Abstract
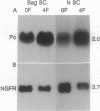
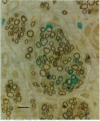
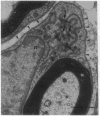
We have developed a method for genetically modifying Schwann cells (SCs) in vitro and then assessed whether these SCs could interact normally with axons in vivo. Rat SCs were transduced in vitro with the lacZ gene by using a retroviral vector and then expanded with the SC mitogens forskolin and glial growth factor. These mitogen-expanded SCs had an abnormal phenotype as compared to both SCs in vivo and primary SCs in vitro, yet when they were introduced into a regenerating rat sciatic nerve, they formed morphologically normal myelin sheaths around the axons. These results demonstrate that SCs can be genetically altered, their numbers expanded in culture, and yet respond appropriately to axonal signals in the peripheral nervous system. This approach offers a plausible way to manipulate genes involved in axon-SC interactions, including genes that may be defective in some inherited peripheral neuropathies.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray G. M., Rasminsky M., Aguayo A. J. Interactions between axons and their sheath cells. Annu Rev Neurosci. 1981;4:127–162. doi: 10.1146/annurev.ne.04.030181.001015. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Fields K. L., Raff M. C. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979 Apr 6;165(1):105–118. doi: 10.1016/0006-8993(79)90048-9. [DOI] [PubMed] [Google Scholar]
- Brockes J. P., Lemke G. E., Balzer D. R., Jr Purification and preliminary characterization of a glial growth factor from the bovine pituitary. J Biol Chem. 1980 Sep 25;255(18):8374–8377. [PubMed] [Google Scholar]
- Buchberg A. M., Moskow J. J., Buckwalter M. S., Camper S. A. Mouse chromosome 11. Mamm Genome. 1991;1(Spec No):S158–S191. doi: 10.1007/BF00656492. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Eccleston P. A., Mirsky R., Jessen K. R. Spontaneous immortalisation of Schwann cells in culture: short-term cultured Schwann cells secrete growth inhibitory activity. Development. 1991 May;112(1):33–42. doi: 10.1242/dev.112.1.33. [DOI] [PubMed] [Google Scholar]
- Franks R. R., Anderson R., Moore J. G., Hough-Evans B. R., Britten R. J., Davidson E. H. Competitive titration in living sea urchin embryos of regulatory factors required for expression of the CyIIIa actin gene. Development. 1990 Sep;110(1):31–40. doi: 10.1242/dev.110.1.31. [DOI] [PubMed] [Google Scholar]
- Hough-Evans B. R., Franks R. R., Zeller R. W., Britten R. J., Davidson E. H. Negative spatial regulation of the lineage specific CyIIIa actin gene in the sea urchin embryo. Development. 1990 Sep;110(1):41–50. doi: 10.1242/dev.110.1.41. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Schwann cell precursors and their development. Glia. 1991;4(2):185–194. doi: 10.1002/glia.440040210. [DOI] [PubMed] [Google Scholar]
- Langford L. A., Owens G. C. Resolution of the pathway taken by implanted Schwann cells to a spinal cord lesion by prior infection with a retrovirus encoding beta-galactosidase. Acta Neuropathol. 1990;80(5):514–520. doi: 10.1007/BF00294612. [DOI] [PubMed] [Google Scholar]
- Lemke G., Axel R. Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin. Cell. 1985 Mar;40(3):501–508. doi: 10.1016/0092-8674(85)90198-9. [DOI] [PubMed] [Google Scholar]
- Lemke G., Chao M. Axons regulate Schwann cell expression of the major myelin and NGF receptor genes. Development. 1988 Mar;102(3):499–504. doi: 10.1242/dev.102.3.499. [DOI] [PubMed] [Google Scholar]
- Lupski J. R., de Oca-Luna R. M., Slaugenhaupt S., Pentao L., Guzzetta V., Trask B. J., Saucedo-Cardenas O., Barker D. F., Killian J. M., Garcia C. A. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991 Jul 26;66(2):219–232. doi: 10.1016/0092-8674(91)90613-4. [DOI] [PubMed] [Google Scholar]
- Matsuoka I., Meyer M., Thoenen H. Cell-type-specific regulation of nerve growth factor (NGF) synthesis in non-neuronal cells: comparison of Schwann cells with other cell types. J Neurosci. 1991 Oct;11(10):3165–3177. doi: 10.1523/JNEUROSCI.11-10-03165.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokuno K., Kamholz J., Behrman T., Black C., Sessa M., Feinstein D., Lee V., Pleasure D. Neuronal modulation of Schwann cell glial fibrillary acidic protein (GFAP). J Neurosci Res. 1989 Aug;23(4):396–405. doi: 10.1002/jnr.490230405. [DOI] [PubMed] [Google Scholar]
- Morgan L., Jessen K. R., Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol. 1991 Feb;112(3):457–467. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. C., Bunge R. P. Evidence for an early role for myelin-associated glycoprotein in the process of myelination. Glia. 1989;2(2):119–128. doi: 10.1002/glia.440020208. [DOI] [PubMed] [Google Scholar]
- Owens G. C., Bunge R. P. Schwann cells depleted of galactocerebroside express myelin-associated glycoprotein and initiate but do not continue the process of myelination. Glia. 1990;3(2):118–124. doi: 10.1002/glia.440030205. [DOI] [PubMed] [Google Scholar]
- Porter S., Glaser L., Bunge R. P. Release of autocrine growth factor by primary and immortalized Schwann cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7768–7772. doi: 10.1073/pnas.84.21.7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeke M. J., Misko T. P., Hsu C., Herzenberg L. A., Shooter E. M. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987 Feb 12;325(6105):593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Stoll G., Griffin J. W., Li C. Y., Trapp B. D. Wallerian degeneration in the peripheral nervous system: participation of both Schwann cells and macrophages in myelin degradation. J Neurocytol. 1989 Oct;18(5):671–683. doi: 10.1007/BF01187086. [DOI] [PubMed] [Google Scholar]
- Trapp B. D., Quarles R. H., Suzuki K. Immunocytochemical studies of quaking mice support a role for the myelin-associated glycoprotein in forming and maintaining the periaxonal space and periaxonal cytoplasmic collar of myelinating Schwann cells. J Cell Biol. 1984 Aug;99(2):594–606. doi: 10.1083/jcb.99.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizoso A. D., Young J. Z. Internode length and fibre diameter in developing and regenerating nerves. J Anat. 1948 Apr;82(Pt 1-2):110–134.1. [PMC free article] [PubMed] [Google Scholar]
- Weinberg H. J., Spencer P. S. Studies on the control of myelinogenesis. II. Evidence for neuronal regulation of myelin production. Brain Res. 1976 Aug 27;113(2):363–378. doi: 10.1016/0006-8993(76)90947-1. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Engel E. L. Peripheral nerve glycoproteins and myelin fine structure during development of rat sciatic nerve. J Neurocytol. 1976 Oct;5(8):605–615. doi: 10.1007/BF01175573. [DOI] [PubMed] [Google Scholar]