Although it is well accepted that type 2 diabetes pathophysiology involves decreases in β-cell function and mass, their relative importance has been debated (1–3). Moreover, it is unclear whether β-cells adapt to insulin resistance by secreting more insulin per β-cell (increased function) or by increasing β-cell mass.
A major reason why these questions remain unanswered is the lack of an animal model where dynamic changes in islet mass and islet function can be noninvasively monitored. Current approaches for optical monitoring of β-cell mass in vivo lack sufficient spatial resolution, and histological measurements in ex vivo pancreatic sections do not allow function and mass to be measured simultaneously.
In this issue of Diabetes, Chen et al. (4) transplanted isolated islets into the anterior chamber of the eye of a recipient mouse. This enabled longitudinal and simultaneous monitoring of β-cell mass and β-cell function. Although transplanting islets into a foreign milieu such as the eye is unusual and imperfect, this ingenious approach allowed the investigators to exploit the immunologically privileged eye environment (5–7) and the ability to image engrafted islets through the crystalline lens using confocal microscopy. Previous work from the group demonstrated the feasibility of the approach for exploring a range of questions related to diabetes (5). In the current study (4), attention was focused on whether β-cell mass or β-cell function preferentially mediates adaptation to insulin resistance and hyperglycemia. This is a major question in the field, and one for which the novel approach used is well suited to provide important answers.
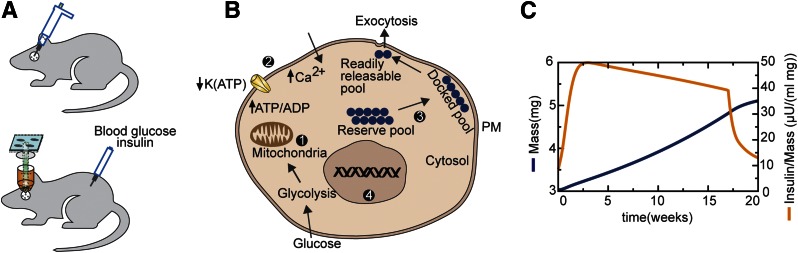
Placing mice with transplanted islets on a high-fat diet (HFD) for 17 weeks caused weight gain and, presumably, a decrease in insulin sensitivity. Chen et al. (4) combined high-resolution islet mass measurements based on laser light backscattering (8) with in vivo insulin and glucagon measurements. Transducing the donor islets with a genetically encoded Ca2+-sensing probe (GCaMP) permitted the measurement of β-cell Ca2+ levels and Ca2+ oscillations in real time. Figure 1A depicts the experimental design used.
Figure 1.
A: Schematic of the experimental preparation. Islets transplanted to the anterior chamber of the eye can be evaluated through the lens for β-cell mass and calcium dynamics, while whole-body glucose and insulin are sampled from the circulation. B: Proposed hierarchy of β-cell adaptations to insulin resistance 1: Normal exercise of the glucose dose-response curve: glucose metabolism increases ATP/ADP ratio and closes K(ATP) channels. 2: Persistent hyperglycemia leads to reduced K(ATP) channel density, shifting the dose-response curve to the left (3). If 2 is insufficient, calcium efficacy is increased by enhanced trafficking of vesicles to the plasma membrane (PM) (3). If 2 and 3 are insufficient, β-cell replication (4) is stimulated. C: Simulations of β-cell response to reduced insulin sensitivity using the model of Ha et al. (10). β-Cell mass (blue) rises slowly, whereas the functional index (stimulated plasma insulin over β-cell mass [orange]) rises rapidly. At 17 weeks, returning mice to normal chow reverses augmented β-cell function, while β-cell mass approaches a plateau.
Chen et al. (4) found that although the HFD was associated with changes in both islet function (insulin secretion as well as altered Ca2+) and mass, the changes in function occurred considerably earlier than changes in mass, with changes evident as early as 1 week after the HFD commenced. In contrast, changes in mass occurred after 4 weeks. Similar behavior was previously observed in rats (9). The good quality of the islet images used to estimate islet volume and the observed robust functional changes strongly support the conclusions drawn to a greater degree than previous studies using more conventional, less robust techniques. This makes the current study very important.
It is instructive to compare the results obtained to the predictions of a recent mathematical model of type 2 diabetes and β-cell adaptation recently published by our group (10), which is an extension of the work by Topp et al. (11). In the model, increased β-cell mass is driven by an increased rate of proliferation, but this occurs more slowly than changes in function, so it becomes substantial only when β-cell function is unable to compensate for the declining insulin sensitivity. Compensation of mass in the model is driven by increased β-cell “workload” or secretion per cell, in accordance with the work of Dor and colleagues (12). Increased workload is in turn driven by increased glucose and two complementary forms of functional compensation. First, the glucose sensitivity of the islet shifts to the left (Fig. 1B). Such a shift has been observed in a variety of diabetes models (reviewed in 13), and the increased basal calcium reported in Chen et al. (4) (see Fig. 6A in ref. 4) is suggestive that it occurred in their experiments as well. Second, there is an increase in the amplification factor that is distal to a rise in β-cell Ca2+ but increases β-cell exocytosis for a given level of Ca2+. This increase, which corresponds to what Chen et al. (4) call “calcium efficacy,” may reflect the augmentation of the insulin granule docked pool (14) (Fig. 1B).
If these two compensatory mechanisms fail to restrain plasma glucose, then β-cell workload remains elevated and promotes an increased β-cell proliferation rate (P). The balance of P and the rate of apoptosis (A) determines net β-cell mass. If P > A, then mass expands. If the increased number of secreting β-cells is sufficient, euglycemia may be restored. However, if mass expansion is insufficient due to, for example, genetic impairments in P or A or a too rapid loss of insulin sensitivity, blood glucose may rise further and lead to “glucotoxicity” and A being greater than P. From this point on, β-cell mass will decrease. Noticeably reduced mass does not generally occur until diabetes is well established, but the preceding failure to expand rapidly enough already seals the diabetic fate. In the study by Chen et al. (4), the HFD-fed mice only progressed to prediabetes, consistent with numerous other studies (15–17), so mass did not fall.
Thus, there is a multitiered defense against hyperglycemia in the face of insulin resistance, with compensation in two forms of function preceding compensation in mass. This hierarchy of defenses is regulated by the progressively slower time constants of each layer, with slower processes taking over when the faster ones are inadequate to control glucose. The main result of experiments in the study by Chen et al. (4), i.e., function adapts before mass does, is demonstrated by the rise of plasma insulin during an intraperitoneal glucose tolerance test (Fig. 1D in ref. 4), which is faster than the rise of islet cell volume (Fig. 2C in ref. 4). Further, the authors’ index of β-cell function, the ratio of increased insulin to the augmentation of islet volume (Fig. 4 in ref. 4), plateaued 1 week after the initiation of the HFD. These findings are strikingly consistent with the model simulations shown in Fig. 1C.
Although the study by Chen et al. (4) represents an important advance for the β-cell field and offers new insights into diabetes, it is not without weaknesses. The evidence from the ex vivo experiments with palmitate implicating Epac as a major player is indirect. It is not clear how palmitate increases Epac; it would have been better perhaps to measure plasma glucagon-like peptide 1. The specific role or necessity of palmitate in increasing calcium and insulin secretion could have been probed by exposing islets overnight to serum without added palmitate.
Earlier reports from the group showed that transplanted islets secrete insulin and that islets transplanted into the eye changed their mass in parallel with those residing in pancreas (5,8). To quantify differences in calcium oscillations between transplanted and control islets, the authors used “calcium dynamics,” or mean glucose-stimulated increases in mean calcium. It might have been better to report changes in plateau fraction of the Ca2+ oscillations, as in the study by Glynn et al. (13). Also, one wonders how faithfully glucose in the eye tracks glucose concentration in the plasma. The approach is finally subject to the general criticism that islets placed into the eye are unlikely to fully replicate the physiological milieu of the pancreas.
Hopefully, the study by Chen et al. (4) will turn out to be one of many newly emerging animal models of diabetes that can allow dynamic changes in β-cell mass and function to be determined simultaneously and longitudinally. As these new models appear, experimentalists will potentially have an expanding number of systems with which to better establish the time dependence and mechanistic relationships between β-cell mass and function. This will help us to more clearly understand how β-cells compensate and then sometimes fail to compensate for insulin resistance and rising hyperglycemia.
Article Information
Acknowledgments. The authors are grateful to Mariana R. Ortiz of the Department of Molecular & Integrative Physiology of the University of Michigan for her expert help with the figures.
Funding. L.S.S. was supported by a grant from the National Institutes of Health (RO1 DK46409). J.H. and A.S.S. were supported by the Intramural Research Program of the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 2676.
References
- 1.Kahn SE, Zraika S, Utzschneider KM, Hull RL. The beta cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia 2009;52:1003–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science 2005;307:380–384 [DOI] [PubMed] [Google Scholar]
- 3.Halban PA, Polonsky KS, Bowden DW, et al. β-Cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 2014;37:1751–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C, Chmelova H, Cohrs CM, et al. Alterations in β-cell calcium dynamics and efficacy outweigh islet mass adaptation in compensation of insulin resistance and prediabetes onset. Diabetes 2016;65:2676–2685 [DOI] [PubMed] [Google Scholar]
- 5.Speier S, Nyqvist D, Köhler M, Caicedo A, Leibiger IB, Berggren PO. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat Protoc 2008;3:1278–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speier S, Nyqvist D, Cabrera O, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nat Med 2008;14:574–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilegems E, Dicker A, Speier S, et al. Reporter islets in the eye reveal the plasticity of the endocrine pancreas. Proc Natl Acad Sci U S A 2013;110:20581–20586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilegems E, van Krieken PP, Edlund PK, et al. Light scattering as an intrinsic indicator for pancreatic islet cell mass and secretion. Sci Rep 2015;5:10740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Topp BG, Atkinson LL, Finegood DT. Dynamics of insulin sensitivity, -cell function, and -cell mass during the development of diabetes in fa/fa rats. Am J Physiol Endocrinol Metab 2007;293:E1730–E1735 [DOI] [PubMed] [Google Scholar]
- 10.Ha J, Satin LS, Sherman AS. A mathematical model of the pathogenesis, prevention, and reversal of type 2 diabetes. Endocrinology 2016;157:624–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Topp B, Promislow K, deVries G, Miura RM, Finegood DT. A model of beta-cell mass, insulin, and glucose kinetics: pathways to diabetes. J Theor Biol 2000;206:605–619 [DOI] [PubMed] [Google Scholar]
- 12.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PubMed] [Google Scholar]
- 13.Glynn E, Thompson B, Vadrevu S, et al. Chronic glucose exposure systematically shifts the oscillatory threshold of mouse islets: experimental evidence for an early intrinsic mechanism of compensation for hyperglycemia. Endocrinology 2016;157:611–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YD, Wang S, Sherman A. Identifying the targets of the amplifying pathway for insulin secretion in pancreatic beta-cells by kinetic modeling of granule exocytosis. Biophys J 2008;95:2226–2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winzell MS, Ahrén B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004;53(Suppl. 3):S215–S219 [DOI] [PubMed] [Google Scholar]
- 16.Peyot M-L, Pepin E, Lamontagne J, et al. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes 2010;59:2178–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab 2002;282:E834–E842 [DOI] [PubMed] [Google Scholar]