Abstract
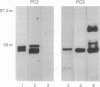
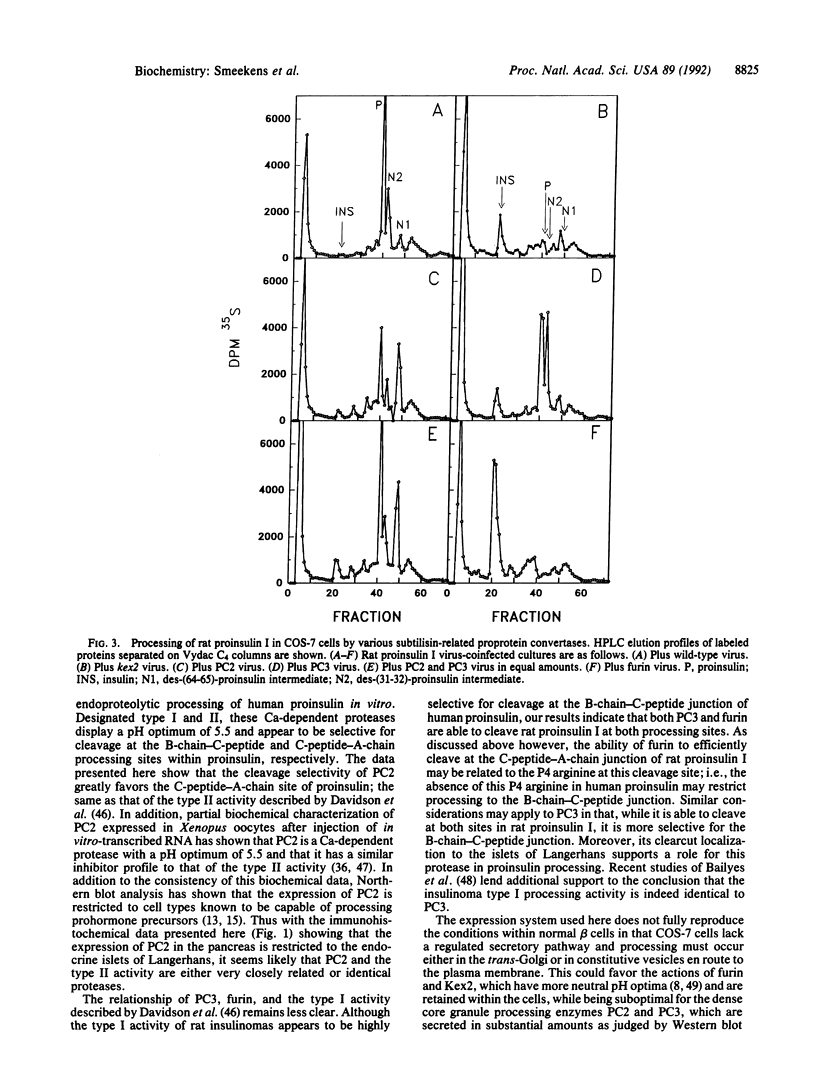
Experiments using recombinant vaccinia viruses expressing rat proinsulin I coinfected into COS-7 cells with recombinant vaccinia virus expressing human furin, human PC2, mouse PC3 (subtilisin-related proprotein convertases 1-3, respectively), or yeast Kex2 indicate that in this system both Kex2 and furin produce mature insulin, whereas PC2 selectively cleaves proinsulin at the C-peptide-A-chain junction. This is a property consistent with its probable identity with the rat insulinoma granule type II proinsulin processing activity as described by Davidson et al. [Davidson, H. W., Rhodes, C. J. & Hutton, J. C. (1988) Nature (London) 333, 93-96]. PC3 generates mature insulin but cleaves preferentially at the proinsulin B-chain-C-peptide junction. This pattern of cleavage by PC3 is similar, but not identical, to that of the highly B-chain-C-peptide junction-selective type I activity as described by Davidson et al., perhaps due to the presence of a P4 arginine residue near the C-peptide-A-chain junction unique to the rat proinsulins. These results along with data presented on the expression of both PC2 and PC3 in islet beta cells strongly support the conclusion that these proteases are involved in the conversion of proinsulin to insulin in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailyes E. M., Shennan K. I., Seal A. J., Smeekens S. P., Steiner D. F., Hutton J. C., Docherty K. A member of the eukaryotic subtilisin family (PC3) has the enzymic properties of the type 1 proinsulin-converting endopeptidase. Biochem J. 1992 Jul 15;285(Pt 2):391–394. doi: 10.1042/bj2850391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr P. J., Mason O. B., Landsberg K. E., Wong P. A., Kiefer M. C., Brake A. J. cDNA and gene structure for a human subtilisin-like protease with cleavage specificity for paired basic amino acid residues. DNA Cell Biol. 1991 Jun;10(5):319–328. doi: 10.1089/dna.1991.10.319. [DOI] [PubMed] [Google Scholar]
- Benjannet S., Rondeau N., Day R., Chrétien M., Seidah N. G. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist B. T., Eipper B. A., Mains R. E. Prohormone-converting enzymes: regulation and evaluation of function using antisense RNA. Mol Endocrinol. 1991 Dec;5(12):2014–2024. doi: 10.1210/mend-5-12-2014. [DOI] [PubMed] [Google Scholar]
- Brenner C., Fuller R. S. Structural and enzymatic characterization of a purified prohormone-processing enzyme: secreted, soluble Kex2 protease. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):922–926. doi: 10.1073/pnas.89.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnahan P. A., Leduc R., Thomas L., Thorner J., Gibson H. L., Brake A. J., Barr P. J., Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990 Dec;111(6 Pt 2):2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., Noyes B. E., Agarwal K. L., Steiner D. F. Construction and selection of recombinant plasmids containing full-length complementary DNAs corresponding to rat insulins I and II. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5036–5040. doi: 10.1073/pnas.76.10.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. J., Oliva A. A., Jr, LaMendola J., Grens A., Bode H., Steiner D. F. Conservation of the prohormone convertase gene family in metazoa: analysis of cDNAs encoding a PC3-like protein from hydra. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6678–6682. doi: 10.1073/pnas.89.15.6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson H. W., Rhodes C. J., Hutton J. C. Intraorganellar calcium and pH control proinsulin cleavage in the pancreatic beta cell via two distinct site-specific endopeptidases. Nature. 1988 May 5;333(6168):93–96. doi: 10.1038/333093a0. [DOI] [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Germain D., Zollinger L., Racine C., Gossard F., Dignard D., Thomas D. Y., Crine P., Boileau G. The yeast KEX-2-processing endoprotease is active in the Golgi apparatus of transfected NIH 3T3 fibroblasts. Mol Endocrinol. 1990 Oct;4(10):1572–1579. doi: 10.1210/mend-4-10-1572. [DOI] [PubMed] [Google Scholar]
- Hakes D. J., Birch N. P., Mezey A., Dixon J. E. Isolation of two complementary deoxyribonucleic acid clones from a rat insulinoma cell line based on similarities to Kex2 and furin sequences and the specific localization of each transcript to endocrine and neuroendocrine tissues in rats. Endocrinology. 1991 Dec;129(6):3053–3063. doi: 10.1210/endo-129-6-3053. [DOI] [PubMed] [Google Scholar]
- Hayflick J. S., Wolfgang W. J., Forte M. A., Thomas G. A unique Kex2-like endoprotease from Drosophila melanogaster is expressed in the central nervous system during early embryogenesis. J Neurosci. 1992 Mar;12(3):705–717. doi: 10.1523/JNEUROSCI.12-03-00705.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M., Nagahama M., Kim W. S., Watanabe T., Hatsuzawa K., Ikemizu J., Murakami K., Nakayama K. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem. 1991 Jul 5;266(19):12127–12130. [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Kiefer M. C., Tucker J. E., Joh R., Landsberg K. E., Saltman D., Barr P. J. Identification of a second human subtilisin-like protease gene in the fes/fps region of chromosome 15. DNA Cell Biol. 1991 Dec;10(10):757–769. doi: 10.1089/dna.1991.10.757. [DOI] [PubMed] [Google Scholar]
- Korner J., Chun J., Harter D., Axel R. Isolation and functional expression of a mammalian prohormone processing enzyme, murine prohormone convertase 1. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6834–6838. doi: 10.1073/pnas.88.15.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J., Chun J., O'Bryan L., Axel R. Prohormone processing in Xenopus oocytes: characterization of cleavage signals and cleavage enzymes. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11393–11397. doi: 10.1073/pnas.88.24.11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg I. The new eukaryotic precursor processing proteinases. Mol Endocrinol. 1991 Oct;5(10):1361–1365. doi: 10.1210/mend-5-10-1361. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Oda K., Fujiwara T., Takami N., Tashiro K., Ikehara Y. Functional expression of furin demonstrating its intracellular localization and endoprotease activity for processing of proalbumin and complement pro-C3. J Biol Chem. 1991 Sep 5;266(25):16954–16959. [PubMed] [Google Scholar]
- Mizuno K., Nakamura T., Ohshima T., Tanaka S., Matsuo H. Yeast KEX2 genes encodes an endopeptidase homologous to subtilisin-like serine proteases. Biochem Biophys Res Commun. 1988 Oct 14;156(1):246–254. doi: 10.1016/s0006-291x(88)80832-5. [DOI] [PubMed] [Google Scholar]
- Molloy S. S., Bresnahan P. A., Leppla S. H., Klimpel K. R., Thomas G. Human furin is a calcium-dependent serine endoprotease that recognizes the sequence Arg-X-X-Arg and efficiently cleaves anthrax toxin protective antigen. J Biol Chem. 1992 Aug 15;267(23):16396–16402. [PubMed] [Google Scholar]
- Nakayama K., Hosaka M., Hatsuzawa K., Murakami K. Cloning and functional expression of a novel endoprotease involved in prohormone processing at dibasic sites. J Biochem. 1991 Jun;109(6):803–806. doi: 10.1093/oxfordjournals.jbchem.a123461. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Schekman R. Clathrin: a role in the intracellular retention of a Golgi membrane protein. Science. 1989 Sep 22;245(4924):1358–1365. doi: 10.1126/science.2675311. [DOI] [PubMed] [Google Scholar]
- Schalken J. A., Roebroek A. J., Oomen P. P., Wagenaar S. S., Debruyne F. M., Bloemers H. P., Van de Ven W. J. fur gene expression as a discriminating marker for small cell and nonsmall cell lung carcinomas. J Clin Invest. 1987 Dec;80(6):1545–1549. doi: 10.1172/JCI113240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Marcinkiewicz M., Benjannet S., Gaspar L., Beaubien G., Mattei M. G., Lazure C., Mbikay M., Chrétien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991 Jan;5(1):111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Shennan K. I., Seal A. J., Smeekens S. P., Steiner D. F., Docherty K. Site-directed mutagenesis and expression of PC2 in microinjected Xenopus oocytes. J Biol Chem. 1991 Dec 15;266(35):24011–24017. [PubMed] [Google Scholar]
- Shennan K. I., Smeekens S. P., Steiner D. F., Docherty K. Characterization of PC2, a mammalian Kex2 homologue, following expression of the cDNA in microinjected Xenopus oocytes. FEBS Lett. 1991 Jun 24;284(2):277–280. doi: 10.1016/0014-5793(91)80703-6. [DOI] [PubMed] [Google Scholar]
- Siezen R. J., de Vos W. M., Leunissen J. A., Dijkstra B. W. Homology modelling and protein engineering strategy of subtilases, the family of subtilisin-like serine proteinases. Protein Eng. 1991 Oct;4(7):719–737. doi: 10.1093/protein/4.7.719. [DOI] [PubMed] [Google Scholar]
- Sizonenko S. V., Halban P. A. Differential rates of conversion of rat proinsulins I and II. Evidence for slow cleavage at the B-chain/C-peptide junction of proinsulin II. Biochem J. 1991 Sep 15;278(Pt 3):621–625. doi: 10.1042/bj2780621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Steiner D. F., Michael J., Houghten R., Mathieu M., Gardner P. R., Ravazzola M., Orci L. Use of a synthetic peptide antigen to generate antisera reactive with a proteolytic processing site in native human proinsulin: demonstration of cleavage within clathrin-coated (pro)secretory vesicles. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6184–6188. doi: 10.1073/pnas.84.17.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thim L., Hansen M. T., Norris K., Hoegh I., Boel E., Forstrom J., Ammerer G., Fiil N. P. Secretion and processing of insulin precursors in yeast. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6766–6770. doi: 10.1073/pnas.83.18.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Herbert E., Hruby D. E. Expression and cell type--specific processing of human preproenkephalin with a vaccinia recombinant. Science. 1986 Jun 27;232(4758):1641–1643. doi: 10.1126/science.3754979. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thorne B. A., Thomas L., Allen R. G., Hruby D. E., Fuller R., Thorner J. Yeast KEX2 endopeptidase correctly cleaves a neuroendocrine prohormone in mammalian cells. Science. 1988 Jul 8;241(4862):226–230. doi: 10.1126/science.3291117. [DOI] [PubMed] [Google Scholar]
- Thomas L., Leduc R., Thorne B. A., Smeekens S. P., Steiner D. F., Thomas G. Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5297–5301. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varandani P. T. A convenient preparation of reduced and S-sulfonated A and B chains of insulin. Biochim Biophys Acta. 1966 Sep 26;127(1):246–249. doi: 10.1016/0304-4165(66)90498-3. [DOI] [PubMed] [Google Scholar]
- Vindrola O., Lindberg I. Biosynthesis of the prohormone convertase mPC1 in AtT-20 cells. Mol Endocrinol. 1992 Jul;6(7):1088–1094. doi: 10.1210/mend.6.7.1508222. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Nakagawa T., Ikemizu J., Nagahama M., Murakami K., Nakayama K. Sequence requirements for precursor cleavage within the constitutive secretory pathway. J Biol Chem. 1992 Apr 25;267(12):8270–8274. [PubMed] [Google Scholar]
- Wise R. J., Barr P. J., Wong P. A., Kiefer M. C., Brake A. J., Kaufman R. J. Expression of a human proprotein processing enzyme: correct cleavage of the von Willebrand factor precursor at a paired basic amino acid site. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9378–9382. doi: 10.1073/pnas.87.23.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasa Y., Paul J. I., Whittaker J., Steiner D. F. Effects of amino acid replacements within the tetrabasic cleavage site on the processing of the human insulin receptor precursor expressed in Chinese hamster ovary cells. J Biol Chem. 1990 Oct 5;265(28):17230–17237. [PubMed] [Google Scholar]
- van de Ven W. J., Voorberg J., Fontijn R., Pannekoek H., van den Ouweland A. M., van Duijnhoven H. L., Roebroek A. J., Siezen R. J. Furin is a subtilisin-like proprotein processing enzyme in higher eukaryotes. Mol Biol Rep. 1990 Nov;14(4):265–275. doi: 10.1007/BF00429896. [DOI] [PubMed] [Google Scholar]