Abstract
OBJECTIVE
β-Cell autoantibodies are a feature of the preclinical phase of type 1 diabetes. Here, we asked how frequently they revert in a cohort of children at risk for type 1 diabetes and whether reversion has any effect on type 1 diabetes risk.
RESEARCH DESIGN AND METHODS
Children were up to 10 years of age and screened more than once for insulin autoantibody, GAD antibody, and insulinoma antigen-2 antibodies. Persistent autoantibody was defined as an autoantibody present on two or more consecutive visits and confirmed in two reference laboratories. Reversion was defined as two or more consecutive negative visits after persistence. Time-dependent Cox regression was used to examine how reversion modified the risk of development of multiple autoantibodies and type 1 diabetes.
RESULTS
Reversion was relatively frequent for autoantibodies to GAD65 (19%) and insulin (29%), but was largely restricted to children who had single autoantibodies (24%) and rare in children who had developed multiple autoantibodies (<1%). Most (85%) reversion of single autoantibodies occurred within 2 years of seroconversion. Reversion was associated with HLA genotype, age, and decreasing titer. Children who reverted from single autoantibodies to autoantibody negative had, from birth, a risk for type 1 diabetes of 0.14 per 100 person-years; children who never developed autoantibodies, 0.06 per 100 person-years; and, children who remained single-autoantibody positive, 1.8 per 100 person-years.
CONCLUSIONS
Type 1 diabetes risk remained high in children who had developed multiple β-cell autoantibodies even when individual autoantibodies reverted. We suggest that monitoring children with single autoantibodies for at least 1 year after seroconversion is beneficial for stratification of type 1 diabetes risk.
Introduction
β-Cell autoantibodies are significant predictors of type 1 diabetes risk (1,2). The presence of two or more autoantibodies (insulin autoantibody [IAA], GAD antibody [GADA], insulinoma antigen-2 [IA-2A], and zinc transporter type 8 autoantibodies) and the associated titer have been shown to confer the highest risk of type 1 diabetes (3,4). β-Cell autoantibodies develop before type 1 diabetes, are still detectable on onset of clinical diabetes, and usually persist over time in the progression to type 1 diabetes. However, β-cell autoantibody titers can fluctuate, and some autoantibody-positive individuals can revert to autoantibody negative (5,6).
Persistent β-cell autoantibodies (i.e., positive at consecutive visits) are associated with high-risk type 1 diabetes HLA genotypes, and transient autoantibody expression with lower genetic risk (4,7). Previous findings in the Finnish Type 1 Diabetes Prediction and Prevention (DIPP) and Diabetes Autoimmunity Study in the Young (DAISY) studies showed that ∼50% of autoantibody-positive subjects at a single visit revert to negative within 2 years (5,7). Although little is known about the effects of the variable presence of β-cell autoantibodies on the risk of type 1 diabetes, transience may be true remission of autoimmunity, humoral markers only, or just assay variability. Assessing autoantibody expression may clarify the natural progression of the disease and assist in the identification of factors associated with different progression rates. Such knowledge will assist in risk profiling as well as in reducing the cost burden of repeat testing of autoimmunity.
The aim of this study was to determine whether the persistence of β-cell autoantibodies over time could stratify the risk for type 1 diabetes and further clarify the natural progression of the disease and its association with different progression rates in The Environmental Determinants of Diabetes in the Young (TEDDY) study of genetically high-risk children.
Research Design and Methods
Study Population
TEDDY is a prospective cohort study of children at genetic high risk for type 1 diabetes, funded by the National Institutes of Health, which seeks to identify environmental causes of type 1 diabetes. There are six clinical research centers—three in the U.S.: Colorado, Georgia/Florida, Washington, and three in Europe: Finland, Germany, and Sweden. The high-risk genotypes for subjects screened from the general population with no family history of type 1 diabetes (89%) were as follows: DRB1*04-DQA1*03-DQB1*03:02/DRB1*03-DQA1*05-DQB1*02:01 (DR3/4-Q2/8), DRB1*04-DQA1*03-DQB1*03:02/DRB1*04-DQA1*03-DQB1*03:02 (DR4/4-DQ8/8), DRB1*04-DQA1*03-DQB1*03:02/DRB1*08-DQA1*04-DQB1*04:02 (DR4/8-DQ8/4), and DRB1*03-DQA1*05-DQB1*02:01/DRB1*03-DQA1*05-DQB1*02:01 (DR3/3-DQ2/2) and six additional genotypes in first-degree relatives of those with a family history of type 1 diabetes, as previously described (8).
Children enrolled are monitored prospectively from 3 months to 15 years with study visits every 3 months until 4 years and every 3 or 6 months thereafter, depending on autoantibody positivity. All children who are persistently positive for any autoantibody are monitored every 3 months until the age of 15 years or onset of type 1 diabetes. If remission of all autoantibodies occurs at any time during follow-up for a period of four consecutive visits or 1 year, a follow-up interval of 6 months becomes effective. Detailed study design and methods have been previously published (9,10). The protocol was approved by institutional review boards at participating centers, and all participants provided written informed consent before participation in the genetic screening and enrollment.
β-Cell Autoantibodies
β-Cell autoantibodies IAA, GADA, or IA-2A were measured in two laboratories by radiobinding assays (9,10). In the U.S., all sera were assayed at the Barbara Davis Center for Childhood Diabetes at the University of Colorado Denver; in Europe, all sera were assayed at the University of Bristol, U.K. Both laboratories reported high sensitivity, specificity, and concordance (11). All positive β-cell autoantibodies and 5% of negative samples were retested in the other reference laboratory and deemed confirmed if concordant. Autoantibody determination of maternal acquired or de novo appearance was based on the presence of both maternal and child autoantibodies over the first 18 months of age. De novo production was designated if the mother was negative for autoantibodies and the child was positive. If the mother was positive, then the child was deemed negative for autoantibodies unless the child had a negative sample before the first positive sample or the autoantibody persisted beyond 18 months of age. Persistent β-cell autoimmunity was defined as autoantibody presence on two or more consecutive visits 3 months apart and confirmed in two TEDDY laboratories. Reversion was defined as two or more consecutive negative visits after persistence. Age of seroconversion was the age of the child on the initial date of seroconversion to persistent β-cell autoimmunity. The specific autoantibody result on the initial date was defined as the baseline measure. Type 1 diabetes was defined according to American Diabetes Association criteria for diagnosis (12). HLA-eligible subjects were <10 years of age and screened a minimum of four times during follow-up for IAA, GADA, and IA-2A. Children who were ineligible for this analysis included those who did not have an autoantibody test result (n = 55) and were HLA ineligible (n = 118). Children who developed type 1 diabetes within 3 months of the last autoantibody negative (n = 6) or first positive test result (n = 19) were defined as not developing persistent autoantibodies before type 1 diabetes. Other children who developed type 1 diabetes more than 3 months after the last autoantibody test (n = 14, min–max time after last test 0.8–8.3 years) were censored at the time of the last test because it was not possible to determine persistent autoantibodies before diagnosis. Autoantibody-positive children who were lost to follow-up immediately after the persistent sample were censored at the time of the initial seroconversion because they were never observed at risk for reversion (n = 14).
Autoantibody titers were converted to z-scores due to different autoantibody cutoff values in the two reference laboratories (Bristol and Denver) and change in type of assay over time from the original TEDDY standard assay to the harmonized assay. Autoantibody titers were converted to SD units centered on the assay threshold for analysis. The harmonized assay was used for IA-2A and GADA; if a measure was missing from the harmonized assay, the TEDDY assay transformed to the harmonized assay was used. The Bristol z-score was used for IAA (not harmonized), and, if missing, the Denver z-score was used. Change in z-score over time had a symmetrical distribution and was untransformed. The z-score will be denoted as titer.
Statistical Analysis
Data were analyzed using SAS 9.4 software (SAS Institute Inc., Cary, NC), and GraphPad PRISM 5.03 software (GraphPad Software Inc., La Jolla, CA) was used for figures. The Mann-Whitney U test was used to assess differences in time to reversion among children who had reverted. Time-dependent Cox regression examined how reversion modified risk of both development of multiple autoantibodies and type 1 diabetes in two nonmutually exclusive cohorts defined by number of autoantibodies at the time of seroconversion (single autoantibody or multiple autoantibodies). Kaplan-Meier curves were used to descriptively show the cumulative incidence of multiple autoantibodies and reversion separately as well as the cumulative risk of type 1 diabetes for children who did and did not revert (hazard ratios and P values from time-dependent Cox proportional hazards models were used to evaluate statistical relevance). Multivariable analyses were adjusted for HLA-DR-DQ, sex, age of first autoantibody seroconversion, family history of type 1 diabetes, country of residence, and, when appropriate, presence of other autoantibodies, baseline autoantibody titer at the time of seroconversion, and change in autoantibody titers from the initial sample to consecutive samples. The incidence of type 1 diabetes was described as a rate per 100 person-years from birth. Exact 95% CI in incidence rates were calculated using the χ2 relationship to the Poisson distribution. All analyses were preplanned. P values of <0.05 were considered significant and two-sided.
Results
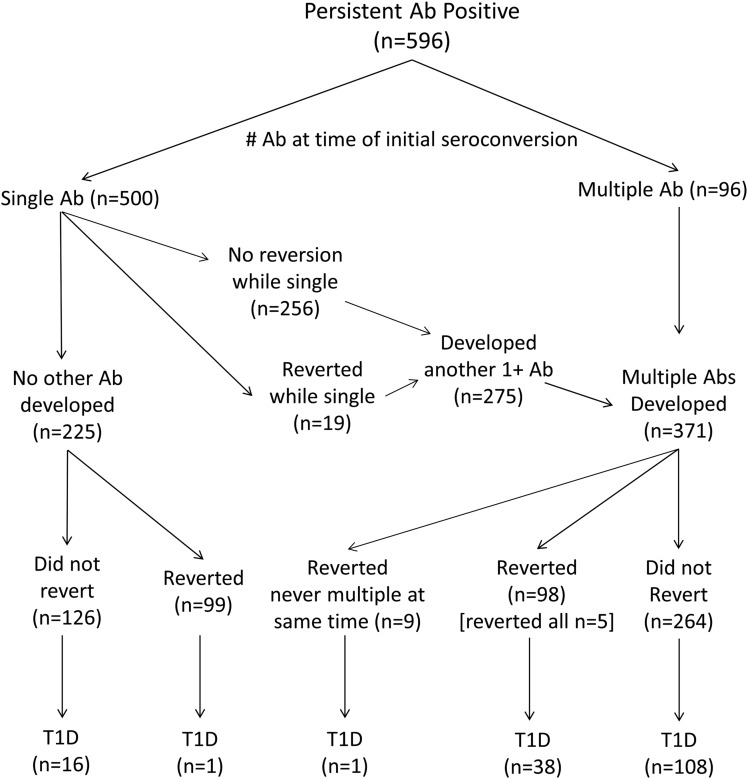
Overall, 596 of 8,503 (7%) of the eligible enrolled HLA high-risk children in TEDDY developed one or more persistent autoantibodies (remained single, n = 225; developed multiple, n = 371) and were monitored until 31 March 2015 for the development of type 1 diabetes (Fig. 1). Median (interquartile range) age at initial seroconversion was 27.7 (15.2–48.3) months. Of these 596 children, 164 (28%) developed type 1 diabetes, and by the time of diagnosis, 146 of 164 (89%) were positive for two or more autoantibodies at least once during follow-up. The current analysis examined both the single autoantibody– and multiple autoantibody–positive children for changes in autoantibody expression and the effect of those changes on the development of type 1 diabetes.
Figure 1.
Flowchart of β-cell autoantibody (Ab) persistent positivity (n = 596), number of autoantibodies at time of initial seroconversion (single autoantibody, n = 500; multiple autoantibodies, n = 96), number that remained positive or became negative, and how many developed type 1 diabetes (T1D).
Factors Associated With Persistence/Reversion
Descriptive statistics for those autoantibody-positive children who had single or multiple persistent autoantibodies are reported in Supplementary Table 1. In addition, these data specify the distribution of known risk factors and their influence on the risk of autoimmune reversion. At the time of the initial seroconversion, 500 children presented with only one persistent autoantibody (242 GADA, 245 IAA, 13 IA-2A), 84 presented with two autoantibodies, and 12 presented with three autoantibodies. Among the children with a single autoantibody, 24% reverted: 19% (45 of 242) for GADA (median time: 8.8 months), 29% (72 of 245) for IAA (median time: 10.1 months), and 8% (1 of 13) for IA-2A (median time: 6.1 months). Reversion primarily occurred within 1 year of the initial seroconversion (69% of GADA reversions, 64% of IAA reversions, 100% of IA-2A reversions). Of the children who reverted for their single autoantibody, 19% seroconverted back to positive: 18% (8 of 45) for GADA, 19% (14 of 72) for IAA, and 0% (0 of 1) for IA-2A. Children who were initially multiple autoantibody positive (n = 96) or who developed persistent multiple autoantibodies after initial seroconversion to a single autoantibody (n = 275) were less likely to revert to negative; 1.2% when two autoantibodies were present, and 0.5% when three autoantibodies were present.
Risk of reversion of a single GADA or IAA was associated with the change from baseline titer of the autoantibody to next consecutive sample titer (Supplementary Table 1). An increase from the baseline autoantibody titer to the defining persistent sample (consecutive) titer decreased the risk of reversion (GADA: HR 0.18, 95% CI 0.09–0.35, P < 0.001; and, IAA: HR 0.32, 95% CI 0.19–0.56, P < 0.001), such that an increase compared with a decrease in titer reduced the risk of GADA reversion by 82% and IAA by 68%. IAA was more likely to revert in children with HLA-DR3/3-DQ2/2 compared with HLA-DR3/4-DQ2/8 (HR 2.67, 95% CI 1.14–6.24, P = 0.03) and less likely to revert when baseline levels were very high (≥3 SD above cutoff). Age at seroconversion was strongly associated with GADA reversion; each year older in early life reduced the risk of reversion by 4% (HR 0.96, 95% CI 0.94–0.97, P < 0.001). Sex, family history of type 1 diabetes, and country did not predict GADA, IAA, or IA-2A reversion.
Risk of Developing Other Autoantibodies and Persistence/Reversion
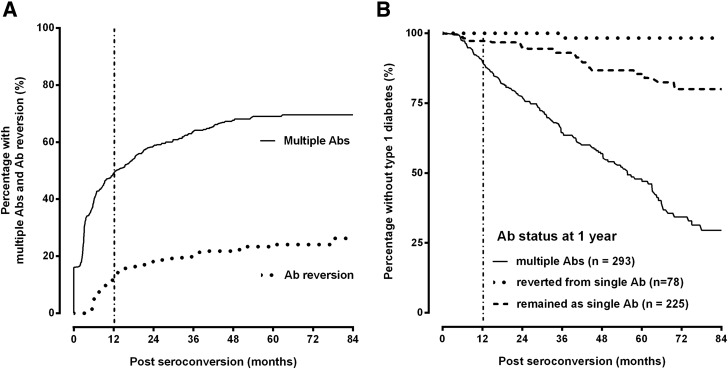
Reversion of autoantibodies affected the risk of developing other autoantibodies (Table 1). After adjusting for known risk factors associated with type 1 diabetes autoimmunity, GADA reversion, when presenting as a single autoantibody, reduced the risk of developing multiple autoantibodies by 90% (HR 0.10, 95% CI 0.03–0.29, P < 0.0001) compared with children with a single persistent autoantibody; IAA reversion reduced the risk of developing multiple autoantibodies by 76% (HR 0.24, 95% CI 0.13–0.44, P < 0.0001). Reversion of IA-2A was rare (3% [9 of 290]). Development of another autoantibody essentially precluded reversion. The cumulative percentage of children who developed multiple autoantibodies was 50% within 1 year (median survival time, 12.7 months) (Fig. 2A). The cumulative percentage for the children who reverted was 14% during the same follow-up time (1 year).
Table 1.
Reversion on the risk of multiple autoantibodies and/or type 1 diabetes based on the type and order of islet autoantibodies initially developed
| Risk | Ab(s) developed |
Follow-up pattern of specific Ab |
Outcome developed |
Reversion on outcome |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Ab number through follow-up & order developed relative to specific Ab | Total (N) | Reversion or persistence of specific Ab group | N | Percent of total (%) | N | Percent of group (%) | Months to outcome (median) | Incidence per 100 person-years | Adjusted for age of seroconversion HR (95% CI) | Fully adjusted* HR (95% CI) | P value |
| Multiple Abs | Single, IAA first | 245 | Persistent IAA | 173 | 70.6 | 139 | 80.4 | 5.1 | 92.9 | ref | ref | |
| Reverted IAA | 72 | 29.4 | 16 | 22.2 | 23.1 | 5.2 | 0.25 (0.14–0.44) | 0.24 (0.14–0.44) | <0.0001 | |||
| Single, GADA first | 242 | Persistent GADA | 197 | 81.4 | 111 | 56.4 | 8.3 | 33.8 | ref | ref | ||
| Reverted GADA | 45 | 18.6 | 3 | 6.7 | 33.6 | 1.6 | 0.07 (0.02–0.21) | 0.10 (0.03–0.29) | 0.0001 | |||
| Type 1 diabetes | Single, IAA first | 90 | Persistent IAA | 34 | 37.8 | 10 | 29.4 | 11.5 | 13.6 | ref | ref | |
| Reverted IAA | 56 | 62.2 | 1 | 1.8 | 45.0 | 0.4 | 0.05 (0.01–0.44) | 0.02 (0.00–0.42) | 0.02 | |||
| Multiple, IAA first | 139 | Remained persistent IAA | 114 | 82.0 | 56 | 49.1 | 19.3 | 17.7 | ref | ref | ||
| Reverted IAA after multiple | 25 | 18.0 | 9 | 36.0 | 33.4 | 7.6 | 0.72 (0.34–1.51) | 1.18 (0.54–2.57) | 0.68 | |||
| Multiple, IAA after other Abs | 186 | Persistent IAA | 141 | 75.8 | 61 | 43.3 | 17.4 | 19.2 | ref | ref | ||
| Reverted IAA | 45 | 24.2 | 15 | 33.3 | 48.3 | 8.3 | 0.78 (0.42–1.44) | 0.95 (0.48–1.88) | 0.88 | |||
| Single, GADA first | 128 | Persistent GADA | 86 | 67.2 | 4 | 4.7 | 37.8 | 1.8 | ref | ref | ||
| Reverted GADA | 42 | 32.8 | 0 | 0.0 | N/A | 0.0 | 0.00 | 0.00 | ||||
| Multiple, GADA first | 111 | Remained persistent GADA | 105 | 94.6 | 28 | 26.7 | 24.8 | 10.0 | ref | ref | ||
| Reverted GADA after multiple | 6 | 5.4 | 4 | 66.7 | 25.5 | 21.0 | 2.21 (0.76–6.36) | 2.34 (0.55–10.4) | 0.25 | |||
| Multiple, GADA after other Abs | 214 | Persistent GADA | 192 | 89.7 | 73 | 38.0 | 18.6 | 12.6 | ref | ref | ||
| Reverted GADA | 22 | 10.3 | 17 | 77.3 | 31.3 | 23.0 | 2.70 (1.57–4.63) | 3.77 (2.04–6.96) | <0.0001 | |||
| Single or multiple IA-2A anytime | 290 | Persistent IA-2A | 281 | 96.9 | 126 | 44.8 | 20.9 | 18.3 | ref | ref | ||
| Reverted IA-2A | 9 | 3.1 | 2 | 22.2 | 48.8 | 4.8 | 0.26 (0.06–1.05) | 0.35 (0.08–1.46) | 0.15 | |||
Ab, autoantibody; N/A, not applicable given the numerator and denominator are 0.
*Adjusted for HLA-DR-DQ, sex, age of autoantibody seroconversion, family history of type 1 diabetes, country of residence, presence of other autoantibodies, baseline autoantibody level at time of seroconversion, and change in autoantibody levels from the initial to the consecutive sample.
Figure 2.
A: Cumulative incidence of development of multiple autoantibodies (Abs) after initial seroconversion and cumulative incidence of autoantibody reversion. B: Risk of progression to type 1 diabetes by autoantibody persistence (single and multiple) and reversion.
Risk of Type 1 Diabetes and Persistence/Reversion
Reversion of a specific autoantibody on risk of type 1 diabetes after development of multiple autoantibodies was also examined. The seroconversion and reversion of GADA or IAA in the presence of multiple autoantibodies appeared to modify the risk of type 1 diabetes compared with those with persistent autoantibodies (Table 1) after adjustment for known type 1 diabetes risk factors. Among children who seroconverted for GADA at the time of or after the appearance of another autoantibody, GADA reversion (10% [22 of 214]) was associated with a greater risk of progression to type 1 diabetes (adjusted HR 3.77, 95% CI 2.04–6.96, P < 0.0001). In contrast, IAA reversion (21% [70 of 341]) when another autoantibody was present had little effect on risk of type 1 diabetes accounting for known risk factors (IAA appeared in the presence [adjusted HR 0.95, 95% CI 0.48–1.88, P = 0.88] or before the appearance of other autoantibodies [adjusted HR 1.18, 95% CI 0.54–2.57, P = 0.68]).
The incidence (95% CI) of type 1 diabetes from birth up to 10 years was calculated by the child’s autoantibody seroconversion and reversion pattern during follow-up. Among children who seroconverted for a single autoantibody without reverting, the incidence (95% CI) of type 1 diabetes was 1.83 (1.05–2.97) per 100 person-years (Table 2). Those who remained single IAA without reverting had a 5.48 (2.63–10.10) per 100 person-years risk of type 1 diabetes; whereas, those with a single nonreverting GADA had a risk of 0.62 (0.17–1.59) per 100 person-years. The children who seroconverted and reverted for a sole autoantibody (0.14 [0.00–0.79] per 100 person-years, n = 1 of 99) had a relatively similar risk of type 1 diabetes as the HLA high-risk children who had not seroconverted (0.06 [0.04–0.09] per 100 person-years, n = 25 of 7,907). The incidence (95% CI) of type 1 diabetes for those children who developed multiple autoantibodies over time and never reverted for any autoantibody was 7.19 (5.90–8.68) per 100 person-years. Among children who developed two or more autoantibodies over time, the incidence of type 1 diabetes changed depending on the combination of autoantibodies and the specific autoantibody that reverted. If GADA reverted in the presence of IAA, the incidence (95% CI) of type 1 diabetes remained high (GADA reversion: 13.7 [1.66–49.48] per 100 person-years); whereas, if IAA reverted in the presence of GADA, the incidence was similar to that of a single persistent autoantibody (0.88 [0.11–3.19] per 100 person-years).
Table 2.
Number of autoantibodies the child developed and reverted during follow-up from birth to ≤10 years
| Reversion pattern |
Developed type 1 diabetes |
||||||
|---|---|---|---|---|---|---|---|
| Maximum number of persistent Abs developed during follow-up | Total N | Ab reversion pattern during follow-up | N (% of total) | Fluctuated* after reversion N (% of total) | Person-time follow-up for type 1 diabetes (years) | N | Incidence (95% CI) per 100 person-years |
| None | 7,907 | 40,001.6 | 25 | 0.06 (0.04–0.09) | |||
| Single (1 Ab) | 225 | Remained persistent | 126 (56.0) | 874.7 | 16 | 1.83 (1.05–2.97) | |
| IAA | 34 (15.1) | 182.5 | 10 | 5.48 (2.63–10.1) | |||
| GADA | 86 (38.2) | 645.8 | 4 | 0.62 (0.17–1.59) | |||
| Reverted | 99 (44.0) | 14 (14.1) | 701.3 | 1 | 0.14 (0.00–0.79) | ||
| Reverted IAA | 56 (24.9) | 7 (12.5) | 405.9 | 1 | 0.25 (0.01–1.37) | ||
| Reverted GADA | 42 (18.7) | 7 (16.7) | 289.3 | 0 | 0 | ||
| Multiple (2 Abs) | 161 | Remained persistent 2 Abs | 113 (70.2) | 663.0 | 39 | 5.88 (4.18–8.04) | |
| IAA & GADA | 53 (32.9) | 303.2 | 15 | 4.95 (2.77–8.16) | |||
| IAA & IA-2A | 32 (19.9) | 147.6 | 20 | 13.55 (8.28–20.93) | |||
| GADA & IA-2A | 28 (17.4) | 212.1 | 4 | 1.89 (0.51–4.83) | |||
| Reverted 1 Ab | 44 (27.3) | 21 (47.7) | 322.6 | 9 | 2.79 (1.28–5.30) | ||
| Reverted IAA, persistent GADA | 29 (18.0) | 14 (48.3) | 226.8 | 2 | 0.88 (0.11–3.19) | ||
| Reverted IAA, persistent IA-2A | 10 (6.2) | 5 (50.0) | 63.6 | 4 | 6.29 (1.71–16.10) | ||
| Reverted GADA, persistent IAA | 3 (1.9) | 1 (33.3) | 14.6 | 2 | 13.70 (1.66–49.48) | ||
| Reverted GADA, persistent IA-2A | 1 (1.2) | 0 (0) | 7.5 | 0 | 0 | ||
| Reverted 2 Abs | 4 (2.5) | 3 (75.0) | 22.9 | 2 | 8.73 (1.06–31.55) | ||
| Reverted GADA & IAA | 3 (1.9) | 2 (66.7) | 16.1 | 2 | 12.42 (1.50–44.87) | ||
| Multiple (3 Abs) | 210 | Remained persistent 3 Abs | 151 (71.9) | 839.2 | 69 | 8.22 (6.4–10.41) | |
| Reverted 1 Ab | 46 (21.9) | 19 (41.3) | 315.9 | 21 | 6.65 (4.12–10.16) | ||
| Reverted IAA | 30 (14.3) | 15 (50.0) | 218.9 | 10 | 4.57 (2.19–8.40) | ||
| Reverted GADA | 15 (7.1) | 3 (20.0) | 91.1 | 10 | 10.98 (5.26–10.19) | ||
| Reverted 2 Abs | 12 (5.7) | 9 (75.5) | 78.2 | 6 | 7.67 (2.82–16.70) | ||
| Reverted GADA & IAA | 8 (<0.1) | 5 (62.5) | 47 | 6 | 12.77 (4.68–27.79) | ||
| Reverted 3 Abs | 1 (0.5) | 1 (100) | 5.84 | 1 | 17.12 (0.43–95.4) | ||
Expression patterns involving the reversion of IA-2A are not shown specifically but are included in the overall reversion group.
Ab, autoantibody.
*If reverted for greater than 1 Ab, fluctuate refers to at least 1 Ab repeat seroconverts.
The cumulative risk of developing type 1 diabetes by three groups that depict the change in autoantibody status up to 1 year after initial seroconversion is illustrated in Fig. 2B. Group 1 were the children who developed multiple autoantibodies (n = 293), group 2 were the children who reverted from a single autoantibody (n = 78), and group 3 were the remaining children who neither reverted for the single autoantibody nor developed multiple autoantibodies during the first year (n = 225). Progression to type 1 diabetes was very low in children who reverted up to 1 year (1 of 78 [1.3%]; HR 0.14, 95% CI 0.04–0.59, P = 0.007) compared with the children who remained single persistent for the first year (24 of 225 [10.7%]). The children who reverted in the first year and later developed type 1 diabetes were part of a group (9 of 78 [11.5%]) who developed another autoantibody after reversion but never had multiple autoantibodies at the same time (Fig. 1). Of the 24 of 225 (10.7%) who remained single persistent for the first year and later developed type 1 diabetes, 7 of 24 belonged to a group (n = 67) who developed multiple autoantibodies after the first year, and 17 of 24 belonged to a group (n = 158) who never developed multiple antibodies. The percentage developing type 1 diabetes was similar (1 of 9 [11.5%], 7 of 67 [10.4%], and 17 of 158 [11.1%]); irrespective of whether the child reverted and developed another autoantibody (n = 9), was persistent for the first year and developed another antibody (n = 67), or was persistent only the first year without developing another autoantibody (n = 158). In contrast, of the remaining children developing multiple autoantibodies in the first year after seroconversion (139 of 293 [47%]), there was a high risk of progression to type 1 diabetes (HR 5.72, 95% CI 3.44–9.52, P < 0.0001) compared with the children who remained single persistent. Further stratification by continent (U.S., Europe) showed no difference in risk of type 1 diabetes by the three autoantibody profiles.
Conclusions
Reversion of diabetes-associated persistent autoantibodies varied by specific autoantibody and usually occurred within 2 years after seroconversion. Reversion was infrequent among those with multiple autoantibodies. A younger age at seroconversion for GADA conferred a higher risk of reversion; whereas, IAA was more likely to revert in children with HLA-DR3/3-DQ2/2 compared with HLA-DR3/4-DQ2/8. An important risk factor associated with reversion was an increase in autoantibody titer from seroconversion, which reduced the risk of reversion. Reversion of IAA and GADA both decreased the risk of further development of other autoantibodies. This study showed that reversion is linked to HLA-genotype, age, and decreasing titer and is less likely after the development of multiple autoantibodies. The risk of type 1 diabetes in children who reverted for single persistent GADA or IAA was similar to those TEDDY children who have not developed autoantibodies. However, there are different implications on the risk of type 1 diabetes when GADA or IAA reverted in the presence of another autoantibody. The observation that the incidence of type 1 diabetes remained high when GADA reverted in the presence of IAA would be consistent with a more aggressive β-cell autoimmunity at an earlier age associated with persistent IAA and DR4-DQ8. The IAA reversion in the presence of persistent GADA may be explained by a less aggressive β-cell autoimmunity associated with older age and DR3-DQ2. Knowledge of such diversity in risk allows for better risk profiling and affects study design and cohort selection.
Previous studies have primarily focused on number of autoantibodies (4,13), combinations of autoantibodies appearance (3,7,14,15), magnitude of autoantibody titer (1,16,17), and age at initial seroconversion to determine type 1 diabetes risk (4,14,18,19). Little has been published on the transient nature of autoantibodies and the associated risk of development of other autoantibodies and/or type 1 diabetes. Such studies have suggested that reversion of autoantibodies was likely due to assay variability/error (5,7), maternal autoantibody exposure in utero (20), and possibly an indicator of β-cell defects (7). It is clear that reversion most likely occurs in those with a single autoantibody. The TEDDY study findings of 24% transience are consistent with the DAISY study (7), which showed 20% transience in those with a single confirmed autoantibody (i.e., positive on two or more blinded aliquots) at more than one sample/visit, and the 16% transience reported in the DIPP study (5). All three studies ensured minimal error or assay variation. TEDDY used two reference laboratories to confirm each positive sample and required positivity at both laboratories over two consecutive screening visits. Consistent with similar study populations, IAA had the highest incidence of reversion (5,21). Although the reason for this higher incidence is unclear, it has been suggested that this might be due to maternal autoantibody exposure; however, determination of de novo production was not a major issue because this study’s definition of persistent positive excluded maternal acquired autoantibodies, as defined previously (22). Nevertheless, maternal acquired autoantibodies could not be determined for 0.4% of the enrolled children, which may account for the 3 of 10 GADA-only children who reverted at a very early age (<1 year). Assessment of risk associated with further development of other autoantibodies and/or type 1 diabetes has been based on case-observation (5,7,22) or univariate in nature (23). This study provides evidence that a single transient autoantibody has limited influence on risk of type 1 diabetes; whereas, a single nontransient autoantibody confers a risk of 1.8 per 100 person-years.
Type 1 diabetes is a complex and heterogeneous disease process, as evidenced by geographical differences in incidence, progression rates, and genetics. The TEDDY study offers a unique opportunity in children with four major type 1 diabetes HLA-DR-DQ risk genotypes to dissect the etiopathogenesis of the appearance of a first autoantibody (14) and progression to clinical onset in relation to the dynamics of these autoantibodies. Transience in autoantibodies that are assumed indicators of the progression of β-cell autoimmunity to type 1 diabetes can support the notion that β-cell autoimmunity is a waxing-and-waning disease process. A better understanding on the development of known preclinical markers provides a chance to improve risk profiles and allow for identification of homogenous risk groups. These more specific risk groups can improve the prediction of those with imminent risk of clinical type 1 diabetes onset, define autoantibody screening intervals, and identify potential windows where environmental exposures may enhance or perhaps reduce risk.
Progression to type 1 diabetes is very low (risk) in those who only develop a single autoantibody (15%); whereas, 70% of those who develop multiple autoantibodies progress to type 1 diabetes, as reported from a geographically diverse pooled cohort of children (4). Autoantibody presentation is heterogeneous, with some acquiring multiple autoantibodies at relatively the same time, others developing multiple autoimmunity over months/years, some only developing one autoantibody, and others transient. This study identified three autoantibody profiles that have differential progression patterns within 1 year of initial seroconversion: 1) reversion, 2) multiple autoantibodies, and 3) single autoantibody. Integration of these three groups into the current schematic of risk profiling, which includes HLA genotype and first-degree relative, may reduce the apparent heterogeneity in risk status that has affected intervention trials (24).
The TEDDY study cohort is at high genetic risk for type 1 diabetes, and thus, all inferences are subject to such high-risk characteristics. However, this study’s primary focus was on characteristics of appearance of β-cell autoimmunity and associated risk with type 1 diabetes independent of known risk factors. The TEDDY cohort is still very young, with the median follow-up age of only 5.7 years (interquartile range 2.7–7.6), so extrapolation of findings to adolescents and adults is limited. Nevertheless, compared with older study populations, TEDDY has a unique strength of capturing those children who rapidly develop diabetes at very young ages; as such, there are relatively few children developing type 1 diabetes with only one autoantibody (25). TEDDY’s short screening interval permitted better capture of those who revert; thus, an ideal screening interval design would continue to screen those subjects who seroconvert and revert negative for at least an additional year after reversion. Future analyses in TEDDY will include zinc transporter 8 autoantibody as it becomes available.
This work provides a benchmark for using specific autoantibodies as more than just positive thresholds in assessing risk. Autoantibody titers as markers of disease have been assessed for many years, but sample size limitations have reduced their use to number/combinations of autoantibodies and whether an assay threshold has been met for risk assessment. The varying appearance of autoantibodies provides valuable information that can assist in profiling risk of progression to type 1 diabetes independent of genetics, family history, and age. The sheer size and follow-up of the TEDDY study has provided a unique opportunity to explore the intricate details of autoantibody appearance, autoantibody patterns conditioned on other autoantibody titers, and their association with known risk factors, windows upon which “triggering events” potentially occur, environmental factors, and interactions with other biomarkers (“omics”). On the basis of these findings, further testing for β-cell autoantibodies does not add further stratification to type 1 diabetes risk once children have developed multiple autoantibodies but is important for assessing the type 1 diabetes risk in children who present with a single autoantibody.
Supplementary Material
Article Information
Funding. This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, JDRF, and Centers for Disease Control and Prevention grants U01-DK-63829, U01-DK-63861, U01-DK-63821, U01-DK-63865, U01-DK-63863, U01-DK-63836, U01-DK-63790, UC4-DK-63829, UC4-DK-63861, UC4-DK-63821, UC4-DK-63865, UC4-DK-63863, UC4-DK-63836, UC4-DK-95300, and UC4-DK-100238 and Contract No. HHSN267200700014C. This work was partly supported by the National Institutes of Health/National Center for Advancing Translational Sciences Clinical and Translational Science Awards to the University of Florida (UL1- TR-000064) and the University of Colorado (UL1-TR-001082).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.V. contributed to discussion and wrote, reviewed, and edited the manuscript. K.F.L. researched data and reviewed and edited the manuscript. B.A., W.H., M.R., J.-X.S., O.S., J.T., and A.-G.Z. reviewed and edited the manuscript. D.A.S., Å.L., E.B., and J.P.K. contributed to discussion and reviewed and edited the manuscript. K.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. A portion of this work was presented at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-0181/-/DC1.
A complete list of the TEDDY Study Group can be found in the Supplementary Data online.
References
- 1.Bingley PJ, Bonifacio E, Williams AJ, Genovese S, Bottazzo GF, Gale EA. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes 1997; 46:1701–1710 [DOI] [PubMed]
- 2.Bonifacio E, Bingley PJ. Islet autoantibodies and their use in predicting insulin-dependent diabetes. Acta Diabetol 1997;34:185–193 [DOI] [PubMed] [Google Scholar]
- 3.Orban T, Sosenko JM, Cuthbertson D, et al.; Diabetes Prevention Trial-Type 1 Study Group . Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimpimäki T, Kulmala P, Savola K, et al. Natural history of beta-cell autoimmunity in young children with increased genetic susceptibility to type 1 diabetes recruited from the general population. J Clin Endocrinol Metab 2002;87:4572–4579 [DOI] [PubMed] [Google Scholar]
- 6.Spencer KM, Tarn A, Dean BM, Lister J, Bottazzo GF. Fluctuating islet-cell autoimmunity in unaffected relatives of patients with insulin-dependent diabetes. Lancet 1984;1:764–766 [DOI] [PubMed] [Google Scholar]
- 7.Barker JM, Barriga KJ, Yu L, et al.; Diabetes Autoimmunity Study in the Young . Prediction of autoantibody positivity and progression to type 1 diabetes: Diabetes Autoimmunity Study in the Young (DAISY). J Clin Endocrinol Metab 2004;89:3896–3902 [DOI] [PubMed] [Google Scholar]
- 8.Hagopian WA, Erlich H, Lernmark A, et al.; TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes 2011;12:733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann N Y Acad Sci 2008;1150:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007;8:286–298 [DOI] [PubMed] [Google Scholar]
- 11.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 13.Krischer JP, Cuthbertson DD, Yu L, et al. Screening strategies for the identification of multiple antibody-positive relatives of individuals with type 1 diabetes. J Clin Endocrinol Metab 2003;88:103–108 [DOI] [PubMed] [Google Scholar]
- 14.Krischer JP, Lynch KF, Schatz DA, et al.; TEDDY Study Group. The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015;58:980–987 [DOI] [PMC free article] [PubMed]
- 15.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 1999;48:460–468 [DOI] [PubMed]
- 16.Achenbach P, Warncke K, Reiter J, et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 2004;53:384–392 [DOI] [PubMed]
- 17.Bonifacio E, Bingley PJ, Shattock M, et al. Quantification of islet-cell antibodies and prediction of insulin-dependent diabetes. Lancet 1990;335:147–149 [DOI] [PubMed] [Google Scholar]
- 18.Vehik K, Haller MJ, Beam CA, et al.; DPT-1 Study Group . Islet autoantibody seroconversion in the DPT-1 study: justification for repeat screening throughout childhood. Diabetes Care 2011;34:358–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vehik K, Beam CA, Mahon JL, et al.; TrialNet Natural History Study Group . Development of autoantibodies in the TrialNet Natural History Study. Diabetes Care 2011;34:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Achenbach P, Bonifacio E, Koczwara K, Ziegler AG. Natural history of type 1 diabetes. Diabetes 2005;54(Suppl. 2):S25–S31 [DOI] [PubMed]
- 21.Colman PG, Steele C, Couper JJ, et al. Islet autoimmunity in infants with a type I diabetic relative is common but is frequently restricted to one autoantibody. Diabetologia 2000;43:203–209 [DOI] [PubMed]
- 22.Naserke HE, Bonifacio E, Ziegler AG. Prevalence, characteristics and diabetes risk associated with transient maternally acquired islet antibodies and persistent islet antibodies in offspring of parents with type 1 diabetes. J Clin Endocrinol Metab 2001;86:4826–4833 [DOI] [PubMed] [Google Scholar]
- 23.Steck AK, Vehik K, Bonifacio E, et al.; TEDDY Study Group . Predictors of progression from the appearance of islet autoantibodies to early childhood diabetes: The Environmental Determinants of Diabetes in the Young (TEDDY). Diabetes Care 2015;38:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bollyky JB, Xu P, Butte AJ, Wilson DM, Beam CA, Greenbaum CJ; Type 1 Diabetes TrialNet Study Group . Heterogeneity in recent-onset type 1 diabetes–a clinical trial perspective. Diabetes Metab Res Rev 2015;31:588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hummel M, Bonifacio E, Schmid S, Walter M, Knopff A, Ziegler AG. Brief communication: early appearance of islet autoantibodies predicts childhood type 1 diabetes in offspring of diabetic parents. Ann Intern Med 2004;140:882–886 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.