Abstract
No imaging methodology currently exists to monitor viable islet mass after clinical intraportal islet transplantation. We investigated the potential of the endocrine positron emission tomography (PET) marker [11C]5-hydroxytryptophan ([11C]5-HTP) for this purpose. In a preclinical proof-of-concept study, the ex vivo and in vivo [11C]5-HTP signal was compared with the number of islets transplanted in rats. In a clinical study, human subjects with an intraportal islet graft (n = 8) underwent two [11C]5-HTP PET and MRI examinations 8 months apart. The tracer concentration in the liver as a whole, or in defined hotspots, was correlated to measurements of islet graft function. In rat, hepatic uptake of [11C]5-HTP correlated with the number of transplanted islets. In human subjects, uptake in hepatic hotspots showed a correlation with metabolic assessments of islet function. Change in hotspot standardized uptake value (SUV) predicted loss of graft function in one subject, whereas hotspot SUV was unchanged in subjects with stable graft function. The endocrine marker [11C]5-HTP thus shows a correlation between hepatic uptake and transplanted islet function and promise as a tool for noninvasive detection of viable islets. The evaluation procedure described can be used as a benchmark for novel agents targeting intraportally transplanted islets.
Introduction
In many countries, intraportal islet transplantation is an established treatment strategy for the most severe forms of type 1 diabetes (T1D). The function and survival of transplanted islets are known to be compromised during the acute peritransplant period (1–3) and long term (4) as assessed by islet [18F]fluorodeoxyglucose labeling pretransplantation and positron emission tomography (PET)/computed tomography (CT) imaging and indirect metabolic tests. Preloading of islets with paramagnetic material has been used in a similar fashion for MRI assessment of posttransplantation graft viability (5,6).
The glucose-potentiated arginine test has been validated for the assessment of functional β-cell mass after islet autotransplantation in recipients with euglycemia (7,8). However, for a given number of islets, transplanted autoislets have four- to fivefold greater functional capacity than allogeneic islets. In addition, in subjects with failing islet grafts, hyperglycemia may cause degranulation of the insulin-producing cells. Direct in situ targeting of islets would overcome these limitations, enabling longitudinal noninvasive assessment of viable islet mass, and would further help to optimize transplantation protocols and improve patient management.
We have previously reported the use of the serotonin precursor [11C]5-hydroxytryptophan ([11C]5-HTP) as a surrogate imaging marker for islet mass in the human pancreas (9,10). Because [11C]5-HTP is routinely used in the clinic for localizing ectopic foci of β-cells (in the form of insulinoma metastasis in the liver), transplanted islets also could conceivably be visualized with the same technology (11).
In the current study, we aimed to assess the feasibility of the approach in a preclinical intraportal islet transplantation rat model. Subsequently, a prospective study in subjects with T1D who already underwent intraportal islet transplantation was performed, correlating the [11C]5-HTP uptake in liver with metabolic function during stimulation with a mixed-meal tolerance test (MMTT).
Research Design and Methods
Radiochemistry
The tracer [11C]5-HTP was produced according to a published method (12) at the Uppsala University Hospital PET center, with >95% purity. Clinical grade tracer deliveries were used for both the preclinical and the clinical studies.
Preclinical Study
All animals were housed under standard laboratory conditions and had free access to food and water. All handling procedures and experiments were performed in accordance with national guidelines and were approved by the local ethics committee for animal research.
Intraportal Transplantation
Nondiabetic Sprague Dawley rats (n = 22) were intraportally transplanted with 0 (mock transplant, n = 7), 300 (n = 7), 600 (n = 7), or 1,200 (n = 7) rat islets. The transplanted rats were housed for 6 weeks to allow for engraftment and revascularization before PET studies.
Organ Distribution Study
Each animal was administered 2–20 MBq [11C]5-HTP corresponding to <1 μg tracer mass. Two to three rats in each group were euthanized by CO2 30 min after tracer administration. The remaining rats were examined by PET (see next section) before being euthanized 60 min after tracer administration. The liver of each animal was then resected, weighed, and measured for radioactive uptake in a well counter. The radioactive uptake was decay corrected to the time of injection and further corrected for total amount of tracer administered and animal weight. The radioactive uptake in tissue was reported as the standardized uptake value (SUV) (Eq. 1) to allow for comparison between mice. The hepatic uptake in each mouse was plotted against the number of transplanted islets.
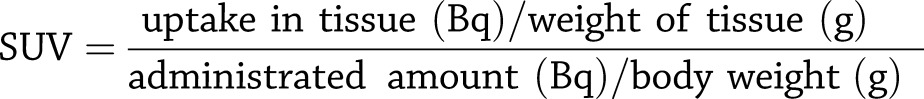 |
Eq. 1 |
Small Animal PET
After tracer administration, rats were kept under 1.5–2.5% isoflurane anesthesia blended with 450 mL/min air/O2 delivered through a face mask for 60 min and examined by a small animal PET/CT scanner (Triumph trimodality system; Trifoil Imaging, Northridge, CA). A heated bed supported body temperature. After the PET examination, the rats were euthanized, and organ distribution examination was performed as previously described.
The data sets were reconstructed into a static 60-min image by using a maximum likelihood, expectation-maximization three-dimensional algorithm (10 iterations). Images were normalized to SUV using PMOD version 3.508 software (PMOD Technologies, Zurich, Switzerland) to enable direct comparison between animals.
Retrospective Study of Nontransplanted Subjects With T1D and Healthy Volunteers
For comparison of the hepatic radiotracer background uptake in nontransplanted subjects with T1D, we analyzed the uptake of [11C]5-HTP in subjects with T1D (n = 8) who were enrolled in a previous study on [11C]5-HTP uptake and retention in the pancreas (8). In that study, dynamic PET examinations were performed for 60 min (30 frames: 12 × 10 s, 6 × 30 s, 5 × 120 s, 5 × 300 s, 2 × 600 s) over the abdominal region, with the pancreas in the center of the field of view. The observable regions of liver uptake of [11C]5-HTP were measured by delineating the liver on sequential coregistered CT images using PMOD. CT-derived volumes of interest (VOIs) were transferred to [11C]5-HTP PET images. Liver uptake during the entire examination was expressed as mean SUV. The subjects with T1D were not age matched with the subjects who had undergone intraportal islet graft (IPX) enrolled in the current study.
Prospective Studies of Subjects With Intraportal Islet Transplants
The study is a part of the CIT-09 (Clinical Islet Transplantation) trial. The regional ethics board and the regional radiation committee for diagnostic and radionuclide therapy in Uppsala approved the study protocol. All subjects provided informed written consent before participation.
Study Subjects
All subjects included in the prospective study had received an islet transplant within the Nordic network at either Karolinska University Hospital (Stockholm, Sweden) or Uppsala University Hospital (Uppsala, Sweden). Subjects with IPX (n = 8) underwent two [11C]5-HTP PET/CT examinations 8 months (±4 weeks) apart (Table 1). The study included subjects with expected stable graft function over the 8-month period.
Table 1.
Overview of the study subjects
| Subject group | Age (years) | Sex |
|---|---|---|
| IPX | ||
| IPX1 | 50 | F |
| IPX2 | 57 | F |
| IPX3 | 53 | F |
| IPX4 | 57 | M |
| IPX5 | 59 | M |
| IPX6 | 67 | M |
| IPX7 | 64 | F |
| IPX8 | 50 | M |
| T1D* | ||
| T1D1 | 29 | F |
| T1D2 | 28 | M |
| T1D3 | 27 | M |
| T1D4 | 25 | M |
| T1D5 | 25 | F |
| T1D6 | 28 | F |
| T1D7 | 23 | F |
| T1D8 | 26 | M |
*Retrospective analysis from a previous study (8).
All PET/CT examinations were performed at the PET center at Uppsala University Hospital. MRI examinations were performed at the radiology departments of Uppsala University Hospital (n = 4) and Karolinska University Hospital (n = 4). One subject (IPX8) did not undergo a second PET examination. Table 2 shows the number of infused islets and the number of transplants for each subject.
Table 2.
Overview of the number of transplantations and islet equivalents for each subject with IPX
| Islet equivalents |
|||||||
|---|---|---|---|---|---|---|---|
| No. of Tx | IPX1 | IPX2 | IPX3 | IPX4 | IPX5 | IPX6 | IPX7 |
| 1 | 693,587 | 310,869 | 800,010 | 427,609 | 237,935 | 300,000 | 339,979 |
| 2 | 445,435 | 336,696 | 600,000 | 680,435 | 334,239 | 300,000 | 332,935 |
| 3 | 364,978 | 250,022 | 325,000 | 471,739 | 400,000 | 210,326 | |
| 4 | 520,000 | 203,261 | 450,000 | ||||
| 5 | 379,565 | ||||||
Tx, transplant.
PET Examination
Before the PET/CT examination, all subjects had fasted >4 h. Female subjects had a negative pregnancy test. The subjects were positioned on the bed of a Discovery ST PET/CT scanner (GE Healthcare) to include the liver in the field of view. A low-dose CT scout view (140 kV, 10 mA) was performed. Attenuation correction CT examination over the abdomen, including the entire liver, was acquired during exhalation by a 140-kV, AutomA 10–80 mA.
One hundred fifty to 400 MBq [11C]5-HTP (∼2–5 MBq/kg) was administered manually as an intravenous bolus through a catheter in the arm. Dynamic scanning for 60 min was initiated at administration (33 frames: 1 × 10 s, 8 × 5 s, 4 × 10 s, 2 × 15 s, 6 × 30 s, 5 × 120 s, 5 × 300 s, 2 × 600 s).
Image acquisition was performed in three dimensions and reconstructed by using an iterative VuePoint order subset expectation-maximization algorithm (4-mm Hann filter, two iterations, 21 subsets) in a 128 × 128 matrix (zoom 50 cm). Reconstructed data were analyzed using PMOD. Whole liver and other organs of interest (pancreas as well as erector spinae muscle, which was used as a negative reference) were delineated on sequential coregistered CT images. CT VOIs were transferred to [11C]5-HTP PET images, and organ uptake of tracer was expressed as mean SUV over 60 min for each subject and organ (Eq. 1). The same total scanning time (60 min) was used to calculate mean SUV for the subjects with IPX as well as for subjects in the retrospective study to make them comparable.
Analysis of PET Hotspots in Liver
A small volume of transplanted islets and uptake of [11C]5-HTP are potentially drowned in the hepatic background because of the sheer size of the liver (2,000 mL) compared with the transplanted islets (1–5 mL). Hence, the uptake of [11C]5-HTP in the liver of nontransplanted subjects with T1D from the retrospective study were chosen as reference. When examining the mean SUV uptake, values 2 × SD above the average tracer uptake (SUV = 1.68) in this group, few hotspots were seen. Therefore, the average plus 2 × SD (SUV = 2.09) was assumed to represent the maximal natural background uptake in subjects with T1D. All areas with a mean SUV >2.09 in subjects with IPX, therefore, were considered to represent hotspots, which could represent accumulation in islets rich in serotonergic biosynthesis.
Hotspot maps were generated in each subject’s liver by applying the isocontouring operation in the whole-bounding liver VOI in PMOD, with the following settings: algorithm, 3D; search mode, hot; inner holes; multiple contours; mode of searching, automatic; and absolute threshold lower cutoff value, 2.09.
These hotspots or isocontoured VOIs were generated for the contouring of structures on the basis of an intensity threshold. [11C]5-HTP uptake in all hotspots in aggregate were calculated in terms of mean SUV. Additionally, the number of individually resolved hotspots was recorded. Either the hotspot SUV or the number of hotspots was used for correlation with graft function.
MRI Examination
MRIs were implemented after respective [11C]5-HTP PET/CT scans to complement the information received from the scans. MRIs were performed at 1.5 T with chemical shift gradient echo in-phase and opposed-phase image acquisition. Steatosis in the liver was assessed, and if present, steatotic foci were correlated with PET hotspots.
Measurement of β-Cell Function
Each imaging examination was linked to a metabolic evaluation (performed at the local hospital). The metabolic evaluations were performed ±10 days from each PET/CT examination.
All subjects underwent an MMTT, which was used to determine basal (fasting) and stimulated glucose and C-peptide levels. MMTTs were performed after overnight fasting and while on bed rest. For the MMTT, all subjects received 6 mL/kg body weight (to a maximum of 360 mL) of resource protein drink (Boost; Nestlé) consumed in 5 min, starting at time 0. The B-glucose and C-peptide concentrations were then measured after 15, 30, 60, 90, and 120 min.
The area under the curve (AUC) for C-peptide was generated for each subject using Prism 5.04 software (GraphPad Software, La Jolla, CA). The zero-point C-peptide was used as baseline for AUC calculations. We also used the peak C-peptide value as well as the C-peptide value immediately following the first B-glucose >8 mmol/L as measurements of graft function. Each of these three measures of islet graft function was correlated against the PET output. The change in graft function between examinations (change in AUC C-peptide or C-peptide immediately following the first B-glucose >8 mmol/L) was calculated by subtracting the parameter value at examination 1 from the parameter value at examination 2. Kidney clearance was not corrected for because subject creatinine levels were similar (131 ± 40 μmol/L, range 92–202 μmol/L) and stable over the duration of the study.
Statistical Analysis
All data were expressed as average ± SEM. The correlation charts among the subjects with IPX, subjects with T1D, and healthy subjects were acquired with a parametric one-way ANOVA analysis. Linear regression was performed with Prism 5.04 software to assess the correlation between PET measurements (whole-liver SUV or hotspot SUV) and measures of graft function.
Results
Preclinical Study
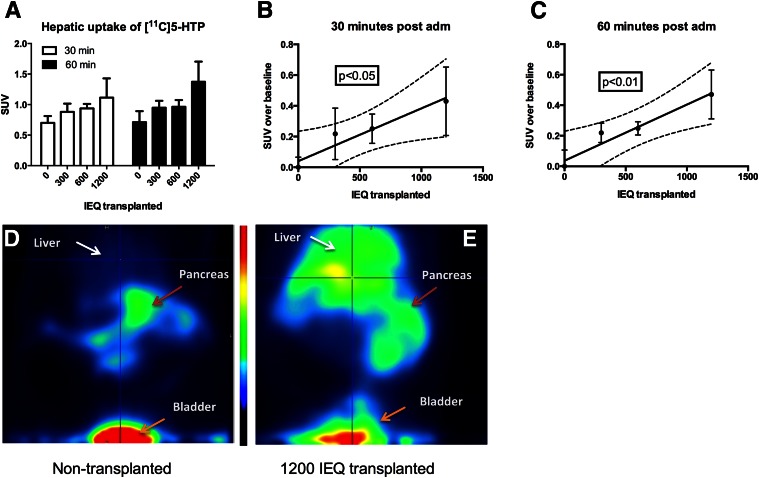
Ex vivo radiotracer uptake as assessed by sensitive organ distribution measurements showed a relatively low hepatic background SUV of ∼0.6 in nontransplanted rats (Fig. 1). A progressive increase in hepatic SUV was observed in rats transplanted with 300, 600, and 1,200 islets (Fig. 1A). SUV above the nontransplanted liver baseline correlated with transplanted islet mass both 30 min (Fig. 1B) and 60 min (Fig. 1C) after tracer administration. Figure 1D shows representative abdominal biodistribution of [11C]5-HTP, with high retention seen in the pancreas, weak retention in the liver, and excretion through the bladder. In an animal transplanted with 1,200 islets, there was an increased retention in liver, whereas the remaining biodistribution was unchanged (Fig. 1E).
Figure 1.
Preclinical evaluation of [11C]5-HTP in a rat model of intraportal islet allotransplantation. A progressive increase in hepatic SUV was observed in rats transplanted with 300, 600, and 1,200 islets (A) when measured ex vivo 30 min (B) or 60 min (C) after tracer administration. PET imaging in representative subjects demonstrates low hepatic background in a nontransplanted subject (D). In an animal transplanted with 1,200 islets, there was an increased hepatic retention (E). adm, administration; IEQ, islet equivalent.
Clinical Study
Correlation Between Transplanted Islet Mass and Current Metabolic Function
The metabolic function of the transplanted islets, as assessed during an MMTT by either AUC C-peptide, peak C-peptide, or immediate C-peptide response at first B-glucose >8 mmol/L, did not correlate with total number of transplanted islets, number of islets transplanted during the past 2 years, or time since last islet transplantation (data not shown).
Comparison Between Transplanted and Nontransplanted Subjects
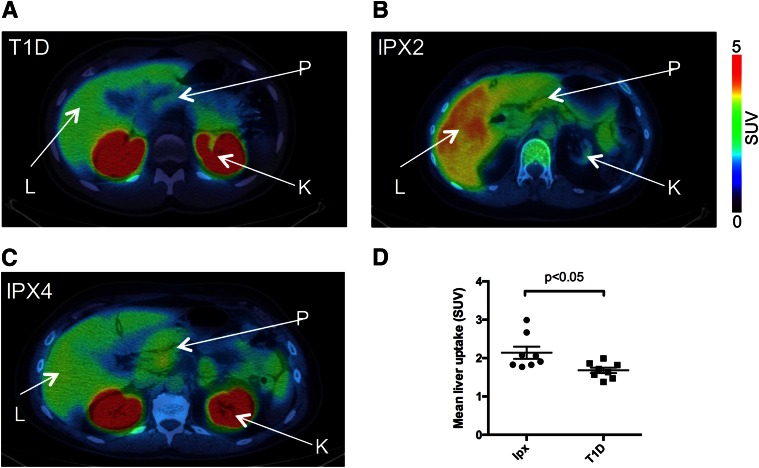
In nontransplanted subjects with T1D, a higher background signal was observed than in the rat model, with an average SUV >1.5 (Fig. 2A and D). Subjects with IPX, as measured during the first PET examination, had higher tracer uptake than nontransplanted subjects (Fig. 2B–D). In nontransplanted subjects, the hepatic uptake corresponded to an SUV of 1.68 ± 0.205.
Figure 2.
Comparison of hepatic uptake of [11C]5-HTP between nontransplanted subjects with T1D (A) and subjects with IPX with good function (B) and low function (C). The renal [11C]5-HTP uptake is absent in B due to renal failure (subject IPX2 was transplanted with islets after receiving a kidney transplant). The renal uptake, although high in comparison, does not affect the liver measurement due to spillover effects. The average hepatic background uptake was SUV = 1.68 in nontransplanted subjects, which was lower than the average SUV = 2.14 in subjects with a hepatic islet graft (D). K, kidney; L, liver; P, pancreas.
Correlation Between [11C]5-HTP PET Output and Metabolic Function
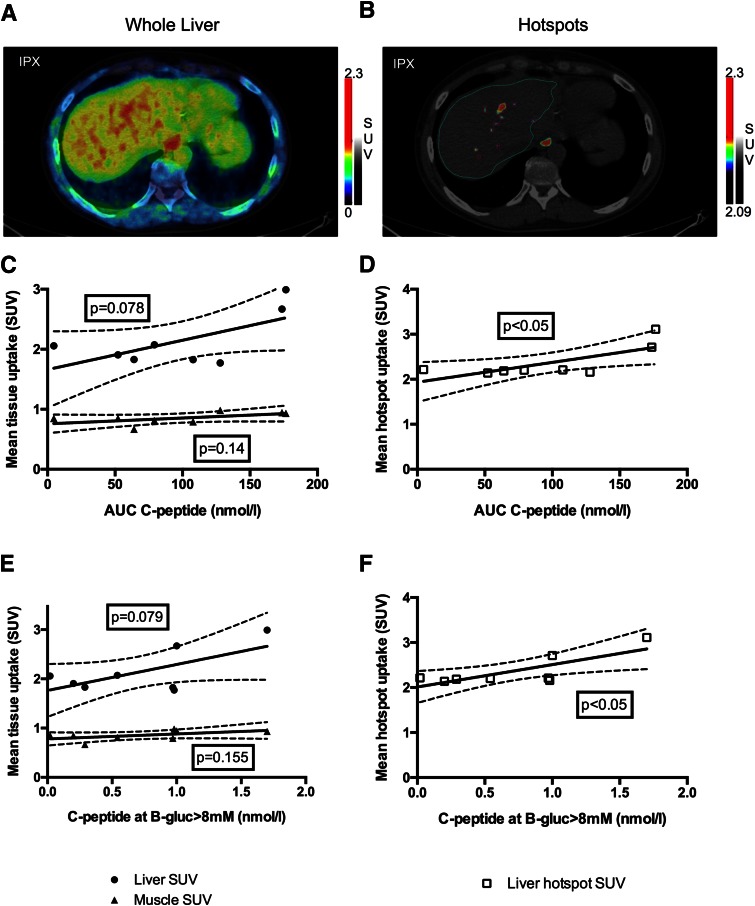
The results of the first round of PET examinations are shown in Figs. 2B–D and 3. Figure 3A and B shows an example of how the whole liver and liver hotspots were delineated. A weak correlation (P < 0.1) was observed between the average whole-liver concentration of [11C]5-HTP and islet function as assessed by AUC C-peptide (Fig. 3C) and immediate C-peptide response at first B-glucose >8 mmol/L (Fig. 3E) after an MMTT challenge to graft insulin secretion. Peak C-peptide response after the same MMTT challenge did not correlate to whole-liver SUV. The correlation to the negative control muscle tissue was lower than for liver.
Figure 3.
Mean SUV in whole liver and in hepatic hotspots as measured by [11C]5-HTP PET and their correlation to the metabolic function of intraportally transplanted islets. PET output was measured either as whole-liver mean SUV (A) or as mean SUV in hotspots 2 × SD above the expected hepatic background in subjects with T1D (B). Whole-liver mean SUV correlated weakly with graft function as assessed by AUC C-peptide and C-peptide response after first B-glucose >8 mmol/L (C and E). Using tracer uptake concentration in hotspots to remove noise caused by background uptake (mean SUV in all hotspots in aggregate in each subject) improved correlation with graft function (D and F). gluc, glucose.
SUV in defined hepatic hotspots correlated to both AUC C-peptide (Fig. 3D) and C-peptide response at first B-glucose >8 mmol/L (Fig. 3F) but not peak C-peptide. The number of hotspots did not correlate with any measures of islet graft function. Steatosis of the liver, as assessed by visual inspection of the in-phase and opposed-phase MRI protocol images, was not detected in any subjects in either examination (data not shown).
Longitudinal PET to Assess the Outcome of Intraportal Islet Transplantation
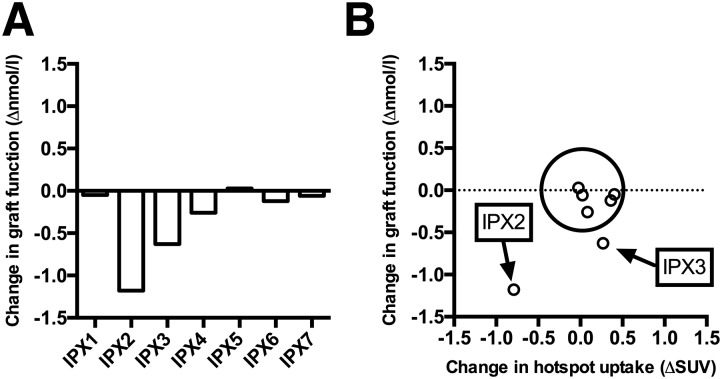
Another round of PET examinations and metabolic tests were performed 8 months after the first. Figure 4 presents the change in both metabolic function and PET output. Change in graft function as assessed by immediate C-peptide response after first B-glucose >8 mmol/L after administration of MMTT is shown in Fig. 4A. Graft function was largely unchanged in five subjects with IPX. Two subjects (IPX2 and IPX3) unexpectedly exhibited a decrease in graft function at the second examination, despite the relatively short duration (8 months) between examinations. The PET output, as measured as hotspot SUV, was unchanged in the five subjects with stable graft function (average coefficient of variation 7.8%) (Fig. 4B). The change in hotspot SUV was also markedly decreased in subject IPX2, who had the largest loss in graft function. However, the loss in graft function for subject IPX3 was not reflected in the PET output. Based on linear regression, a weak correlation was found between hotspot SUV and change in C-peptide response at first B-glucose >8 mmol/L (P = 0.059), which is mostly explained by the changes in subject IPX2.
Figure 4.
A: Change in graft function as assessed by immediate C-peptide response after first B-glucose >8 mmol/L after administration of an MMTT. Graft function was largely unchanged in five subjects with IPX. Two subjects (IPX2 and IPX3) unexpectedly exhibited a marked decrease in graft function at the second examination. B: The PET output, measured as hotspot SUV, was unchanged in the five subjects with stable graft function (average coefficient of variation 7.8%) (circled). The change in hotspot SUV was also markedly decreased in subject IPX2, who had the largest loss in graft function. However, the loss in graft function for subject IPX3 was not reflected in the PET output.
Discussion
Preclinical and clinical evidence is presented to support the notion that [11C]5-HTP can be used as a quantitative imaging biomarker for intraportally transplanted islets. As an illustration of the need for this type of methodology, no correlations were found between transplantation procedure parameters related to viable islet mass (given islet mass, number of transplantations, time since last transplantation) and islet function as assessed by current indirect plasma markers. This state of affairs makes validation of putative imaging biomarkers highly challenging because we have no a priori information on the actual amount of islets in the subjects’ liver and, thus, no gold standard to which to calibrate the scans.
Preclinical data can partially explain this problem, but we know that even our best rodent models have limited translational capability. In this study, we used an allogeneic transplantation model in Sprague Dawley rats. Nondiabetic animals were used to optimize engraftment and to prevent differences in metabolic burden on the transplanted islets. There was a distinct increase in hepatic signal in transplanted animals compared with the nontransplanted animals (Fig. 1D and E). This can be partly attributed to the low background signal (Fig. 1D), which greatly increases the possibility of detecting ectopic tissue containing the molecular machinery for biosynthesis of serotonin as opposed to the hepatocytes. The increased hepatic background in humans is presumably due to species differences in the hepatocyte capacity for metabolizing [11C]5-HTP. The hepatic background uptake of [11C]5-HTP can be partly reduced by oral pretreatment with the DOPA decarboxylase inhibitor carbidopa (100–200 mg), which increases the sensitivity for localization of neuroendocrine tumors (11). However, the effect of carbidopa varies between individuals as well as between repeated examinations in an individual. This addition of another variable lessens the possibility of absolute quantification of [11C]5-HTP uptake in the hepatic islet graft, and carbidopa, therefore, was not administered in this study.
In the clinical study, we chose to use measurements of metabolic functions in response to MMTT as an indirect assessment of the number of viable islets. AUC C-peptide reflects the total insulin secretory capacity of the graft but does not explain the dynamics of response. Instead, peak C-peptide indicates whether insulin release occurs as a bolus, as in normal physiology, or as a dysfunctional, protracted process. Immediate C-peptide response after first B-glucose >8 mmol/L partly explains the dynamics or timeliness of the C-peptide response (i.e., whether insulin secretion occurs as a nondelayed response to changes in B-glucose) and is independent of physiological factors unrelated to islet graft function, such as intestinal glucose absorption.
Measurement of whole-liver SUV or other concentration measurements in humans unlikely can reliably detect transplanted islets mainly because of two factors: the dilution of target tissue as <5 mL of islets administered into 2,000 mL liver (a dilution of >1:400), this irrespective of the molecular tracer used, and the nonendocrine background signal encountered by [11C]5-HTP itself in the human liver. It should be noted that this assay better predicts metabolic function than actual transplantation procedure parameters, such as time since last transplantation and transplanted islet mass.
We present a potentially more sensitive method of analysis: the uptake of [11C]5-HTP in hotspots 2 × SD above the expected normal physiological background. We used the livers of nontransplanted subjects with T1D as a control for the IPX population because of their shared history of T1D and associated metabolic changes as opposed to nontransplanted subjects without diabetes. Of note, the nontransplanted subjects with T1D was not age matched because of an absence of PET/CT scans, including those of the liver, at our center in Uppsala.
The hotspot SUV analysis, which decreases the influence of islet dilution as well as hepatic background to at least detect focal accumulations of islets, correlated better with metabolic function during the initial examination. Eight months later, five of the subjects with IPX retained stable graft function. Accordingly, the PET output measured as hotspot SUV was largely unchanged. The repeatability of the [11C]5-HTP PET examination, therefore, seems promising. Hotspot SUV predicted the unexpected decrease in graft function in subject IPX2 but not in subject IPX3.
Subject IPX3 exhibited good C-peptide stimulatory capacity at the first PET examination. The stimulatory capacity was markedly poorer at the time of the second PET examination 8 months later (Fig. 4). However, 4 months after the second PET examination, the C-peptide stimulatory capacity was again showing good graft function (fasting C-peptide 0.42 nmol/L, stimulated C-peptide 1.5 nmol/L).
During the study, subject IPX3 gained weight and around the time of the second PET examination started exogenous insulin (8 IU, previously insulin independent). It is possible that the increase in body weight increased insulin resistance in subject IPX3, exhausting the islet graft. The reduction of C-peptide secretory capacity at the time of the second PET examination, therefore, most likely reflects degranulation rather than loss of islet viability. After starting exogenous insulin, the metabolic load (β-cell exhaustion, glucotoxicity) on the islet graft was likely reduced, and the C-peptide secretory capacity was almost normalized 4 months later, indicating persisting viable islet graft mass throughout the study despite a further increase in body weight. Retrospectively, this individual gained 10 kg in body weight. Thus, the stable liver uptake in the PET examination indicates that [11C]5-HTP provides an improved measurement of islet viability compared with metabolic examination because it demonstrated remaining islet mass despite confounding factors such as high metabolic load and degranulation of the insulin producing cells. A larger study with more subjects with unstable, decreasing graft function is required to further examine whether the [11C]5-HTP PET hotspot SUV truly correlates with graft function.
We know from quantitative prelabeling techniques that islets tend to cluster in small hepatic foci rather than spread out homogenously across the liver in humans, which is possibly due to thrombosis (2,3). Furthermore, transplanted islet homogeneity in hepatic distribution can be shown to differ between human subjects (13). Therefore, the hotspot SUV readout from the [11C]5-HTP examination may be biased toward identifying more islets in subjects with IPX with more pronounced hepatic heterogeneity, which further points to the importance of low hepatic background for this type of application.
We attempted to visualize the islet distribution in the liver by in- and out-of-phase MRI. The rationale was that a higher fat content in the islets would appear dark against the surrounding liver in out-of-phase images. However, the islets demonstrated by hotspot SUV at the [11C]5-HTP examination could not be visualized on MRI. Hence, in- and out-of-phase 1.5-T MRI does not appear to be a feasible technique to identify intraportally transplanted islets.
In conclusion, the endocrine marker [11C]5-HTP shows a correlation between hepatic uptake and transplanted islet mass in both the preclinical and the clinical setting. The evaluation procedure described here can be used as a benchmark for novel agents targeting intraportally transplanted islets.
Article Information
Acknowledgments. The authors acknowledge all personnel involved in the Nordic Network for Islet Transplantation and the National Institutes of Health Clinical Islet Transplantation Consortium for making the study possible.
Funding. The study was funded by JDRF (17-2012-540), National Institutes of Health (2U01-AI-065192-06), Swedish Medical Research Council (K2015-54X-12219-19-4 and K2013-64X-08268-26-3), Barndiabetesfonden, Diabetesfonden, and EXODIAB.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. O.E. and O.K. contributed to the study design, preclinical and clinical studies, data analysis and interpretation, and writing of the manuscript. R.S. contributed to the preclinical and clinical studies, data analysis, and writing of the manuscript. T.E. contributed to the preclinical study and writing of the manuscript. M.W. contributed to the clinical study and writing of the manuscript. T.B.B., L.C., and H.A. contributed to the clinical MRI study and writing of the manuscript. G.T. and T.L. contributed to the clinical study design and performance and writing of the manuscript. O.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT02689479, clinicaltrials.gov.
References
- 1.Toso C, Zaidi H, Morel P, et al. . Positron-emission tomography imaging of early events after transplantation of islets of Langerhans. Transplantation 2005;79:353–355 [DOI] [PubMed] [Google Scholar]
- 2.Eriksson O, Eich T, Sundin A, et al. . Positron emission tomography in clinical islet transplantation. Am J Transplant 2009;9:2816–2824 [DOI] [PubMed] [Google Scholar]
- 3.Eich T, Eriksson O, Lundgren T; Nordic Network for Clinical Islet Transplantation . Visualization of early engraftment in clinical islet transplantation by positron-emission tomography. N Engl J Med 2007;356:2754–2755 [DOI] [PubMed] [Google Scholar]
- 4.Ryan EA, Paty BW, Senior PA, et al. . Five-year follow-up after clinical islet transplantation. Diabetes 2005;54:2060–2069 [DOI] [PubMed] [Google Scholar]
- 5.Toso C, Vallee JP, Morel P, et al. . Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling. Am J Transplant 2008;8:701–706 [DOI] [PubMed] [Google Scholar]
- 6.Malosio ML, Esposito A, Brigatti C, et al. . MR imaging monitoring of iron-labeled pancreatic islets in a small series of patients: islet fate in successful, unsuccessful, and autotransplantation. Cell Transplant 2015;24:2285–2296 [DOI] [PubMed] [Google Scholar]
- 7.Rickels MR, Liu C, Shlansky-Goldberg RD, et al. . Improvement in β-cell secretory capacity after human islet transplantation according to the CIT07 protocol. Diabetes 2013;62:2890–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson RP, Bogachus LD, Oseid E, et al. . Assessment of β-cell mass and α- and β-cell survival and function by arginine stimulation in human autologous islet recipients. Diabetes 2015;64:565–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson O, Selvaraju RK, Johansson L, et al. . Quantitative imaging of serotonergic biosynthesis and degradation in the endocrine pancreas. J Nucl Med 2014;55:460–465 [DOI] [PubMed] [Google Scholar]
- 10.Eriksson O, Espes D, Selvaraju RK, et al. . Positron emission tomography ligand [11C]5-hydroxy-tryptophan can be used as a surrogate marker for the human endocrine pancreas. Diabetes 2014;63:3428–3437 [DOI] [PubMed] [Google Scholar]
- 11.Orlefors H, Sundin A, Garske U, et al. . Whole-body (11)C-5-hydroxytryptophan positron emission tomography as a universal imaging technique for neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and computed tomography. J Clin Endocrinol Metab 2005;90:3392–3400 [DOI] [PubMed] [Google Scholar]
- 12.Bjurling P, Watanabe Y, Tokushige M, Oda T, Långström B. Synthesis of B-11C-labeled L-tryptophan and 5-hydroxy-L-tryptophan using a multi-enzymatic reaction route. J Chem Soc, Perkin Trans 1 1989;1331–1334 [Google Scholar]
- 13.Eriksson O. Imaging Islets of Langerhans by Positron Emission Tomography: Quantification of Beta-Cell Mass in the Native Pancreas and the Islet Graft. Uppsala, Sweden, Acta Universitatis Upsaliensis, 2011 [Google Scholar]