Abstract
OBJECTIVE
This study assessed the efficacy and safety of LixiLan, a fixed-ratio, titratable, combination of 2 units insulin glargine (Gla-100) and 1 μg lixisenatide administered once daily via a single pen, versus Gla-100 in insulin-naïve type 2 diabetes on metformin.
RESEARCH DESIGN AND METHODS
Participants were randomized to once-daily LixiLan (n = 161) or Gla-100 (n = 162) for 24 weeks, while continuing metformin. LixiLan and Gla-100 were started at 10 units/5 μg and 10 units, respectively, and titrated based on the Gla-100 requirement according to fasting plasma glucose levels. The primary objective was to test noninferiority (upper bound of the 95% CI ≤0.4%) of LixiLan in reducing HbA1c; if met, statistical superiority was tested. Secondary objectives included body weight changes, hypoglycemia, and safety.
RESULTS
Baseline characteristics (mean age 57 years, diabetes duration 6–7 years, BMI 32 kg/m2) were similar between groups. At week 24, mean HbA1c was reduced from 8.0% (64 mmol/mol) at baseline to 6.3% (45 mmol/mol) and 6.5% (48 mmol/mol) with LixiLan and Gla-100, respectively, establishing statistical noninferiority and superiority of LixiLan (least-squared mean [95% CI] difference: −0.17% [−0.31, −0.04] {−1.9 mmol/mol [−3.4, −0.4]}; P = 0.01). HbA1c <7.0% (<53 mmol/mol) was achieved in 84% and 78% of participants (nonsignificant), respectively. LixiLan improved 2-h postmeal plasma glucose versus Gla-100 (least-squared mean difference: –3.17 mmol/L [–57 mg/dL]; P < 0.0001). Body weight was reduced with LixiLan (–1 kg) and increased with Gla-100 (+0.5 kg; P < 0.0001), with no increase in hypoglycemic events (∼25% in each group). The incidence of nausea (7.5%) and vomiting (2.5%) was low with LixiLan.
CONCLUSIONS
LixiLan achieved statistically significant reductions to near-normal HbA1c levels with weight loss and no increased hypoglycemic risk, compared with insulin glargine alone, and a low incidence of gastrointestinal adverse events in type 2 diabetes inadequately controlled on metformin.
Introduction
Basal insulin is one of the suggested treatment options in the 2015 recommendations of the American Diabetes Association/European Association for the Study of Diabetes for patients with metformin-treated type 2 diabetes who do not achieve individualized HbA1c targets (1). Indeed, the best outcomes in HbA1c reduction, to levels usually in the range of 6.8–7.1% (2–4), are achieved with basal insulin after failure on metformin monotherapy, whereas modestly higher levels are achieved when basal insulin is added after failure of a combination of oral agents. This is indicative of an earlier stage in the disease process that may have better responses to insulin therapy than later stages (2,5). However, owing to the fear of hypoglycemia and concerns with weight gain and treatment complexity (6,7), most patients and physicians in clinical practice prefer to add a second or even a third oral agent, delaying the start of insulin therapy by 8–12 years (4,8–11). Insulin is then initiated at a more advanced disease stage, in which smaller proportions of patients, usually in the range of 45–55%, achieve HbA1c <7% (<53 mmol/mol), only if basal insulin is properly titrated (4,12,13). Furthermore, because basal insulin therapy improves glycemic control predominantly by reducing nocturnal and fasting plasma glucose (FPG), and many patients with type 2 diabetes exhibit substantial postprandial glucose (PPG) excursions, ∼40–50% of patients require additional treatment with prandial insulin or a glucagon-like peptide 1 receptor agonist (GLP-1 RA) to control PPG (1).
Short-acting GLP-1 RAs, in a glucose-dependent fashion, stimulate postprandial insulin secretion, suppress glucagon release, and delay gastric emptying (14,15). Hence, the clinical rationale for the combination of basal insulin with a GLP-1 RA is based on the complementary and additive effects of these individual therapies: basal insulin improves FPG, whereas the short-acting GLP-1 RA decreases PPG (14,16–20).
Lixisenatide (Lyxumia; Sanofi, Paris, France) is a once-daily, prandial GLP-1 RA with a predominant PPG-lowering effect brought about mainly by delaying gastric emptying and reducing glucagon release (6). The complementary effects of lixisenatide, when administered in combination with basal insulin, were demonstrated in the GetGoal-L and GetGoal Duo-1 trials, which showed statistically superior PPG and HbA1c reductions compared with those reported with basal insulin alone and without weight gain or increased hypoglycemic risk (18,19).
Lixisenatide and insulin glargine (Gla-100) have similar physicochemical features, such as good solubility at low pH, allowing both components to be mixed at a defined fixed-ratio formulation and delivered via a single, daily injection (LixiLan).
The main objective of this proof-of-concept study was to compare the effects of LixiLan (a 2 units/1 µg fixed-ratio combination of 2 units Gla-100 and 1 μg lixisenatide) versus Gla-100 alone (both treatments added to metformin) on glycemic control over 24 weeks, as evaluated by HbA1c reduction, with particular focus on changes in body weight, hypoglycemic risk, and the gastrointestinal safety profile.
Research Design and Methods
Study Design and Interventions
This Phase 2, proof-of-concept, randomized, open-label, active-controlled, two-arm parallel-group, 24-week duration study was conducted at 67 centers in 13 countries (Chile, Czech Republic, Germany, Denmark, France, Hungary, Lithuania, Mexico, Poland, Romania, Slovakia, Sweden, and the U.S.). It was initiated (first patient enrolled) on 21 November 2011 and ended (last patient completed) on 17 December 2012. The study comprised three periods (Supplementary Fig. 1): an up to 2-week screening period, a 24-week treatment period, and a 3-day safety follow-up period. Inclusion criteria included adult patients with type 2 diabetes diagnosed ≥1 year before the screening visit, HbA1c of ≥7% (≥53 mmol/mol) to ≤10% (≤86 mmol/mol), screening FPG ≤13.9 mmol/L (≤250 mg/dL), and treatment with metformin at a stable dose of ≥1.5 g/day for ≥3 months before the screening visit. The main exclusion criteria included treatment with glucose-lowering agent(s) other than metformin during the 3 months before the screening visit, any use of insulin within the last 6 months before screening, and use of insulin ≥6 months before screening, except for episode(s) of short-term treatment caused by intercurrent illness. The trial protocol complied with the recommendations of the Declaration of Helsinki and was approved by an independent ethics committee. All participants provided written informed consent.
Patients were randomized 1:1 to receive once-daily LixiLan or once-daily Gla-100. Randomization and treatment allocation were performed centrally by an interactive voice/Web response system (IVRS/IWRS). LixiLan and Gla-100 were provided in open-label boxes and identified with batch numbers. For each randomized patient, the IVRS/IWRS allocated a batch number and quantity of kit to be dispensed.
Both treatments were administered in the morning within 1 h before breakfast. LixiLan was supplied as a sterile aqueous solution for subcutaneous injection in 3-mL cartridges that were used in a reusable self-injector pen (TactiPen; Sanofi, Paris, France), and Gla-100 was supplied as a sterile, aqueous solution in a Lantus SoloSTAR pen (Sanofi). Patients in the LixiLan and Gla-100 treatment groups continued metformin at a stable dose throughout the study period, unless there was a specific safety issue related to this treatment.
The initial daily dose was 10 units Gla-100/5 µg lixisenatide in the LixiLan group and 10 units in the Gla-100 group. The titration of Gla-100 (alone and in the LixiLan combination) was based on plasma glucose levels; plasma glucose was measured, and corresponding dose changes were made to allow patients to achieve FPG targets of 4.4–5.6 mmol/L (80–100 mg/dL). For LixiLan, the dose of lixisenatide followed the Gla-100 dose according to the 2 units/1 µg fixed ratio. The maximum daily dose for LixiLan was 60 units Gla-100 corresponding to 30 µg lixisenatide. No upper limit of titration was set for the Gla-100 group.
All glucose measurements were performed with capillary blood glucose and were automatically converted to plasma-equivalent glucose values. These were shown on the glucometer display and recorded in the patients’ diaries.
At baseline and week 24, a standardized liquid breakfast was administered (600 kcal: 50–55% carbohydrate; 17–20% protein; 25–30% fat). At week 24, randomized treatment was administered within 30 min before the standardized breakfast.
End Points and Assessments
The primary efficacy end point was the change in HbA1c from baseline to week 24. Secondary end points were percentage of patients achieving HbA1c <7% (<53 mmol/mol) or ≤6.5% (≤48 mmol/mol); change from baseline to week 24 for body weight, 2-h PPG, and 2-h plasma glucose excursion during the standardized breakfast, 7-point self-monitored plasma glucose (SMPG) profiles (at each daily time point and mean daily value), and FPG; and average daily Gla-100 dose at week 24.
Safety variables included documented symptomatic hypoglycemia, defined as an event with typical symptoms of hypoglycemia accompanied by a measured plasma glucose concentration of ≤3.9 mmol/L (≤70 mg/dL), and the occurrence of adverse events (AEs) that developed, worsened, or became serious during treatment.
Two composite end points were assessed at week 24: the percentage of patients achieving HbA1c <7% (<53 mmol/mol) with no documented symptomatic hypoglycemia (≤3.9 mmol/L [≤70 mg/dL]), and HbA1c <7% and no weight gain. One exploratory composite end point was assessed at week 24: the percentage of patients achieving HbA1c <7% (<53 mmol/mol) with no documented symptomatic hypoglycemia and no weight gain.
Statistical Methods
For this noninferiority trial, a sample size of 310 patients (155 patients per treatment group) was required to ensure that the upper CL of the two-sided 95% CI for the adjusted mean difference between LixiLan and Gla-100 would not exceed HbA1c 0.4% (4.4 mmol/mol) with ≥80% power. The calculation assumed a common SD of 1.2% (13.1 mmol/mol) and a true difference in HbA1c between the treatment groups of 0.
The study was designed to establish the noninferiority of LixiLan compared with Gla-100 on the primary end point of change from baseline to week 24 in HbA1c. Noninferiority was established if the upper bound of the two-sided 95% CI of the difference between the treatments groups of the modified intention-to-treat (mITT) population was ≤0.4% (≤4.4 mmol/mol). If noninferiority was established, then statistical superiority was evaluated. This was achieved if the upper bound of the two-sided 95% CI was less than zero. If noninferiority was demonstrated for the primary end point, the superiority of key secondary end points was tested sequentially in a predetermined, stepwise manner to control the type I error, with the testing procedure being stopped as soon as an end point was found not to be statistically significant.
The safety population comprised all patients who were randomized to treatment and who received 1 dose or more of the study drug. All safety analyses were performed on the safety population. The mITT population comprised all patients who were randomized to treatment and who received one dose or more of the study drug and had both a baseline and one or more postbaseline assessments of the primary or any secondary end point, irrespective of compliance with the study protocol and procedures. All efficacy analyses were performed on this mITT population.
The primary end point was analyzed using an ANCOVA model with treatment, randomization strata of screening HbA1c (<8, ≥8% [<64, ≥64 mmol/mol]), BMI (<30, ≥30 kg/m2), and country as fixed effects; the baseline HbA1c value was used as a covariate. Missing end point values were imputed from the last available on-treatment value using the last observation carried forward (LOCF). The on-treatment period for efficacy end points was defined as the time from the first injection of the open-label investigational medicinal product up to 14 days for HbA1c; 0 days for standardized meal test parameters, 7-point SMPG, and insulin glargine dose; 1 day for FPG and hypoglycemia; and 3 days for body weight; after the last injection of the investigational medicinal product or up to the introduction of rescue therapy, whichever was the earliest. All continuous, secondary efficacy end points were analyzed for superiority of LixiLan versus Gla-100 using the same primary ANCOVA model described above with the corresponding baseline value as a covariate (except for average daily Gla-100 dose at week 24, for which this was not applicable). Categorical secondary efficacy end points were analyzed using a Cochran-Mantel-Haenszel method stratified by randomization strata of screening HbA1c (<8, ≥8% [<64, ≥64 mmol/mol]) and BMI (<30, ≥30 kg/m2). Continuous data are summarized using descriptive statistics, and categorical data are summarized using counts and percentages.
Results
Patient Disposition and Baseline Characteristics
A total of 520 patients were screened and, of these, 323 patients were randomized and treated (161 in the LixiLan group and 162 in the Gla-100 group; Supplementary Fig. 2). Screening and baseline demographics, history of diabetes, and disease characteristics were generally similar across treatment groups (Table 1). Mean baseline HbA1c and FPG ranged from 8.0 to 8.1% (64 to 65 mmol/mol) and from 9.5 to 9.8 mmol/L (170 to 176 mg/dL), respectively.
Table 1.
Screening or baseline demographics, history of diabetes, and disease characteristics (randomized patients)
| Parameters | LixiLan(n = 161) | Gla-100(n = 162) |
|---|---|---|
| Age, years | 56.9 ± 9.5 | 56.6 ± 9.4 |
| Male, n (%) | 80 (49.7) | 85 (52.5) |
| White, n (%) | 158 (98.1) | 160 (98.8) |
| Duration of type 2 diabetes, years | 6.3 ± 4.3 | 7.1 ± 5.3 |
| Duration of metformin treatment, years | 4.1 ± 3.6 | 4.3 ± 3.9 |
| Baseline measurements | ||
| Metformin dose, mg/day | 2,076 ± 441 | 2,094 ± 416 |
| Weight, kg | 90.1 ± 17.6 | 91.6 ± 16.7 |
| BMI, kg/m2 | 32.2 ± 4.8 | 32.0 ± 4.4 |
| HbA1c, % | 8.1 ± 0.8 | 8.0 ± 0.8 |
| HbA1c, mmol/mol | 64 ± 8.7 | 64 ± 8.7 |
| 2-h PPG, mmol/L | 16.0 ± 3.7 | 15.6 ± 3.9 |
| 2-h PPG, mg/dL | 288 ± 67 | 281 ± 70 |
| FPG, mmol/L | 9.8 ± 2.2 | 9.5 ± 2.2 |
| FPG, mg/dL | 176 ± 39 | 170 ± 39 |
Data are mean ± SD at screening or baseline, unless indicated otherwise.
Efficacy
Primary End Point
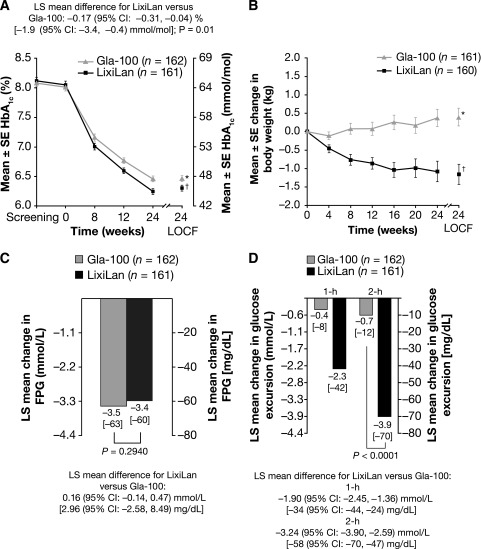
Treatment with LixiLan and Gla-100 resulted in marked reductions in HbA1c from baseline to week 24 that were significantly greater with LixiLan (Fig. 1A). The least-squared (LS) mean changes from baseline to week 24 LOCF in HbA1c were –1.82% (–19.9 mmol/mol) for the LixiLan group and –1.64% (–17.9 mmol/mol) for the Gla-100 group, reaching a mean HbA1c level of 6.3% (45 mmol/mol) and 6.5% (48 mmol/mol), respectively. The LS mean (95% CI) difference between mean changes from baseline for the LixiLan and Gla-100 groups was –0.17% (−0.31, −0.04) (–1.9 mmol/mol [–3.4, –0.4]), establishing noninferiority and then statistical superiority for LixiLan treatment (P = 0.01).
Figure 1.
A: Mean ± SE HbA1c by study visit until week 24 and week 24 LOCF. *6.5% (48 mmol/mol); –1.64% (–17.9 mmol/mol) LS mean change from baseline; †6.3% (45 mmol/mol); –1.82% (–19.9 mmol/mol) LS mean change from baseline. B: Mean ± SE change in body weight by study visit from baseline to week 24 and week 24 LOCF. *0.39 kg; †–1.16 kg. C: LS mean FPG change from baseline to week 24. D: LS mean 1- and 2-h glucose excursion change from baseline to week 24 (glucose excursion was calculated by subtracting plasma glucose 30 min before the meal test [before study drug administration] from 2-h PPG). All data shown are for the mITT population.
Secondary End Points
A numerically higher percentage of patients in the LixiLan group reached target HbA1c <7% (84 vs. 78%; difference LixiLan vs. Gla-100: 6.2%; 95% CI –2.2, 14.5) or ≤6.5% (72 vs. 65%; difference LixiLan vs. Gla-100: 7.3%; 95% CI –2.6, 17.3) compared with the Gla-100 group.
Treatment with LixiLan significantly improved postprandial glycemic control compared with Gla-100, as shown by the greater reduction from baseline to week 24 observed for 2-h PPG (LS mean difference of –3.17 mmol/L [–57 mg/dL]; P < 0.0001; data not shown) and 2-h glucose excursion (LS mean difference of –3.24 mmol/L [–58 mg/dL]; P < 0.0001; Fig. 1D) after a standardized breakfast meal. Patients treated with LixiLan had a statistically significantly greater decrease from baseline to week 24 in average 7-point SMPG profile compared with patients treated with Gla-100 (LS mean difference of –0.30 mmol/L [–5.5 mg/dL]; P = 0.015; data not shown). A significant difference was reported in the mean change in body weight, which decreased in the LixiLan group and increased in the Gla-100 group (Fig. 1B), with a between-group LS mean (95% CI) difference of –1.44 kg (–2.1, –0.8) (P < 0.0001). Similar reductions in mean FPG from baseline to week 24 LOCF (LS mean changes of –3.35 mmol/L [–60 mg/dL] in the LixiLan group and –3.51 mmol/L [–63 mg/dL] in the Gla-100 group) were observed (Fig. 1C). The average daily Gla-100 doses were similar up to week 12 in both treatment groups and then started to diverge from weeks 12 to 24, with a trend for lower doses with LixiLan (36 units) than in the Gla-100 group (39 units). The distribution of the final daily dose of Gla-100 in both the LixiLan and the Gla-100 groups at week 24 LOCF is reported in Supplementary Table 1. These results, together with the available safety information for each component, were carefully considered when the ratio of lixisenatide versus Gla-100 for further development of LixiLan in Phase 3 studies was selected. Future studies will provide the flexibility of using a pen with a fixed 2 units/1 µg ratio, delivering 10–40 units Gla-100 with an equivalent dose of 5–20 μg lixisenatide, or, for those requiring a higher insulin dose, a pen with a fixed 3 units/1 µg ratio, delivering 30–60 units Gla-100 with an equivalent dose of 10–20 μg lixisenatide. One patient in the Gla-100 group qualified as having received rescue therapy, given that his metformin dose was increased from 1,700 to 2,550 mg; no patients in the LixiLan group required rescue therapy.
Composite End Points
A significantly higher proportion of patients receiving LixiLan treatment compared with Gla-100 reached the composite end points of HbA1c <7% (<53 mmol/mol) with no weight gain (56 vs. 37%; difference 19.0%; 95% CI 8.6, 29.5), and of HbA1c <7% with no documented symptomatic hypoglycemia and no weight gain (46 vs. 29%; difference 17.7%; 95% CI 7.5, 28.0) at week 24. The proportion of patients reaching HbA1c <7% and no documented symptomatic hypoglycemia was numerically higher for the LixiLan group than for the Gla-100 group (68 vs. 59%; difference 8.5%; 95% CI –1.9, 18.9).
Safety
During the 24-week treatment period, the proportion of patients experiencing documented symptomatic hypoglycemia was comparable between the two treatment groups (Table 2). No severe hypoglycemia occurred in either group. Although AEs and serious AEs were present in a slightly higher proportion of patients in the LixiLan group compared with the Gla-100 group (Table 2), this difference was mainly due to the modestly higher percentage of patients who experienced treatment-related gastrointestinal AEs (mainly nausea and vomiting) in the LixiLan group. Only two patients (1.2%) receiving LixiLan withdrew from the study due to gastrointestinal symptoms (one patient experienced nausea and vomiting, and the other reported only nausea). No pancreatitis was reported in this study.
Table 2.
Summary of AEs (safety population)
| LixiLan (n = 161) | Gla-100 (n = 162) | |
|---|---|---|
| AEs* | n (%) | n (%) |
| Patients with documented symptomatic hypoglycemia† | 35 (21.7) | 37 (22.8) |
| Patients with any TEAE | 86 (53.4) | 82 (50.6) |
| Patients with serious TEAEs | 9 (5.6) | 6 (3.7) |
| Deaths | 0 (0.0) | 0 (0.0) |
| Patients with TEAEs leading to discontinuation | 6 (3.7) | 0 (0.0) |
| Nausea | 2 (1.2) | 0 (0.0) |
| Vomiting | 1 (0.6) | 0 (0.0) |
| Hypersensitivity‡ | 1 (0.6) | 0 (0.0) |
| Confusional state | 1 (0.6) | 0 (0.0) |
| Dizziness | 1 (0.6) | 0 (0.0) |
| Headache | 1 (0.6) | 0 (0.0) |
| Ovarian cancer | 1 (0.6) | 0 (0.0) |
| Gastrointestinal TEAEs | 25 (15.5) | 15 (9.3) |
| Nausea | 12 (7.5) | 0 (0.0) |
| Diarrhea | 5 (3.1) | 6 (3.7) |
| Vomiting | 4 (2.5) | 1 (0.6) |
| Constipation | 3 (1.9) | 0 (0.0) |
| Dyspepsia | 2 (1.2) | 1 (0.6) |
| Abdominal distension | 1 (0.6) | 2 (1.2) |
TEAE, treatment-emergent AE.
*AEs listed are TEAEs. More than one AE could be listed as a reason for discontinuation of an individual patient.
†Documented symptomatic hypoglycemia was an event during which typical symptoms of hypoglycemia were accompanied by a measured plasma glucose concentration of ≤3.9 mmol/L (≤70 mg/dL).
‡Not positively adjudicated as an allergic reaction by an independent Allergic Reaction Assessment Committee.
Conclusions
The primary objective of this proof-of-concept study was to demonstrate that, in insulin-naïve patients with type 2 diabetes inadequately controlled on metformin, LixiLan, the combination of Gla-100 and the prandial GLP-1 RA lixisenatide in a titratable, fixed-ratio coformulation (2 units/1 µg), is not only well tolerated but also effective in achieving glycemic targets compared with Gla-100 alone. HbA1c levels decreased in both groups to unprecedented levels, with a larger mean reduction from 8.1% at baseline to 6.3% at week 24, with weight loss despite such a robust HbA1c lowering and without increased hypoglycemic risk in the LixiLan group. Furthermore, LixiLan not only reached the noninferiority objective but also proved to be more efficacious compared with Gla-100 (P = 0.0130) in reducing HbA1c over the 24-week study period.
As expected with LixiLan and Gla-100 optimally titrated to the same FPG targets following the same algorithm, the reductions seen in FPG over the 24-week study period were similar between the two treatment groups. However, treatment with LixiLan significantly improved postprandial glycemic control compared with Gla-100, as shown by the mean change in 2-h PPG and 2-h glucose excursion (Fig. 1D). These results confirm that lixisenatide exerts glycemic control primarily by lowering PPG at meals after its administration, in agreement with findings from previous studies (18–24).
Weight gain concern is one of the main reasons for delaying insulin initiation in insulin-naïve patients (25). In the current study, LixiLan treatment was associated with a weight reduction despite major improvements in HbA1c to nearly normal levels, whereas patients in the Gla-100 group showed a modest increase (P < 0.0001 for a 1.4-kg treatment difference). These results indicate that the addition of lixisenatide to Gla-100 in a fixed-ratio combination can attenuate the body weight gain associated with basal insulin initiation and optimal titration.
As with other studies of GLP-1 RAs, gastrointestinal disorders were among the most common AEs reported by patients receiving LixiLan treatment, with nausea (7.5%), vomiting (2.5%), and diarrhea (3.1%) occurring most frequently. However, these rates were much lower than those reported in previous studies of lixisenatide and the prandial GLP-1 RA exenatide when administered alongside basal insulin, with nausea, vomiting, and diarrhea reported by 26–40%, 8–18%, and 7–11% of patients, respectively (18–20,26).
In a dose-ranging study of four doses (from 5 to 30 µg) of lixisenatide once and twice daily, the 20 µg once-daily dose demonstrated the best efficacy-to-tolerability ratio (27). Because the dose levels of lixisenatide >20 µg in that study were still relatively well tolerated, it was deemed acceptable in this proof-of-concept study to investigate the 2 units/1 µg dose ratio in the LixiLan group exploring up to Gla-100 60 units/lixisenatide 30 µg.
The favorable gastrointestinal-tolerability profile of LixiLan in the current study is supported by the low withdrawal rate caused by gastrointestinal symptoms (1.2%). The rate of discontinuations because of nausea and vomiting in other studies of lixisenatide, when added to basal insulin in a separate injection, was higher, ranging from 2.7 to 6.5% (18–20). The gradual increase of lixisenatide with LixiLan treatment, which is closely linked to the titration of Gla-100, likely mitigated the adverse gastrointestinal effects of lixisenatide and contributed to the low incidence of nausea, vomiting, and diarrhea and to the low rates of study withdrawal.
Similar marked efficacy and low rates of gastrointestinal disorders demonstrated in this LixiLan proof-of-concept trial were reported in the DUAL I study of IDegLira (28), which combined the basal insulin degludec and the long-acting GLP-1 RA liraglutide in a single injection, in patients with type 2 diabetes on metformin with or without pioglitazone. IDegLira was started at 10 dose steps (10 units insulin degludec plus 0.36 mg liraglutide, once daily). The daily dose of IDegLira could be titrated to 50 dose steps (50 units insulin degludec plus 1.8 mg liraglutide). After 26 weeks, the final HbA1c level achieved was 6.4% (46 mmol/mol) in patients on IDegLira and 6.9% (52 mmol/mol) for those on insulin degludec only. The rates of nausea, vomiting, and diarrhea (9%, 4%, and 8%, respectively) were lower than in previous studies in which basal insulin and liraglutide were administered separately (22–23%, 3–11%, and 11%, respectively) (6,29).
Because the main target of the lixisenatide component of LixiLan is PPG, and Gla-100 in this study was being titrated based on FPG targets, patients in both the LixiLan and Gla-100 treatment groups were receiving comparable doses of Gla-100. In the DUAL I study (28), the liraglutide portion of IDegLira exerted its effect predominantly on FPG. Hence, because the basal insulin and the GLP-1 RA used in that combination both have FPG-lowering effects, it is conceivable that the dose of basal insulin required was comparably lower.
To confirm and expand further the findings presented in this proof-of-concept trial and to address its limitations, further large-scale studies are needed in different populations. Two 30-week Phase 3 trials of the safety and efficacy of LixiLan in patients with type 2 diabetes were completed in 2015: LixiLan-O (NCT02058147) compared LixiLan with Gla-100 and lixisenatide (metformin was a mandatory background therapy in all three treatment groups) (30), and LixiLan-L (NCT02058160) compared LixiLan with Gla-100 (with or without metformin in both treatment groups) in patients uncontrolled on ≥6 months of basal insulin (31).
Current guidelines (1) suggest that injectable therapy, such as basal insulin or a GLP-1 RA, is appropriate in patients with type 2 diabetes when therapy with metformin, alone or combined with other oral agents, has failed. Often, however, both of these injectable options are insufficient to achieve individualized glycemic targets and are limited by their respective barriers and AE profiles. Weight gain and hypoglycemia often impede optimal basal insulin titration, while the addition of prandial insulin exacerbates these limitations, and the increased complexity in the treatment regimen leads to low adherence and compliance. GLP-1 RAs are hindered by gastrointestinal AEs and variable glucose-lowering and body weight responses, leading to a high discontinuation rate in clinical practice. Titratable, fixed-ratio coformulations of a basal insulin with a GLP-1 RA represent a new treatment paradigm, encouraging the complementary action of these two therapies, both in efficacy, with robust HbA1c reductions to levels previously unattainable through treatment with the individual components, and in safety, without increased hypoglycemic risk and with some modest weight loss. The gradual, incremental titration with LixiLan represents a more rational way to deliver a peptide such as a GLP-1 RA, with slow adjustments, based on efficacy response and tolerance, resulting in a marked mitigation of gastrointestinal AEs, including nausea and vomiting.
In conclusion, these findings support the concept of combining prandial lixisenatide with basal insulin glargine in a titratable, single-injection combination as a valid and highly effective treatment option for patients with early type 2 diabetes on metformin monotherapy with HbA1c levels between 7 and 10% (53 and 86 mmol/mol).
Supplementary Material
Article Information
Acknowledgments. The authors thank the trial participants, trial staff, and investigators for their participation.
Funding. Editorial assistance for this publication was provided by Christina Holleywood, PhD, of Caudex (Oxford, U.K.) and was funded by Sanofi. V.F. is supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. No payment was received to any of the authors for preparation of this manuscript.
Duality of Interest. The LixiLan proof-of-concept trial (NCT01476475) was sponsored by Sanofi. J.R. received research support from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Hanmi, Intarcia, Janssen, Lexicon, MannKind, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi, and Takeda and served on advisory boards of or received consulting honoraria from Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Intarcia, Janssen, Lexicon, Merck, Novo Nordisk, Sanofi, and Takeda. V.R.A. received research support from Amylin, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eisai, GI Dynamics, GlaxoSmithKline, Halozyme, Hanmi, Intarcia, Janssen, Novo Nordisk, Sanofi, and Takeda; received consulting honoraria from Janssen, Novo Nordisk, and Sanofi; and is an employee of MedStar Health Research Institute. L.S. was an employee of Sanofi and is a shareholder of Sanofi. E.S., T.Z., and R.P. are employees of and own stock/shareholdings in Sanofi. V.F. received research support (to Tulane) from Abbott, Asahi, Eli Lilly, Endo Barrier, Gilead Sciences, and Novo Nordisk and received honoraria for consulting and lectures from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, Pamlabs, Sanofi Aventis, and Takeda. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. J.R. outlined the manuscript and was involved in the writing and discussion of the manuscript, analyses and interpretation of the data, and all critical revisions of the manuscript. M.D. was involved in the writing, discussion, and review of the manuscript and analyses and interpretation of the data and contributed to the original outline of the manuscript. V.R.A. researched the data and was involved in the writing, discussion, reviewing, and editing of the manuscript. E.S. contributed to the design of the study, wrote the study protocol, was the medical supervisor for the study sponsor, contributed to the analysis and interpretation of the data, and was involved with writing, discussion, and critical revisions of the manuscript. L.S., T.Z., R.P., and V.F. were involved in the writing, discussion, and review of the manuscript. J.R. is the guarantor of this work and, as such, had full access to all of the data in the study and with T.Z. takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Portions of these data were presented at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014, and at the 50th Annual Meeting of the European Association for the Study of Diabetes, Vienna, Austria, 15–19 September 2014. The data were also presented in brief at the 75th Scientific Sessions of the American Diabetes Association, Boston, MA, 5–9 June 2015, and at the 51st Annual Meeting of the European Association for the Study of Diabetes, Stockholm, Sweden, 14–18 September 2015.
Footnotes
Clinical trial reg. no. NCT01476475, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-0046/-/DC1.
Deceased.
Principal investigators at the clinical sites are listed in the Supplementary Data online.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. . Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2015;58:429–442 [DOI] [PubMed] [Google Scholar]
- 2.Aschner P, Chan J, Owens DR, et al.; EASIE investigators . Insulin glargine versus sitagliptin in insulin-naive patients with type 2 diabetes mellitus uncontrolled on metformin (EASIE): a multicentre, randomised open-label trial. Lancet 2012;379:2262–2269 [DOI] [PubMed] [Google Scholar]
- 3.Bolli GB, Riddle MC, Bergenstal RM, et al.; on behalf of the EDITION 3 study investigators . New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab 2015;17:386–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riddle MC, Rosenstock J, Gerich J; Insulin Glargine 4002 Study Investigators . The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 2003;26:3080–3086 [DOI] [PubMed] [Google Scholar]
- 5.Fonseca V, Gill J, Zhou R, Leahy J. An analysis of early insulin glargine added to metformin with or without sulfonylurea: impact on glycaemic control and hypoglycaemia. Diabetes Obes Metab 2011;13:814–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier JJ, Rosenstock J, Hincelin-Méry A, et al. . Contrasting effects of lixisenatide and liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin glargine with or without metformin: a randomized, open-label trial. Diabetes Care 2015;38:1263–1273 [DOI] [PubMed] [Google Scholar]
- 7.Raccah D, Bretzel RG, Owens D, Riddle M. When basal insulin therapy in type 2 diabetes mellitus is not enough--what next? Diabetes Metab Res Rev 2007;23:257–264 [DOI] [PubMed] [Google Scholar]
- 8.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 2006;29:1269–1274 [DOI] [PubMed] [Google Scholar]
- 9.Raskin P, Allen E, Hollander P, et al.; INITIATE Study Group . Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care 2005;28:260–265 [DOI] [PubMed] [Google Scholar]
- 10.Raskin P, Gylvin T, Weng W, Chaykin L. Comparison of insulin detemir and insulin glargine using a basal-bolus regimen in a randomized, controlled clinical study in patients with type 2 diabetes. Diabetes Metab Res Rev 2009;25:542–548 [DOI] [PubMed] [Google Scholar]
- 11.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 2008;51:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenstock J, Sugimoto D, Strange P, Stewart JA, Soltes-Rak E, Dailey G. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patients. Diabetes Care 2006;29:554–559 [DOI] [PubMed] [Google Scholar]
- 13.Swinnen SG, Dain MP, Aronson R, et al. . A 24-week, randomized, treat-to-target trial comparing initiation of insulin glargine once-daily with insulin detemir twice-daily in patients with type 2 diabetes inadequately controlled on oral glucose-lowering drugs. Diabetes Care 2010;33:1176–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutniak M, Orskov C, Holst JJ, Ahrén B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 1992;326:1316–1322 [DOI] [PubMed] [Google Scholar]
- 15.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 1993;36:741–744 [DOI] [PubMed] [Google Scholar]
- 16.Charbonnel B, Bertolini M, Tinahones FJ, Domingo MP, Davies M. Lixisenatide plus basal insulin in patients with type 2 diabetes mellitus: a meta-analysis. J Diabetes Complications 2014;28:880–886 [DOI] [PubMed] [Google Scholar]
- 17.Raccah D, Lin J, Wang E, et al. . Once-daily prandial lixisenatide versus once-daily rapid-acting insulin in patients with type 2 diabetes mellitus insufficiently controlled with basal insulin: analysis of data from five randomized, controlled trials. J Diabetes Complications 2014;28:40–44 [DOI] [PubMed] [Google Scholar]
- 18.Riddle MC, Aronson R, Home P, et al. . Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (GetGoal-L). Diabetes Care 2013;36:2489–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddle MC, Forst T, Aronson R, et al. . Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care 2013;36:2497–2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators . Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab 2012;14:910–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahrén B, Leguizamo Dimas A, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once-daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal-M). Diabetes Care 2013;36:2543–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE; EFC6018 GetGoal-Mono Study Investigators . Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care 2012;35:1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu Pan C, Han P, Liu X, et al. . Lixisenatide treatment improves glycaemic control in Asian patients with type 2 diabetes mellitus inadequately controlled on metformin with or without sulfonylurea: a randomized, double-blind, placebo-controlled, 24-week trial (GetGoal-M-Asia). Diabetes Metab Res Rev 2014;30:726–735 [DOI] [PubMed] [Google Scholar]
- 24.Rosenstock J, Hanefeld M, Shamanna P, et al. . Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S). J Diabetes Complications 2014;28:386–392 [DOI] [PubMed] [Google Scholar]
- 25.Korytkowski M. When oral agents fail: practical barriers to starting insulin. Int J Obes Relat Metab Disord 2002;26(Suppl. 3):S18–S24 [DOI] [PubMed] [Google Scholar]
- 26.Diamant M, Nauck MA, Shaginian R, et al.; 4B Study Group . Glucagon-like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care 2014;37:2763–2773 [DOI] [PubMed] [Google Scholar]
- 27.Ratner RE, Rosenstock J, Boka G; DRI6012 Study Investigators . Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with Type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled trial. Diabet Med 2010;27:1024–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gough SC, Bode B, Woo V, et al.; NN9068-3697 (DUAL-I) trial investigators . Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol 2014;2:885–893 [DOI] [PubMed] [Google Scholar]
- 29.Ahmann AJ, Rodbard HW, Rosenstock J, et al. . Efficacy and safety of liraglutide vs. placebo when added to basal insulin analogs in patients with type 2 diabetes (LIRAADD2BASAL). Diabetes 2014;63:A87 [Google Scholar]
- 30.Rosenstock J, Aronson R, Hanefeld M, et al. Clinical impact of titratable fixed-ratio combination of insulin glargine/lixisenatide vs each component alone in type 2 diabetes inadequately controlled on oral agents: LixiLan-O trial. Presented at the 76th Scientific Sessions of the American Diabetes Association (ADA), 10–14 June 2016, New Orleans, Louisiana, 186-OR [Google Scholar]
- 31.Aroda V, Rosenstock J, Wysham C, et al. Efficacy and safety of the insulin glargine/lixisenatide fixed-ratio combination versus insulin glargine in patients with T2DM: the LixiLan-L trial (NCT02058160). Presented at the 76th Scientific Sessions of the American Diabetes Association (ADA), 10–14 June 2016, New Orleans, Louisiana, 238-OR [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.