Abstract
Study Design.
A retrospective study.
Objective.
This study aims to develop a new scoring system that can guild surgeons to select the best candidates for decompressive surgery in patients with metastatic spinal cord compression (MSCC).
Summary of Background Data.
Predicting survival and functional outcome is essential when selecting the individual treatment for patients with MSCC. The criteria for identifying MSCC patients who are most likely to benefit from decompressive surgery remain unclear.
Methods.
We retrospectively analyzed 12 preoperative characteristics for postoperative survival in a series of 206 patients with MSCC who were operated with decompressive surgery and spine stabilization. Characteristics significantly associated with survival in the multivariate analysis were included in the scoring system. Postoperative function outcome was also analyzed on the basis of the scoring system.
Results.
According to the multivariate analysis, primary site (P < 0.01), preoperative ambulatory status (P < 0.01), visceral metastases (P < 0.01), preoperative chemotherapy (P = 0.02), and bone metastasis at cancer diagnosis (P = 0.03) had a significant impact on postoperative survival and were included in the scoring system. According to the prognostic scores, which ranged from 0 to 10 points, three risk groups were designed: 0 to 2, 3 to 5, and 6 to 10 points. The corresponding 6 months survival rates were 8.2%, 56.5%, and 91.5%, respectively (P < 0.01), and postoperative ambulatory rates were 35.7%, 73.3%, and 95.9%, respectively (P < 0.01).
Conclusion.
We present a new scoring system for predicting survival and function outcome of MSCC patients after surgical decompression and spine stabilization. This new scoring system can help surgeons select the best candidates for surgical treatment.
Level of Evidence: 4
Keywords: scoring system, spinal cord compression, spine metastasis, surgical decompression and spine stabilization, survival prognosis
Metastatic spinal cord compression (MSCC) is one of the most serious complications of metastatic cancers and occurs in up to 10% of malignant cancer patients, which, if left untreated, can result in relentless and progressive pain, sphincter dysfunction, and even paralysis.1 The growing literature demonstrated that direct decompressive surgery followed radiotherapy was superior to radiotherapy alone in terms of postoperative survival prognosis, function status, and pain outcome.2–4 Thus, surgical decompression and spine stabilization along with radiotherapy have become one of the most widely used modality for MSCC patients in recent years.5,6
A generally accepted benchmark for a surgical intervention is an expected remaining survival time of greater than 3 to 6 months.7–9 With lower life expectancy, radiation alone or even best supportive cares would do more for the patient's quality of remaining life, while for patients with an expected survival of more than 3 to 6 months, surgical intervention could remarkably improve the patient's symptoms.10,11 Accurate survival estimation, therefore, is prerequisite to determine the most appropriate treatment for patients with MSCC.
Several scoring systems were developed to estimate the survival outcome of each patient and select the optimal treatment strategy, and perhaps the Tokuhashi scores7,8 and Tomita score12 were the most representative and commonly used scores among them. Unfortunately, the most of available scoring systems were designed in the 1990s and early 2000s, while the majority of the recent anticancer agents, such as the anti-VEGF therapy, were available from 2005. Thus, those scoring system did not take the effectiveness of new therapeutic strategies on survival into consideration, contributing to a progressive loss of accuracy.13–20 Moreover, function outcome after treatments was not taken into account in all above-mentioned scores. To our knowledge, function outcome after surgery plays an important role in patient's quality of remaining life.21
Therefore, our present study is designed to develop a new survival score and analyze the function outcome for MSCC patients after decompressive surgery. Notably, the gain in survival time induced by recent new anticancer drugs was also considered in our scoring system.
MATERIALS AND METHODS
Patients
Two hundred six patients with MSCC who were operated with decompressive surgery were retrospectively analyzed in the study between May 2005 and September 2015. The diagnosis of bone metastasis was confirmed histologically, and adequate diagnostic imaging including spinal computed tomography (CT) or magnetic resonance imaging (MRI), as well as bone scan. Patients with an estimated survival less than 3 months or health too poor to undergo surgery were excluded. The data were collected from patients, their family members, treating surgeons, and patients’ files. The Medical Research Ethics Board of the Affiliated Hospital of Academy of Military Medical Sciences approved this retrospective study and required neither patient approval nor informed consent for review of patients’ images and medical records. The data were retrospective in nature and anonymized by the Medical Research Ethics Board.
Survival Analysis
We retrospectively analyzed 12 preoperative characteristics for postoperative survival, including age (≤56 vs. >56 yrs; median age: 56 yrs), gender (female vs. male), primary site (slow growth vs. moderate growth vs. rapid growth), preoperative ambulatory status (ambulatory vs. not ambulatory), Eastern Cooperative Oncology Group (ECOG) performance status (1–2 vs. 3–4), number of involved vertebrae (1–2 vs. ≥3, conformed to previous studies), visceral metastases (no vs. yes), preoperative chemotherapy (no vs. yes), bone metastasis at cancer diagnosis (no vs. yes), the time developing motor deficits (≤14 vs. >14 days, median time: 14 days), preoperative albumin (≤35 vs. >35 g/L, conformed to previous studies), and radical surgery at primary site (no vs. yes).
Primary cancer was classified into three groups, namely, tumors that exhibited slow growth, including hormone-dependent breast cancer, hormone-dependent prostate cancer, thyroid cancer, multiple myeloma, and malignant lymphoma, moderate growth, including lung cancer treated with molecularly targeted drugs, hormone-independent breast cancer, hormone-independent prostate cancer, renal cell carcinoma, endometrial cancer, ovarian cancer, and sarcoma, or rapid growth, including lung cancer without molecularly targeted drugs, colorectal cancer, gastric cancer, pancreatic cancer, esophageal cancer, hepatocellular carcinoma, head and neck cancer, melanoma, malignant thymoma, and cancers of unknown origin, which was developed from Katagiri et al.22 Head and neck cancer mainly included nasopharyngeal carcinoma and squamous cell carcinoma in the study.
Time-developing motor deficits was defined as the time between deterioration of motor function to disability or surgery. Deterioration of motor function was defined as a change of at least one Frankel grade. The postoperative survival was defined as the time between the date of surgery and death or the latest follow-up, and patients who were alive at the last follow-up were censored in the postoperative survival analysis. In patients who had surgery for more than one metastasis, all sites were included in the analysis. However, only the first surgical procedure was accounted for in the survival analysis.
Surgery and Function Analysis
The indication for surgery was neurological deficit due to spinal cord compression. Patients were operated with decompressive surgery (Case report was seen in Figure 1. Figure 1A: preoperative X-ray presented vertebral collapse at T12. Figure 1B: preoperative MRI showed spinal cord compression at T12. Figure 1C: preoperative CT showed bone destruction at T12. Figure 1D: preoperative MRI showed spinal cord compression at T12. Figure 1E and F: following laminectomy at T11 and T12, and pedicle screw fixation was conducted to spine stabilization.). Local radiotherapy, systemic chemotherapy, endocrine therapy, and targeted therapy were routinely performed after the wound healed, about 3 to 4 weeks after the surgery, if applicable. Postoperative function outcome was also analyzed according to the scoring system. Neurological function was graded on the basis of Frankel grades preoperatively and about 4 weeks postoperatively (Patients with Frankel D and E have the ability to walk).
Figure 1.
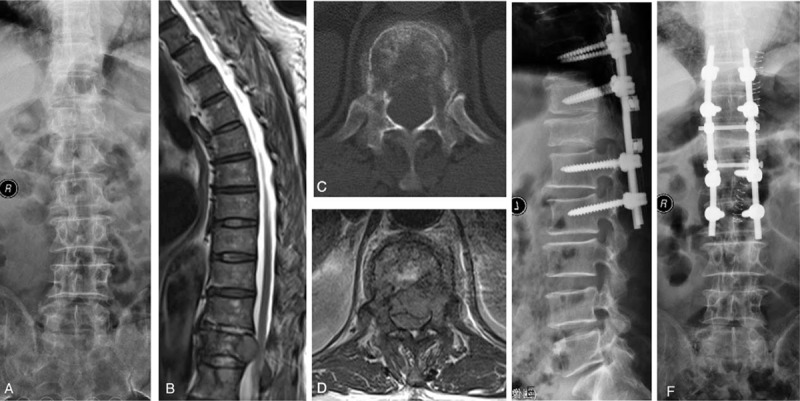
A 58-year-old man who was unable to walk due to metastatic spinal cord compression (MSCC) resulted from lung cancer. A, Preoperative X-ray presented vertebral collapse at T12. B, Preoperative MRI showed spinal cord compression at T12. C, Preoperative CT showed bone destruction at T12. D, Preoperative MRI showed spinal cord compression at T12. E, F, Following laminectomy at T11 and T12, and pedicle screw fixation was conducted to spine stabilization. Postoperative motor function was improved from Frankel C to D 4 weeks after operation. He died at postoperative 6 months and spine stability was maintained throughout the survival period.
Statistical Analysis
The univariate and multivariate analysis of postoperative survival was estimated by the simple and multiple Cox proportional hazards regression models, respectively. Characteristics significantly associated with postoperative survival in the multivariate analysis were included in the scoring system. The scoring point for each significant factor was derived from the hazard ratios on multiple Cox proportional hazards regression model. The total prognostic score for each patient was determined by adding the scoring points of every significant factor. Regarding postoperative function outcome, the ambulatory status in prognostic groups was compared with Chi-square test. A P value of 0.05 or less was considered statistically significant. Statistical analysis was performed using SAS 9.2 software for windows XP (SAS Institute Inc., Cary, NC).
RESULTS
Patient Characteristics
In the entire cohort of 206 patients, 40 patients with slow growth cancer, 60 patients with moderate growth cancer, and 106 patients with rapid growth cancer. Lung and breast were the most common primary site: 44.2% (91/206) patients with lung cancer (lung cancer treated with molecularly targeted drugs: 32 cases, and lung cancer treated without molecularly targeted drugs: 59 cases) and 18.9% (39/206) patients with breast cancer (hormone-dependent breast cancer: 25 cases, and hormone-independent breast cancer: 14 cases). The median overall survival was 7.3 months [95% confidence interval (95% CI), 6.4–9.3 months], and 6-month and 12-month survival rates were 59.7% and 32.7%, respectively. At the latest follow up, 24 patients were alive with a mean follow-up of 11.5 months (range, 1.0–67.2 months).
Scoring System
In the univariate analysis, primary site [hazard ratio (HR), 1.78, 95% CI: 1.46–2.17; P < 0.01], preoperative ambulatory status (HR, 1.94, 95% CI: 1.43–2.64; P < 0.01), ECOG performance status (HR, 1.66, 95% CI: 1.22–2.25; P < 0.01), visceral metastases (HR, 2.21, 95% CI: 1.63–3.00; P < 0.01), preoperative chemotherapy (HR, 2.14, 95% CI: 1.56–2.94; P < 0.01), bone metastasis at cancer diagnosis (HR, 1.77, 95% CI: 1.30–2.41; P < 0.01), time-developing motor deficits (HR, 1.64, 95% CI: 1.21–2.22; P < 0.01), and radical surgery at primary site (HR, 2.07, 95% CI: 1.50–2.86; P < 0.01) were significantly associated with postoperative survival (Table 1). According to the multiple Cox proportional hazards regression model, five of above eight factors, namely, primary site (HR, 1.74, 95% CI: 1.41–2.15; P < 0.01), preoperative ambulatory status (HR, 2.04, 95% CI: 1.48–2.80; P < 0.01), visceral metastases (HR, 3.00, 95% CI: 2.17–4.13; P < 0.01), preoperative chemotherapy (HR, 1.53, 95% CI: 1.08–2.16; P = 0.02), and bone metastasis at cancer diagnosis (HR, 1.46, 95%CI: 1.05–2.02; P = 0.03) maintained significant impact on survival and were included in the survival scoring system (Table 1). The scoring points for each of the five significant characteristics obtained from the hazard ratios based on the multiple Cox proportional hazards regression model are seen in Table 2. The prognostic score for each patient was calculated by adding the scoring points of the five significant characteristics. The addition resulted in prognostic scores of 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 points, and the corresponding 6-month survival rates, median survival time, and the total number of patients of each prognostic score are seen in Figure 2. Taking into account the 6-month survival rate and median survival time of each prognostic score, patients were divided into three prognostic groups: 0 to 2 points (group A, n = 42), 3 to 5 points (Group B, n = 90), and 6 to 10 points (group C, n = 74). The corresponding median survival was 3.3 months (95% CI, 2.5–4.4 mnths), 6.6 months (95% CI, 5.8–7.3 mnths), and 16.4 months (95% CI, 13.7–21.4 mnths), respectively; the 6-month survival rates were 8.2%, 56.5%, and 91.5%, respectively, and the 12-month survival rates were 2.7%, 15.1%, and 68.9%, respectively (P < 0.01, Figure 3).
TABLE 1.
Univariate and Multivariate Analysis of Preoperative Characteristics for Postoperative Survival in Patients with MSCC
| Characteristics | Patients (n) | MOS (m) | Simple Cox Regression | Multiple Cox Regression | ||
| HR (95% CI) | P | HR (95% CI) | P | |||
| Age | ||||||
| ≤56 yrs | 106 | 8.8 | 1.21 (0.90–1.64) | 0.21 | Not included | |
| >56 yrs | 100 | 7.1 | ||||
| Gender | ||||||
| Female | 101 | 8.4 | 1.30 (0.96–1.76) | 0.09 | Not included | |
| Male | 105 | 7.1 | ||||
| Primary site | ||||||
| Slow growth | 40 | 18.3 | 1.78 (1.46–2.17) | <0.01 | 1.74 (1.41–2.15) | <0.01 |
| Moderate growth | 60 | 8.2 | ||||
| Rapid growth | 106 | 5.3 | ||||
| Preoperative ambulatory status | ||||||
| Ambulatory | 118 | 10.9 | 1.94 (1.43–2.64) | <0.01 | 2.04 (1.48–2.80) | <0.01 |
| Not Ambulatory | 88 | 5.6 | ||||
| ECOG performance status | ||||||
| 1–2 | 105 | 10.9 | 1.66 (1.22–2.25) | <0.01 | Not included | |
| 3–4 | 101 | 6.0 | ||||
| Number of involved vertebrae | ||||||
| 1–2 | 110 | 8.4 | 1.21 (0.89–1.63) | 0.22 | Not included | |
| ≥3 | 96 | 6.4 | ||||
| Visceral metastases | ||||||
| No | 107 | 11.7 | 2.21 (1.63–3.00) | <0.01 | 3.00 (2.17–4.13) | <0.01 |
| Yes | 99 | 4.5 | ||||
| Preoperative chemotherapy | ||||||
| No | 117 | 5.8 | 2.14 (1.56–2.94) | <0.01 | 1.53 (1.08–2.16) | 0.02 |
| Yes | 89 | 11.5 | ||||
| Bone metastasis at cancer diagnosis | ||||||
| No | 99 | 10.8 | 1.77 (1.30–2.41) | <0.01 | 1.46 (1.05–2.02) | 0.03 |
| Yes | 107 | 5.9 | ||||
| Time-developing motor deficits | ||||||
| ≤14 d | 105 | 6.2 | 1.64 (1.21–2.22) | <0.01 | Not included | |
| >14 d | 101 | 10.8 | ||||
| Preoperative albumin | ||||||
| ≤35 g/L | 88 | 7.1 | 1.26 (0.93–1.71) | 0.13 | Not included | |
| >35 g/L | 118 | 7.7 | ||||
| Radical surgery at primary site | ||||||
| No | 127 | 6.0 | 2.07 (1.50–2.86) | <0.01 | Not included | |
| Yes | 79 | 11.7 | ||||
CI indicates confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; m, months; MOS, median overall survival; MSCC, Metastatic spinal cord compression.
TABLE 2.
A New Scoring System for Patients With MSCC After Surgical Decompression and Spine Stabilization
| Prognostic Factors | Hazard Ratio | Scores |
| Primary site | ||
| Slow growth | 1.74 | 2 |
| Moderate growth | 1 | |
| Rapid growth | 0 | |
| Preoperative ambulatory status | ||
| Ambulatory | 2.04 | 2 |
| Not Ambulatory | 0 | |
| Visceral metastases | ||
| No | 3.00 | 3 |
| Yes | 0 | |
| Preoperative chemotherapy | ||
| No | 1.53 | 0 |
| Yes | 2 | |
| Bone metastasis at cancer diagnosis | ||
| No | 1.46 | 1 |
| Yes | 0 | |
| Prognostic groups | Patients (n) | |
| Group A | 42 | 0–2 |
| Group B | 90 | 3–5 |
| Group C | 74 | 6–10 |
Slow growth: hormone-dependent breast cancer, hormone-dependent prostate cancer, thyroid cancer, multiple myeloma, and malignant lymphoma.
Moderate growth: lung cancer treated with molecularly targeted drugs, hormone-independent breast cancer, hormone-independent prostate cancer, renal cell carcinoma, endometrial cancer, ovarian cancer, and sarcoma.
Rapid growth: lung cancer without molecularly targeted drugs, colorectal cancer, gastric cancer, pancreatic cancer, esophageal cancer, other urological cancers, hepatocellular carcinoma, head and neck cancer, melanoma, malignant thymoma, and cancers of unknown origin.
MSCC indicates metastatic spinal cord compression.
Figure 2.
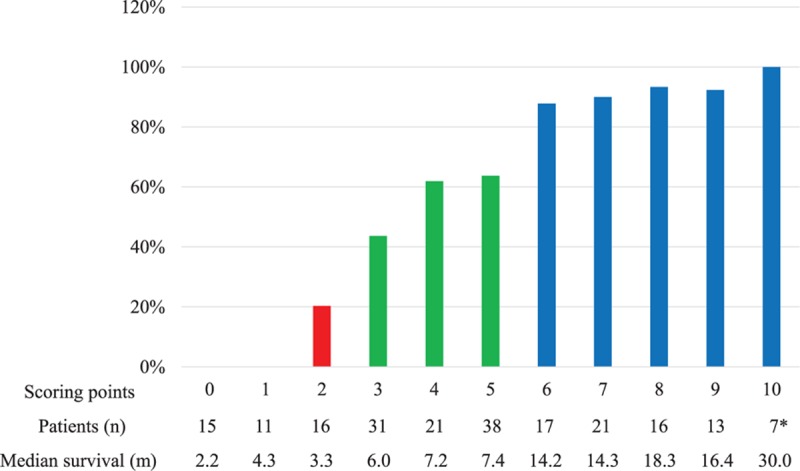
The 6-month survival rates (%), the total number of patients, and the median survival time of each score. (∗Five patients were still alive).
Figure 3.
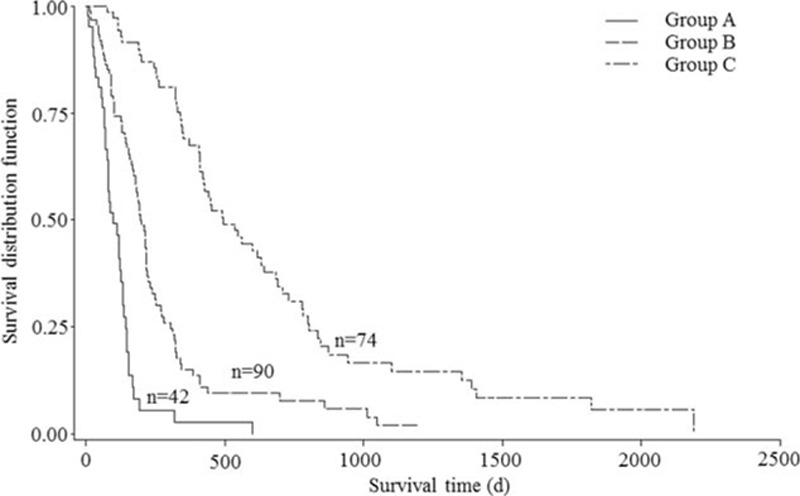
A new score for patient with metastatic spinal cord compression: survival curves for the three prognostic groups (P < 0.01, log-rank test).
Function Outcome
The postoperative ambulatory rates were 35.7% (15/42) in patients with 0 to 2 points, 73.3% (66/90) in patients with 3 to 5 points, and 95.9% (71/74) in patients with 6 to 10 points, respectively (P < 0.01, Table 3). In the entire cohort of 206 patients, 73.8% (152/206) patients had the ability to walk after surgery, 55.7% (49/88) of nonambulatory patients before operation became ambulatory after surgery, and 87.3% (103/118) of ambulatory patients maintained their neurological status.
TABLE 3.
Neurological Status of the Patients in Three Prognostic Groups 4 Weeks After Decompressive Surgery
| Groups | Scores | Patients (n) | Neurological Status | P | ||
| Not Ambulatory | Ambulatory | |||||
| A | 0–2 | 42 | 27 | 15 | <0.01 | |
| B | 3–5 | 90 | 24 | 66 | ||
| C | 6–10 | 74 | 3 | 71 | ||
P value was obtained from Chi-square test.
DISCUSSION
Nowadays, who are the best candidates for decompressive surgery in patients MSCC remains unclear. Generally speaking, selection of patients for surgery in MSCC patients depends on several factors, including the patient's expected survival prognosis and function outcome after surgery, general performance status, the nature and severity of symptoms, and the biological behavior and metastatic extent of the cancer. Of these, the patient's survival prognosis7,8 and function outcome should be of the utmost important considerations. Previously, in order to avoid under- or overtreatment, we developed scoring systems to enable physicians to identify patients with MSCC who may be candidates for decompressive surgery, supportive care alone, or more aggressive surgery, such as excisional procedures, based on survival prognosis and function outcome after decompressive surgery.10,11 However, those scoring systems were especially for lung cancer or nonsmall cell lung cancer patients, making it difficult to draw conclusions on patients with MSCC resulted from other primary cancers.
Fortunately, several prognostic scoring systems, including the Tokuhashi and revised Tokuhashi scores, Tomita score, Van der Linden score, Sioutos score, Bauer score, and Bauer modified score, have been proposed to predict survival time, to help surgeon select appropriate candidates for surgical intervention, and to provide guidelines for the type and extent of surgery to be performed in the selected patients with spinal metastasis from various primary tumors. However, aside from Bauer and Bauer modified scores, the above-mentioned scores were not reliable in predicting survival in patients with spinal metastasis.13,14 Lee et al.15 estimated the prognostic accuracy of Tokuhashi and Tomita scores in a meta-analysis and concluded that both scores had weak diagnostic evidence due to low sensitivity. Another literature review was conducted to evaluate the accuracy of the revised Tokuhashi score by Zoccali et al.,16 the authors showed that only 63% patients actually followed the survivorship pattern. More importantly, the accuracy of the revised Tokuhashi score was decreasing over time.16 Pointillart et al.17 found that the original and revised Tokuhashi scores were accurate in predicting survival in less than 67% of cases in a total of 142 patients. Other studies also have reported moderate-to-low overall precision between 33% and 57%.18,19 Moreover, function outcome after treatments was not taken into account in all above-mentioned scores. Notably, function outcome after surgery plays an important role in patient's quality of remaining life. Thus, revision of those scoring system or a relatively more precious new score is really needed. Besides, function outcome after surgery should be considered in the scoring system.
In a retrospective study by Lee et al.20 in 2013, the authors found the observed survival to be much longer than the survival predicted by the revised Tokuhashi score. Such underestimation of life expectancy of scores was also observed in other studies.15,16 To our knowledge, the Tokuhashi score and other prognostic scoring systems were designed in the 1990s and early 2000s, while the majority of the recent anticancer agents, such as the anti-VEGF therapy, were available from 2005. Patients with spine metastasis are living longer due to the development of targeted therapy, new chemotherapeutic agents, and hormonal therapies.23,24 The underestimation of life expectancy of scores and subsequent inadequate treatment of spinal metastases for a long-term survival patients may be insufficient to assure a good quality of life over an extended period of time. Therefore, the gain in survival produced by recent anticancer agents should be considered in a new scoring system.
In the present study, the scoring system was developed on the basis of the data derived from 206 patients with MSCC who were treated with decompressive surgery along with spine stabilization. Lung cancer patients were divided into two subgroups, namely, lung cancer patients with molecularly targeted agents and other lung cancer, and so were breast and prostate cancers that were divided on the basis of their sensitivity to hormonal therapy. Thus, the different survival period induced by recent anticancer drugs was taken into consideration in our scoring system. Further, functional outcome was also considered according to the scoring system. The patient's individual situation, therefore, was taken more into account in the present scoring system. Five preoperative prognostic factors were included in the new scoring system: primary site, preoperative ambulatory status, visceral metastases, preoperative chemotherapy, and bone metastasis at cancer diagnosis. The scoring points for each of the five significant characteristics were obtained from the hazard ratios, and the hazard ratios were rounded off to the nearest integer. Regarding primary site, the rapid growth cancer was given two points and the moderate growth cancer was given one point. Preoperative ambulation and chemotherapy were each given two points. Without bone metastasis at cancer diagnosis was given one point. Notably, without visceral metastases was given the highest points. Thus, visceral metastases should be carefully evaluated before surgery. According to the new scoring system, patients with scores of 0 to 2 points, who had a median survival time of 3.3 months and ambulatory rate of 35.7%, appeared to be best treated with radiotherapy or best supportive care alone. Patients with scores of 6 to 10, who survived more than 12 months in a median time and were ambulatory in 95.9% patients after surgery, more radical surgery, such as widely excision of vertebra metastasis, can be considered in order to realize better local control of disease and prevent the occurrence of local disease. The remaining patients (3–5 points) with a median survival of greater than 6 months and ambulatory rate of 73.3% should be the best surgical candidates, because survival prognosis and functional outcome were acceptable after surgery.
The median overall survival time was 7.3 months in the entire cohort of 206 patients, 7.7 months was reported in a prospective study,21 and 5 to 11 months was showed in other retrospective studies.17,25 Regarding function outcome in all patients, 73.8% patients had the ability to walk after surgery, 55.7% nonambulatory patients before operation became ambulatory after surgery, and 87.3% ambulatory patients maintained their neurological status. Other studies reported that 67% to 82%25,26 patients were able to walk after surgery and 29% to 62% nonambulatory patients became ambulatory again.3,26
We acknowledged the limitations of our study. First of all, this was a retrospective study, patients with incomplete data for analysis had to be excluded, and systematic treatment was not carefully recorded. Second, patients with myeloma and lymphoma were included in our study. Their inclusion in research has been a matter of debate. Tokuhashi scores excluded them, while Bauer scores included them. And then, preoperative chemotherapy and without bone metastasis at cancer diagnosis had similar hazard ratios, while both were given different points, which might result in bias in the study. Finally, there is always patient's hope for an intervention that might preserve ambulation and drastically improve quality of remaining life, despite poor prognosis predicted by some clinical scores. Therefore, the decision regarding treatment of MSCC patients is complicated, should take into account patient's personal opinions or concerns, and should not merely rely on clinical scores. Besides, the score still warrants a prospective study to be confirmed.
In conclusion, we present a new scoring system for predicting survival and function outcome of MSCC patients after surgical decompression and spine stabilization. This new scoring system can help surgeons select the best candidates for surgical treatment. Patients with scores of 3 to 5 points should be the best surgical candidates, because survival prognosis and function outcome are preferable after surgery, while patients with scores of 0 to 2 points, who have the shortest survival time and poorest function outcome, appear best treated with radiotherapy or best supportive care alone, and patients with scores of 6 to 10 points, who have the most favorable survival prognosis and function outcome, can be treated with more radical surgery to realize better local control of disease and prevent the occurrence of local disease.
Key Points
A new scoring system was developed to guild surgeons to select the best candidates for decompressive surgery in patients with metastatic spinal cord compression.
Primary site, preoperative ambulatory status, visceral metastases, preoperative chemotherapy, and bone metastasis at cancer diagnosis were included in the new scoring system.
Both survival and function outcome were considered in the new scoring system.
Patients with scores of 3 to 5 points should be the best surgical candidates, as survival prognosis and function outcome were preferable after surgery.
Footnotes
Both Mingxing Lei and Jianjie Li are the first authors.
The manuscript submitted does not contain information about medical device(s)/drug(s).
Beijing Municipal Science and Technology Commission (No. Z131107002213052) funds were received in support of this work.
No relevant financial activities outside the submitted work.
References
- 1.Shiue K, Sahgal A, Chow E, et al. Management of metastatic spinal cord compression. Expert Rev Anticancer Ther 2010; 10:697–708. [DOI] [PubMed] [Google Scholar]
- 2.Kim JM, Losina E, Bono CM, et al. Clinical outcome of metastatic spinal cord compression treated with surgical excision ± radiation versus radiation therapy alone: a systematic review of literature. Spine 2012; 37:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005; 366:643–648. [DOI] [PubMed] [Google Scholar]
- 4.Lee CH, Kwon JW, Lee J, et al. Direct decompressive surgery followed by radiotherapy versus radiotherapy alone for metastatic epidural spinal cord compression: a meta-analysis. Spine 2014; 39:E587–E592. [DOI] [PubMed] [Google Scholar]
- 5.Nemelc RM, Stadhouder A, van Royen BJ, et al. The outcome and survival of palliative surgery in thoraco-lumbar spinal metastases: contemporary retrospective cohort study. Eur Spine J 2014; 23:2272–2278. [DOI] [PubMed] [Google Scholar]
- 6.Chong S, Shin SH, Yoo H, et al. Single-stage posterior decompression and stabilization for metastasis of the thoracic spine: prognostic factors for functional outcome and patients’ survival. Spine J 2012; 12:1083–1092. [DOI] [PubMed] [Google Scholar]
- 7.Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine 1990; 15:1110–1113. [DOI] [PubMed] [Google Scholar]
- 8.Tokuhashi Y, Matsuzaki H, Oda H, et al. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005; 30:2186–2191. [DOI] [PubMed] [Google Scholar]
- 9.L’Espérance S, Vincent F, Gaudreault M, et al. Treatment of metastatic spinal cord compression: CEPO review and clinical recommendations. Current Oncol 2012; 19:e478–e490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lei M, Liu Y, Yan L, et al. A validated preoperative score predicting survival and functional outcome in lung cancer patients operated with posterior decompression and stabilization for metastatic spinal cord compression. Eur Spine J 2015; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 11.Lei M, Liu Y, Tang C, et al. Prediction of survival prognosis after surgery in patients with symptomatic metastatic spinal cord compression from non-small cell lung cancer. BMC Cancer 2015; 15:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine 2001; 26:298–306. [DOI] [PubMed] [Google Scholar]
- 13.Wibmer C, Leithner A, Hofmann G, et al. Survival analysis of 254 patients after manifestation of spinal metastases: evaluation of seven preoperative scoring systems. Spine 2011; 36:1977–1986. [DOI] [PubMed] [Google Scholar]
- 14.Dardic M, Wibmer C, Berghold A, et al. Evaluation of prognostic scoring systems for spinal metastases in 196 patients treated during 2005–2010. Eur Spine J 2014; 2014:1–9. [DOI] [PubMed] [Google Scholar]
- 15.Lee CH, Chung CK, Jahng TA, et al. Which one is a valuable surrogate for predicting survival between Tomita and Tokuhashi scores in patients with spinal metastases? A meta-analysis for diagnostic test accuracy and individual participant data analysis. J Neurooncol 2015; 123:267–275. [DOI] [PubMed] [Google Scholar]
- 16.Zoccali C, Skoch J, Walter CM, et al. The Tokuhashi score: effectiveness and pitfalls. Eur Spine J 2015; 1–6. [DOI] [PubMed] [Google Scholar]
- 17.Pointillart V, Vital JM, Salmi R, et al. Survival prognostic factors and clinical outcomes in patients with spinal metastases. J Cancer Res Clin Oncol 2011; 137:849–856. [DOI] [PubMed] [Google Scholar]
- 18.Gakhar H, Swamy GN, Bommireddy R, et al. A study investigating the validity of modified Tokuhashi score to decide surgical intervention in patients with metastatic spinal cancer. Eur Spine J 2013; 22:565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SJ, Lee CS, Chung SS, et al. How accurately can Tokuhashi score system predict survival in the current practice for spinal metastases? J Spinal Disord Tech 2015; 28:E219–E224. [DOI] [PubMed] [Google Scholar]
- 20.Lee BH, Kim TH, Chong HS, et al. Prognostic factor analysis in patients with metastatic spine disease depending on surgery and conservative treatment: review of 577 cases. Ann Surg Oncol 2013; 20:40–46. [DOI] [PubMed] [Google Scholar]
- 21.Fehlings MG, Nater A, Tetreault L, et al. Survival and clinical outcomes in surgically treated patients with metastatic epidural spinal cord compression: results of the prospective multicenter AOSpine study. J Clin Oncol 2016; 34:268–276. [DOI] [PubMed] [Google Scholar]
- 22.Katagiri H, Okada R, Takagi T, et al. New prognostic factors and scoring system for patients with skeletal metastasis. Cancer Med 2014; 3:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arana E, Kovacs FM, Royuela A, et al. Agreement in the assessment of metastatic spine disease using scoring systems. Radiother Oncol 2015; 115:135–140. [DOI] [PubMed] [Google Scholar]
- 24.Kaloostian PE, Yurter A, Zadnik PL, et al. Current paradigms for metastatic spinal disease: an evidence-based review. Ann Surg Oncol 2014; 21:248–262. [DOI] [PubMed] [Google Scholar]
- 25.Tancioni F, Navarria P, Lorenzetti MA, et al. Multimodal approach to the management of metastatic epidural spinal cord compression (MESCC) due to solid tumors. Int J Radiat Oncol Biol Phys 2010; 78:1467–1473. [DOI] [PubMed] [Google Scholar]
- 26.Rades D, Huttenlocher S, Bajrovic A, et al. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. Int J Radiat Oncol Biol Phys 2011; 81:e861–e868. [DOI] [PubMed] [Google Scholar]


