Abstract
OBJECTIVE
We tested whether an elevation in the serum proinsulin–to–C-peptide ratio (PI:C), a biomarker of β-cell endoplasmic reticulum (ER) dysfunction, was associated with progression to type 1 diabetes.
RESEARCH DESIGN AND METHODS
Fasting total PI and C levels were measured in banked serum samples obtained from TrialNet Pathway to Prevention (PTP) participants, a cohort of autoantibody-positive relatives without diabetes of individuals with type 1 diabetes. Samples were obtained ∼12 months before diabetes onset from PTP progressors in whom diabetes developed (n = 60), and were compared with age-, sex-, and BMI-matched nonprogressors who remained normoglycemic (n = 58). PI:C ratios were calculated as molar ratios and were multiplied by 100% to obtain PI levels as a percentage of C levels.
RESULTS
Although absolute PI levels did not differ between groups, PI:C ratios were significantly increased in antibody-positive subjects in whom there was progression to diabetes compared with nonprogressors (median 1.81% vs. 1.17%, P = 0.03). The difference between groups was most pronounced in subjects who were ≤10 years old, where the median progressor PI:C ratio was nearly triple that of nonprogressors; 90.0% of subjects in this age group within the upper PI:C quartile progressed to the development of diabetes. Logistic regression analysis, adjusted for age and BMI, demonstrated increased odds of progression for higher natural log PI:C ratio values (odds ratio 1.44, 95% CI 1.02, 2.05).
CONCLUSIONS
These data suggest that β-cell ER dysfunction precedes type 1 diabetes onset, especially in younger children. Elevations in the serum PI:C ratio may have utility in predicting the onset of type 1 diabetes in the presymptomatic phase.
Introduction
Type 1 diabetes is defined classically as autoimmune-mediated destruction of the insulin-producing β-cells. However, recent rodent and human studies (1,2) have identified an increasing role for pathways intrinsic to the β-cell during the development of type 1 diabetes. These data suggest that processes such as β-cell calcium dyshomeostasis, altered protein folding, and oxidative stress become activated early in the evolution of type 1 diabetes and may act to augment autoimmune-mediated β-cell death, in part, via the triggering of β-cell endoplasmic reticulum (ER) dysfunction and ER stress (1).
As a highly secretory endocrine cell, the β-cell requires a robust and functional ER to ensure that proteins, including insulin, are efficiently produced and properly folded. Under conditions that impair ER health, insulin demand may exceed the ability of the ER to process newly translated proteins, a transition referred to as ER stress (3). Whereas unchecked ER stress ultimately leads to β-cell death, noninvasive identification of this process may also provide a means to monitor disease evolution and identify individuals at risk for developing type 1 diabetes at time points prior to the onset of massive β-cell destruction (3). A hallmark of β-cell ER dysfunction is the accumulation and secretion of inadequately processed proinsulin (PI) molecules (3). Therefore, β-cell ER stress may be detectable noninvasively via measurement of the serum PI-to-serum C-peptide (PI:C) ratio, the latter of which is released in a 1:1 molar ratio with mature and fully processed insulin (4,5). We and others have previously demonstrated (5–9) elevations in the PI:C ratio around the time of clinical onset of type 1 diabetes in murine models and humans, suggesting that β-cell ER dysfunction is a feature of the autoimmune process. We hypothesized that an elevation in the PI:C ratio may also exist in high-risk subjects, even before the onset of clinically significant hyperglycemia, and serve to predict type 1 diabetes development. Elevations in random PI:C ratios were previously shown to predict subsequent type 1 diabetes in a Belgian cohort at varying times before diabetes development (10). However, we endeavored to test this relationship in a large cohort of genetically diverse individuals, using newer PI and C assays and in fasting samples to avoid variability related to varying degrees of nutrient stimulation in randomly collected samples.
To this end, banked serum samples were obtained from the TrialNet Pathway to Prevention (PTP) cohort, a longitudinal study of well-characterized autoantibody-positive individuals without diabetes who are observed for the development of dysglycemia and diabetes. Fasting serum PI:C ratios were measured in TrialNet “progressors” ∼12 months before the onset of type 1 diabetes, and were compared with age-, sex-, and BMI-matched nonprogressors, who remained normoglycemic while being observed in the study for a comparable time period. Our results confirm higher PI:C ratios in progressors a year before diabetes onset, suggesting that there is a role for altered β-cell ER function during evolving type 1 diabetes and a utility for the PI:C ratio as a biomarker of diabetes risk.
Research Design and Methods
Biobanked Samples
Type 1 Diabetes TrialNet is an ongoing clinical trial with centers located in the U.S., Canada, the U.K., Germany, Italy, Australia, and New Zealand. In the TrialNet PTP study (TN01; Clinical trial reg. no. NCT00097292, clinicaltrials.gov), first-, second-, or third-degree blood relatives without diabetes of individuals with type 1 diabetes, who are confirmed to be positive for at least one pancreatic autoantibody, are observed longitudinally for changes in antibody status, dysglycemia, and diabetes (11).
Banked fasting serum samples from 60 autoantibody-positive PTP participants without diabetes who progressed to type 1 diabetes were obtained ∼12 months prior to diabetes onset (median 12.4 months, interquartile range [IQR] 11.1, 14.0 months). Diabetes was defined according to American Diabetes Association criteria (12). To approximate prepubertal, peripubertal, and postpubertal age groups, progressors were equally chosen from three age ranges (≤10, 11–18, and >18 years of age). Fasting serum samples were also obtained from nonprogressors in the PTP who were matched for age, sex, and BMI z score, and in whom diabetes did not develop while being observed for a comparable time period. Sample size determination was based on data from the study by Snorgaard et al. (13), in which median differences in PI:C between subjects with recent-onset diabetes and control subjects of 2.5% and 0.7%, respectively, were seen (common SD 1.5 [estimated from the percentiles given]). For normally distributed data, a two-sided two-sample test with a 0.05 level of significance has 80% power to detect this difference with n = 12 per group. We conservatively enrolled 20 subjects per age and progressor/nonprogressor group to account for non-normality and differences in PI and C assays, and since our time point was 12 months prior to disease onset (14). At the time of unblinding, nonprogressors had been observed for a range of 19–109 months (median 46.5 months). Two subjects originally classified as nonprogressors when samples were received were excluded from analysis because their conditions progressed to diabetes between the time of sample distribution and unblinding.
Other metabolic variables previously linked to diabetes development were also analyzed. C and glucose values were obtained from an oral glucose tolerance test performed at the same monitoring visit as the PI analysis. Glucose sum was calculated as the sum of the post–glucose challenge time points (30–120 min). Early insulin response was calculated as the Δinsulin 0–30 min/Δglucose 0–30 min, and the oral disposition index was calculated as the product of 1/fasting insulin × (Δinsulin 0–30/Δglucose 0–30) (15,16). Index60 was calculated as follows: 0.3695(log fasting C) + 0.0165(60 min glucose) − 0.3644(60 min C) (17). Diabetes Prevention Trial–Type 1 Risk Scores (DPTRSs) were calculated as previously described (18). The HOMA model of β-cell function (HOMA%B) was calculated using the University of Oxford HOMA calculator (19). HOMA-insulin resistance (IR) was calculated as follows: fasting insulin (milliunits/L) × fasting glucose (mmol/L)/22.5 (20).
Assays
Total serum PI levels were measured in a blinded fashion using a capture ELISA (ALPCO). This assay detected PI levels in the range of 2.5–180 pmol/L, with a reported sensitivity of 1.25 pmol/L, and a cross-reactivity of <0.01% with human C and 0.1% with human insulin. For samples with a serum PI level below the assay lower limit of detection (n = 11 progressors and 12 nonprogressors), a value of one-half the lower limit of detection was assigned. Autoantibodies against GAD65 or GAD65H, microinsulin antibodies (mIAAs), islet cell antibodies (ICAs [ICA512]), or IA-2H were measured using procedures outlined by the Diabetes Antibody Standardization Program and described in detail in previous publications (11). Because TrialNet transitioned from GAD65 and ICA512 to GAD65H and IA-2H assays in 2010, subjects with positive results for either assay were considered to be GAD or ICA positive. Zinc transporter 8 antibody status was not included because results were only available for a small subset of our population (n = 11). The TrialNet central laboratory measured C and insulin levels using Tosoh immunoassays (21,22). PI:C ratios were calculated as a molar ratio multiplied by 100% to obtain PI as a percentage of C.
Statistical Analyses
Because all patient variables except natural log (Ln) PI:C ratio (LnPI:C) and Index60 were not normally distributed, medians (IQRs) were calculated and compared between progressors and nonprogressors using Wilcoxon rank sum tests. Fisher exact tests were used for categorical variables. Kruskal-Wallis tests with Dunn multiple-comparison tests were used to compare PI:C ratios for progressors and nonprogressors, and the months between blood draw and diagnosis for progressors across age groups. Spearman correlations were used to measure monotonic relationships between LnPI:C and key subject variables. Cochran-Mantel-Haenszel χ2 tests were used to assess increasing percentages of progression by PI:C ratio quartile. Logistic regression was performed to determine the effect of Lnfasting C, Lnfasting PI, and LnPI:C on the progression to diabetes adjusted for age group and BMI. For bivariate plots and logistic regression analysis, LnPI:C, PI, and fasting C were used to adjust for skewness. SAS version 9.4 (SAS Inc., Cary, NC) and GraphPad Prism version 6.00 (GraphPad Software, La Jolla, CA) were used for statistical analyses. For all analyses, a P value of ≤0.05 was considered to be significant.
Results
Baseline characteristics for 60 progressor and 58 nonprogressor subjects are shown in Table 1. No differences in age, sex, or BMI z scores existed between groups. As expected, the fasting C and peak C levels were lower in progressors compared with nonprogressors (P = 0.04 and P < 0.001, respectively), whereas fasting glucose levels and glucose sums were significantly higher (P = 0.05 and P < 0.001, respectively). Consistent with this, DPTRSs were higher in progressors compared with nonprogressors (median 7.69 vs. 6.23, P < 0.001) (Table 1).
Table 1.
Baseline characteristics of study population
| Variable | Nonprogressors | Progressors | P value |
|---|---|---|---|
| Age (years) | 12.1 (8.1, 25.0) | 11.6 (7.8, 26.2) | 0.84 |
| Male sex (%) | 48.28 | 48.33 | 1.00 |
| BMI (kg/m2) | 20.21 (16.54, 26.15) | 19.80 (16.56, 25.07) | 0.89 |
| BMI-for-age (z score) | 0.75 (0.04, 1.30) | 0.68 (−0.12, 1.40) | 0.84 |
| Multiple antibody positive (%) | 32.76 | 71.67 | <0.001 |
| Fasting PI (pmol/L) | 6.91 (1.69, 10.91) | 7.49 (4.25, 13.76) | 0.24 |
| Fasting C (pmol/L) | 532.95 (409.20, 712.80) | 427.35 (288.75, 617.10) | 0.04 |
| Fasting insulin (pmol/L) | 52.43 (34.03, 71.53) | 38.89 (23.13, 56.95) | 0.02 |
| PI:C ratio (%) | 1.17 (0.46, 1.76) | 1.81 (0.81, 3.09) | 0.03 |
| LnPI:C | −4.45 (−5.38, −4.04) | −4.01 (−4.81, −3.48) | 0.03 |
| 30-min C difference | 1,338.15 (1,034.55, 1,626.90) | 693.00 (452.10, 1,075.80) | <0.001 |
| Peak C | 2,636.70 (1,950.30, 3,260.40) | 1,775.40 (1,364.55, 2,412.30) | <0.001 |
| Total C AUC | 713.95 (577.10, 880.15) | 490.65 (374.55, 622.25) | <0.001 |
| Peak C after 30 min (%) | 96.55 | 98.33 | 0.62 |
| Peak C after 60 min (%) | 75.86 | 93.33 | 0.01 |
| Peak C after 90 min (%) | 48.28 | 83.33 | <0.001 |
| Peak C after 120 min (%) | 31.03 | 33.33 | 0.85 |
| Hemoglobin A1c, %; mmol/mol | 5.0 (4.8, 5.2); 31 (29, 33) | 5.4 (5.2, 5.6); 36 (33, 38) | <0.001 |
| Fasting blood glucose | 91 (86, 96) | 95 (89, 103.5) | 0.05 |
| Blood glucose, 30 min | 144.5 (130, 164.5) | 162 (145, 182) | <0.001 |
| Blood glucose, 60 min | 136 (114.5, 172) | 180 (155, 215) | <0.001 |
| Blood glucose, 90 min | 120 (104, 150.5) | 178 (156, 202) | <0.001 |
| Blood glucose, 120 min | 120.5 (99.5, 135.5) | 154 (128, 182) | <0.001 |
| Glucose sum | 506.5 (470, 642) | 667 (592, 772) | <0.001 |
| Early insulin response | 1.00 (0.68, 1.58) | 0.30 (0.20, 0.63) | <0.001 |
| Oral disposition index | 0.12 (0.09, 0.19) | 0.06 (0.03, 0.09) | <0.001 |
| HOMA%B | 90.8 (74.7, 114.70) | 69.45 (56.25, 84.55) | <0.001 |
| HOMA-IR | 1.72 (1.12, 2.52) | 1.38 (0.91, 2.04) | 0.08 |
| Index60 | 0.10 (−0.77, 0.55) | 1.46 (0.59, 1.99) | <0.001 |
| DPTRS | 6.23 (5.50, 6.74) | 7.69 (6.85, 8.59) | <0.001 |
Values are reported as the median (IQR), unless otherwise indicated. n = 58 for nonprogressors and n = 60 for progressors for all variables except the following: fasting insulin level (n = 50 for nonprogressors and n = 48 for progressors); 30-min C difference (n = 56 for nonprogressors and n = 59 for progressors); total C AUC (n = 54 for nonprogressors and n = 55 for progressors); glucose level during oral glucose tolerance test time points (n = 56 for nonprogressors and n = 59 for progressors); early insulin response (n = 46 for nonprogressors and n = 46 for progressors); oral disposition index (n = 46 for nonprogressors and n = 46 for progressors); HOMA%B (n = 50 for nonprogressors and n = 48 for progressors); HOMA-IR (n = 50 for nonprogressors and n = 48 for progressors); Index60 (n = 56 for nonprogressors and n = 59 for progressors); and DPTRS (n = 58 for nonprogressors and n = 59 for progressors). AUC, area under the curve. Boldface type indicates P ≤ 0.05.
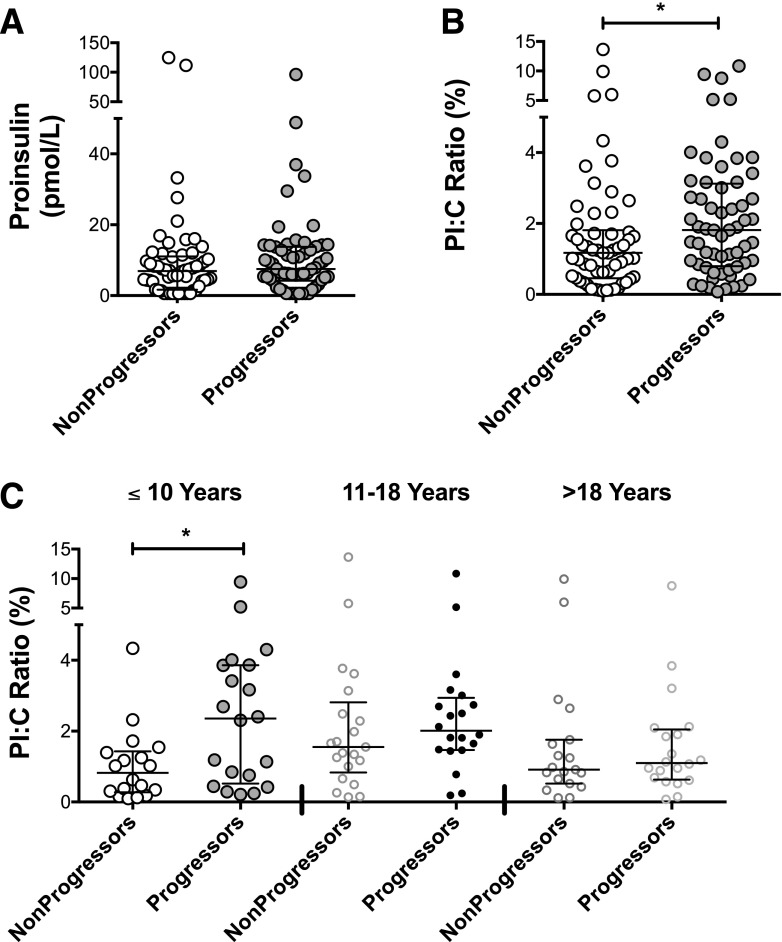
Although no significant difference in absolute PI values was observed between groups, the median (IQR) PI:C ratio was significantly increased in progressors compared with nonprogressors (1.81% [IQR 0.81%, 3.09%] vs. 1.17% [IQR 0.46%, 1.76%], P = 0.03). The distribution of PI and PI:C ratios in nonprogressors and progressors is presented graphically in Fig. 1A and B. Comparison of PI:C ratios in male and female subjects revealed no differences between sexes in either the progressor (P = 0.30) or nonprogressor groups (P = 0.41) (Supplementary Fig. 1).
Figure 1.
PI:C ratios in nonprogressors and progressors. A: Scatterplot of absolute PI values for all nonprogressor and progressor subjects. A Wilcoxon rank sum test revealed no significant differences between groups. B: Scatterplot of PI:C ratios for all nonprogressors and progressor subjects. Wilcoxon rank sum test demonstrated higher PI:C values in progressors (P = 0.03). For both PI and PI:C ratios, n = 58 for nonprogressors and n = 60 for progressors. C: PI:C ratios by age categories, as follows: ≤10 years old (n = 38), 11–18 years of age (n = 41), and >18 years of age (n = 39). Kruskal-Wallis test demonstrated significant differences between groups (P = 0.02). Dunn multiple-comparisons test revealed that only differences between nonprogressors and progressors in the group of subjects ≤10 years of age reached statistical significance (P < 0.05). *P ≤ 0.05.
Analysis of PI:C ratios by age group and progressor/nonprogressor group revealed significant differences (Kruskal-Wallis test, P = 0.02). Median PI:C ratios by age category for nonprogressors were as follow: 0.8% (IQR 0.3, 1.4) in subjects ≤10 years of age, 1.6% (IQR 1.0, 2.5) in subjects 11–18 years of age, and 0.9% (IQR 0.5, 1.8) in subjects >18 years of age. For progressors, the median PI:C ratios were as follows: 2.4% (IQR 0.6, 3.9) in subjects ≤10 years of age, 2.0% (IQR 1.5, 2.9) in subjects 11–18 years of age, and 1.1% (0.7, 2.0) in subjects >18 years of age. Interestingly, the median PI:C ratio was nearly triple in progressors compared with nonprogressors in the youngest age group of subjects ≤10 years of age (Fig. 1C). Using Dunn multiple-comparison tests, progressors were significantly different from nonprogressors only in the group of subjects ≤10 years of age. Among nonprogressors, PI:C ratios tended to be increased in the group of adolescents 11–18 years of age, but this difference did not reach statistical significance (P = 0.09). Furthermore, although no correlation with age was detected in nonprogressors, a trend toward a significant negative correlation existed between age and LnPI:C in progressors (rs = −0.22, P = 0.09) (Supplementary Fig. 1). No significant difference in the time from blood draw to diabetes diagnosis was present between age groups (Supplementary Fig. 1).
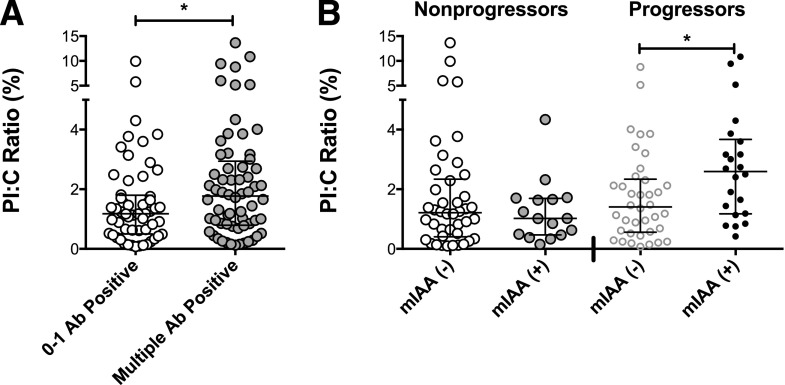
To evaluate a possible link between PI:C ratio and islet autoimmunity, the relationships of PI:C ratio with autoantibody positivity were assessed. Among the entire population, 62 subjects with multiple antibody positivities (two or more) had higher PI:C ratios (median 1.77%, IQR 0.78, 3.01) compared with 56 subjects who reverted to antibody-negative status or had one positive autoantibody at the time of analysis (median 1.18%, IQR 0.51, 1.78, P = 0.049) (Fig. 2A). This relationship was no longer significant when analyzed within progressor and nonprogressor subgroups. Analysis of PI:C ratios with respect to individual autoantibody positivity revealed that mIAA+ progressors (n = 22) had higher PI:C ratios than mIAA− progressors (n = 38) (median 2.60% [IQR 1.187, 3.60] vs. 1.41% [IQR 0.575, 2.31], P = 0.02) (Fig. 2B). No relationship between mIAA and PI:C ratio was detected in nonprogressors, and no significant relationships with other autoantibodies were detected.
Figure 2.
Relationship of PI:C ratios with islet autoimmunity. A: Comparison of PI:C ratios among subjects testing positive for 0–1 islet autoantibody (Ab) (n = 56) vs. testing positive for multiple Abs (n = 62). Wilcoxon rank sum test demonstrated increased PI:C ratios in multiple Ab-positive subjects (P = 0.049). B: PI:C ratios of subjects by mIAA status and progressor status. Wilcoxon rank sum test revealed significantly higher PI:C ratios in progressors with mIAA positivity (n = 22) than those negative for mIAA (n = 38). No difference was detected in mIAA+ and mIAA− nonprogressors (n = 16 and n = 42, respectively). *P ≤ 0.05.
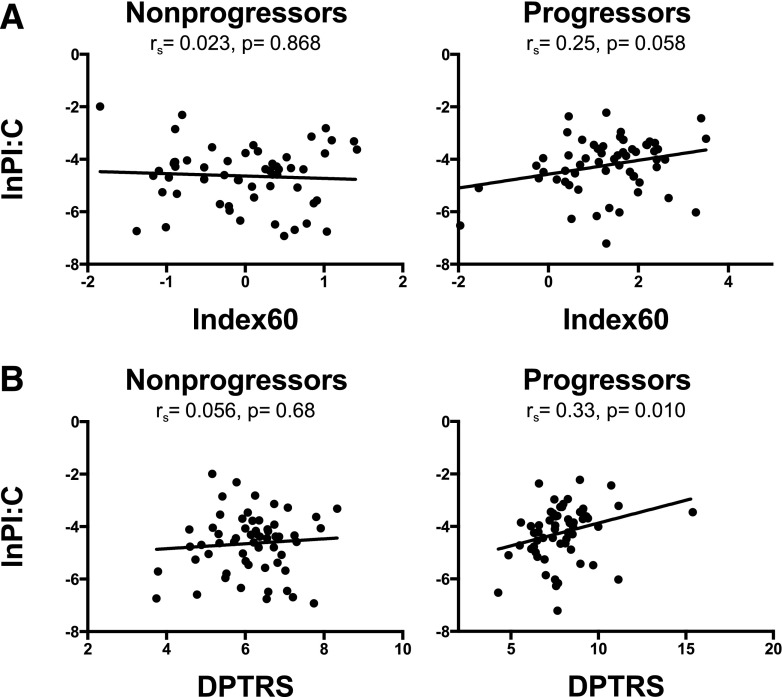
Correlations of LnPI:C with BMI and other metabolic parameters were evaluated. No significant correlation was seen with the BMI-for-age z score in either the nonprogressor or the progressor group (Supplementary Fig. 1). In progressors, a trend toward correlation between LnPI:C and Index60 findings was observed (Fig. 3A) (rs = 0.25, P = 0.06), although a significant correlation with DPTRS was present (Fig. 3B) (rs = 0.33, P = 0.01). No correlations with C peak, Lnfasting C, fasting insulin level, the 30-min C difference, C area under the curve, C or glucose level at any individual time point, hemoglobin A1c level, early insulin response, oral disposition index, HOMA%B, or HOMA-IR were present in either group.
Figure 3.
Correlations of PI:C with type 1 diabetes risk prediction scores. Spearman correlation coefficients were generated to test correlation of LnPI:C with Index60 (A) and DPTRS (B) (for Index60 n = 56 for nonprogressors and n = 59 for progressors, and for DPTRS n = 58 for nonprogressors and n = 59 for progressors).
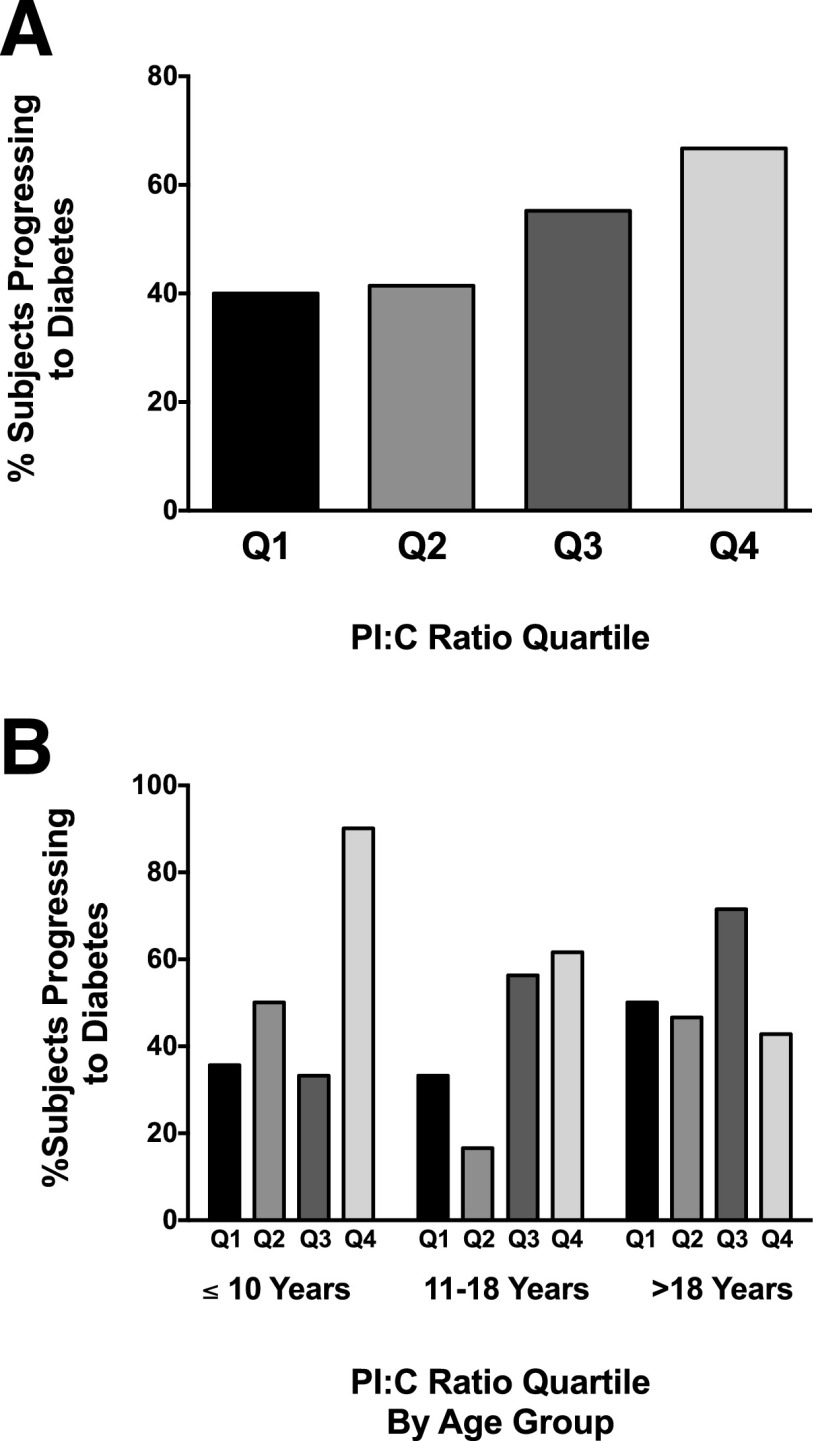
The percentage of subjects whose conditions progressed to type 1 diabetes as determined by PI:C ratio quartile was calculated (Fig. 4A) and revealed a progressively increased likelihood of progression to diabetes with increasing PI:C quartile overall (P = 0.02). Specifically, 66.7% of subjects with ratios in quartile 4 progressed to diabetes compared with 40.0% in quartile 1. Analysis by age group again demonstrated that this phenomenon was most pronounced in the group of subjects ≤10 years of age, in which 90.0% of those with PI:C ratios in quartile 4 progressed to diabetes compared with only 35.7% of those with ratios in quartile 1 (P = 0.02) (Fig. 4B). This association was not significant in the groups of subjects 11–18 years of age (P = 0.10) or >18 years of age (P = 0.91). Finally, logistic regression with adjustment for age group and BMI was performed to determine whether elevations in the PI:C ratio were associated with progression to type 1 diabetes. Indeed, increased LnPI:C significantly increased the odds of diabetes progression (odds ratio [OR] 1.44 [95% CI 1.02, 2.05], P = 0.04). Increased LnPI alone did not predict diabetes development (OR 1.19 [95% CI 0.87, 1.64], P = 0.28), but reductions in Lnfasting C levels were also significantly associated with diabetes progression (OR 0.18 [95% CI 0.05, 0.59], P = 0.005).
Figure 4.
Percentage type 1 diabetes progression by PI:C ratio quartile. A: Percentage of all subjects whose conditions progressed to diabetes for each PI:C ratio quartile. Cochran-Mantel-Haenszel χ2 test revealed a progressively increased likelihood of progression to type 1 diabetes with increasing PI:C quartile overall (P = 0.02). For Quartile (Q) 1 n = 30, for Q2 n = 29, for Q3 n = 29, for Q4 n = 30. B: Percentage of subjects in each age category progressing to diabetes for each PI:C ratio quartile. Cochran-Mantel-Haenszel χ2 test revealed a significant association in the group of subjects ≤10 years of age (P = 0.02) but not in the group of subjects 11–18 years of age (P = 0.10) or >18 years of age (P = 0.91). For subjects ≤10 years of age: Q1 n = 14, Q2 n = 8, Q3 n = 6, Q4 n = 10. For subjects 11–18 years of age: Q1 n = 6, Q2 n = 6, Q3 n = 16, Q4 n = 13. For subjects >18 years of age: Q1 n = 10, Q2 n = 15, Q3 n = 7, Q4 n = 7.
Conclusions
In this case-control analysis of data collected from the TrialNet PTP Study, we demonstrate that an elevation in the PI:C ratio, a reported marker of β-cell ER dysfunction, precedes the development of type 1 diabetes in high-risk subjects at least 12 months before diabetes onset. We also found that elevations in the PI:C ratio were greatest in children ≤10 years of age. However, even after adjustment for age and BMI, an elevated PI:C ratio was associated with diabetes development.
A growing number of preclinical studies suggest that cell intrinsic stress pathways that lead to β-cell ER stress contribute to the pathogenesis of type 1 diabetes. In this regard, elevated PI-to-insulin ratios and islet ER dysfunction have been identified in the NOD mouse model of type 1 diabetes prior to the onset of hyperglycemia (5,7). Furthermore, activation of ER stress signaling has been observed in islets from the virus-inducible BioBreeding diabetes resistant (BBDR) rat model of autoimmune diabetes as early as 4 days after virus injection. These changes preceded the development of insulitis, which occurred 11 days after viral stress induction (23). In two murine models of type 1 diabetes, treatment with a chemical chaperone to reduce ER stress in the prediabetic period subsequently reduced lymphocytic infiltration of islets and the development of diabetes (7). Importantly, similar findings have been shown in human disease, where immunostaining of islets from individuals with type 1 diabetes revealed abnormal expression of multiple ER stress markers (6,7). In combination with studies focusing on the activation of alternative β-cell stress pathways, these works collectively support the idea that intrinsic β-cell dysfunction plays an important role in type 1 diabetes pathogenesis (24,25).
In human subjects, elevations in the PI:C ratio are present in those with type 1 and type 2 diabetes (8,26). Moreover, reductions in the PI:C ratio have been associated with improved β-cell function in both disorders. In subjects with new-onset type 1 diabetes, cyclosporine increased the rates of noninsulin requiring remission and prevented an increase in the PI:C ratio observed with diabetes progression in placebo-treated control subjects (13). Interleukin-1 receptor antagonist treatment in subjects with type 2 diabetes improved insulin secretion and similarly led to reduced PI-to-insulin ratios (26). Finally, in a cohort of obese adolescents who underwent laparoscopic Roux-en-Y gastric bypass, reductions in PI:C ratios were observed concomitant with marked weight loss and improvements in the glucose disposition index (27).
A small number of previous studies have examined PI:C ratios in subjects at risk for type 1 diabetes. In Finland, an increased PI:C ratio was observed in 11 antibody-positive siblings of persons with type 1 diabetes who exhibited reduced first-phase insulin responses during an intravenous glucose tolerance test (9). Fasting PI-immunoreactive material increased during the 6 months prior to diabetes onset in seven of nine siblings of type 1 diabetes probands who were monitored longitudinally for type 1 diabetes development (28). An elevated random PI:C ratio was also present in Belgian autoantibody positive first-degree relatives in whom type 1 diabetes developed within 11–50 months (10).
Although our data are consistent with those of previous studies, our work also provides several novel contributions to the field. It is the first study to describe fasting PI:C ratios in a relatively large and well-characterized high-risk multinational cohort of subjects whose conditions progressed to type 1 diabetes. We examined the PI:C ratio 12 months prior to type 1 diabetes onset, whereas other studies have focused on baseline PI:C ratios relative to variable times until diabetes development, which likely has effects on the degree of PI:C ratio elevation. Furthermore, several important differences that likely increase assay specificity exist between current PI detection kits and ELISAs that were previously used (10). These include the following: 1) a capture antibody that specifically binds to PI; 2) a directly labeled detecting antibody; and 3) lack of requirement for serum dilution and prolonged incubations or washing steps (29). Perhaps most importantly, our study was designed to evaluate prepubertal, peripubertal, and postpubertal age groups, allowing for the observation that elevated PI:C ratios were most pronounced in children ≤10 years of age.
The pronounced increase in PI:C ratios in younger at-risk children could be due to multiple etiologies. As no significant difference in the time from serum collection until diagnosis existed among age groups (Fig. 1D), this phenomenon is unlikely to be related to proximity to diabetes development. This age group has consistently demonstrated a more fulminant form of type 1 diabetes, with faster rates of C decline postdiagnosis (30). Therefore, increased PI:C ratios could reflect more severe autoimmune-mediated inflammation. In support of this, we found that increased autoantibody positivity in the entire cohort was associated with higher PI:C values.
Alternatively, subjects with a higher PI:C ratio may also have inherited a predisposition toward increased β-cell inflammation, altered insulin processing, specific autoimmune targeting of insulin-processing enzymes, or increased ER dysfunction, each of which may result in more rapid β-cell failure in the face of immune activation. This idea of a susceptible β-cell is supported by recent data identifying variations in the Xrcc4 and Glis3 genes within the NOD mouse model. These variations alter the β-cell response to ER dysfunction, leading to apoptosis and senescence in situations of increased cellular stress, and therefore to increased susceptibility to β-cell failure in the context of autoimmune inflammation (31). Similarly, a human type 1 diabetes kindred was found to express a mutated form of the histone deacetylase SIRT1, which was thought to lead to amplified production of inducible nitric oxide synthase in the β-cell in response to cytokine signaling (32).
Because PI is metabolized by the liver, higher circulating PI levels could reflect reduced insulin clearance in progressors (33). This seems unlikely, because similar HOMA-IR values between groups point away from differences in insulin sensitivity. In fact, HOMA-IR values tended to be higher in nonprogressors. Furthermore, if hepatic metabolism was impaired in progressors, decreased clearance of insulin would also be expected (34). To the contrary, the magnitude of reduction in fasting insulin and C levels among progressors was similar (26% and 20%, respectively; shown in Table 1).
The predictive value of PI:C ratios could be driven, in part, by significant reductions in C among progressors, as absolute PI levels were not significantly different between groups. Along these lines, LnPI alone was not associated with diabetes development, whereas both Lnfasting C and LnPI:C were significant predictors. However, if our findings merely reflected a global insulin secretory defect, progressors should exhibit decreased absolute PI levels in addition to decreased C. Because PI secretion is maintained and, more importantly, relative PI secretion is increased even in the setting of decreased secretion of C and insulin, we maintain that PI:C ratios offer valuable insight into the state of β-cell health in presymptomatic type 1 diabetes progressors. Along these lines, we previously reported elevated PI:C ratios at the time of type 1 diabetes diagnosis, which persisted during the honeymoon period, despite increased C production. Here, PI:C ratios yielded additional insight into continued β-cell stress that was not evident based on the evaluation of C levels alone (8).
Although PI:C ratios were significantly associated with progression to type 1 diabetes in the entire cohort, considerable overlap existed between progressors and nonprogressors, even among the group of subjects ≤10 years of age. Although we studied a fairly robust number of progressors (n = 60), only 20 subjects were available for each age cohort, a number that may have limited our power to study correlations of PI:C ratios within different age categories and to model the predictive ability of this biomarker by age category. Future studies will use increased numbers within younger age groups to better ascertain predictive accuracy. Moreover, because our analysis was performed on a single time point, no definitive conclusions can be drawn regarding longitudinal changes in PI:C ratios over time prior to the development of diabetes. Studies following the natural history of changes in PI:C ratios in at-risk progressors as well as normal control subjects, with more subjects ≤10 years old, are indicated to better understand the timing and potential contribution of β-cell dysfunction and death during type 1 diabetes development.
Increased PI:C ratios were also seen in nonprogressor adolescent subjects. Presumably, based on the distribution of age groups, this could reflect an effect of puberty and could be related to increased IR during this time period (35). Although this increase in nonprogressor adolescents reduces the predictive value of the PI:C ratio in this age group, these data could have important implications for the underlying pathophysiology of incident diabetes around the onset of puberty and deserves further study (36). Therefore, prospective studies in normal control subjects, without any islet autoimmunity, using Tanner Staging and biochemical measures of puberty will also be important to understand the relationship between PI:C ratio and pubertal development.
In conclusion, our findings confirm the relevance of β-cell ER dysfunction as a potential contributor to the development of type 1 diabetes in humans. Regardless of the etiology of the observed increases in the PI:C ratio, monitoring this index as an indicator of β-cell stress may prove useful as a surrogate or intermediate outcome measure in treatment trials, signifying the need to escalate or change therapy. Moreover, the PI:C ratio may have additional use in dissecting the heterogeneity observed in type 1 diabetes cohorts, identifying subjects who would benefit from novel therapeutic approaches, including those that target β-cell ER stress and dysfunction in combination with immunomodulation (30). Our results also suggest that biomarkers of intrinsic β-cell stress may be useful in the evaluation of type 1 diabetes risk. Future studies will evaluate PI:C ratios, in combination with other novel biomarkers of developing diabetes, and as a component of a composite risk score of diabetes progression, similar to the DPTRS, which includes a combination of C measures and BMI (18).
Supplementary Material
Article Information
Acknowledgments. The authors thank Ms. Kathy Day of Indiana University for technical assistance.
Funding. Samples from at-risk subjects were obtained through a TrialNet ancillary study to the TN-01 Pathway to Prevention study funded by National Institutes of Health (NIH) grants U01-DK-061010, U01-DK-061034, U01-DK-061042, U01-DK-061058, U01-DK-085465, U01-DK-085453, U01-DK-085461, U01-DK-085463, U01-DK-085466, U01-DK-085499, U01-DK-085504, U01-DK-085505, U01-DK-085509, U01-DK-103180, U01-DK-103153, U01-DK-085476, and U01-DK-103266 and JDRF. The manuscript was also supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants K08-DK-103983 (to E.K.S.), UC4-DK-104166 (to R.G.M. and C.E.-M.), R01-DK-093954 (to C.E.-M.), and P30-DK-097512; a Pediatric Endocrine Society Clinical Scholar Award (to E.K.S.); U.S. Department of Veterans Affairs Merit Award I01BX001733 (to C.E.-M.); JDRF Pioneer Award and Strategic Research Agreement (to J.B., L.A.D., and C.E.-M.); JDRF grants 47-2012-744 (to L.A.D.), 2-SRA-2014-299-Q-R, SRA-2014-41, and 47-2012-744; and NIH National Center for Advancing Translational Sciences Clinical and Translational Sciences Award UL1TR000006 UO1 (to R.W.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.K.S. planned analyses, evaluated and interpreted the data, and wrote the manuscript. Z.C., J.B., and R.G.M. interpreted the data and edited the manuscript. R.W. assisted with acquisition of the samples and edited the manuscript. F.S. and F.O. evaluated the data and edited the manuscript. S.M.P. evaluated and interpreted the data and edited the manuscript. J.S. planned the analyses, interpreted the data, and edited the manuscript. L.A.D. planned the analyses, assisted with acquisition of the samples, interpreted the data, and edited the manuscript. C.E.-M. planned the analyses, interpreted the data, and wrote the manuscript. C.E.-M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014; Pediatric Academic Societies Meeting, Baltimore, MD, 30 April–3 May 2016; and the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-2849/-/DC1.
References
- 1.Atkinson MA, Bluestone JA, Eisenbarth GS, et al. How does type 1 diabetes develop?: the notion of homicide or β-cell suicide revisited. Diabetes 2011;60:1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soleimanpour SA, Stoffers DA. The pancreatic β cell and type 1 diabetes: innocent bystander or active participant? Trends Endocrinol Metab 2013;24:324–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eizirik DL, Miani M, Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia 2013;56:234–241 [DOI] [PubMed] [Google Scholar]
- 4.Steiner DF. On the discovery of precursor processing. Methods Mol Biol 2011;768:3–11 [DOI] [PubMed] [Google Scholar]
- 5.Tersey SA, Nishiki Y, Templin AT, et al. Islet β-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes 2012;61:818–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marhfour I, Lopez XM, Lefkaditis D, et al. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia 2012;55:2417–2420 [DOI] [PubMed] [Google Scholar]
- 7.Engin F, Yermalovich A, Nguyen T, et al. Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes. Sci Transl Med 2013;5:211ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watkins RA, Evans-Molina C, Terrell JK, et al. Proinsulin and heat shock protein 90 as biomarkers of beta-cell stress in the early period after onset of type 1 diabetes. Transl Res 2016;168:96–106.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Røder ME, Knip M, Hartling SG, Karjalainen J, Akerblom HK, Binder C; The Childhood Diabetes in Finland Study Group . Disproportionately elevated proinsulin levels precede the onset of insulin-dependent diabetes mellitus in siblings with low first phase insulin responses. J Clin Endocrinol Metab 1994;79:1570–1575 [DOI] [PubMed] [Google Scholar]
- 10.Truyen I, De Pauw P, Jørgensen PN, et al.; Belgian Diabetes Registry . Proinsulin levels and the proinsulin:c-peptide ratio complement autoantibody measurement for predicting type 1 diabetes. Diabetologia 2005;48:2322–2329 [DOI] [PubMed] [Google Scholar]
- 11.Mahon JL, Sosenko JM, Rafkin-Mervis L, et al.; TrialNet Natural History Committee; Type 1 Diabetes TrialNet Study Group . The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes 2009;10:97–104 [DOI] [PubMed] [Google Scholar]
- 12.American Diabetes Association (2) Classification and diagnosis of diabetes. Diabetes Care 2015;38(Suppl.):S8–S16 [DOI] [PubMed] [Google Scholar]
- 13.Snorgaard O, Hartling SG, Binder C. Proinsulin and C-peptide at onset and during 12 months cyclosporin treatment of type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1990;33:36–42 [DOI] [PubMed] [Google Scholar]
- 14.Lehmann EL. Nonparametrics: Statistical Methods Based on Ranks. New York, Springer, 1998 [Google Scholar]
- 15.Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med 1994;11:286–292 [DOI] [PubMed] [Google Scholar]
- 17.Sosenko JM, Skyler JS, DiMeglio LA, et al.; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial-Type 1 Study Group . A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 2015;38:271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sosenko JM, Skyler JS, Mahon J, et al.; Type 1 Diabetes TrialNet and Diabetes Prevention Trial-Type 1 Study Groups . Use of the Diabetes Prevention Trial-Type 1 Risk Score (DPTRS) for improving the accuracy of the risk classification of type 1 diabetes. Diabetes Care 2014;37:979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21:2191–2192 [DOI] [PubMed] [Google Scholar]
- 20.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 21.Little RR, Rohlfing CL, Tennill AL, et al. Standardization of C-peptide measurements. Clin Chem 2008;54:1023–1026 [DOI] [PubMed] [Google Scholar]
- 22.Manley SE, Stratton IM, Clark PM, Luzio SD. Comparison of 11 human insulin assays: implications for clinical investigation and research. Clin Chem 2007;53:922–932 [DOI] [PubMed] [Google Scholar]
- 23.Yang C, Diiorio P, Jurczyk A, O’Sullivan-Murphy B, Urano F, Bortell R. Pathological endoplasmic reticulum stress mediated by the IRE1 pathway contributes to pre-insulitic beta cell apoptosis in a virus-induced rat model of type 1 diabetes. Diabetologia 2013;56:2638–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beales PE, Pozzilli P. Thiazolidinediones for the prevention of diabetes in the non-obese diabetic (NOD) mouse: implications for human type 1 diabetes. Diabetes Metab Res Rev 2002;18:114–117 [DOI] [PubMed] [Google Scholar]
- 25.Karunakaran U, Park SJ, Jun do Y, et al. Non-receptor tyrosine kinase inhibitors enhances beta-cell survival by suppressing the PKCdelta signal transduction pathway in streptozotocin-induced beta-cell apoptosis. Cell Signal 2015;27:1066–1074 [DOI] [PubMed] [Google Scholar]
- 26.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med 2007;356:1517–1526 [DOI] [PubMed] [Google Scholar]
- 27.Inge TH, Prigeon RL, Elder DA, et al. Insulin sensitivity and beta-cell function improve after gastric bypass in severely obese adolescents. J Pediatr 2015;167:1042–1048.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartling SG, Knip M, Røder ME, Dinesen B, Akerblom HK, Binder C; Study Group on Childhood Diabetes in Finland . Longitudinal study of fasting proinsulin in 148 siblings of patients with insulin-dependent diabetes mellitus. Eur J Endocrinol 1997;137:490–494 [DOI] [PubMed] [Google Scholar]
- 29.Kjems LL, Røder ME, Dinesen B, Hartling SG, Jørgensen PN, Binder C. Highly sensitive enzyme immunoassay of proinsulin immunoreactivity with use of two monoclonal antibodies. Clin Chem 1993;39:2146–2150 [PubMed] [Google Scholar]
- 30.Ludvigsson J, Carlsson A, Deli A, et al. Decline of C-peptide during the first year after diagnosis of Type 1 diabetes in children and adolescents. Diabetes Res Clin Pract 2013;100:203–209 [DOI] [PubMed] [Google Scholar]
- 31.Dooley J, Tian L, Schonefeldt S, et al. Genetic predisposition for beta cell fragility underlies type 1 and type 2 diabetes. Nat Genet 2016;48:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biason-Lauber A, Böni-Schnetzler M, Hubbard BP, et al. Identification of a SIRT1 mutation in a family with type 1 diabetes. Cell Metab 2013;17:448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubenstein AH, Pottenger LA, Mako M, Getz GS, Steiner DF. The metabolism of proinsulin and insulin by the liver. J Clin Invest 1972;51:912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vauhkonen IK, Niskanen LK, Mykkänen L, Haffner SM, Uusitupa MI, Laakso M. Hyperproinsulinemia is not a characteristic feature in the offspring of patients with different phenotypes of type II diabetes. Eur J Endocrinol 2000;143:251–260 [DOI] [PubMed] [Google Scholar]
- 35.Hannon TS, Janosky J, Arslanian SA. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr Res 2006;60:759–763 [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association Type 2 diabetes in children and adolescents. Pediatrics 2000;105:671–680 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.