Abstract
OBJECTIVE
We compared 3-year achievement of an American Diabetes Association composite treatment goal (HbA1c <7.0%, LDL cholesterol <100 mg/dL, and systolic blood pressure <130 mmHg) after 2 years of intensive lifestyle-medical management intervention, with and without Roux-en-Y gastric bypass, with one additional year of usual care.
RESEARCH DESIGN AND METHODS
A total of 120 adult participants, with BMI 30.0–39.9 kg/m2 and HbA1c ≥8.0%, were randomized 1:1 to two treatment arms at three clinical sites in the U.S. and one in Taiwan. All patients received the lifestyle-medical management intervention for 24 months; half were randomized to also receive gastric bypass.
RESULTS
At 36 months, the triple end point goal was met in 9% of lifestyle-medical management patients and 28% of gastric bypass patients (P = 0.01): 10% and 19% lower than at 12 months. Mean (SD) HbA1c values at 3 years were 8.6% (3.5) and 6.7% (2.0) (P < 0.001). No lifestyle-medical management patient had remission of diabetes at 36 months, whereas 17% of gastric bypass patients had full remission and 19% had partial remission. Lifestyle-medical management patients used more medications than gastric bypass patients: mean (SD) 3.8 (3.3) vs. 1.8 (2.4). Percent weight loss was mean (SD) 6.3% (16.1) in lifestyle-medical management vs. 21.0% (14.5) in gastric bypass (P < 0.001). Over 3 years, 24 serious or clinically significant adverse events were observed in lifestyle-medical management vs. 51 with gastric bypass.
CONCLUSIONS
Gastric bypass is more effective than lifestyle-medical management intervention in achieving diabetes treatment goals, mainly by improved glycemic control. However, the effect of surgery diminishes with time and is associated with more adverse events.
Introduction
Treatment of type 2 diabetes is aimed at reducing complications, particularly micro- and macrovascular disease. The best means of achieving this goal is expressed in a consensus statement of the American Diabetes Association (ADA), which recommends therapy to correct hyperglycemia, hypertension, and dyslipidemia (1). In the U.S., achievement of this composite end point (HbA1c <7.0%, LDL cholesterol <100 mg/dL, and systolic blood pressure [SBP] <130 mmHg) has proven frustratingly difficult (2–4). Bariatric surgery has been proposed as a strategy to better correct these metabolic disturbances and is thought to act through greater weight loss or altered gut function and hormone release.
Several small randomized trials have demonstrated improvement of glycemic control with addition of bariatric surgery to medical management (5–11). Post hoc analysis from a long-term prospective study showed that bariatric surgery improved glycemic control but had less benefit on blood pressure and dyslipidemia (12). The Diabetes Surgery Study (DSS) is the only randomized trial designed to study the effect of the Roux-en-Y gastric bypass (gastric bypass) on the ADA composite end point (glycemia, blood pressure, and lipidemia) in patients with type 2 diabetes. We previously reported that the gastric bypass was associated with 1-year and 2-year improvements in rates of achieving the composite end point, balanced by an increased number of nutritional abnormalities and adverse events associated with gastric bypass (8,13).
In this report we describe the interventions’ effectiveness and risks 3 years after randomization. The study was designed to provide 2 years of intense lifestyle and medical management in all subjects with three additional years of observation while on usual medical care, so the results at 3 years reflect the first year of usual medical care after the first 2 study years. We had hypothesized that addition of gastric bypass to intense lifestyle and medical management would substantially improve the achievement of the ADA composite end point of metabolic control of type 2 diabetes. We also assessed potential baseline predictors of successful surgical outcomes in order to better inform patients about treatment alternatives.
Research Design and Methods
This 5-year study is being conducted at the University of Minnesota, Columbia University Medical Center in New York, National Taiwan University Hospital and Min Sheng General Hospital (together referred to here as “Taiwan”), and the Mayo Clinic. Institutional Review Board approval and written informed consent from each patient were obtained at all sites. The study design calls for 2 years of intense behavioral and medical management in all subjects, followed by 3 additional years of follow-up in all subjects as they receive standard medical care.
The complete list of inclusion and exclusion criteria has been published (13). Briefly, key inclusion criteria included HbA1c ≥8.0% despite at least 6 months under a provider’s care for type 2 diabetes, BMI 30.0–39.9 kg/m2, C-peptide >1.0 ng/mL, and stated willingness and ability to accept randomization and follow the full treatment protocol. Recruitment efforts have previously been described (14).
The 2-year lifestyle intervention was based on protocols from two successful clinical trials: the Diabetes Prevention Program (DPP) and the Look AHEAD (Action for Health in Diabetes) study (15,16). Over the first 12 months the median number of lifestyle modules delivered was 32 for lifestyle-medical management intervention and 27 for gastric bypass. Between 12 and 24 months the median number of modules was five and seven for lifestyle-medical management intervention and gastric bypass, respectively. Visits with an endocrinologist occurred monthly for 6 months, then quarterly (or monthly if not at ADA treatment goal) for the next 6 months, and then quarterly through the second year. The intensive medical management protocol for both treatment groups aimed to optimize drug therapy to control hyperglycemia, cholesterol, and hypertension.
After 24 months, all study interventions ceased and patients returned to usual care with their primary physician. Each subject’s primary physician received a letter describing the study, the subject’s current status, medications, and goals of care. The primary physicians also received recommendations about medications and information about the need for nutritional supplementation as appropriate. The study coordinator contacted participants at 30 months to maintain their connection with the study and to obtain interim data on adverse events. Study endocrinologists evaluated patients during a clinic visit at 36 months but did not modify medications. The visit included a collection of blood pressure, weight, and waist circumference data and laboratory studies. Participants were encouraged to increase medication compliance, nutritional supplementation, and dietary control if adherence was deemed to be an issue.
Gastric bypass was laparoscopically performed in a standardized fashion with construction of a 20-mL lesser curvature gastric pouch and a 100-cm biliopancreatic limb. Study surgeons performed all postoperative surgical interventions (13,17).
The primary outcome was assessed at the 12-month visit. It was met if patients achieved the prespecified composite end point conforming to the ADA recommendations at the time of study inception (18): HbA1c <7.0%, LDL cholesterol <100 mg/dL (to convert to millimoles per liter, multiply by 0.0259), and SBP <130 mmHg. Secondary outcomes included durability of the triple end point and continuous measures of HbA1c, LDL cholesterol, and SBP, as well as weight loss, HDL, diastolic blood pressure (DBP), medication usage, and adverse events.
Full remission at the 3-year visit, as defined using the ADA consensus statement, required HbA1c <6.0% at 24 and 36 months, with no use of antihyperglycemic medication from 24 to 36 months (19). Partial remission was defined as HbA1c <6.5% at both visits, without antihyperglycemic medication, and not meeting criteria for full remission (19).
In reports of 1-year and 2-year outcomes, we presented all clinically important adverse events and all selected nutritional abnormalities. However, in the third year of the study, reduced visits and transfer of patients back to primary care meant that only data on serious adverse events were collected. Serious adverse events were defined using standard U.S. Food and Drug Administration criteria and include death, hospitalization, life-threatening complications, disability or permanent damage, the need for intervention to prevent permanent damage, and other important medical events.
Randomization was stratified by site, and clinical center personnel were masked to aggregated outcomes data. Details of the randomization process have been published (13). Clinical personnel were provided with study-wide statistical summaries after each year’s follow-up data were complete.
Except for exploration of potential predictors, all effectiveness analyses are done on an intention-to-treat basis, with multiple imputations used for missing data. One patient who died of pancreatic cancer after the 12-month visit is excluded from all effectiveness analyses. Directly observed data for all 120 patients are included in the analysis of adverse events and are reported on an as-treated basis. Potential predictors of 36-month outcomes were analyzed on an as-treated basis, using only directly observed data and excluding patients who crossed over after the first 6 months.
Multiple imputation was used to address the issue of missing data (20). Data included in the multiple imputation were for the outcomes reported in Table 2 and the time points shown in Fig. 2A–D. All values reported in Table 2 (outcomes) were modeled together using PROC MI in SAS 9.3 (SAS Institute). Information on crossover was also included when developing imputed data. Forty imputations were done. Regressions were done separately for each of the 40 imputed datasets and then summarized using PROC MIANALYZE. The analysis of key outcomes was repeated on an as-treated basis as validation and for sensitivity testing. Graphs indicating means and 95% CI are based on imputed data.
Table 2.
Outcome values
| Dichotomous outcomes |
||||||
|---|---|---|---|---|---|---|
| Lifestyle/medical management |
RYGB |
Treatment difference at 36 months |
||||
| 12 months | 36 months | 12 months | 36 months | OR (95% CI) | P | |
| Meets triple end point | 11 (19) | 5 (9) | 28 (47) | 17 (28) | 4.0 (1.3, 12.1) | 0.01 |
| HbA1c <7.0% | 19 (31) | 13 (22) | 44 (73) | 35 (58) | 4.9 (2.0, 11.7) | 0.0004 |
| LDL cholesterol <100 mg/dL | 41 (70) | 33 (55) | 47 (78) | 42 (69) | 1.8 (0.8, 3.9) | 0.14 |
| SBP <130 mmHg | 45 (76) | 30 (50) | 49 (81) | 43 (72) | 2.5 (1.1, 5.6) | 0.02 |
| HbA1c <6.0% | 5 (9) | 4 (7) | 25 (42) | 16 (27) | 5.1 (1.2, 21.5) | 0.03 |
| Full remission* | n/a | 0 (0) | n/a | 10 (17) | n/a | 0.001 |
| Full or partial remission** | n/a | 0 (0) | n/a | 22 (36) | n/a | <0.0001 |
| SBP <140 mmHg | 53 (90) | 43 (73) | 55 (92) | 55 (91) | 4.3 (1.3, 14.4) | 0.02 |
| Use of prescription medication | ||||||
| Insulin | 26 (44) | 28 (47) | 12 (20) | 10 (16) | 0.19 (0.07, 0.48) | 0.0005 |
| Other glycemic medicines | 57 (96) | 47 (80) | 21 (35) | 25 (42) | 0.16 (0.06, 0.41) | 0.0002 |
| LDL medicines | 38 (65) | 32 (54) | 23 (38) | 22 (37) | 0.50 (0.23, 1.07) | 0.07 |
| Blood pressure medications | 42 (72) | 34 (57) | 23 (39) | 24 (39) | 0.44 (0.19, 1.02) | 0.055 |
| At goal without medication | ||||||
| Triple end point | 0 (0) | 1 (2) | 13 (22) | 8 (14) | 9.1 (1.1, 75.0) | 0.04 |
| HbA1c <7.0% | 0 (0) | 3 (5) | 34 (58) | 30 (52) | 22 (4.6, 105) | 0.0001 |
| LDL cholesterol <100 mg/dL | 15 (26) | 15 (26) | 31 (52) | 24 (41) | 1.9 (0.9, 4.2) | 0.11 |
| SBP <130 mmHg | 15 (25) | 15 (25) | 32 (54) | 28 (52) | 3.1 (1.3, 7.6) | 0.01 |
| Continuous outcomes |
||||||
|---|---|---|---|---|---|---|
| Lifestyle/medical management |
RYGB |
Treatment difference at 36 months |
||||
| 12 months | 36 months | 12 months | 36 months | Estimate (95% CI) | P | |
| HbA1c (%) | 7.8 (2.3) | 8.6 (3.5) | 6.4 (1.6) | 6.7 (2.0) | −1.9 (−2.6, −1.2) | <0.0001 |
| Serum lipids | ||||||
| LDL cholesterol (mg/dL) | 88 (50) | 103 (70) | 83 (40) | 90 (46) | −13.3 (−27.7, 1.0) | 0.07 |
| HDL cholesterol (mg/dL) | 42 (16) | 46 (18) | 51 (20) | 53 (19) | 7.0 (2.5, 11.6) | 0.003 |
| Blood pressure | ||||||
| SBP (mmHg) | 124 (17) | 130 (28) | 117 (22) | 123 (24) | −7.6 (−13.7, −1.5) | 0.01 |
| DBP (mmHg) | 74 (13) | 77 (19) | 68 (14) | 71 (17) | −6.5 (−10.8, −2.3) | 0.003 |
| Weight (kg) | 90.6 (24.3) | 92.1 (29.2) | 73.5 (19.8) | 77.9 (20.9) | −14.3 (−20.1, −8.5) | <0.0001 |
| Percent weight change (%) | −7.3 (12.5) | −6.3 (16.1) | −25.5 (13.5) | −21.0 (14.5) | 14.8 (11.0, 18.7) | <0.0001 |
| BMI (kg/m2) | 31.7 (5.5) | 32.1 (6.8) | 26.0 (5.3) | 27.7 (5.8) | −4.5 (−6.0, −3.0) | <0.0001 |
| Medications prescribed | ||||||
| For glycemia | 2.6 (1.0) | 2.1 (1.3) | 0.8 (1.0) | 0.7 (1.0) | −1.4 (−1.4, −1.3) | <0.0001 |
| For dyslipidemia and blood pressure | 2.4 (1.8) | 1.8 (1.4) | 1.1 (1.1) | 1.1 (1.1) | −0.7 (−1.1, −0.3) | 0.001 |
| Total | 4.9 (3.1) | 3.8 (3.3) | 1.8 (2.7) | 1.8 (2.4) | −2.0 (−2.7, −1.4) | <0.0001 |
Dichotomous outcomes are N (%) with characteristic and OR (95% CI). Continuous outcomes are mean (SD) and estimated difference (95% CI). All values estimated using multiple imputations for missing data. Treatment comparisons adjusted for sites. Boldface type indicates the primary end point. n/a, not applicable; RYGB, Roux-en-Y gastric bypass.
*Full remission: defined as HbA1c <6.0% for a full year (i.e., visits 24 and 36) and no glycemic medications during that time. P value for treatment difference determined by using Fisher exact test.
**Full or partial remission: same as full remission, but replace 6.0% with 6.5%.
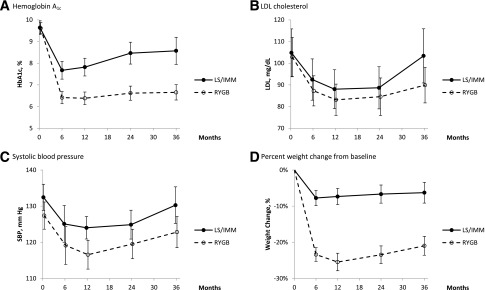
Figure 2.
Key outcomes based on imputed data. Error bars indicate 95% CI. LS/IMM, lifestyle and intense medical management; RYGB, Roux-en-Y gastric bypass.
Dichotomous data were analyzed using logistic regressions stratified by site; continuous data were analyzed using linear regressions adjusted for site. Estimates of counts, means, and SDs were derived from the imputed database. SDs reflected estimated population variations and also variability associated with imputations.
For dichotomous variables, analyses of 36-month data were based on logistic regressions with the 36-month value as the outcome and the starting value as an adjusting variable. For continuous variables, the change from baseline was used as the outcome in linear regressions.
The relationship between 3-year weight loss and triple end point attainment was evaluated graphically on an as-treated basis. Loess curves with 95% CI were used to represent the proportion of triple end point successes in relation to percent weight loss. The graphical analysis was done in SAS 9.3, using PROC SGPLOT. The relationship between weight regain and 3-year HbA1c was examined using multivariate linear regressions adjusted for site and variables related to glycemia only if they remained significant in multivariate regressions including weight loss.
We tested the difference in reported adverse event rates using a negative binomial model with an offset for person-years of exposure. Adverse events were examined on an as-treated basis, with adverse events attributed to the treatment group at the time of the event and with person-years of exposure allocated based on the timing of the crossover. SAS procedure GENMOD was used, with repeated measures within subject (necessary to correctly model crossovers and represent within-person correlation). An adjustment by site was tested and removed to optimize overall model fit criteria. The P value was estimated using score statistics for type 3 general estimating equation analyses.
Results
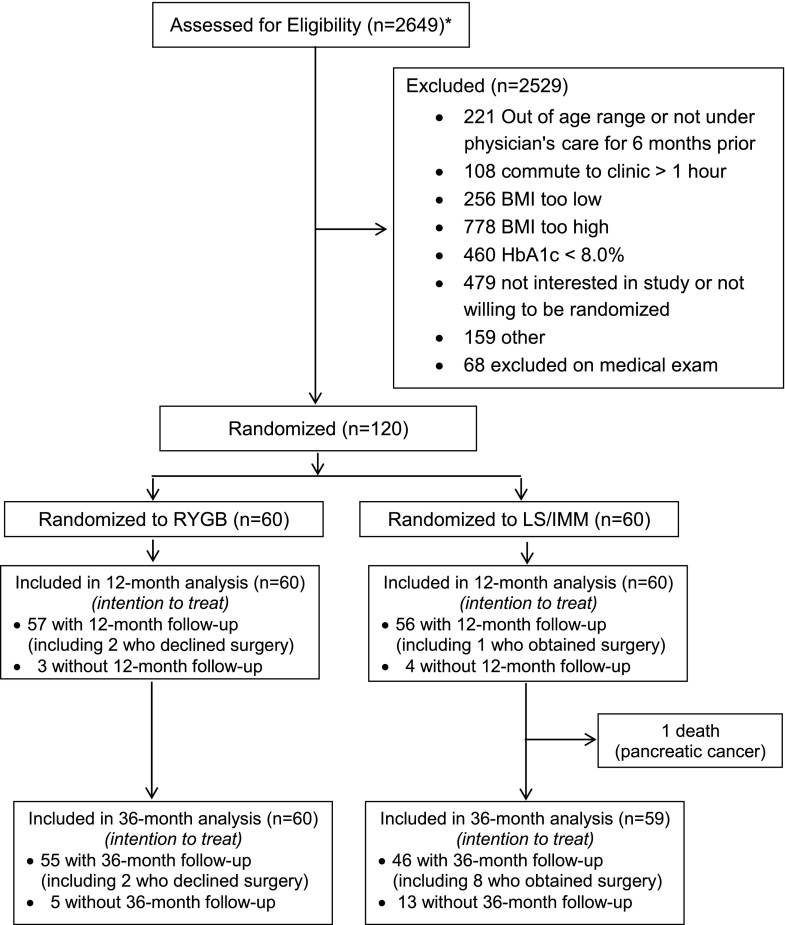
Between April 2008 and December 2011, 120 patients were randomized: 60 to lifestyle-medical management intervention and 60 to lifestyle-medical management intervention with gastric bypass (Fig. 1). Baseline characteristics (Table 1) were similar between groups except that insulin use was less in lifestyle-medical management patients (42%) versus gastric bypass (62%). In total, 1 lifestyle-medical management patient died, 13 of the 59 remaining lifestyle-medical management patients were lost to follow-up, and 5 of 60 gastric bypass patients were lost to follow-up. There were 10 crossovers through 36 months, including 8 participants randomized to lifestyle-medical management and 2 randomized to gastric bypass. One of the lifestyle-medical management participants who crossed over elected to undergo gastric bypass after 3 months; the others underwent surgery between the 12-month and 36-month visits. The two patients randomized to gastric bypass who crossed over declined surgery at the outset but received the lifestyle-medical management protocol.
Figure 1.
Diabetes surgery study consort diagram. *Patients were recruited using mailings, radio messages, clinic referrals, and posters. LS/IMM, lifestyle and intense medical management; RYGB, Roux-en-Y gastric bypass.
Table 1.
Baseline data by treatment group
| Lifestyle/medical management (n = 59) | RYGB (n = 60) | |
|---|---|---|
| Demographics | ||
| Age (years) | 49 (8) | 49 (9) |
| Female, n (%) | 34 (57) | 38 (63) |
| Race/ethnicity, n (%) | ||
| Non-Hispanic white | 30 (50) | 33 (55) |
| East Asian | 17 (28) | 16 (27) |
| Non-Hispanic black | 6 (10) | 5 (8) |
| Hispanic | 4 (7) | 4 (7) |
| Native American | 1 (2) | 2 (3) |
| Other | 2 (3) | 0 (0) |
| General medical information | ||
| BMI (kg/m2) | 34.3 (3.1) | 34.9 (3.0) |
| BMI 30.0–34.9 kg/m2, n (%) | 35 (58) | 36 (60) |
| Waist circumference (cm) | 113 (12) | 114 (10) |
| SBP (mmHg) | 132 (14) | 127 (15) |
| DBP (mmHg) | 79 (10) | 78 (12) |
| Years since diabetes diagnosis | 9.1 (5.7) | 8.9 (6.1) |
| Laboratory values (serum) | ||
| HbA1c (%) | 9.6 (1.2) | 9.6 (1.0) |
| LDL cholesterol (mg/dL) | 105 (43) | 103 (36) |
| HDL cholesterol (mg/dL) | 42 (9) | 41 (1) |
| Triglycerides (mg/dL) | 197 (82) | 187 (79) |
| Total cholesterol (mg/dL) | 189 (46) | 182 (39) |
| Creatinine (mg/dL) | 0.79 (0.19) | 0.81 (0.20) |
| Fasting C-peptide (ng/mL) | 3.0 (1.4) | 2.7 (1.4) |
| Postmeal C-peptide (ng/mL) | 4.7 (2.1) | 4.3 (2.0) |
| Fasting glucose (mg/dL) | 206 (52) | 214 (57) |
| Medicines | ||
| Taking insulin, n (%) | 25 (42) | 37 (62) |
| Taking other glycemic medicines, n (%) | 56 (95) | 52 (87) |
| Taking dyslipidemia medicines, n (%) | 40 (68) | 39 (65) |
| Taking blood pressure medicines, n (%) | 43 (73) | 41 (68) |
| Number of medications for control of glycemia, dyslipidemia, and blood pressure | 4.4 (1.5) | 4.1 (1.9) |
Data are means (SD) unless otherwise indicated. RYGB, Roux-en-Y gastric bypass.
At 36 months, 5 (9%) lifestyle-medical management participants and 17 (28%) gastric bypass participants achieved the composite triple end point (P < 0.001; odds ratio [OR] 4.0; 95% CI 1.3, 12.6) (Table 2). Success rates were down from their 12-month apex of 11 (19%) in lifestyle-medical management and 28 (49%) in gastric bypass participants and from the 24-month achievement rates of 8 (14%) in lifestyle-medical management and 26 (43%) in the gastric bypass group. The treatment difference provided by gastric bypass on the composite end point was reduced from 30 to 27% and then 19% over the first, second, and third years after randomization. With the individual triple end point components considered as dichotomous variables, statistically significant treatment effects were seen at 36 months for HbA1c and SBP but not for LDL cholesterol (Table 2). Thirteen (22%) lifestyle-medical management subjects vs. 35 (58%) in the gastric bypass group obtained HbA1c <7.0% (OR 4.9; 95% CI 2.0, 11.7). Thirty (50%) lifestyle-medical management patients and 43 (72%) in the gastric bypass group achieved SBP <130 mmHg (OR 2.5; 95% CI 1.1, 5.6).
For the lifestyle-medical management group, the mean (SD) percent weight lost (Table 2 and Fig. 2D) was 7.3% (12.5) at 12 months, 7.3% (14.9) at 24 months, and 6.3% (16.1%) at 36 months. Weight loss in gastric bypass patients was 25.5% (13.5), 23.8% (13.9), and 21.0% (14.5%) at 12, 24, and 36 months, respectively. The two groups’ weight loss differed by 14.8% at 36 months (95% CI 11.0, 18.7; P < 0.0001).
Mean (SD) HbA1c for lifestyle-medical management rose from 7.8% (2.3) at 12 months to 8.6% (3.5) at 36 months. Mean HbA1c for gastric bypass was 6.4% (1.6) at 12 months and 6.7% (2.0) at 36 months (Table 2 and Fig. 2A). Twenty-two (36%) subjects randomized to gastric bypass had either full or partial remission at 36 months, including 10 (17%) with full remission and 12 (19%) with partial remission. We observed five gastric bypass subjects who had an HbA1c <6.0% and were off diabetes medications at 2 years but not at 36 months and three participants who had an HbA1c <6.0% and were off diabetes medications at 36 but not 24 months (Table 3). Remission of diabetes (either full or partial) was not observed in lifestyle-medical management participants at any time point. Among the eight participants who crossed over from lifestyle-medical management, five did so after the 24-month visit, and of the three earlier crossovers, only one was close to remission.
Table 3.
Adverse events on an as-treated basis*
| Lifestyle/medical management |
RYGB |
|||||||
|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Total | Year 1 | Year 2 | Year 3 | Total | |
| Surgical complications | ||||||||
| Anastomotic leak | 2 | 2 | ||||||
| Anastomotic ulcer | 1 | 3 | 4 | |||||
| Anastomotic stricture | 2 | 2 | ||||||
| Wound infection | 1 | 1 | ||||||
| Wound hematoma | 1 | 1 | ||||||
| Pouch gastritis | 1 | 1 | ||||||
| Small bowel obstruction | 2 | 2 | ||||||
| Gastrointestinal | ||||||||
| Acute pancreatitis | 1 | 2 | 1 | 4 | ||||
| Pancreatic carcinoma | 2 | 2 | ||||||
| Cholelithiasis | 1 | 1 | 1 | 1 | ||||
| Abdominal pain | 1 | 1 | 4 | 4 | 2 | 10 | ||
| Reflux esophagitis | 2 | 2 | 3 | 3 | ||||
| Duodenitis | 1 | 1 | ||||||
| Cardiovascular | ||||||||
| Deep venous thrombosis | 1 | 1 | ||||||
| Congestive heart failure | 1 | 1 | ||||||
| Acute myocardial infarction | 1 | 1 | ||||||
| Renal | ||||||||
| Nephrolithiasis | 1 | 1 | 1 | 2 | 3 | |||
| Metabolic | ||||||||
| Diabetic ketoacidosis | 1 | 1 | ||||||
| Musculoskeletal | ||||||||
| Below-the-knee amputation | 1 | 1 | ||||||
| Toe amputation | 1 | 1 | 2 | |||||
| Foot amputation | 1 | 1 | ||||||
| Neurologic | ||||||||
| Herniated spinal disc | 1 | 1 | ||||||
| Multiple sclerosis | 1 | 1 | ||||||
| Partial 3rd cranial nerve palsy | 1 | 1 | ||||||
| Psychiatric | ||||||||
| Depression | 1 | 1 | ||||||
| Suicide attempt | 1 | 1 | ||||||
| Miscellaneous | ||||||||
| Fall with fracture or injury | 3 | 3 | 3 | 4 | 7 | |||
| Hypertension w/hospitalization | 1 | 1 | ||||||
| Unwanted pregnancy | 1 | 1 | ||||||
| Abnormal uterine bleeding | 1 | 1 | ||||||
| Retinal vascular occlusion | 1 | 1 | ||||||
| Abdominoplasty | 1 | 1 | ||||||
| Infection | 2 | 2 | 2 | 2 | ||||
| Totals | 14 | 5 | 5 | 24 | 23 | 17 | 10 | 50 |
Data are counts. Years 1–2: clinically important events; year 3: serious events. RYGB, Roux-en-Y gastric bypass.
*Two patients were randomized to lifestyle and intense medical management but elected to undergo Roux-en-Y gastric bypass surgery elsewhere and then subsequently had reportable adverse events. These two adverse events (anastomotic ulcer and abdominal pain) are reported under the Roux-en-Y gastric bypass heading.
Gastric bypass participants achieved significantly lower mean SBP and DBP than lifestyle-medical management participants at 3 years (Table 2 and Fig. 2C). LDL cholesterol levels were not different between groups at 36 months.
At 36 months, gastric bypass was associated with 2.0 (95% CI 1.4, 2.7) fewer medications to manage glycemia, hypertension, and dyslipidemia (Table 2). This difference was found despite a reduction in glycemic medication among the lifestyle-medical management group from 2.6 (SD 1.8) at 12 months to 2.1 (SD 1.3) at 36 months. Overall, 1 (2%) lifestyle-medical management participant and 8 (14%) gastric bypass participants obtained the triple end point at 36 months without related medications. Insulin was used by 28 (47%) lifestyle-medical management participants vs. 10 (16%) gastric bypass participants (OR 0.19; 95% CI 0.07, 0.48). The SBP goal was obtained without medications in 15 (25%) lifestyle-medical management and 28 (52%) gastric bypass subjects at 36 months (OR 3.1; 95% CI 1.3, 7.6). The treatment difference in attainment of LDL cholesterol goal without medication was not statistically significant. In the lifestyle-medical management group, the number of medications for LDL and SBP combined dropped from 2.6 (SD 1.8) at 12 months to 2.0 (SD 1.3) at 36 months. Gastric bypass participants reported taking calcium supplements at 96%, multivitamins at 95%, and sublingual or injected vitamin B12 supplements at 94% of annual visits. Comparable rates in the lifestyle-medical management participants were 8%, 36%, and 0%, respectively. Some calcium supplements contain vitamin D, which was not separately tracked, but 40% of gastric bypass subjects compared with 15% of lifestyle-medical management subjects were taking additional vitamin D, and 37% of gastric bypass participants compared with 2% of lifestyle-medical management participants were taking additional iron.
Serious adverse events were tallied between 24 and 36 months (Table 3), and 5 events occurred in lifestyle-medical management and 10 in gastric bypass in that third year. Adding these serious adverse events to the clinically significant events found in the first 24 months yielded a total of 24 reported adverse events in the lifestyle-medical management group and 51 in the gastric bypass group (P = 0.16). The six subjects who crossed over from lifestyle-medical management to surgery had a total of six adverse events; only two (anastomotic leak and abdominal pain) occurred after surgery.
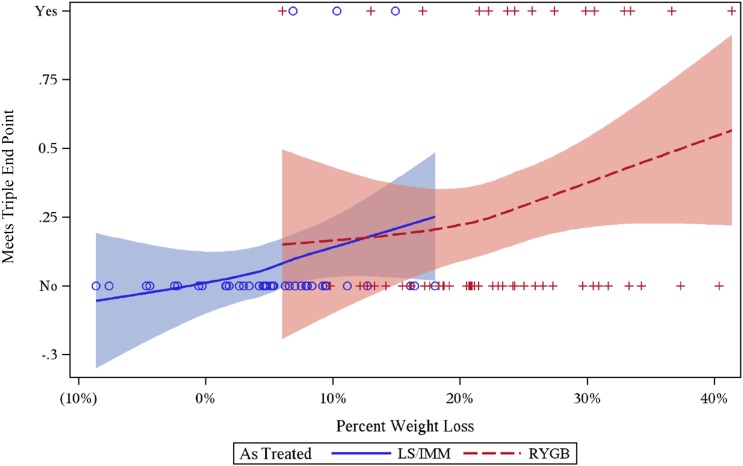
Logistic regressions of as-treated data on 101 participants with full data, stratified by site, were used to identify predictors of obtaining the triple end point at 36 months. In univariate analyses, both treatment group and percent weight loss were significant predictors of triple end point success at 36 months (P = 0.02 for treatment group; P = 0.003 for percent weight loss). The best fit multivariate model included percent weight loss, SBP, and LDL cholesterol. Figure 3 illustrates the relationship between weight loss and triple end point attainment using a Loess curve and indicates the strong effect of weight loss among and within both treatment groups. When only baseline variables were included, the best fit model included treatment with surgery as well as SBP and LDL cholesterol.
Figure 3.
DSS 36-month outcomes. Treatment and triple end point success versus percent weight loss. Loess curves approximate average percent at goal (and 95% CI). As-treated analysis. LS/IMM, lifestyle and intense medical management; RYGB, Roux-en-Y gastric bypass.
Analyses were conducted examining predictors of full or partial diabetes remission. Since there were no remissions in the lifestyle-medical management group, the analysis included only the gastric bypass group, and weight loss had limited variability within that group, making it difficult to assess as a predictor in single-arm analyses. In addition, multivariate models could not be fit because of the small number of positive outcomes. Statistically significant univariate predictors of diabetes remission, either full or partial, included baseline values for C-peptide but did not include baseline measures of BMI, HbA1c, blood pressure, lipids, or duration of diabetes.
Durability of effect may also be evaluated by evaluating variables at 12 months that predict 36-month outcomes. In the gastric bypass group, significant 12-month predictors include lower HbA1c, not taking oral hypoglycemic medication, lower medication count, lower fasting glucose, and lower percent weight loss in the first year. In the lifestyle-medical management group, low success rate prevented identification of significant predictors. Exploratory analyses revealed that weight regain from 24 to 36 months was associated with higher HbA1c at 36 months for each treatment group separately and for all patients combined (point estimates ranged from 0.2 to 0.3 increase in HbA1c per 3% weight regain; all P values ≤0.04) in models adjusted for 24-month HbA1c and BMI. No association was found for 12–36 month weight changes. Weight regain between either 12 or 24 months and the 36 month weight was not significantly associated with the 36-month triple end point.
Conclusions
The DSS tested Roux-en-Y gastric bypass as an adjunct to intensive lifestyle and medical management for obtaining the composite treatment end point in patients with poorly controlled type 2 diabetes. Gastric bypass substantially improved the likelihood of triple end point success at 1 year (13) and 2 years (8), but durability of this effect remains an important question. In these results, gastric bypass significantly improved composite end point attainment at 3 years, but the clinical effect was reduced in both groups over the course of the 3 years. At 36 months, the proportion of gastric bypass patients meeting the triple end point had fallen from nearly half to approximately one-quarter of the participants.
Triple end point goals are more difficult to reach, as each of three dichotomous variables must be simultaneously attained, and multiplying across those variables puts pressure on full achievement of the composite. Even so, it is useful to look at which variables are contributing most to the reduction in triple end point achievement with time, and it appears most associated with reduction in glycemic control. Although there are a substantial number of patients with improved glycemic control, the number of bypass surgery patients with HbA1c <7.0% declined from 73% at 1 year to 58% at 3 years. There are indications that deteriorations in glycemic control, especially in the 24- to 36-month interval, are associated with weight regain. Reductions in meeting goals for SBP, 81% to 72%, and LDL cholesterol, 78% to 69%, were less substantial. Since both treatment groups had reductions in triple end point achievement that appeared more prominent between the second and third years, it is possible that transition to usual care and withdrawal of intense medical management reduced impact of the surgical treatment, or the natural history of diabetes may have contributed to the fall off in surgical benefit. Reductions in glycemic control with time from bariatric surgery have previously been seen (11,21), but the importance of reduction in treatment effect when considered in the full metabolic portfolio of the triple end point is noteworthy.
In analysis of the first-year experience of the DSS, weight loss was identified as the primary predictor of triple end point achievement (13). At 3 years, the best-fitting models for predicting triple end point attainment included weight loss or if, using baseline variables only, the decision to add bariatric surgery. Together, these predictive models indicate that the effect of treatment group appears to be mostly due to weight loss. In either case, the other variables in the final models were blood pressure and LDL cholesterol. Notably, we did not find duration of diabetes, C-peptide values, HbA1c, or BMI to add much to predicting triple end point attainment. More needs to be known about the possibility of additional mechanisms involved in metabolic benefits observed after bariatric surgery (22,23), as well as the importance of diabetes duration and severity in determining diabetes outcomes after weight loss or bariatric surgery (21).
Considering the individual components of the primary end point, there were differences between lifestyle-medical management and gastric bypass on several measures of glucose control. At 36 months, 22% of patients in the medical group achieved an HbA1c <7.0%, while 58% of surgical patients achieved this target. Remission of diabetes has been widely used to judge the efficacy of bariatric surgery. Four randomized studies have reported 2 or more years of data, finding full remission occurring in 25–35% and partial or full remission in 46–70% with gastric bypass (9–11,24). Our rates of 17% full remission and 36% partial or full remission are lower than those studies but similar to the findings of another recently reported study (5). We observed loss of remission in five participants between 24 and 36 months, while three participants remitted between 24 and 36 months. Relapse of diabetes has been reported in another study among subjects after gastric bypass who were successful in achieving biochemical correction of diabetes at 12 months (HbA1c <6.0% without diabetes medications) (11). Similarly, a large observational study showed recurrence of diabetes in 35% of patients after bariatric surgery (12). Our analyses using baseline covariates, which by definition could not include weight loss, indicated that only baseline β-cell function, indicated by fasting and stimulated C-peptide, contributed to predicting remission, whereas diabetes duration, BMI, and HbA1c at baseline did not. This outcome is similar to an analysis of a subset of the DSS in which more complete serum measures were available, which found that baseline C-peptide levels predicted HbA1c levels at 1 year postrandomization (23). These predictor analyses of the DSS differ from larger observational studies where baseline HbA1c and diabetes duration have been identified as a possible factors in remission (21) or where a predictor composite of age, HbA1c, and treatment type could predict remission (25). Based on this analysis, lower remission rates in this study as well as the diminishing benefit on the primary triple end point may relate to baseline β-cell status, more severe diabetes, or other, as yet unidentified, participant factors, since weight loss from surgery is similar across trials.
Mean SBP was significantly lower in the surgical group at 3 years, but there was no significant improvement in LDL cholesterol with surgery. In both treatment groups, SBP and LDL cholesterol increased somewhat from their nadir at 12 months. There was an increase in HDL in both groups by 36 months, more so in gastric bypass, consistent with other reports (11).
At 3 years, the gastric bypass group used half as many medications for control of hyperglycemia, hypertension, and dyslipidemia. This may be interpreted as underscoring the effectiveness of gastric bypass in affecting the underlying biology related to each end point. It may also reflect reluctance on patients’ and providers’ part to add back medications (26). It should be noted that need for nutritional supplements in gastric bypass patients means that the total number of daily pills did not decrease.
Serious and clinically significant adverse events were more common in the gastric bypass group compared with the lifestyle-medical management group. The difference in adverse event rates was not statistically significant, despite a large difference in count. The lack of significance may be attributed to clustering within patients. Assessment of the burden of complications remains one of the greatest challenges facing adoption of bariatric surgery as a primary therapy for diabetes. Previous reports of complications from single-site randomized trials have been lower (10,11) and could reflect different methodology in monitoring adverse events, differences in the treatment protocols, and/or characteristics of the subject population.
Sustained achievement of all three ADA therapeutic targets for patients with diabetes is a daunting task. This study demonstrates that augmenting lifestyle-medical management with gastric bypass significantly improves triple end point attainment, and multivariate analyses showed that either weight loss or assignment to surgery was the most important of predictors of triple end point achievement. Gastric bypass exerts most of its triple end point benefit by significantly improving glycemic control, with modest effect on blood pressure and no effect on LDL cholesterol. Overall, at 3 years, gastric bypass produced significant and sustained weight loss and significantly improved glycemic control, but a minority of participants received the benefit of triple end point attainment or remission of diabetes. Benefits of gastric bypass in management of type 2 diabetes are mainly on improved blood glucose, offset by a higher rate of adverse events. Longer follow-up in this and other studies is greatly needed, as it appears that the benefits of surgery may diminish with time.
Article Information
Acknowledgments. Study coordinators were Nyra Wimmergren, Joyce Schone, and Colet Landvik, University of Minnesota; Heather Bainbridge, Columbia University Medical Center; Amy Olofson, Mayo Clinic; and Shu-Chun Chen, Linda Huang, and Meng-Jie Chen, National Taiwan University.
Funding and Duality of Interest. The DSS was supported by Covidien, Dublin, Ireland. Covidien provided funds for the clinical locations: University of Minnesota, Mayo Clinic, Columbia University, National Taiwan University Hospital, and Min-Sheng General Hospital. These studies were supported in part by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Nutrition and Obesity Research Center grant no. P30-DK-050456. This publication was also supported in part by National Center for Advancing Translational Sciences, National Institutes of Health, formerly the National Center for Research Resources, grants UL1 TR000040 and UL1 RR024156 to Columbia University. S.I. serves as an advisory board member for Novo Nordisk, USGI Medical, and Medica; consults for Metamodix; and receives grant support from Covidien, EnteroMedics, USGI Medical, and ReShape Medical. J.K. reports receiving institutional grant support from Covidien and receiving personal support for expert testimony and participation on the speakers bureau for Takeda. J.P.B. reports receiving institutional support for the Look AHEAD study. A.J.T. and Q.W. report receiving salary support from Covidien for the DSS, as well as supplemental salary support from the Minnesota Obesity Center. J.E.C. reports receiving institutional and personal grant support from Covidien, National Institutes of Health grant support, and travel expenses and personal support for the U.S. Food and Drug Administration Advisory Panel on Pulmonary Drugs. M.D.J. reports serving on an advisory board for Vivus and receiving institutional grant support from Aspire Bariatrics. A.V. reports receiving consulting support from Sanofi, Roche, and Novartis; institutional consulting support from Merck; and institutional grant support from Covidien, Daiichi-Sankyo, Merck, and GI Dynamics. L.A. reports receiving institutional grant support from Covidien. C.J.B. reports receiving grant support from Covidien and personal support for consultancy from EnteroMedics and Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
Covidien had no control over manuscript content or submission.
Author Contributions. S.I., J.K., W.-J.L., J.P.B., A.J.T., J.E.C., D.B.L., W.B.I., R.W.J., L.A., K.B., and C.J.B. developed the study concept and design. S.I., J.K., J.P.B., A.J.T., J.E.C., D.B.L., R.W.J., M.D.J., and C.J.B. drafted the manuscript. S.I. obtained funding. S.I., J.K., J.P.B., A.J.T., J.E.C., L.-M.C., M.D.J., A.V., and C.J.B. supervised the study. J.K., W.-J.L., J.P.B., A.J.T., W.B.I., K.C., L.-M.C., M.D.J., A.V., L.A., and C.J.B. acquired data. J.K., J.P.B., A.J.T., J.E.C., D.B.L., W.B.I., Q.W., M.D.J., A.V., L.A., and C.J.B. analyzed and interpreted data. J.K., W.-J.L., J.P.B., A.J.T., J.E.C., D.B.L., W.B.I., K.C., L.-M.C., M.D.J., A.V., L.A., and C.J.B. critically revised the manuscript for important intellectual content. J.K., W.-J.L., J.P.B., A.J.T., J.E.C., D.B.L., W.B.I., K.C., L.-M.C., M.D.J., A.V., L.A., A.E.O., H.A.B., and C.J.B. provided administrative, technical, or material support. A.J.T., J.E.C., and Q.W. performed statistical analysis. S.I., A.J.T., J.E.C., Q.W., and C.J.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00641251, clinicaltrials.gov.
References
- 1.American Diabetes Association Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes 2015;33:97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004;291:335–342 [DOI] [PubMed] [Google Scholar]
- 3.Bertoni AG, Clark JM, Feeney P, et al.; Look AHEAD Research Group . Suboptimal control of glycemia, blood pressure, and LDL cholesterol in overweight adults with diabetes: the Look AHEAD Study. J Diabetes Complications 2008;22:1–9 [DOI] [PubMed] [Google Scholar]
- 4.Wong K, Glovaci D, Malik S, et al. . Comparison of demographic factors and cardiovascular risk factor control among U.S. adults with type 2 diabetes by insulin treatment classification. J Diabetes Complications 2012;26:169–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courcoulas AP, Belle SH, Neiberg RH, et al. Three-year outcomes of bariatric surgery vs lifestyle intervention for type 2 diabetes mellitus treatment: a randomized clinical trial. JAMA Surg 2015;150:931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon JB, O’Brien PE, Playfair J, et al. . Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 2008;299:316–323 [DOI] [PubMed] [Google Scholar]
- 7.Halperin F, Halperin F, Ding S-A, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014;149:716–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikramuddin S, Billington CJ, Lee W-J, et al. Roux-en-Y gastric bypass for diabetes (the Diabetes Surgery Study): 2-year outcomes of a 5-year, randomised, controlled trial. Lancet Diabetes Endocrinol 2015;3:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang Z, Wu Q, Chen B, Yu P, Zhao H, Ouyang X. Effect of laparoscopic Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus with hypertension: a randomized controlled trial. Diabetes Res Clin Pract 2013;101:50–56 [DOI] [PubMed] [Google Scholar]
- 10.Mingrone G, Panunzi S, De Gaetano A, et al. . Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 2012;366:1577–1585 [DOI] [PubMed] [Google Scholar]
- 11.Schauer PR, Schauer PR, Bhatt DL, et al. Bariatric surgery versus intensive medical therapy for diabetes--3-year outcomes. In N Engl J Med 2014;370:2002–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–2304 [DOI] [PubMed] [Google Scholar]
- 13.Ikramuddin S, Korner J, Lee W-J, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA 2013;309:2240–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas AJ, Thomas AJ, Bainbridge HA, et al. Recruitment and screening for a randomized trial investigating Roux-en-Y gastric bypass versus intensive medical management for treatment of type 2 diabetes. Obes Surg 2014;24:1875–1880 [DOI] [PubMed] [Google Scholar]
- 15.Diabetes Prevention Program (DPP) Research Group The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care 2002;25:2165–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wadden TA, West DS, Delahanty L, et al.; Look AHEAD Research Group . The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikramuddin S, Kendrick ML, Kellogg TA, Sarr MG: Open and laparoscopic Roux-en-Y gastric bypass: our techniques. J Gastrointest Surg 2007;11:217–228 [DOI] [PubMed]
- 18.Association AD; American Diabetes Association . Standards of medical care in diabetes--2009. Diabetes Care 2009;32(Suppl. 1):S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buse JB, Caprio S, Cefalu WT, et al. . How do we define cure of diabetes? Diabetes Care 2009;32:2133–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Little RJA, Rubin DB. Statistical Analysis With Missing Data. Hoboken, NJ, Wiley, 2002 [Google Scholar]
- 21.Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 2013;23:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laferrère B. Do we really know why diabetes remits after gastric bypass surgery? Endocrine 2011;40:162–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen KT, Billington CJ, Vella A, et al. Preserved insulin secretory capacity and weight loss are the predominant predictors of glycemic control in patients with type 2 diabetes randomized to Roux-en-Y gastric bypass. Diabetes 2015;64:3104–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wentworth JM, Playfair J, Laurie C, et al. Multidisciplinary diabetes care with and without bariatric surgery in overweight people: a randomised controlled trial. Lancet Diabetes Endocrinol 2014;2:545–552 [DOI] [PubMed] [Google Scholar]
- 25.Still CD, Wood GC, Benotti P, Petrick AT. Preoperative prediction of type 2 diabetes remission after Roux-en-Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol 2014;2:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serrot FJ, Dorman RB, Miller CJ, et al. . Comparative effectiveness of bariatric surgery and nonsurgical therapy in adults with type 2 diabetes mellitus and body mass index <35 kg/m2. Surgery 2011;150:684–691 [DOI] [PubMed] [Google Scholar]