and causes platelet clearance via hepatic asialoglycoprotein (Ashwell-Morell) receptors 6. Whether these observations are relevant to the clearance of endogenous senescent platelets is not clear because their clearance has been attributed to RE rather than hepatic cells. However depletion of hepatic and splenic macrophages has little effect on the lifespan of freshly transfused platelets, suggesting that desialylation as platelets age could play a role in the recognition of senescent platelets 10.
Proteolysis of GPIbα by the membrane-associated metalloproteinase ADAM17 occurs continuously as platelets circulate, releasing the 130 kDa N-terminal ectodomain fragment glycocalicin into the plasma11, 12. GPIbα ectodomain shedding also occurs after platelet stimulation by agonists and when platelet are exposed to W7, a compound that sequesters calmodulin, to CCCP, a drug that damages mitochondria and induces apoptosis, and to the protein kinase C activator PMA 11. Mouse platelets stored in vitro shed substantial amounts of GPIbα and are rapidly cleared when re-infused 13. Because platelet recovery and survival are improved when ADAM17 activity is inhibited 13, 14, Chen at al postulated that blocking GPIbα shedding could inhibit the clearance of stored platelets. Previously, they had produced a monoclonal antibody 5G6 that recognizes the ADAM17 cleavage site between residues Gly464 and Val465 in human GPIbα, thereby inhibiting GPIba ectodomain shedding 11. Here, they formally tested their hypothesis by comparing the recovery and survival of platelets stored at room temperature in the presence or absence of 5G6 Fab fragments. Because 5G6 only binds to human GPIbα, they measured its effect using human platelets transfused into SCID mice or in transgenic mice whose platelets expressed human rather than mouse GPIbα. In SCID mice, 5G6 Fab improved platelet recovery and lifespan when platelets were stored in vitro for 8 days, whereas recovery and lifespan were the same as controls when platelets were stored for 4 days. In the transgenic model, storage with 5G6 Fab improved platelet recovery, but did so without affecting platelet lifespan. Importantly, 5G6 Fab did not exacerbate the impaired platelet responses to ristocetin, ADP, and collagen that result from in vitro storage and it preserved the ability of stored platelets to shorten the prolonged bleeding times of mice in whom the ectodomain of GPIbα was replaced with the ectodomain of the IL4 receptor.
Based on these results, it is likely that inhibiting GPIbα shedding may be a useful way to optimize platelet storage conditions. Nonetheless, the results raise interesting questions about the accelerated clearance of stored platelets after transfusion. First, GPIbα ectodomain shedding removes the glycans recognized by αMβ2 and the asialoglycoprotein receptor. Thus, what receptor is responsible for the clearance of glycocalicin-depleted platelets? Second, what is the relative contribution of the GPIbα glycan modification versus ectodomain shedding to the accelerated platelet clearance of stored platelets. Lastly, it is noteworthy that the amount of GPIbα shed during storage did not decrease substantially when platelets were incubated with 5G6 . However, this small difference was sufficient to rescue platelet survival. Thus, how much glycan cleavage and/or GPIbα cleavage is required to accelerate platelet cleavage?
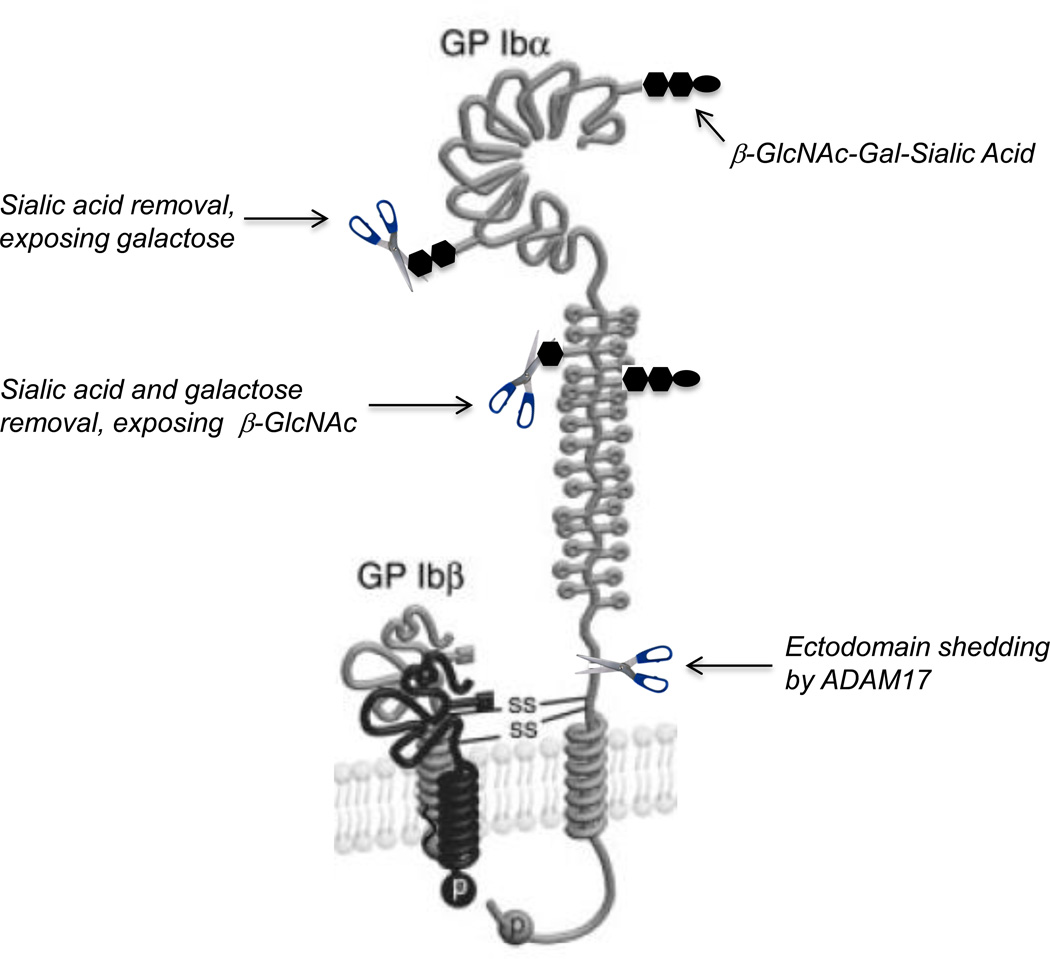
Figure 1.
Mechanisms for the GPIbα-mediated clearance of stored platelets following platelet transfusion. Removal of sialic acid from N-linked glycans by sialidases when refrigerated platelets are rewarmed exposes galactose resides, leading to the clearance of transfused platelets by hepatic asialoiglycoprotein receptors. Removal of both sialic acid and galactose after platelet refrigeration exposes β-N-acetylglucosamine, causing the clearance of transfused platelets by the integrin αMβ2 on hepatic macrophages. Shedding of the GPIbα ectodomain by the metalloproteinase ADAM17 during platelet storage also causes the accelerated clearance of transfused platelets by as a yet unidentified receptor. β-GlcNAc, β-N-acetylglucosamine; Gal, galactose.
REFERENCES
- 1.Mason KD, Carpinelli MR, Fletcher JI, Collinge JE, Hilton AA, Ellis S, Kelly PN, Ekert PG, Metcalf D, Roberts AW, Huang DC, Kile BT. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 2.Dowling MR, Josefsson EC, Henley KJ, Hodgkin PD, Kile BT. Platelet senescence is regulated by an internal timer, not damage inflicted by hits. Blood. 2010;116:1776–1778. doi: 10.1182/blood-2009-12-259663. [DOI] [PubMed] [Google Scholar]
- 3.Vanags DM, Orrenius S, Aguilar-Santelises M. Alterations in Bcl-2/Bax protein levels in platelets form part of an ionomycin-induced process that resembles apoptosis. Br J Haematol. 1997;99:824–831. doi: 10.1046/j.1365-2141.1997.4813284.x. [DOI] [PubMed] [Google Scholar]
- 4.Bertino AM, Qi XQ, Li J, Xia Y, Kuter DJ. Apoptotic markers are increased in platelets stored at 37 degrees C. Transfusion. 2003;43:857–866. doi: 10.1046/j.1537-2995.2003.t01-4-00431.x. [DOI] [PubMed] [Google Scholar]
- 5.Birge RB, Boeltz S, Kumar S, Carlson J, Wanderley J, Calianese D, Barcinski M, Brekken RA, Huang X, Hutchins JT, Freimark B, Empig C, Mercer J, Schroit AJ, Schett G, Herrmann M. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016;23:962–978. doi: 10.1038/cdd.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmeister KM. The role of lectins and glycans in platelet clearance. J Thromb Haemost. 2011;9(Suppl 1):35–43. doi: 10.1111/j.1538-7836.2011.04276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wandall HH, Hoffmeister KM, Sorensen AL, Rumjantseva V, Clausen H, Hartwig JH, Slichter SJ. Galactosylation does not prevent the rapid clearance of long-term, 4 degrees C-stored platelets. Blood. 2008;111:3249–3256. doi: 10.1182/blood-2007-06-097295. [DOI] [PubMed] [Google Scholar]
- 8.Grozovsky R, Hoffmeister KM, Falet H. Novel clearance mechanisms of platelets. Curr Opin Hematol. 2010;17:585–589. doi: 10.1097/MOH.0b013e32833e7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen AJ, Josefsson EC, Rumjantseva V, Liu QP, Falet H, Bergmeier W, Cifuni SM, Sackstein R, von Andrian UH, Wagner DD, Hartwig JH, Hoffmeister KM. Desialylation accelerates platelet clearance after refrigeration and initiates GPIbalpha metalloproteinase-mediated cleavage in mice. Blood. 2012;119:1263–1273. doi: 10.1182/blood-2011-05-355628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rumjantseva V, Grewal PK, Wandall HH, Josefsson EC, Sorensen AL, Larson G, Marth JD, Hartwig JH, Hoffmeister KM. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nat Med. 2009;15:1273–1280. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang X, Russell SR, Estelle S, Jones LH, Cho S, Kahn ML, Berndt MC, Bunting ST, Ware J, Li R. Specific inhibition of ectodomain shedding of glycoprotein Ibalpha by targeting its juxtamembrane shedding cleavage site. J Thromb Haemost. 2013;11:2155–2162. doi: 10.1111/jth.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardiner EE, Karunakaran D, Shen Y, Arthur JF, Andrews RK, Berndt MC. Controlled shedding of platelet glycoprotein (GP)VI and GPIb-IX-V by ADAM family metalloproteinases. J Thromb Haemost. 2007;5:1530–1537. doi: 10.1111/j.1538-7836.2007.02590.x. [DOI] [PubMed] [Google Scholar]
- 13.Bergmeier W, Burger PC, Piffath CL, Hoffmeister KM, Hartwig JH, Nieswandt B, Wagner DD. Metalloproteinase inhibitors improve the recovery and hemostatic function of in vitro-aged or -injured mouse platelets. Blood. 2003;102:4229–4235. doi: 10.1182/blood-2003-04-1305. [DOI] [PubMed] [Google Scholar]
- 14.Canault M, Duerschmied D, Brill A, Stefanini L, Schatzberg D, Cifuni SM, Bergmeier W, Wagner DD. p38 mitogen-activated protein kinase activation during platelet storage: consequences for platelet recovery and hemostatic function in vivo. Blood. 2010;115:1835–1842. doi: 10.1182/blood-2009-03-211706. [DOI] [PMC free article] [PubMed] [Google Scholar]