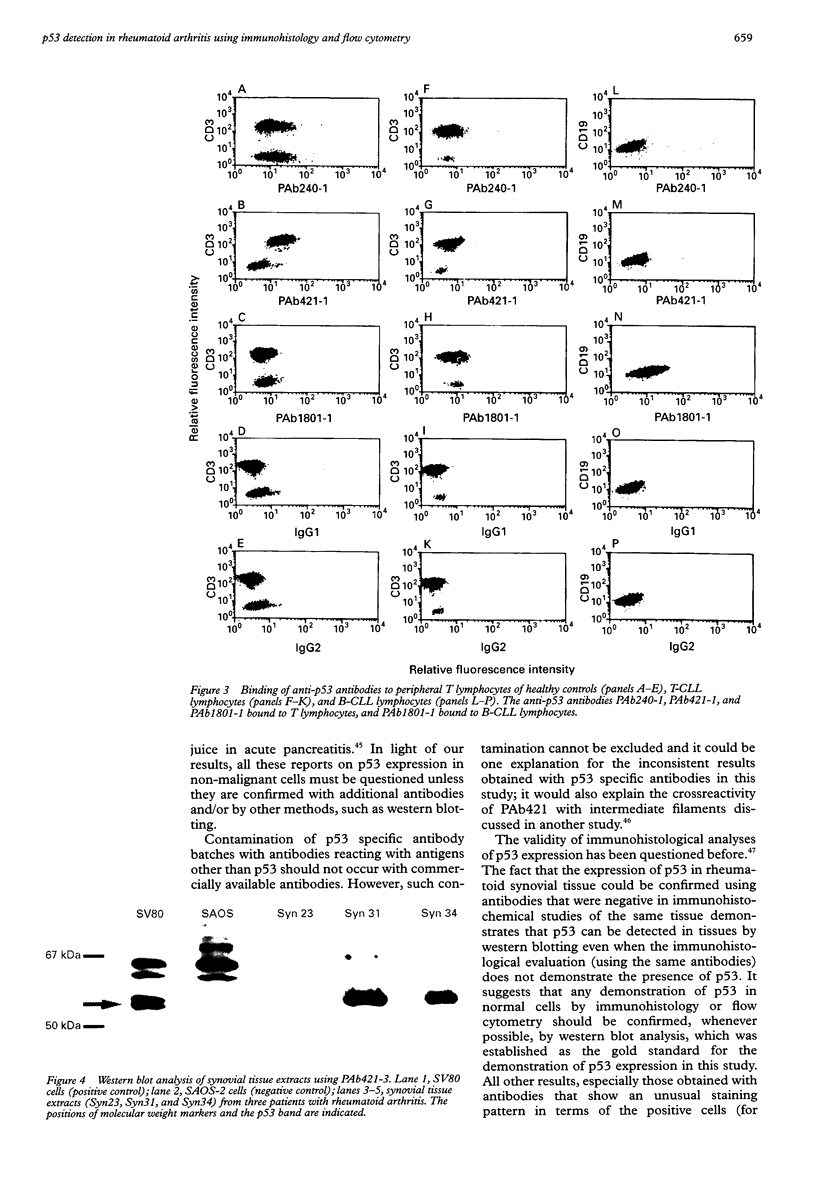
Abstract
AIMS: To analyse the expression of p53 in lymphatic cells found in inflammatory tissues and the peripheral blood by immunological methods. METHODS: Immunohistological analysis of synovial tissues from patients with rheumatoid arthritis and flow cytometric analysis of peripheral blood lymphocytes were performed with anti-p53 antibodies from different sources. RESULTS: The anti-p53 antibodies PAb240, PAb421, and PAb1801 from one supplier bound to the cytoplasm of lymphocytes, fibroblasts, and endothelial cells in rheumatoid synovial tissue, while the same anti-p53 antibodies from other sources and the p53 specific antibodies PAb1620 and DO1 were negative. Using flow cytometry, the antibodies that labelled cells in inflammatory tissues were shown to bind also to peripheral lymphocytes, while the antibodies that were negative in immunohistology did not react with peripheral blood lymphocytes. p53 expression could be confirmed by western blot in rheumatoid synovial tissue, but not in peripheral blood lymphocytes using PAb421 and PAb240 antibodies from our own laboratory, which had been negative in immunohistology. CONCLUSIONS: Demonstration of p53 by western blot is more sensitive and reliable than immunohistology and flow cytometry. Western blot is the gold standard for the demonstration of p53 expression and should be used, whenever possible, to confirm p53 expression in normal tissue shown by immunohistology or flow cytometry. All other reports on p53 expression, especially those obtained using antibodies with an unusual staining pattern must be interpreted with caution.
Full text
PDF

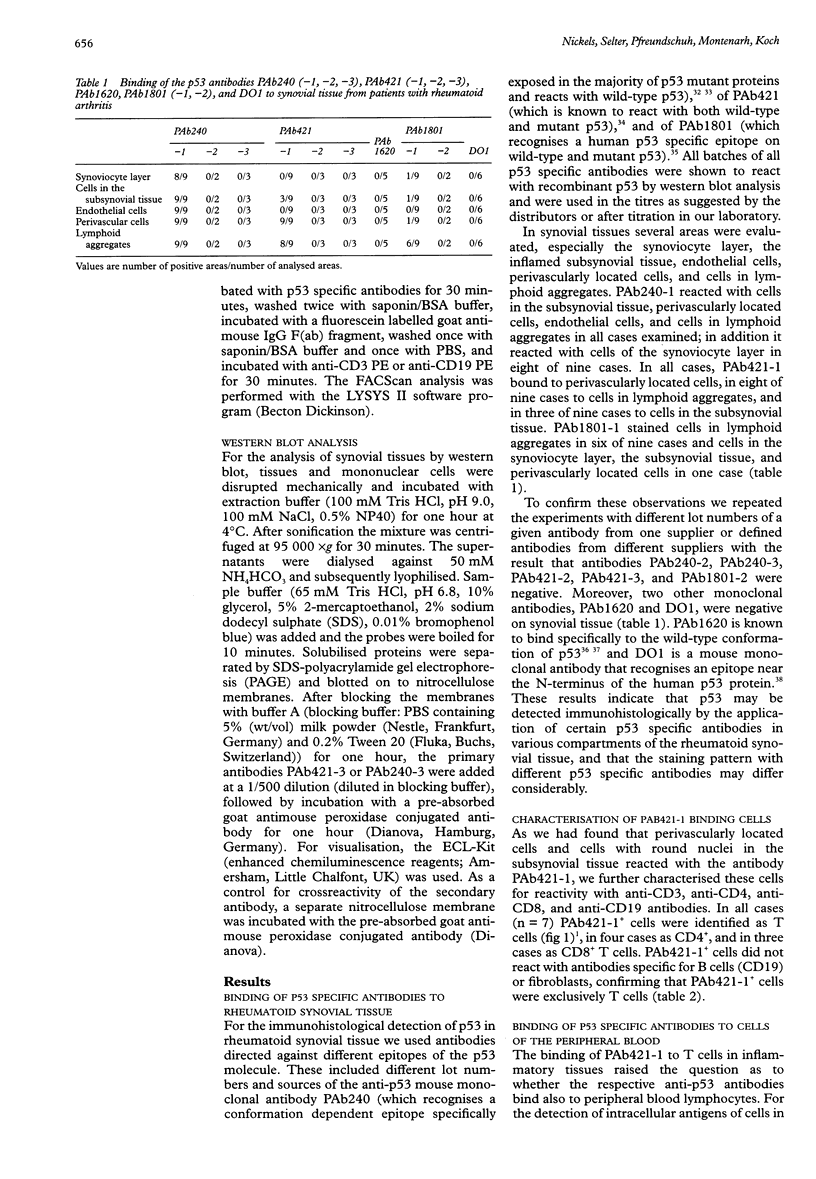
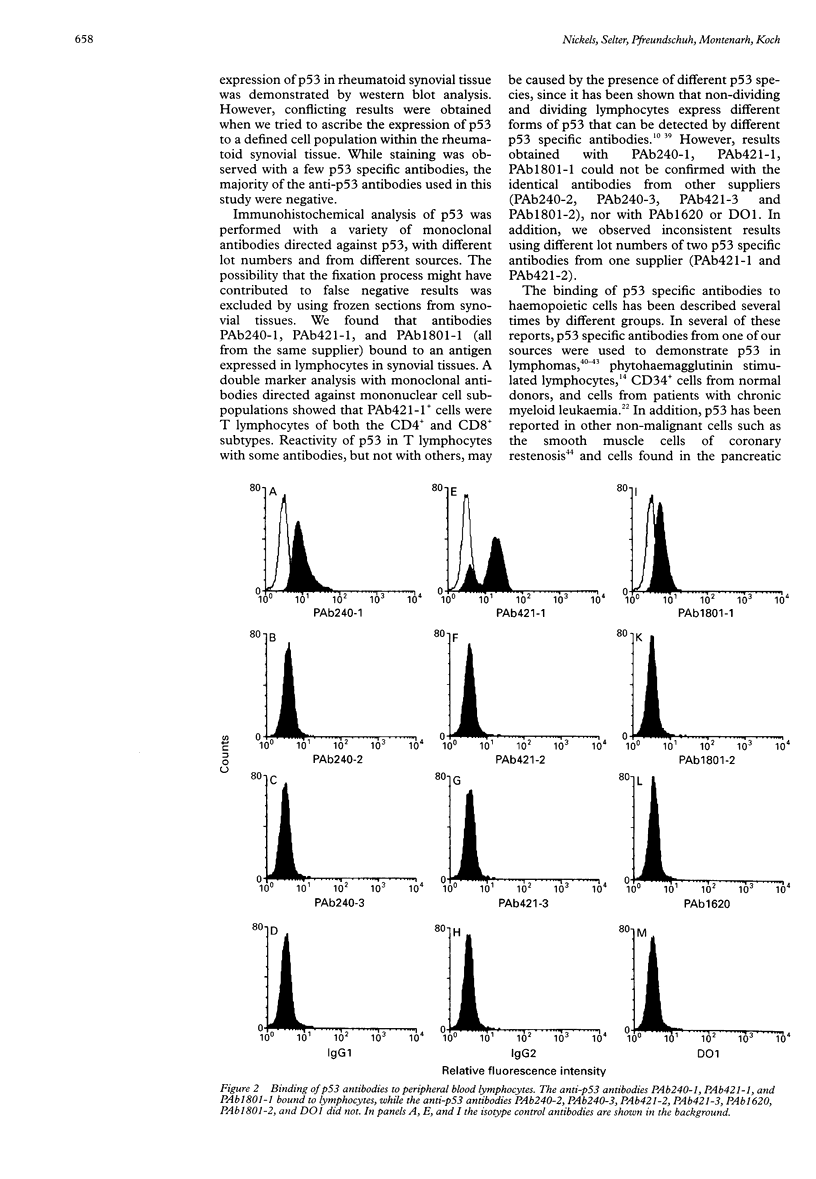

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball R. K., Siegl B., Quellhorst S., Brandner G., Braun D. G. Monoclonal antibodies against simian virus 40 nuclear large T tumour antigen: epitope mapping, papova virus cross-reaction and cell surface staining. EMBO J. 1984 Jul;3(7):1485–1491. doi: 10.1002/j.1460-2075.1984.tb02000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks L., Matlashewski G., Crawford L. Isolation of human-p53-specific monoclonal antibodies and their use in the studies of human p53 expression. Eur J Biochem. 1986 Sep 15;159(3):529–534. doi: 10.1111/j.1432-1033.1986.tb09919.x. [DOI] [PubMed] [Google Scholar]
- Barnes D. M., Hanby A. M., Gillett C. E., Mohammed S., Hodgson S., Bobrow L. G., Leigh I. M., Purkis T., MacGeoch C., Spurr N. K. Abnormal expression of wild type p53 protein in normal cells of a cancer family patient. Lancet. 1992 Aug 1;340(8814):259–263. doi: 10.1016/0140-6736(92)92354-i. [DOI] [PubMed] [Google Scholar]
- Bartkova J., Bartek J., Vojtesek B., Lukas J., Rejthar A., Kovarik J., Millis R. R., Lane D. P., Barnes D. M. Immunochemical analysis of the p53 oncoprotein in matched primary and metastatic human tumours. Eur J Cancer. 1993;29A(6):881–886. doi: 10.1016/s0959-8049(05)80431-9. [DOI] [PubMed] [Google Scholar]
- Bi S., Lanza F., Goldman J. M. The involvement of "tumor suppressor" p53 in normal and chronic myelogenous leukemia hemopoiesis. Cancer Res. 1994 Jan 15;54(2):582–586. [PubMed] [Google Scholar]
- Burmester G. R., Locher P., Koch B., Winchester R. J., Dimitriu-Bona A., Kalden J. R., Mohr W. The tissue architecture of synovial membranes in inflammatory and non-inflammatory joint diseases. I. The localization of the major synovial cell populations as detected by monoclonal reagents directed towards Ia and monocyte-macrophage antigens. Rheumatol Int. 1983;3(4):173–181. doi: 10.1007/BF00541597. [DOI] [PubMed] [Google Scholar]
- Bártek J., Bártková J., Vojtesek B., Stasková Z., Rejthar A., Kovarík J., Lane D. P. Patterns of expression of the p53 tumour suppressor in human breast tissues and tumours in situ and in vitro. Int J Cancer. 1990 Nov 15;46(5):839–844. doi: 10.1002/ijc.2910460515. [DOI] [PubMed] [Google Scholar]
- Cesarman E., Inghirami G., Chadburn A., Knowles D. M. High levels of p53 protein expression do not correlate with p53 gene mutations in anaplastic large cell lymphoma. Am J Pathol. 1993 Sep;143(3):845–856. [PMC free article] [PubMed] [Google Scholar]
- Dameron K. M., Volpert O. V., Tainsky M. A., Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994 Sep 9;265(5178):1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Yeo M., Zvaifler N. J. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995 Sep;96(3):1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald O., Soden M., Yanni G., Robinson R., Bresnihan B. Morphometric analysis of blood vessels in synovial membranes obtained from clinically affected and unaffected knee joints of patients with rheumatoid arthritis. Ann Rheum Dis. 1991 Nov;50(11):792–796. doi: 10.1136/ard.50.11.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche M., Haessler C., Brandner G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene. 1993 Feb;8(2):307–318. [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz C., Montenarh M. p53: DNA damage, DNA repair, and apoptosis. Rev Physiol Biochem Pharmacol. 1996;127:65–95. doi: 10.1007/BFb0048265. [DOI] [PubMed] [Google Scholar]
- Hall P. A., McKee P. H., Menage H. D., Dover R., Lane D. P. High levels of p53 protein in UV-irradiated normal human skin. Oncogene. 1993 Jan;8(1):203–207. [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. C. The 1995 Walter Hubert Lecture--molecular epidemiology of human cancer: insights from the mutational analysis of the p53 tumour-suppressor gene. Br J Cancer. 1996 Feb;73(3):261–269. doi: 10.1038/bjc.1996.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helander S. D., Peters M. S., Pittelkow M. R. Expression of p53 protein in benign and malignant epidermal pathologic conditions. J Am Acad Dermatol. 1993 Nov;29(5 Pt 1):741–748. doi: 10.1016/0190-9622(93)70240-t. [DOI] [PubMed] [Google Scholar]
- Imatani A., Sasano H., Yabuki N., Kato K., Ohara S., Asaki S., Toyota T., Nagura H. In situ analysis of tissue dynamics and p53 expression in human gastric mucosa. J Pathol. 1996 May;179(1):39–42. doi: 10.1002/(SICI)1096-9896(199605)179:1<39::AID-PATH543>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Kalthoff H., Schmiegel W., Roeder C., Kasche D., Schmidt A., Lauer G., Thiele H. G., Honold G., Pantel K., Riethmüller G. p53 and K-RAS alterations in pancreatic epithelial cell lesions. Oncogene. 1993 Feb;8(2):289–298. [PubMed] [Google Scholar]
- Knippschild U., Oren M., Deppert W. Abrogation of wild-type p53 mediated growth-inhibition by nuclear exclusion. Oncogene. 1996 Apr 18;12(8):1755–1765. [PubMed] [Google Scholar]
- Koch B., Locher P., Burmester G. R., Mohr W., Kalden J. R. The tissue architecture of synovial membranes in inflammatory and non-inflammatory joint diseases. II. The localization of mononuclear cells as detected by monoclonal antibodies directed against T-lymphocyte subsets and natural killer cells. Rheumatol Int. 1984;4(2):79–85. doi: 10.1007/BF00541201. [DOI] [PubMed] [Google Scholar]
- Kulju K. S., Lehman J. M. Increased p53 protein associated with aging in human diploid fibroblasts. Exp Cell Res. 1995 Apr;217(2):336–345. doi: 10.1006/excr.1995.1095. [DOI] [PubMed] [Google Scholar]
- Lalor P. A., Mapp P. I., Hall P. A., Revell P. A. Proliferative activity of cells in the synovium as demonstrated by a monoclonal antibody, Ki67. Rheumatol Int. 1987;7(5):183–186. doi: 10.1007/BF00541375. [DOI] [PubMed] [Google Scholar]
- Martinez J. C., Mateo M., Sánchez-Beato M., Villuendas R., Orradre J. L., Algara P., Sánchez-Verde L., García P., López C., Martínez P. MDM2 expression in lymphoid cells and reactive and neoplastic lymphoid tissue. Comparative study with p53 expression. J Pathol. 1995 Sep;177(1):27–34. doi: 10.1002/path.1711770106. [DOI] [PubMed] [Google Scholar]
- Mateo M. S., Sanchez-Beato M., Martinez J. C., Orfao A., Orradre J. L., Piris M. A. p53, Rb and bcl-2 expression during the cell cycle: a study in phytohaemagglutinin stimulated lymphocytes and microwave irradiated lymphoid tissue sections. J Clin Pathol. 1995 Feb;48(2):151–159. doi: 10.1136/jcp.48.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima A. Y., Cesarman E., Chadburn A., Knowles D. M. Post-thymic T cell lymphomas frequently overexpress p53 protein but infrequently exhibit p53 gene mutations. Am J Pathol. 1994 Mar;144(3):573–584. [PMC free article] [PubMed] [Google Scholar]
- McLure K. G., Lee P. W. A PAb240+ conformation of wild type p53 binds DNA. Oncogene. 1996 Sep 19;13(6):1297–1303. [PubMed] [Google Scholar]
- Milner J., Cook A., Sheldon M. A new anti-p53 monoclonal antibody, previously reported to be directed against the large T antigen of simian virus 40. Oncogene. 1987;1(4):453–455. [PubMed] [Google Scholar]
- Milner J. Different forms of p53 detected by monoclonal antibodies in non-dividing and dividing lymphocytes. Nature. 1984 Jul 12;310(5973):143–145. doi: 10.1038/310143a0. [DOI] [PubMed] [Google Scholar]
- Moll U. M., Riou G., Levine A. J. Two distinct mechanisms alter p53 in breast cancer: mutation and nuclear exclusion. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7262–7266. doi: 10.1073/pnas.89.15.7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenarh M. Biochemical, immunological, and functional aspects of the growth-suppressor/oncoprotein p53. Crit Rev Oncog. 1992;3(3):233–256. [PubMed] [Google Scholar]
- Negoescu A., Labat-Moleur F., Lorimier P., Lamarcq L., Guillermet C., Chambaz E., Brambilla E. F(ab) secondary antibodies: a general method for double immunolabeling with primary antisera from the same species. Efficiency control by chemiluminescence. J Histochem Cytochem. 1994 Mar;42(3):433–437. doi: 10.1177/42.3.7508473. [DOI] [PubMed] [Google Scholar]
- Osipovich O. A., Misuno N. I., Kolesnikova T. S., Sudarikov A. B., Voitenok N. N. The difference in p53 antioncogene transcription in human monocytes and lymphocytes. Oncogene. 1992 Mar;7(3):549–552. [PubMed] [Google Scholar]
- Pezzella F., Micklem K., Turley H., Pulford K., Jones M., Kocialkowski S., Delia D., Aiello A., Bicknell R., Smith K. Antibody for detecting p53 protein by immunohistochemistry in normal tissues. J Clin Pathol. 1994 Jul;47(7):592–596. doi: 10.1136/jcp.47.7.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said J. W., Barrera R., Shintaku I. P., Nakamura H., Koeffler H. P. Immunohistochemical analysis of p53 expression in malignant lymphomas. Am J Pathol. 1992 Dec;141(6):1343–1348. [PMC free article] [PubMed] [Google Scholar]
- Spandau D. F. Distinct conformations of p53 are observed at different stages of keratinocyte differentiation. Oncogene. 1994 Jul;9(7):1861–1868. [PubMed] [Google Scholar]
- Speir E., Huang E. S., Modali R., Leon M. B., Shawl F., Finkel T., Epstein S. E. Interaction of human cytomegalovirus with p53: possible role in coronary restenosis. Scand J Infect Dis Suppl. 1995;99:78–81. [PubMed] [Google Scholar]
- Speir E., Modali R., Huang E. S., Leon M. B., Shawl F., Finkel T., Epstein S. E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994 Jul 15;265(5170):391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- Strang P., Nordstöm B., Nilsson S., Bergström R., Tribukait B. Mutant p53 protein as a predictor of survival in endometrial carcinoma. Eur J Cancer. 1996 Apr;32A(4):598–602. doi: 10.1016/0959-8049(95)00636-2. [DOI] [PubMed] [Google Scholar]
- Szekely L., Pokrovskaja K., Jiang W. Q., Selivanova G., Löwbeer M., Ringertz N., Wiman K. G., Klein G. Resting B-cells, EBV-infected B-blasts and established lymphoblastoid cell lines differ in their Rb, p53 and EBNA-5 expression patterns. Oncogene. 1995 May 4;10(9):1869–1874. [PubMed] [Google Scholar]
- Villuendas R., Piris M. A., Algara P., Sánchez-Beato M., Sánchez-Verde L., Martinez J. C., Orradre J. L., García P., Lopez C., Martinez P. The expression of p53 protein in non-Hodgkin's lymphomas is not always dependent on p53 gene mutations. Blood. 1993 Nov 15;82(10):3151–3156. [PubMed] [Google Scholar]
- Villuendas R., Piris M. A., Orradre J. L., Mollejo M., Algara P., Sanchez L., Martinez J. C., Martinez P. P53 protein expression in lymphomas and reactive lymphoid tissue. J Pathol. 1992 Mar;166(3):235–241. doi: 10.1002/path.1711660305. [DOI] [PubMed] [Google Scholar]
- Voelkerding K. V., Steffen D. W., Zaidi S. H., Malter J. S. Post-transcriptional regulation of the p53 tumor suppressor gene during growth-induction of human peripheral blood mononuclear cells. Oncogene. 1995 Feb 2;10(3):515–521. [PubMed] [Google Scholar]
- Vojtesek B., Bártek J., Midgley C. A., Lane D. P. An immunochemical analysis of the human nuclear phosphoprotein p53. New monoclonal antibodies and epitope mapping using recombinant p53. J Immunol Methods. 1992 Jul 6;151(1-2):237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]