Abstract
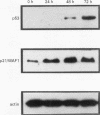
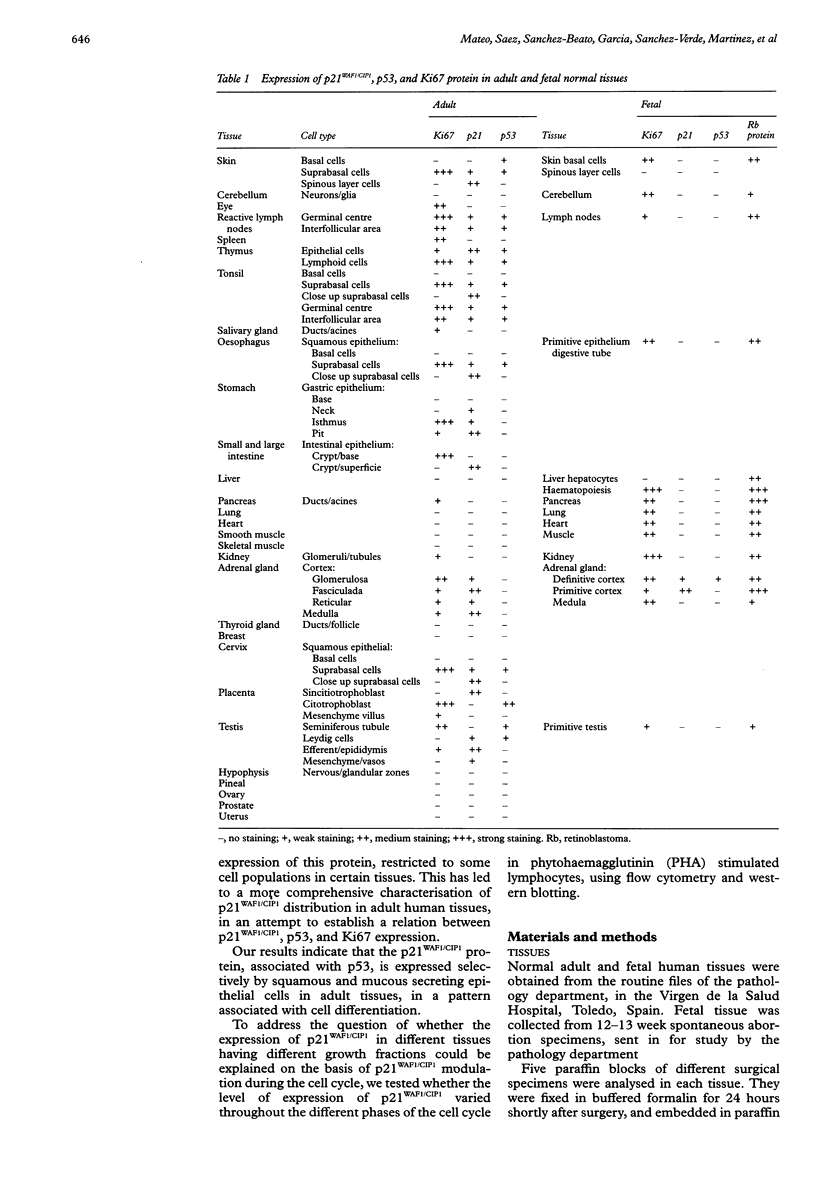
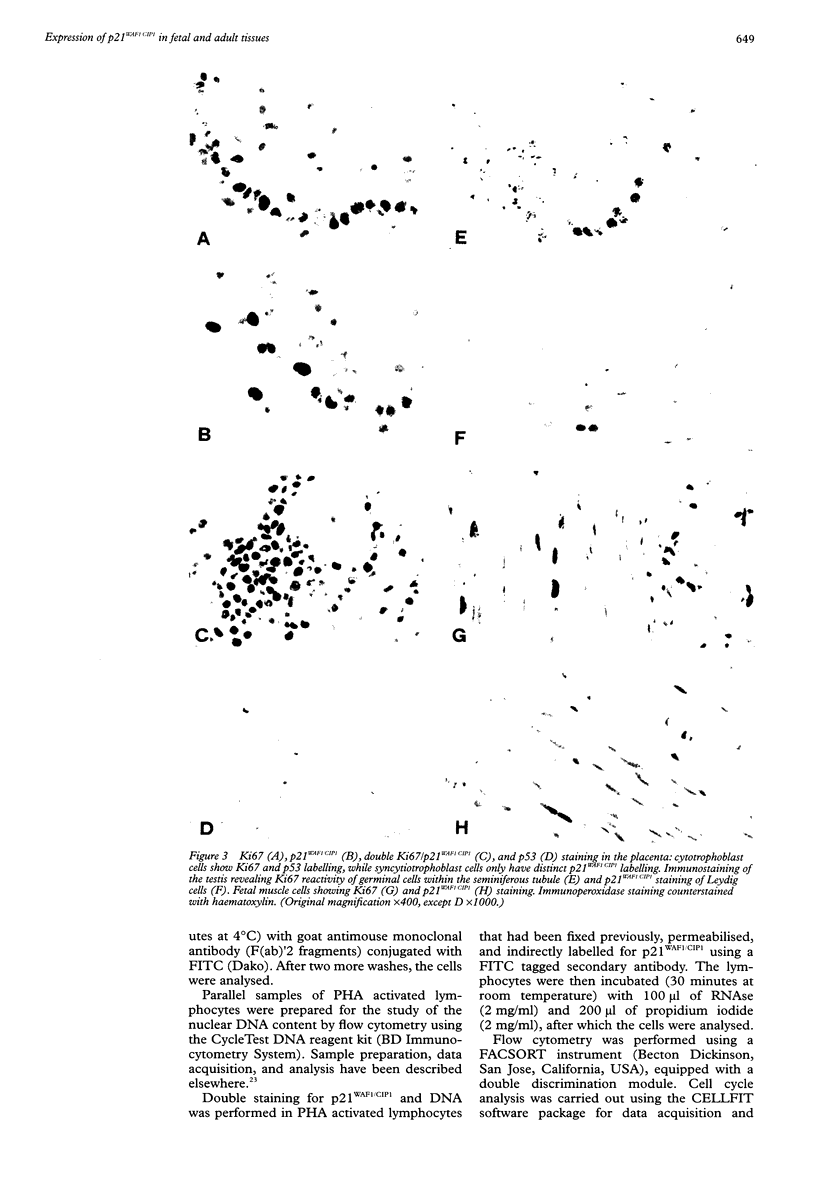
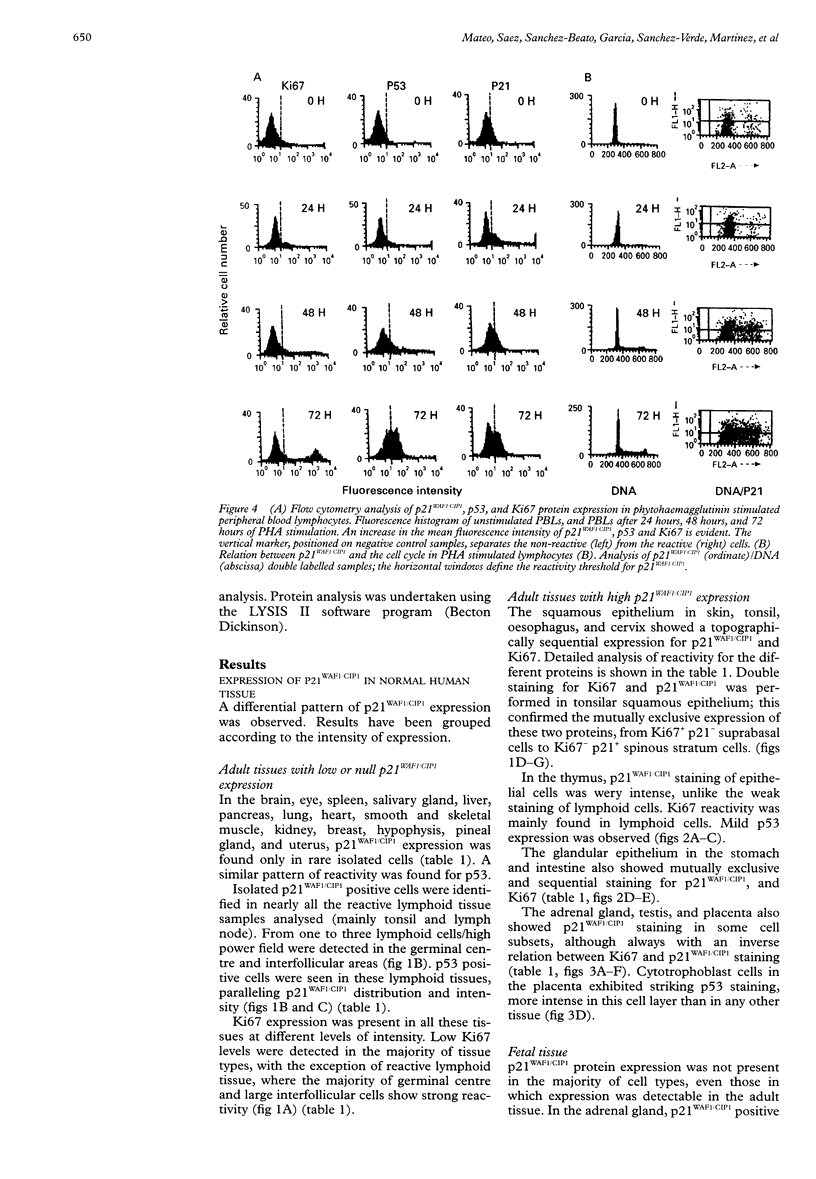
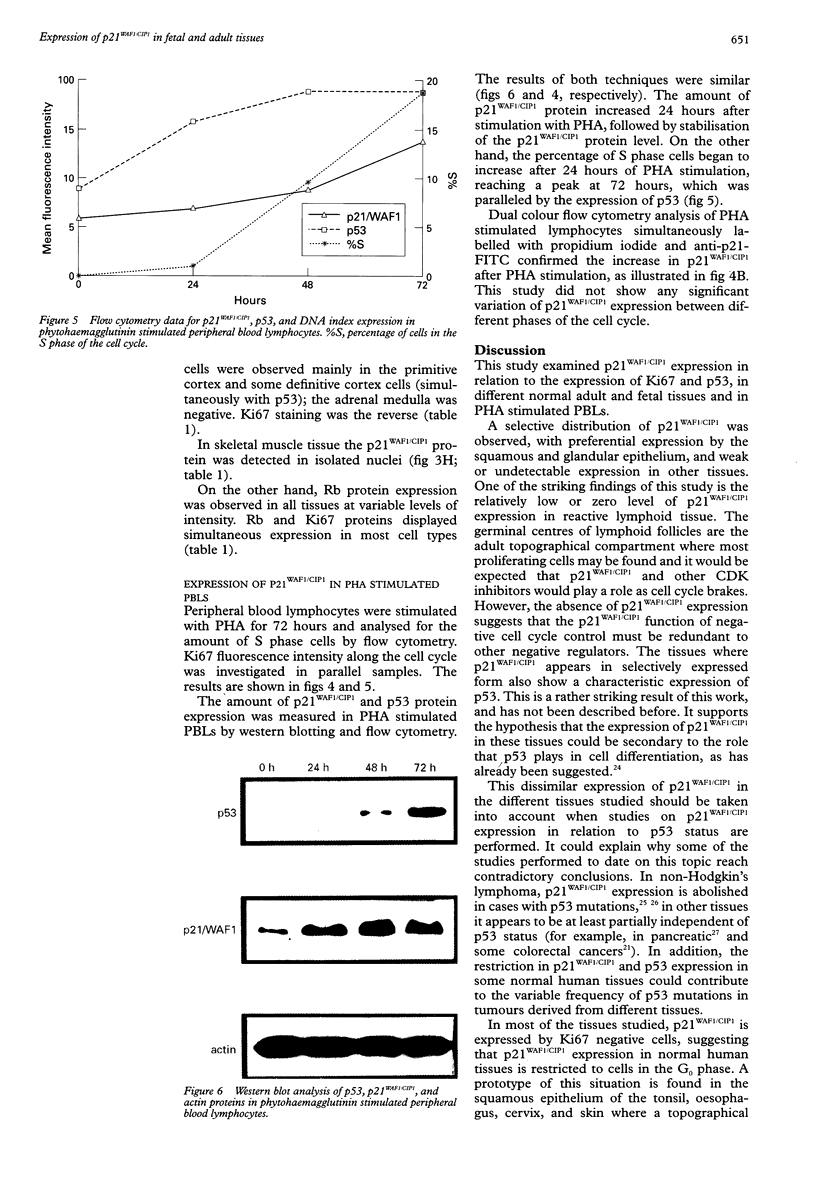
AIMS: To determine the expression of p21WAF1/CIP1 in relation to the expression of Ki67 and p53 in various normal adult and fetal tissues, and to investigate its distribution throughout the cell cycle. METHODS: The expression of p21WAF1/CIP1 in relation to Ki67 and p53 was analysed in adult and fetal tissues using immunohistochemical techniques. Heat induced epitope retrieval techniques were used to characterise the presence of p21WAF1/CIP1 in different tissues, as well as to detect its distribution throughout the cell cycle. In addition, flow cytometry and western blotting were used to test whether the level of p21WAF1/CIP1 expression varied at different phases of the cell cycle in phytohaemagglutinin (PHA) stimulated lymphocytes. RESULTS: p21WAF1/CIP1 expression varied from one tissue to another, and it was restricted mainly to the squamous and glandular epithelium, where it appeared in association with p53. Human tissues in which p21WAF1/CIP1 was found showed a mutually exclusive topographical sequential expression between p21WAF1/CIP1 and Ki67. This was confirmed by double labelling studies, which showed that p21WAF1/CIP1 positive cells were in the G0 phase. Unlike these findings of a decline in p21WAF1/CIP1 expression after the G0 phase, PHA stimulated lymphocytes showed a level of p21WAF1/CIP1 expression that rose as the cell progressed through the cell cycle. CONCLUSIONS: The analysis of p21WAF1/CIP1 expression in relation to the status of p53 should take into account the existence of variable p21WAF1/CIP1 expression in different tissues. This could provide an explanation for the varying frequency of p53 mutations in tumours of different cellular origin. In tissues characterised by regular p21WAF1/CIP1 expression, it appears in a pattern that is consistent with the proposed role of this inhibitor of cyclin dependent kinases in cell cycle arrest-that of inducing cell differentiation. The conflicting results of in vivo and in vitro studies could support the hypothesis that microenvironmental conditions may influence the location of p21WAF1/CIP1 in different phases of the cell cycle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bae I., Smith M. L., Sheikh M. S., Zhan Q., Scudiero D. A., Friend S. H., O'Connor P. M., Fornace A. J., Jr An abnormality in the p53 pathway following gamma-irradiation in many wild-type p53 human melanoma lines. Cancer Res. 1996 Feb 15;56(4):840–847. [PubMed] [Google Scholar]
- Chen J., Jackson P. K., Kirschner M. W., Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995 Mar 23;374(6520):386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- Chilosi M., Doglioni C., Magalini A., Inghirami G., Krampera M., Nadali G., Rahal D., Pedron S., Benedetti A., Scardoni M. p21/WAF1 cyclin-kinase inhibitor expression in non-Hodgkin's lymphomas: a potential marker of p53 tumor-suppressor gene function. Blood. 1996 Nov 15;88(10):4012–4020. [PubMed] [Google Scholar]
- Deng C., Zhang P., Harper J. W., Elledge S. J., Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995 Aug 25;82(4):675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- DiGiuseppe J. A., Redston M. S., Yeo C. J., Kern S. E., Hruban R. H. p53-independent expression of the cyclin-dependent kinase inhibitor p21 in pancreatic carcinoma. Am J Pathol. 1995 Oct;147(4):884–888. [PMC free article] [PubMed] [Google Scholar]
- Doglioni C., Pelosio P., Laurino L., Macri E., Meggiolaro E., Favretti F., Barbareschi M. p21/WAF1/CIP1 expression in normal mucosa and in adenomas and adenocarcinomas of the colon: its relationship with differentiation. J Pathol. 1996 Jul;179(3):248–253. doi: 10.1002/(SICI)1096-9896(199607)179:3<248::AID-PATH571>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Dulić V., Kaufmann W. K., Wilson S. J., Tlsty T. D., Lees E., Harper J. W., Elledge S. J., Reed S. I. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994 Mar 25;76(6):1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Fero M. L., Rivkin M., Tasch M., Porter P., Carow C. E., Firpo E., Polyak K., Tsai L. H., Broudy V., Perlmutter R. M. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell. 1996 May 31;85(5):733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- Fredersdorf S., Milne A. W., Hall P. A., Lu X. Characterization of a panel of novel anti-p21Waf1/Cip1 monoclonal antibodies and immunochemical analysis of p21Waf1/Cip1 expression in normal human tissues. Am J Pathol. 1996 Mar;148(3):825–835. [PMC free article] [PubMed] [Google Scholar]
- Halevy O., Novitch B. G., Spicer D. B., Skapek S. X., Rhee J., Hannon G. J., Beach D., Lassar A. B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995 Feb 17;267(5200):1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell. 1994 Nov 18;79(4):573–582. doi: 10.1016/0092-8674(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H., Kineman R. D., Manova-Todorova K. O., Soares V. C., Hoffman E. S., Ono M., Khanam D., Hayday A. C., Frohman L. A., Koff A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell. 1996 May 31;85(5):721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Chang C. Y., Hu N., Wang Y. C., Lai C. C., Herrup K., Lee W. H., Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992 Sep 24;359(6393):288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- Li R., Waga S., Hannon G. J., Beach D., Stillman B. Differential effects by the p21 CDK inhibitor on PCNA-dependent DNA replication and repair. Nature. 1994 Oct 6;371(6497):534–537. doi: 10.1038/371534a0. [DOI] [PubMed] [Google Scholar]
- Lutzker S. G., Levine A. J. A functionally inactive p53 protein in teratocarcinoma cells is activated by either DNA damage or cellular differentiation. Nat Med. 1996 Jul;2(7):804–810. doi: 10.1038/nm0796-804. [DOI] [PubMed] [Google Scholar]
- Macleod K. F., Sherry N., Hannon G., Beach D., Tokino T., Kinzler K., Vogelstein B., Jacks T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995 Apr 15;9(8):935–944. doi: 10.1101/gad.9.8.935. [DOI] [PubMed] [Google Scholar]
- Michieli P., Chedid M., Lin D., Pierce J. H., Mercer W. E., Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994 Jul 1;54(13):3391–3395. [PubMed] [Google Scholar]
- Parker S. B., Eichele G., Zhang P., Rawls A., Sands A. T., Bradley A., Olson E. N., Harper J. W., Elledge S. J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995 Feb 17;267(5200):1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- Steinman R. A., Hoffman B., Iro A., Guillouf C., Liebermann D. A., el-Houseini M. E. Induction of p21 (WAF-1/CIP1) during differentiation. Oncogene. 1994 Nov;9(11):3389–3396. [PubMed] [Google Scholar]
- Szekely L., Jiang W. Q., Bulic-Jakus F., Rosen A., Ringertz N., Klein G., Wiman K. G. Cell type and differentiation dependent heterogeneity in retinoblastoma protein expression in SCID mouse fetuses. Cell Growth Differ. 1992 Mar;3(3):149–156. [PubMed] [Google Scholar]
- Waga S., Hannon G. J., Beach D., Stillman B. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 1994 Jun 16;369(6481):574–578. doi: 10.1038/369574a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang H., Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992 Oct 30;71(3):505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- Yang Z. Y., Perkins N. D., Ohno T., Nabel E. G., Nabel G. J. The p21 cyclin-dependent kinase inhibitor suppresses tumorigenicity in vivo. Nat Med. 1995 Oct;1(10):1052–1056. doi: 10.1038/nm1095-1052. [DOI] [PubMed] [Google Scholar]
- Zhang H., Hannon G. J., Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994 Aug 1;8(15):1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- Zhang W., Grasso L., McClain C. D., Gambel A. M., Cha Y., Travali S., Deisseroth A. B., Mercer W. E. p53-independent induction of WAF1/CIP1 in human leukemia cells is correlated with growth arrest accompanying monocyte/macrophage differentiation. Cancer Res. 1995 Feb 1;55(3):668–674. [PubMed] [Google Scholar]
- el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994 Mar 1;54(5):1169–1174. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Waldman T., Oliner J. D., Velculescu V. E., Burrell M., Hill D. E., Healy E., Rees J. L., Hamilton S. R. Topological control of p21WAF1/CIP1 expression in normal and neoplastic tissues. Cancer Res. 1995 Jul 1;55(13):2910–2919. [PubMed] [Google Scholar]