Abstract
OBJECTIVE
Standardized, reproducible, and feasible quantification of β-cell function (BCF) is necessary for the evaluation of interventions to improve insulin secretion and important for comparison across studies. We therefore characterized the responses to, and reproducibility of, standardized methods of in vivo BCF across different glucose tolerance states.
RESEARCH DESIGN AND METHODS
Participants classified as having normal glucose tolerance (NGT; n = 23), prediabetes (PDM; n = 17), and type 2 diabetes mellitus (T2DM; n = 22) underwent two standardized mixed-meal tolerance tests (MMTT) and two standardized arginine stimulation tests (AST) in a test-retest paradigm and one frequently sampled intravenous glucose tolerance test (FSIGT).
RESULTS
From the MMTT, insulin secretion in T2DM was >86% lower compared with NGT or PDM (P < 0.001). Insulin sensitivity (Si) decreased from NGT to PDM (∼50%) to T2DM (93% lower [P < 0.001]). In the AST, insulin secretory response to arginine at basal glucose and during hyperglycemia was lower in T2DM compared with NGT and PDM (>58%; all P < 0.001). FSIGT showed decreases in both insulin secretion and Si across populations (P < 0.001), although Si did not differ significantly between PDM and T2DM populations. Reproducibility was generally good for the MMTT, with intraclass correlation coefficients (ICCs) ranging from ∼0.3 to ∼0.8 depending on population and variable. Reproducibility for the AST was very good, with ICC values >0.8 across all variables and populations.
CONCLUSIONS
Standardized MMTT and AST provide reproducible and complementary measures of BCF with characteristics favorable for longitudinal interventional trials use.
Introduction
The pathophysiologic hallmarks of type 2 diabetes mellitus (T2DM) are defects in insulin action and β-cell function (BCF) (1), with the latter manifesting as inadequate insulin secretion in response to hyperglycemia (2). Early intervention may ameliorate these defects (3). However, determination of whether a given intervention has clinically relevant effects on BCF requires long-term testing in large cohorts. Therefore, the ability to simply and reproducibly test BCF is critical. Quantification of BCF would enable evaluation of interventions tailored to specific functional β-cell defects in multiple populations, as well as their potential to alter disease progression.
It is readily apparent that assessment of BCF has been accomplished using different challenge routes (oral vs. intravenous), stimuli (e.g., arginine, glucagon, glucose, mixed meals), and sampling times, often in single-center studies (4–7). Even for the same method, such as the meal tolerance test, the composition and caloric load of the test meals often differ (5,8,9). Additionally, different approaches have been used concurrently to assess insulin secretion and sensitivity. Some reports use simple ratios or calculations for insulin sensitivity (Si) and secretion using measurements under basal (e.g., homeostasis model assessment of BCF and quantitative insulin sensitivity check index) or postchallenge conditions (e.g., Matsuda index and ΔI0–30/ΔG0–30). However, due to the complex physiology of appearance of the nutrient stimuli and the sites and timing of insulin action and clearance, indices obtained using these simple calculations may have limited utility (6,7). Some of the most widely used methods of measuring BCF are described in Table 1, along with a summary of the strengths and limitations of these methods.
Table 1.
Comparison of current methods for measuring BCF
| Test | Description | Advantages | Limitations |
|---|---|---|---|
| Hyperglycemic clamp | A variable IV glucose infusion is administered to maintain the glucose level at a steady state | Provides measures of insulin secretion (first and second phases) and with modeling insulin action | No GI incretin effects |
| Requires continuous adjustment of IV glucose | |||
| Frequent blood sampling and minute-to-minute adjustments of glucose infusion rate at bedside are required | Does not require modeling of data for insulin secretion | Technically challenging to conduct testing | |
| Widely reported and accepted | Expertise limited to select centers | ||
| Graded glucose infusion | IV glucose is administered at progressively increasing rates (each rate maintained for ∼40 min) | Provides measure of insulin secretion over a range of glucose levels | No GI incretin effects |
| Requires frequent blood sampling | Provides measure of β-cell glucose sensitivity | Not as widely studied and reported as hyperglycemic clamp, especially in the context of therapeutic interventions | |
| Data analyses often require expertise in model-based methods | |||
| FSIGT | Rapid IV injection of glucose is followed 20 min later by an IV injection of insulin | Provides insulin secretion and action during rapidly changing glucose levels | No GI incretin effect |
| Requires very frequent blood sampling | Provides first-phase insulin release measures | Technically challenging to conduct | |
| Insulin action results correlate well with those from euglycemic clamp | Expertise to conduct limited to select centers | ||
| Widely used and reported | Requires computer modeling for the outcome measures, requiring specialized expertise | ||
| With C-peptide modeling, provides second-phase insulin release | Requires IV administration of insulin | ||
| AST | IV arginine is administered followed by combined glucose/arginine infusions | Measures of insulin secretion known to correlated with β-cell mass in islet transplant recipients | Mixed effect on incretin response |
| Frequent blood samplings over a short period of time are necessary | Provides a measure of near-maximal insulin secretion (insulin secretory reserve) | Requires IV administration of arginine and glucose | |
| Does not inform on insulin action | |||
| Glucagon stimulation test | IV glucagon is given twice sequentially (at baseline and after glucose has been infused to achieve elevated glucose) | Robust insulin secretory response similar to that of arginine but through different mechanism of action | No oral incretin effect |
| Requires IV administration of glucagon | |||
| Does not inform on insulin action | |||
| Side effects of nausea and vomiting are common and potentially confounding | |||
| MMTT/OGTT | Oral meal or glucose solution is ingested | Easy to administer | Assumptions must be made for rate of nutrient absorption into systemic circulation |
| MMTT physiologically highly relevant, mimicking oral challenges routinely encountered daily | Effect of incretins included | Technically challenging to model outcome measure of insulin secretion of sensitivity, requiring software and expert analysis | |
| Blood samples taken at specified intervals up to 5 h postchallenge | Provides insulin secretion and action during changing glucose levels | Lack of standardized test meal | |
| OGTT standardized and simple as single substrate | MMTT with minimal modeling not as widely reported as the hyperglycemic and euglycemic clamps | ||
| Insulin action and secretion results correlate with those from hyperglycemic and euglycemic clamps |
GI, gastrointestinal; IV, intravenous.
Recognizing the need for more consensus methods for the measurement of BCF in humans, the multistakeholder β-Cell Project Team (BCPT) of the Foundation for the National Institutes of Health (FNIH) Biomarkers Consortium (http://www.biomarkersconsortium.org/) was formed to standardize and characterize select BCF tests. The goal of this consortium-driven work is to enable inclusion of such assessments in future longitudinal clinical trials examining response to therapeutic interventions and disease progression. Members of the BCPT are listed in the appendix. In accordance with the principles of the FNIH partnership, project results are made publically available.
We sought to characterize the reproducibility of two methods that could be used in a multicenter clinical trial and that could quantify insulin secretion across the glucose tolerance spectrum. An important aspect of quantify insulin secretion is to do so as a function of prevailing insulin action. This is the basis of the minimal model as applied to data obtained from an intravenous (frequently sampled intravenous glucose tolerance test [FSIGT]) or oral challenge (mixed-meal tolerance test [MMTT] or oral glucose tolerance test [OGTT]) (10,11). Although the FSIGT is the only in vivo test that potentially replicates the in vitro finding of first-phase insulin secretion, it is more difficult to implement routinely in large multicenter interventional trials (12,13). For the BCPT, this spurred interest in alternative BCF tests, with particular emphasis on operational feasibility and in the context of a physiologically relevant challenge (e.g., meal ingestion) (14).
Additional considerations for BCF testing include attempts to elicit near-maximal stimulation (15) of insulin secretion and determination of insulin secretory reserve. Arginine and glucagon in pharmacologic doses have been used for these purposes, with both stimuli yielding similar responses, although arginine is better tolerated (16). The response to either secretagogue is potentiated by simultaneous administration of glucose. The arginine stimulation test (AST) has been used in islet autotransplantation studies in which the insulin secretory response to arginine correlates with the number of transplanted islets in this patient population (17,18). Although these various tests have been used to detect and quantify phenotypic differences across health and disease, their application to evaluate pharmacologic interventions has been demonstrated in a limited number of studies (19–21). In particular, there is scant information on their variability and reproducibility characteristics.
Following review and discussion of each method, the BCPT selected the MMTT (with minimal model calculations) and AST for further study. The selection was based on both scientific and practical reasons. The MMTT offers a simple-to-administer physiologic test that incorporates the incretin response. When analyzed with the minimal model, the MMTT provides a simultaneous estimate of insulin secretion and sensitivity. The AST generates a supraphysiologic insulin secretory response and, like the MMTT, is far less technically demanding than methodologies such as the hyperglycemic clamp. The BCPT also chose to include the FSIGT as a widely accepted comparator to allow for a reference method, especially as the FSIGT and MMTT both use minimal model approaches for data analysis.
The methodologies for the MMTT, AST, and FSIGT were first standardized. Subsequently, experiments to estimate between and within subject variability (and reproducibility) as well as contrast the means and distributions of BCF parameters across the glucose tolerance spectrum were undertaken. To minimize differences in body composition for comparisons across glucose tolerance groups, all subjects were required to be obese, including those with normal glucose tolerance (NGT).
Research Design and Methods
Subjects
Three groups of subjects classified by their fasting and postchallenge glucose tolerance status (2-h post–75-g OGTT) (and balanced for gender) were studied. NGT subjects had a fasting glucose <100 mg/dL and postchallenge <140 mg/dL; prediabetes mellitus (PDM) subjects had impaired fasting glucose (≥100 and <126 mg/dL) and impaired glucose tolerance (≥140 and <200 mg/dL); subjects with T2DM had fasting glucose values of 126–270 mg/dL and HbA1c 6.5–10.0% on a stable dose of metformin monotherapy (500–2,000 mg/day).
Study Design
After obtaining Institutional Review Board approval, local advertisement was used to recruit for trials, conducted at two study sites (ICON Development Solutions in San Antonio, TX, and Omaha, NE). After written informed consent was obtained and the subjects were screened, all subjects underwent each procedure on separate days. The MMTT and AST were administered twice and the FSIGT once, grouped into three separate visits during which the subject resided at the research center from the evening before the first test until completion of all of the tests that were part of that visit. During each of the first two visits, the overall order of procedures was fixed, with an MMTT on day 1 and AST on day 2, after which the subject was discharged. At the third and separate visit, an FSIGT was performed. All testing was completed within 28 days with at least 5 days between visits.
Procedures
In those subjects with T2DM, metformin was held on the morning of the procedure. After a 10-h overnight fast, a single indwelling intravenous catheter was placed in the forearm for the MMTT, whereas for the AST and FSIGT, indwelling catheters were placed in both upper extremities for infusion and sample acquisition, respectively. The procedures are briefly described below with extensive detail in the Study Operations Manual (available at http://www.fnih.org/what-we-do/current-research-programs/biomarkers-consortium-beta-cell-project).
MMTT
Following baseline sampling (−30, −15, and 0 min), a test meal (470 kcal, ∼66% carbohydrate, 18% fat, and 16% protein) composed of one 8-fluid-ounce Boost nutrition supplement drink (Nestlé Health Science) and one PowerBar (Nestlé Nutrition) was administered. The meal was consumed within 10 min, with the bar consumed first and serial sampling for analytes performed at 10, 15, 20, 30, 60, 90, 120, 180, and 240 min postmeal.
AST
Following baseline sampling (−10, −5, and 0 min), an intravenous injection of 5 g of arginine (given as 10% arginine HCl [as R-Gene; Pfizer]) was administered over 60 s followed by serial sampling at 2, 4, 5, 7, and 10 min. Subsequently, glucose levels were raised by a continuous infusion of glucose (20% dextrose at 900 mg/min) over 60 min with repeat sampling at 50, 55, and 60 min, followed by a second dose of 5 g of arginine at 60 min with sampling at 62, 64, 65, 67, and 70 min.
FSIGT
Following baseline sampling (−30, −15, and 0 min), a 300 mg/kg glucose bolus was administered with sampling at 2, 4, 8, and 19 min. At 20 min, a single dose (0.03 units/kg) of U100 regular human insulin (Humulin R; Eli Lilly and Company, Indianapolis, IN) was administered intravenously with sampling at 22, 30, 40, 50, 70, 100, 180, and 240 min.
Analyte Assays
Samples were assayed in the Immunochemical Core Laboratory, Mayo Clinic (Rochester, MN). Glucose was measured on the Roche Cobas c311 (Roche Diagnostics, Indianapolis, IN) using a hexokinase reagent. C-peptide was measured by a two-site immunometric assay on the Roche Cobas e411 (Roche Diagnostics). Insulin (plasma) was measured by a two-site immunometric (sandwich) assay using electrochemiluminescence detection on the Roche Cobas e411 (Roche Diagnostics). All intra-assay coefficients of variation (CVs) were <3% and interassay CVs <6%.
Data and Statistical Analyses
MMTT
Baseline glucose, insulin, and C-peptide were calculated as the average of −30-, −15-, and 0-min values. Si was estimated using the oral glucose minimal model (22). β-Cell responsivity index (Фtot), a measure of insulin secretion, was estimated from the individual subject plasma glucose and C-peptide concentrations observed during the experiment using the oral C-peptide minimal model (23) incorporating C-peptide kinetics as reported by Van Cauter et al. (24). Disposition index (DI) was calculated as the product of Si and Фtot. For the purposes of this series, a standardized approach to modeling across glucose tolerance populations was used. MMTT analyses were performed using Matlab US R2010B (MathWorks, Natick, MA) with code provided by C. Cobelli. (also available through The Epsilon Group, Charlottesville, VA). No time points were excluded in the derivation of individual subject-level parameters.
FSIGT
Si was calculated by fitting the glucose profiles from the intravenous glucose tolerance tests using Minmod Millennium (Version 6.02; Minmod Inc., Los Angeles, CA). The acute insulin response to glucose (AIRg) was calculated as the area under the curve (AUC) of insulin concentration above the average basal value, from 0 to 10 min after the glucose injection. The DI was calculated as the product of the average Si and AIRg from each experiment.
AST
At basal glucose, the insulin secretory response to arginine at basal glucose (AIRarg) was derived as the mean of the three highest insulin values from minutes 2, 3, 4, and 5 minus baseline insulin at basal glucose (average of −10, −5, and 0 min). At elevated glucose, maximal insulin secretory response to arginine under hyperglycemic conditions (AIRargMAX) was derived as the mean of the three highest insulin values at minutes 62, 63, 64, and 65 minus baseline insulin at elevated glucose (average of 50, 55, and 60 min). Insulin secretory reserve, ISR, represents the difference between insulin secretion at elevated and at basal glucose (AIRargMAX − AIRarg).
Data Conventions and Handling
Analyses for AST and MMTT were performed on natural log-transformed data for subjects having matched pairs (both visits). Final results for transformed parameters were exponentiated and reported on the original scale. The Grubbs’ test for a single outlier was used to assess extreme values, and, if found to be significant at the one-sided, 0.001 significance level, a secondary, sensitivity analysis was performed excluding these data for re-estimation of variance components and intraclass correlation coefficients (ICCs) and presented as secondary analyses. All other analyses, including tests for glucose tolerance population differences, and correlation analyses are presented with all evaluable subjects including extreme values.
Variance Component Estimation
Between- and within-subject variance component estimates were derived using a mixed-effects model, treating gender as a fixed effect, subjects grouped by gender as a random effect, and visits as a repeated effect. Initial modeling allowed for the potential of separate between- and within-subject variances across genders. Log-likelihood ratio tests at the two-sided 0.05 significance level were used to select the most appropriate model. As parameterized, the model accounts for any gender differences in mean response with simultaneous estimation of between- and within-subject variance components. Estimates (95% CI) for log-normally distributed data were reported as geometric CV and model predicted adjusted geometric means (95% CI). Tukey contrasts adjusting for multiplicity were used for comparison of means across glucose tolerance populations at the two-sided 0.05 significance level.
Using the estimated variance components, the ICC was calculated as (σ2between/[σ2between + σ2within]). The ICC is a measure of the degree to which repeated measures within the same subject resemble each other, a measure of relative repeatability (25). ICC values >0.80 were considered highly reproducible. ICC values >0.50 were considered moderately reproducible, whereas those <0.50 were considered weakly reproducible. Overall correlations across populations were derived, including correlations within each population to make a general assessment of the consistency of results (concordance) within and across populations. Correlations among the different parameters are reported as Spearman rank correlations. The MMTT and AST values used were the subject averages.
Results
The evaluable subjects included 23 NGT (12 men/11 women), 17 PDM (6 men/11 women), and 22 T2DM (11 men/11 women), for a total of 62 subjects. Complete demographic characteristics are summarized in Table 2. All groups, including those with NGT, were obese. No serious procedure-emergent adverse events were reported. A complete summary of procedure-emergent adverse events is reported in Supplementary Table 1.1 and 1.2.
Table 2.
Summary demographics and anthropometrics for evaluable subjects by glucose tolerance population
| Parameter/population | NGT | PDM | T2DM |
|---|---|---|---|
| Number of evaluable (paired) observations [N (men/women)] | 23 (12 men/11 women) | 17 (6 men/11 women) | 22 (11 men/11 women) |
| Age (years ± SD) | 41.9 ± 8.4 | 44.8 ± 9.8 | 54.7 ± 8.1 |
| Weight (kg ± SD) | 85.8 ± 14.3 | 97.4 ± 12.9 | 91.1 ± 14.1 |
| BMI (kg/m2 ± SD) | 31.5 ± 2.8 | 35.0 ± 3.8 | 32.8 ± 3.9 |
| Ethnicity distribution | |||
| White, not Hispanic or Latino | 5 | 3 | 3 |
| White, Hispanic, or Latino | 14 | 10 | 16 |
| Black, not Hispanic | 4 | 3 | 2 |
| Other | 1 | 1 | |
| HbA1c | 5.7 ± 0.38% (39 mmol/mol) | 8.28 ± 0.79% (67 mmol/mol) |
For the PDM population, there were 16 evaluable subjects for the AST.
Measured Parameters—Glucose, Insulin, and C-Peptide Concentrations
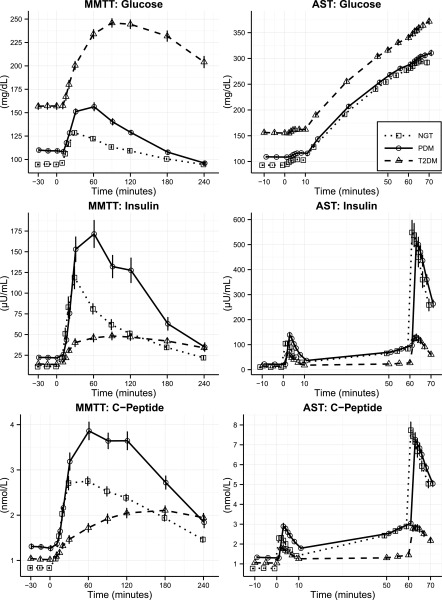
Summary plasma profiles of glucose, insulin, and C-peptide for the MMTT and the AST are shown in Fig. 1, reflecting the mean values of both pairs of tests. Additional figures displaying the excursion of these analytes during each of the two MMTTs, as well as the two ASTs, can be found in Supplementary Fig. 1.1–1.6 (with additional information found in Supplementary Table 2.1–2.3). As expected, fasting glucose rose progressively across NGT, PDM, and T2DM. Fasting insulin and C-peptide were comparable in NGT and T2DM and higher in PDM. In the MMTT, following the meal challenge, glucose rose progressively across populations on both test days, achieving peak levels in T2DM > PDM > NGT, whereas insulin and C-peptide responses were highest in PDM, followed by NGT and then T2DM. AUC for glucose, insulin, and C-peptide all differed among the three groups (Supplementary Table 2.2).
Figure 1.
Mean ± SEM glucose, insulin, and C-peptide concentration time course profiles by stimulation test and glucose tolerance group. Squares, NGT; circles, PDM; triangles, T2DM.
In the AST, in response to arginine, increases over baseline in insulin and C-peptide were observed in all groups on both test days during basal and hyperglycemic conditions. The insulin secretory response to arginine was blunted in those with T2DM compared with PDM and NGT. In the FSIGT, following the glucose bolus, glucose rose progressively over baseline in all groups, achieving peak levels greater in T2DM than in PDM or NGT (Supplementary Fig. 1.7–1.9 and Supplementary Table 2.4). Endogenous insulin and C-peptide responses within the first 19 min were blunted in T2DM. Following exogenous insulin, return to baseline for glucose occurred within 50 min in NGT, but was more delayed in PDM and T2DM.
Indices of BCF From the MMTT, AST, and FSIGT
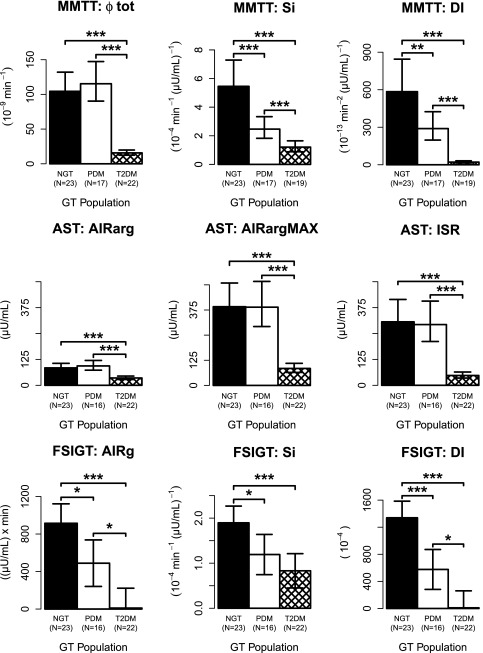
Overall, insulin secretion, as measured by either the MMTT or AST, was similar between the NGT and PDM groups, yet both differed significantly from the T2DM subjects. Fig. 2 summarizes the derived responses to the MMTT and AST, with the actual values presented in Table 3. In the MMTT, β-cell responsivity (Фtot, an index of insulin secretion) in the T2DM group was 86 and 87% lower compared with NGT and PDM (P < 0.001), respectively. Although Фtot was numerically higher in PDM compared with NGT, the difference was not statistically different. Si decreased progressively across glucose tolerance populations, with T2DM being 78 and 52% lower compared with NGT and PDM (P < 0.001), respectively, whereas PDM was 55% lower compared with NGT (P < 0.01). Correspondingly, DI was 51% lower in the PDM group compared with NGT (P < 0.001) and was 93% lower in T2DM compared with PDM (P < 0.001).
Figure 2.
Indices of BCF. Model predicted geometric means (95% CI) for AST and MMTT and arithmetic means (95% CI) for FSIGT. Note: lower CI suppressed for graphical purposes for FSIGT AIRg and DI in T2DM. GT, glucose tolerance. *P < 0.05, **P < 0.01, ***P < 0.001.
Table 3.
Variability and reproducibility metrics: geometric means, between- and within-subject geometric CVs, and ICCs for MMTT and AST and arithmetic means and total CVs for FSIGT
| Stimulation test parameter | Population (N) | Model predicted geometric mean (95% CI) | Geometric CV% between subjects (90% CI) | Geometric CV% within subjects (90% CI) | ICC (90% CI) |
|---|---|---|---|---|---|
| MMTT: Φtot (10−9min−1) | NGT (N = 23) | 104 (83–132) | 27.1 (14.7–51.6) | 41.4 (32–54.1) | 0.31* (0.30–0.77) |
| PDM (N = 17) | 115 (90–147) | 34.8 (24.1–51.1) | 19.6 (14.7– 26.2) | 0.753 (0.70–0.9) | |
| T2DM (N = 22) | 15 (12–20) | 57.8 (41.8–82.1) | 26.1 (20.2–33.8) | 0.814 (0.69–0.96) | |
| MMTT: Si (10−4min−1 [µU/mL]−1) | NGT (N = 23) | 5.5 (4.1–7.3) | 48.2 (34.1–69.8) | 32.6 (25.4–42.3) | 0.674 (0.521–0.874) |
| PDM (N = 17) | 2.5 (1.8–3.3) | 44 (28.4–70.5) | 35.5 (26.5–48.2) | 0.598 (0.469–0.837) | |
| T2DM (N = 19) | 1.2 (0.9–1.6) | 53.5 (26.9–121.5) | 83.2 (60.1–120.6) | 0.323† (0.034–0.772) | |
| MMTT: DI (10−13min−2 [µU/mL]−1) | NGT (N = 23) | 585 (405–845) | 42.3 (22.7–84.9) | 66.1 (50–89.6) | 0.312‡ (0.264–0.769) |
| PDM (N = 17) | 289 (197–424) | 54.4 (35.3–87.9) | 40.3 (29.9–55) | 0.633 (0.543–0.852) | |
| T2DM (N = 19) | 21 (14–31) | 67.3 (35.1–152.2) | 97.1 (69–145) | 0.36§ (0.254–0.754) | |
| AST: AIRarg (µU/mL) | NGT (N = 23) | 84 (67–106) | 38.9 (29.4–52.1) | 11.6 (9.1–14.8) | 0.914 (0.902–0.961) |
| PDM (N = 16) | 94 (73–120) | 38.9 (27.6–55.7) | 11.5 (8.6–15.4) | 0.915 (0.874–0.972) | |
| T2DM (N = 22) | 35 (28–45) | 53.7 (39.1–75.4) | 22.8 (17.7–29.5) | 0.833 (0.734–0.966) | |
| AST: AIRargMAX (µU/mL) | NGT (N = 23) | 391 (301–509) | 57.5 (42.8–79.2) | 16.6 (13–21.2) | 0.914 (0.858–0.967) |
| PDM (N = 16) | 389 (293–517) | 31.2 (22.1–44.5) | 11.2 (8.3–15) | 0.882 (0.815–0.969) | |
| T2DM (N = 22) | 84 (65–110) | 63.1 (46.6–87.9) | 13.8 (10.7–17.7) | 0.947 (0.931–0.982) | |
| AST: ISR (µU/mL) | NGT (N = 23) | 306 (226–414) | 64.1 (47.2–89.6) | 20.1 (15.7–25.7) | 0.897 (0.83–0.96) |
| PDM (N = 16) | 293 (212–406) | 34.4 (24.3–49.3) | 12.4 (9.2–16.6) | 0.88 (0.759–0.976) | |
| T2DM (N = 22) | 47 (35–64) | 79.3 (57.3–114.5) | 19.6 (15.3–25.3) | 0.928 (0.916–0.968) | |
| FSIGT: no within-subject variability estimable, total variance estimated, and analyses on arithmetic scale. | |||||
| • Mean (95% CI) CV% AIRg ([µU/mL]) × min): NGT, 915 (708–1,122), 81%; PDM, 489 (241–737), 77%; and T2DM, 10 (−201 to 222), 413%. | |||||
| • Mean (95% CI) CV% Si (10−4min−1 [µU/mL]−1): NGT, 1.9 (1.5–2.3), 60%; PDM, 1.2 (0.7–1.6), 37%; and T2DM, 0.8 (0.4–1.2) 101%. | |||||
| • Mean (95% CI) CV% DI (10−4): NGT, 1,339 (1,093–1,585), 64%; PDM, 576 (282–871), 90%; and T2DM, 9 (−242 to 261), 557%. | |||||
Exclusion of single-visit extreme value produces good reproducibility results for MMTT across all GT populations: *ICC = 0.59 (0.551–0.801) excluding single-visit extreme value (P < 0.001) from NGT (N = 22); †ICC = 0.559 (0.387–0.795) excluding single-visit extreme value (P < 0.001) from T2DM (N = 18); ‡0.527 (0.476–0.797) excluding single-visit extreme value (P < 0.001) from T2DM (N = 18); §ICC = 0.527 (0.476–0.797) excluding single-visit extreme value (P < 0.001) from T2DM (N = 18).
In 3 of the 44 (∼7%) individual subject visits in the MMTT in the T2DM population, the standardized analytical approach yielded near-zero values for Si. As predefined in the analysis plan, the Si and DI data from these subjects were not included in the primary analyses. An alternate, slightly modified approach to the minimal model as described by Basu et al. (26) (see Supplementary Data) that allowed for inclusion of Si data from these three subjects did not yield values for the point estimates (geometric means and 95% CI) of Si or DI (with these three subjects, Si = 1.2 [0.9–1.6]; DI = 19 [13–38]) that were different from the original analysis (without these three subjects, Si = 1.2 [0.9–1.6] and DI = 21 [14–31]). This was true for the variance component estimates as well.
In the AST, AIRarg, AIRargMAX, and, most notably, the ISR were all lower (P < 0.001) in T2DM compared with NGT and PDM (58 and 63%, 79 and 78%, and 85 and 84% lower, respectively, for NGT and PDM). No significant differences were observed between NGT and PDM.
In the FSIGT, AIRg progressively and significantly decreased across glucose tolerance populations with the mean value for T2DM being nearly zero (Fig. 2). AIRg was statistically separable among the three glucose tolerance groups. Likewise, Si decreased progressively across glucose tolerance populations, although the difference between PDM and T2DM did not reach statistical significance. DI decreased significantly and progressively across groups.
Between- and Within-Subject Variability and Reproducibility for Measured Parameters and Indices From MMTT and AST
Glucose, insulin, and C-peptide responses within the MMTT and AST for each population had good reproducibility (Supplementary Fig. 1.1–1.6 and Supplementary Table 2.1–2.3). The AUCs (0–4 h) for glucose, insulin, and C-peptide from the MMTT generally displayed moderate to high reproducibility (as per ICC values), with the sole exception of glucose in the NGT. Reproducibility, as indexed by the ICC, ranged from weak to strong in the MMTT for all model-based parameters in all populations (Table 3). The inclusion of the three additional subjects with T2DM whose Si was numerically nonidentifiable did not affect that conclusion (not shown). For the AST, reproducibility was strong across all parameters/populations (all values >0.8).
Four extreme values from one of the two visits for two subjects (Φtot and DI in NGT and Si and DI in T2DM) for MMTT parameters were flagged during outlier analyses (P < 0.001). ICCs excluding these points as secondary, sensitivity analyses are provided in the legend for Table 3. When these outliers were excluded, reproducibility for the MMTT (per ICC values) rose considerably.
Comparative Assessment of Model-Based Indices of BCF Across Tests and Metabolic Spectrum
Correlation analyses were undertaken to better understand how these different measures tracked with one another within the same subject populations and across populations. Within the AST, the overall correlation between AIRarg and AIRargMAX was notably high across (0.923) and within (0.794–0.930) all populations (Table 4), indicating concordance in results across populations. In addition, the overall correlation between the AST-derived measures (AIRarg and AIRargMAX) and Фtot from the MMTT was high (0.858) and statistically significant across and within all populations. Overall correlations across glucose tolerance between AIRg from the FSIGT and Фtot from the MMTT as well as that between AIRg and AIRargMAX were high (0.753 and 0.826), whereas within-population correlation was less so, especially in T2DM. Si estimated in the MMTT and FSIGT showed a high overall correlation (0.695), consistent within the three populations. The overall correlation between DI from the MMTT and FSIGT was high (0.779), whereas no relationship for this parameter was observed within groups.
Table 4.
Spearman correlations and significance tests within and across stimulation tests for key parameters
| Parameter association | Across GT populations | Within NGT population | Within PDM population | Within T2DM population |
|---|---|---|---|---|
| MMTT: Φtot (10−9min−1) vs. AST: AIRargMAX (µU/mL) | 0.858*** | 0.492* | 0.638** | 0.799*** |
| AST: AIRarg (µU/mL) vs. AST: AIRargMAX (µU/mL) | 0.923*** | 0.914*** | 0.794*** | 0.930*** |
| MMTT: Φtot (10−9min−1) vs. AST: ISR (µU/mL) | 0.853*** | 0.482* | 0.553* | 0.744*** |
| MMTT: Φtot (10−9min−1) vs. FSIGT: AIRg ([µU/mL] × min) | 0.753*** | 0.394# | 0.279 (NS) | 0.010 (NS) |
| AST: AIRargMAX (µU/mL) vs. FSIGT: AIRg ([µU/mL] × min) | 0.826*** | 0.779*** | 0.379 (NS) | 0.082 (NS) |
| MMTT: Si (10−4min−1 [µU/mL]−1) vs. FSIGT: Si (10−4min−1 [µU/mL]−1) | 0.695*** | 0.827*** | 0.650** | 0.486* |
| MMTT: DI (10−13min−2 [µU/mL]−1) vs. FSIGT: DI (10−4) | 0.779*** | 0.179 (NS) | 0.085 (NS) | −0.284 (NS) |
| AST: ISR (µU/mL) vs. FSIGT: AIRg ([µU/mL] × min) | 0.854*** | 0.824*** | 0.476# | 0.156 (NS) |
GT, glucose tolerance. *P < 0.05; **P < 0.01; ***P < 0.001; #P < 0.1; NS, P ≥ 0.1.
Conclusions
The current series of studies was undertaken to characterize the responses to and reproducibility of a standardized MMTT and AST for assessment of BCF in subjects with NGT, PDM, and T2DM. We report that the MMTT and AST are able to detect differences in BCF across the metabolic spectrum. The reproducibility of the MMTT is, in general, moderate (ranging from weak to strong), depending on parameter and population. The reproducibility of the AST is very strong across all populations. Importantly, for both MMTT and AST, the observed variability predicts reasonably sized clinical studies to detect clinically meaningful changes in insulin secretion. The MMTT- and AST-based measures of BCF are directionally and proportionally concordant and generally concur with indices derived from the reference FSIGT. It should be noted that despite these tests having been in use for quite some time, this is the first report of within- and between-subject variability for outcome parameters from the standardized MMTT and AST, especially in subjects across the metabolic spectrum. These data should be of value for the computation of sample size in interventional studies in relevant populations.
The standardized test meal used in the current series was able to elicit responses in glucose, insulin, and C-peptide during the MMTT that were similar to prior reports in NGT, PDM, and T2DM (27,28). Our findings for between-group differences for model-based estimates of BCF (Φtot) in those with and without diabetes are generally consistent with those reported by Bock et al. (27) and Ferrannini et al. (29), as well as with other methods such as the graded glucose infusion (30), OGTT (29), and hyperglycemic clamp (31). The significant progressive decrease in Si from NGT to T2DM is concordant with prior reports using clamp-based assessments (29). Notably, the DI, a measure of the appropriateness of insulin secretion to prevailing levels of insulin action, decreased across populations, indicating that the methodology was able to detect a progressive decrease in BCF. Together, these observations suggest that the MMTT used in this series recapitulated prior reports of BCF and insulin action measured using different tests.
The AST was able to elicit a β-cell response at baseline glycemia (AIRarg) and hyperglycemia (AIRmax) in all three populations. The responses were similar to those previously reported separately in each population (32,33). AIRarg, AIRmax, and ISR were highly reproducible across populations. The ability of β-cells to respond to arginine appears to be preserved in prediabetes.
An approach to better understand the utility of the reproducibility metrics of the MMTT and AST is to compute the sample sizes required to detect predetermined differences using parameters derived from the current trial. For example, in the MMTT, to detect a 25% increase in Φtot, 18 subjects per group are required for 80% power at a one-sided, 0.05 significance level. Although the variability observed in Si and DI is greater than with Φtot, in the current study, the MMTT detected differences across groups in Si and DI with modest numbers of subjects (∼20) per group. It should be noted that the weaker reproducibility observed in some cases was driven by a single outlier, as shown by the sensitivity analyses. For the AST, five subjects per group are needed at the same power and significance level. Thus, both tests exhibit variances that permit reasonably sized clinical trials in relevant populations.
The glycemic excursions and insulin secretory responses in the FSIGT detected differences in insulin secretion across the metabolic spectrum, which recapitulated previous experimental results (10,34,35). An interesting observation in the current series was that although Фtot in the MMTT did not differ between NGT and PDM, AIRg in the FSIGT was decreased in PDM subjects compared with the NGT subjects. There are two salient points to recognize, however. First, previously published studies have generally made similar observations regarding the MMTT. Previous work from Bock et al. (27) and Ferrannini et al. (29) reported that insulin secretion in subjects with IGT is similar to that of obese subjects with NGT. Distinctions in insulin secretion are detectable, in general, only when contrasted to lean subjects with NGT (29). The subjects with NGT in the current series had a BMI that was close to those of the PDM group and had high normal fasting plasma glucose and insulin levels; they likely had overlap of some aspects of BCF with PDM.
Second, it should be noted that although the MMTT and the FSIGT are different tests, they arrived at the same conclusion—i.e., a progressive decrease in DI from NGT to PDM to T2DM. Ultimately, the DI provided by the minimal model yields an integrated estimate of insulin secretion in the context of insulin action. In the context of a meal, enteral delivery of substrate to subjects with PDM elicits an increase in insulin secretion that, on an absolute basis, is similar to that of obese subjects with NGT. However, insulin secretion is inadequate for the prevailing insulin action, as quantified by the DI and as expected by inspection of the glucose profiles in each group. The FSIGT, in contrast to the MMTT’s mixed substrate delivery and elicitation of incretin (and other) responses, uses intravenous glucose as the sole stimulus that results in a lesser insulin secretory response in PDM than NGT subjects. Consistent with prior reports, the FSIGT in the current series was able to detect differences in acute insulin secretion in response to glucose across the metabolic spectrum (10,34,35). At the same time, the integrated parameter represented in DI showed a progressive decrease from NGT to PDM to T2DM. Thus, with some differences noted, the MMTT and the FSIGT provided the same overall conclusion.
Given the fairly unique circumstance of having access to data from multiple tests of BCF in the same subjects and across the glucose tolerance spectrum, it was informative to ask how the measures of BCF related to one another. Several key findings emerged across tests and within and across populations. In general, correlation across all three glucose tolerance populations was high for every comparison, and comparisons of parameters of BCF obtained with the FSIGT and MMTT showed correlation characteristics consistent with previous reports (36). However, in some instances, within each population, the associations were less prominent. This suggests that some associations may in part be due to significant differences between populations and less so to a correlation of the tests, per se, within a subject. A notable example would be comparisons between Φtot (MMTT) and AIRg (FSIGT), as well as between AIRarg/AIRmax (AST) and AIRg. Although these showed reasonable overall correlations across glucose tolerance groups, they were weaker within each group, especially within T2DM. Taken together, the above observations suggest that although the various indices from each of these tests likely quantify different facets of BCF, there is concordance in the directionality and proportionality across tests (29).
Operationally, the MMTT is simple enough to routinely perform at most clinical research sites and centers. The test meal used in this series had a balanced macronutrient composition, with solid and liquid components, and is readily available from a manufacturer, assuring consistency of the test meal across sites. For similar operational reasons, the AST can be performed at most clinical research sites. Some procedural choices were made to simplify the testing, including sampling of nonarterialized venous blood (i.e., no hot hand). We recognize that the use of the hot hand method may have improved reproducibility. Our approach, however, is similar to previous studies that did not obtain arterialized samples for assessment of insulin secretion and sensitivity (29,37) and avoids potential issues due to the technique (38,39).
Analytically, in contrast to the AST, the MMTT and FSIGT require model-based analyses and occasionally need operator intervention to reconcile near-zero or negative values, respectively, for some parameters (35,40,41). In the current series, we opted for a more standardized analysis strategy that led to nonidentifiable Si parameters for MMTT in three subject visits in the T2DM population. Various methods can mitigate such model-related vulnerabilities (42,43), although inclusion of these three subjects did not change the conclusions. Furthermore, a novel method for assessing parameter reproducibility applied to minimal model data supports the reliability of the MMTT-derived indices (44). Although this series used the Cobelli oral minimal model for MMTT (12), it is important to recognize that there are other methods for estimating BCF from MMTT or OGTT data (37).
In summary, the current series of experiments shows that the variability and reproducibility characteristics of the MMTT and AST are sufficient to detect the types of changes in measured and modeled parameters of BCF that are generally expected in response to therapeutic interventions in relevant patient populations. Although the choice of testing methodology will ultimately be determined by the scientific question, the standardized MMTT and AST can provide reliable, reproducible, and complementary measures of BCF, with characteristics favorable for use in large, longitudinal interventional studies. Further studies are required to evaluate these tests’ performance characteristics in response to a specific pharmacotherapy or lifestyle intervention.
Supplementary Material
Article Information
Acknowledgments. The authors thank Jessica Ratay and Cheryl Melencio for the continuous support in planning, executing, and managing these studies and the overall project; Kathryn Wright for excellent management of the many samples generated in this study; Clay Dehn and Angelica Guerrero for contributions to the study operations manual and operationalizing the methodologies; Hilary Blair and the staff of the Mayo Clinic Endocrine Laboratory; and the research and data management staff at ICON Development Solutions.
Funding. The methodological study described in this report was designed and implemented under the auspices of the FNIH Biomarkers Consortium. The Biomarkers Consortium is a public private partnership that develops and validates biological markers, which speed up the development of therapies for the detection, prevention, diagnosis, and treatment of disease and are ultimately aimed at improving patient care. For this study, the Consortium brought together diabetes experts from leading academic institutions, the U.S. Food and Drug Administration (FDA), the National Institutes of Health (NIH), the nonprofit sector, and the pharmaceutical industry to develop the project. The results of the partnership, discussed in this study, are important because they address a critical unmet medical need and involve key stakeholders in the diabetes treatment field. FNIH has acted as a neutral convener for the partners and provided the project management expertise needed for the execution of the overall project. In the future, this type of partnership can be used as a model for establishing common standards for testing in other therapeutic areas as well. Under the auspices of the FNIH, this project was jointly funded by the NIH/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the FDA and the following participating pharmaceutical companies: Amylin (now AstraZeneca), Janssen, Eli Lilly and Company, Merck, Novartis, Novo Nordisk, Pfizer, Sanofi, and Takeda. An in-kind donation of P800 tubes was made by Becton Dickinson (D. Craft). Additional support was also received from the American Diabetes Association and JDRF.
Duality of Interest. S.S.S. is an employee and shareholder of Eli Lilly and Company. R.H.R. is a shareholder of Bristol-Myers Squibb. R.A.C., D.C., J.Q.D., and D.S.L. are employees and shareholders of Pfizer. R.N.B. is responsible for the MinMod program, which is also marketed. C.Ca. is an employee and shareholder of Takeda Pharmaceuticals. M.D. is an employee and shareholder of Eli Lilly and Company and a member of the American Diabetes Association grant review panel. D.P. is an employee and shareholder of Johnson & Johnson. H.R. is an employee and shareholder of Sanofi. D.A.F. is a shareholder of Pfizer. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.S.S., A.V., and R.H.R. designed the experiment, researched data, wrote, edited, and reviewed the manuscript, and coordinated overall integration of the manuscript. M.A.S., R.A.C., R.N.B., C.Ca., D.C., C.Co., M.D., D.S.L., D.P., R.P.R., H.R., D.S., M.T.V., and G.C.W. designed the experiment, researched data, and wrote, edited, and reviewed the manuscript. C.D.M. and J.Q.D. researched data and reviewed the manuscript. D.A.F. designed the experiment, researched data, oversaw study execution, edited and reviewed the manuscript, and coordinated overall integration of the manuscript. D.A.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, 21–25 June 2013, and at the 29th Annual Meeting of the European Association for the Study of Diabetes, Barcelona, Spain, 23–27 September 2013.
Appendix
Current Members of the β-Cell Project Team. Richard Bergman, PhD, Cedars-Sinai Diabetes & Obesity Research Institute; Roberto Calle, MD, Pfizer; Charlie Cao, PhD, Takeda Development Center Americas; Danny Chen, PhD, Pfizer; Claudio Cobelli, PhD, University of Padova; Mark Farmen, PhD, Eli Lilly and Company; David Fryburg, MD, FNIH and Roi Biopharma Consulting; Atalanta Gosh, PhD, Janssen; Ilan Irony, MD, Center for Drug Evaluation and Research/FDA; David Kelley, MD, Merck; Douglas Lee, PhD, Pfizer; Frank Martin, PhD, JDRF; Henriette Mersebach, MD, Novo Nordisk; Lori Mixson, PhD, Merck; Stephanie Moran, MD, Takeda Development Center Americas; David Polidori, PhD, Janssen; Jessica Ratay, MS, FNIH; Ralph Raymond, MS, FNIH and R-Squared Solutions; R. Paul Robertson, MD, Pacific Northwest Diabetes Research Institute and University of Washington; Hartmut Ruetten, MD, PhD, Sanofi; Peter Savage, MD, NIH/National Institute of Diabetes and Digestive and Kidney Diseases; Sudha Shankar, MD, Eli Lilly and Company; Myrlene Staten, MD, Kelly Government Solutions on contract to NIH/NIDDK; Darko Stefanovski, PhD, University of Pennsylvania; Maria Vassileva, PhD, FNIH; Adrian Vella, MD, Mayo Clinic; Gordon Weir, MD, Joslin Diabetes Center; and Marjorie Zakaria, MD, Novartis.
Previous Members of the β-Cell Project Team Who Contributed to This Work. Richard Chen, PhD, formerly of NIDDK/NIH; Mark Deeg, MD, Eli Lilly and Company; Ying Ding, PhD, formerly of Eli Lilly and Company; Christian Djurhuus, MD, PhD, Novo Nordisk; Cong Han, PhD, Takeda; David Maggs, MD, formerly of Amylin; Mads Rasmussen, MD, PhD, Novo Nordisk; Thomas Strack, MD, formerly of Takeda; Krystyna Tatarkiewicz, PhD, formerly of Amylin; and Adrianne Wong, formerly of JDRF.
Footnotes
Clinical trial reg. nos. NCT01454973, NCT01663207, and NCT01663207, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-0931/-/DC1.
S.S.S. and A.V. share first authorship.
References
- 1.Sathananthan A, Dalla Man C, Zinsmeister AR, et al. . A concerted decline in insulin secretion and action occurs across the spectrum of fasting and postchallenge glucose concentrations. Clin Endocrinol (Oxf) 2012;76:212–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes 1989;38:1512–1527 [DOI] [PubMed] [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic β-cell function: review of methods and clinical applications. Curr Diabetes Rev 2014;10:2–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marena S, Montegrosso G, De Michieli F, Pisu E, Pagano G. Comparison of the metabolic effects of mixed meal and standard oral glucose tolerance test on glucose, insulin and C-peptide response in healthy, impaired glucose tolerance, mild and severe non-insulin-dependent diabetic subjects. Acta Diabetol 1992;29:29–33 [DOI] [PubMed] [Google Scholar]
- 6.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008;294:E15–E26 [DOI] [PubMed] [Google Scholar]
- 7.Pisprasert V, Ingram KH, Lopez-Davila MF, Munoz AJ, Garvey WT. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care 2013;36:845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selimoglu H, Duran C, Kiyici S, et al. . Comparison of composite whole body insulin sensitivity index derived from mixed meal test and oral glucose tolerance test in insulin resistant obese subjects. Endocrine 2009;36:299–304 [DOI] [PubMed] [Google Scholar]
- 9.Wolever TM, Chiasson JL, Csima A, et al. . Variation of postprandial plasma glucose, palatability, and symptoms associated with a standardized mixed test meal versus 75 g oral glucose. Diabetes Care 1998;21:336–340 [DOI] [PubMed] [Google Scholar]
- 10.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 1981;68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes 2014;63:1203–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nesher R, Cerasi E. Modeling phasic insulin release: immediate and time-dependent effects of glucose. Diabetes 2002;51(Suppl. 1):S53–S59 [DOI] [PubMed] [Google Scholar]
- 13.Caumo A, Luzi L. First-phase insulin secretion: does it exist in real life? Considerations on shape and function. Am J Physiol Endocrinol Metab 2004;287:E371–E385 [DOI] [PubMed] [Google Scholar]
- 14.Xiang AH, Watanabe RM, Buchanan TA. HOMA and Matsuda indices of insulin sensitivity: poor correlation with minimal model-based estimates of insulin sensitivity in longitudinal settings. Diabetologia 2014;57:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toschi E, Camastra S, Sironi AM, et al. . Effect of acute hyperglycemia on insulin secretion in humans. Diabetes 2002;51(Suppl. 1):S130–S133 [DOI] [PubMed] [Google Scholar]
- 16.Robertson RP, Raymond RH, Lee DS, et al.; Beta Cell Project Team of the Foundation for the NIH Biomarkers Consortium . Arginine is preferred to glucagon for stimulation testing of β-cell function. Am J Physiol Endocrinol Metab 2014;307:E720–E727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robertson RP, Bogachus LD, Oseid E, et al. . Assessment of β-cell mass and α- and β-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015;64:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teuscher AU, Kendall DM, Smets YF, Leone JP, Sutherland DE, Robertson RP. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes 1998;47:324–330 [DOI] [PubMed] [Google Scholar]
- 19.Derosa G, Carbone A, Franzetti I, et al. . Effects of a combination of sitagliptin plus metformin vs metformin monotherapy on glycemic control, β-cell function and insulin resistance in type 2 diabetic patients. Diabetes Res Clin Pract 2012;98:51–60 [DOI] [PubMed] [Google Scholar]
- 20.Derosa G, Franzetti IG, Querci F, et al. . Exenatide plus metformin compared with metformin alone on β-cell function in patients with Type 2 diabetes. Diabet Med 2012;29:1515–1523 [DOI] [PubMed] [Google Scholar]
- 21.Derosa G, Ragonesi PD, Carbone A, et al. . Vildagliptin added to metformin on β-cell function after a euglycemic hyperinsulinemic and hyperglycemic clamp in type 2 diabetes patients. Diabetes Technol Ther 2012;14:475–484 [DOI] [PubMed] [Google Scholar]
- 22.Dalla Man C, Caumo A, Cobelli C. The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Trans Biomed Eng 2002;49:419–429 [DOI] [PubMed] [Google Scholar]
- 23.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 2001;50:150–158 [DOI] [PubMed] [Google Scholar]
- 24.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 25.Koch GG, Amara IA, Davis GW, Gillings DB. A review of some statistical methods for covariance analysis of categorical data. Biometrics 1982;38:563–595 [PubMed] [Google Scholar]
- 26.Basu A, Dalla Man C, Basu R, Toffolo G, Cobelli C, Rizza RA. Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care 2009;32:866–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bock G, Dalla Man C, Campioni M, et al. . Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 2006;55:3536–3549 [DOI] [PubMed] [Google Scholar]
- 28.Smushkin G, Sathananthan M, Piccinini F, et al. . The effect of a bile acid sequestrant on glucose metabolism in subjects with type 2 diabetes. Diabetes 2013;62:1094–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. beta-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 30.Kjems LL, Holst JJ, Vølund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003;52:380–386 [DOI] [PubMed] [Google Scholar]
- 31.Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. J Pediatr 2012;161:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Zijl NJ, Goossens GH, Moors CC, et al. . Ectopic fat storage in the pancreas, liver, and abdominal fat depots: impact on β-cell function in individuals with impaired glucose metabolism. J Clin Endocrinol Metab 2011;96:459–467 [DOI] [PubMed] [Google Scholar]
- 34.Buchanan TA, Xiang AH, Peters RK, et al. . Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes 2002;51:2796–2803 [DOI] [PubMed] [Google Scholar]
- 35.Mayer-Davis EJ, Monaco JH, Hoen HM, et al.; The Insulin Resistance Atherosclerosis Study (IRAS) . Dietary fat and insulin sensitivity in a triethnic population: the role of obesity. Am J Clin Nutr 1997;65:79–87 [DOI] [PubMed] [Google Scholar]
- 36.Cobelli C, Toffolo GM, Dalla Man C, et al. . Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am J Physiol Endocrinol Metab 2007;293:E1–E15 [DOI] [PubMed] [Google Scholar]
- 37.Utzschneider KM, Prigeon RL, Tong J, et al. . Within-subject variability of measures of beta cell function derived from a 2 h OGTT: implications for research studies. Diabetologia 2007;50:2516–2525 [DOI] [PubMed] [Google Scholar]
- 38.Nauck MA, Blietz RW, Qualmann C. Comparison of hyperinsulinaemic clamp experiments using venous, ‘arterialized’ venous or capillary euglycaemia. Clin Physiol 1996;16:589–602 [DOI] [PubMed] [Google Scholar]
- 39.Petrie JR, Ueda S, Morris AD, Elliott HL, Connell JM. Potential confounding effect of hand-warming on the measurement of insulin sensitivity. Clin Sci (Lond) 1996;91:65–71 [DOI] [PubMed] [Google Scholar]
- 40.Watanabe RM, Valle T, Hauser ER, et al. . Familiality of quantitative metabolic traits in Finnish families with non-insulin-dependent diabetes mellitus. Finland-United States Investigation of NIDDM Genetics (FUSION) Study investigators. Hum Hered 1999;49:159–168 [DOI] [PubMed] [Google Scholar]
- 41.Quon MJ, Cochran C, Taylor SI, Eastman RC. Non-insulin-mediated glucose disappearance in subjects with IDDM. Discordance between experimental results and minimal model analysis. Diabetes 1994;43:890–896 [DOI] [PubMed] [Google Scholar]
- 42.Pillonetto G, Sparacino G, Cobelli C. Numerical non-identifiability regions of the minimal model of glucose kinetics: superiority of Bayesian estimation. Math Biosci 2003;184:53–67 [DOI] [PubMed] [Google Scholar]
- 43.Pillonetto G, Sparacino G, Magni P, Bellazzi R, Cobelli C. Minimal model S(I)=0 problem in NIDDM subjects: nonzero Bayesian estimates with credible confidence intervals. Am J Physiol Endocrinol Metab 2002;282:E564–E573 [DOI] [PubMed] [Google Scholar]
- 44.Dalla Man C, Pillonetto G, Riz M, Cobelli C. An index of parameter reproducibility accounting for estimation uncertainty: theory and case study on β-cell responsivity and insulin sensitivity. Am J Physiol Endocrinol Metab 2015;308:E971–E977 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.