Abstract
Bacteroides fragilis is a common colonic symbiote of which one subtype, enterotoxigenic Bacteroides fragilis (ETBF), causes inflammatory diarrhea. However, asymptomatic ETBF colonization is common. Through its primary virulence factor, B. fragilis toxin (BFT), ETBF causes asymptomatic, chronic colitis in C57BL/6 mice and increased colon tumorigenesis in multiple intestinal neoplasia mice. Human studies suggest an association between ETBF infection, inflammatory bowel disease, and colon cancer. Additional studies on ETBF epidemiology are, therefore, crucial. The goal of this study is to develop a reliable fecal diagnostic for ETBF. To develop a sensitive assay for ETBF, we tested multiple protocols on mouse stools spiked with serially diluted ETBF. Each assay was based on either touchdown or quantitative polymerase chain reaction (qPCR) and used primers targeted to bft to detect ETBF. Using touchdown PCR or qPCR, the mean ETBF detection limit was 1.55 × 106 colony-forming units (CFU)/g stool and 1.33 × 104 CFU/g stool, respectively. Augmentation of Bacteroides spp. growth in fecal samples using PYGB (Peptone Yeast Glucose with Bile) broth enhanced ETBF detection to 2.93 × 102 CFU/g stool using the touchdown PCR method and 2.63 × 102 CFU/g stool using the qPCR method. Fecal testing using combined culture-based amplification and bft touchdown PCR is a sensitive assay for the detection of ETBF colonization and should be useful in studying the role of ETBF colonization in intestinal diseases, such as inflammatory bowel disease and colon cancer. We conclude that touchdown PCR with culture-based amplification may be the optimal ETBF detection strategy, as it performs as well as qPCR with culture-based amplification, but is a less expensive technique.
Introduction
Bacteroides species are common colonic commensals identified in all human hosts and comprise about 30 % of the total enteric flora [1, 2]. Among the at least 20 Bacteroides species [3], enterotoxigenic B. fragilis (ETBF) is unique in its association with human colonic disease. ETBF was first identified as a cause of human diarrheal illness in 1992 [4] and then as an etiology of inflammatory diarrhea in 2008 [5]. Since that time, studies have demonstrated an association between ETBF and both active inflammatory bowel disease (IBD) [6, 7] as well as colorectal cancer [8, 9]. Despite the relevance of this bacterium in acute, and potentially chronic, gastrointestinal diseases, there have been limited efforts to develop a diagnostic protocol for ETBF with well-defined test characteristics.
ETBF is characterized by expression of the B. fragilis toxin (BFT), a ~20-kDa metalloprotease of which there are three known isotypes (i.e., BFT1, BFT2, and BFT3) [10]. Diagnostic assays for ETBF have focused on various ways of identifying the presence of bft in clinical samples. While a number of clinical studies assessing ETBF colonization have not evaluated the testing characteristics of their diagnostic assays, three studies have specifically focused on ETBF diagnostic techniques, each using a variation of a bft polymerase chain reaction (PCR) assay [11–13].
Our study builds upon this literature by analyzing two PCR-based techniques to detect ETBF that may be more easily utilized in a clinical setting. Our hypothesis was that these techniques would have similar or improved detection limits, while at the same time, being time-efficient and/or less costly to perform. The first technique, touchdown PCR, uses a higher initial annealing temperature, promoting high specificity, while still preserving assay sensitivity by incrementally decreasing the annealing temperature throughout the protocol. The second technique, quantitative PCR (qPCR), provides calculable data and can be readily adopted by clinical labs for the rapid testing of samples.
Materials and methods
Sample preparation
One hundred milligrams of C57BL/6 mouse feces was spiked by adding and vortexing 100 µL of 1:10 serial dilutions of a fresh overnight Brain Heart Infusion (BHI) broth culture of ETBF strain 86-5443-2-2. Typically, dilutions corresponding to ~108 or 109 colony-forming units (CFU)/mL to 0 CFU/mL were tested per experiment. DNA was directly extracted from spiked stools (below) or stools were cultured in anaerobic broth media to augment Bacteroides spp. growth. The number of CFU added to mouse stools was determined by plating the serial dilutions of the fresh overnight ETBF culture in duplicate on non-selective agar in an anaerobic chamber for 1–2 days. For each experiment, fecal dilutions yielding 20–100 CFU were counted and used to calculate the CFU spiked into each stool sample. We assume a 1 mL to 1 g conversion to determine our CFU concentration in ETBF culture compared to ETBF-spiked stools.
For studies comparing 1- versus 2-day augmentation, BHI supplemented with hemin, vitamin K1, cysteine +/− clindamycin [14], or PYGB (Peptone Yeast Glucose with Bile; Anaerobe Systems, Morgan Hill, CA) broth media were initially tested. Subsequently, all augmentation was performed in PYGB. Samples were incubated at 37 °C using an AS-580 anaerobic chamber (Anaerobe Systems, Morgan Hill, CA). DNA was extracted from samples using either Omega Biotek’s E.Z.N.A.® Mag-Bind® Stool DNA Kit or QIAcube (Qiagen’s stool DNA protocol for pathogen detection).
Touchdown PCR and qPCR
Primers for touchdown PCR and qPCR were designed to be specific for the bft gene and inclusive of all three known isoforms of BFT (Table 1). Primers for the touchdown PCR were obtained from the literature [15] (Table 1). Each reaction contained a total volume of 25 µL, comprising 23.5 µL of Platinum® PCR Supermix (Invitrogen, Grand Island, NY), 10 µM of each primer, and 1 µL of DNA template. The touchdown PCR protocol utilized a “hot start” step at 94 °C for 2 min; denaturation at 94 °C for 30 s; then annealing steps in which the annealing temperature decreased by 0.5 °C from 60 to 50 °C over 20 cycles of 30 s each; followed by a 20-cycle amplification at the annealing temperature of 50 °C. Elongation after the 50 °C annealing step was 72 °C for 30 s. The final elongation step was at 72 °C for 5 min, with a final hold temperature of 4 °C. Amplicons (10 µL of the reaction mixture) were visualized using 1 % agarose gels stained with ethidium bromide and run in SB™ buffer (Faster Better Media LLC, Hunt Valley, MD). A 100-bp DNA ladder (New England Biolabs, Ipswich, MA) was used as the molecular size marker for all gels. Gels were run at a constant rate of 130 mV and photographed under UV light.
Table 1.
Primers and probe sets for touchdown polymerase chain reaction (PCR) and quantitative PCR (qPCR)
| Primers/probe | Sequence 5′ → 3′ | Reference |
|---|---|---|
| Touchdown PCR – bft F | GAA CCT AAA ACG GTA TAT GT | [15] |
| Touchdown PCR – bft R | GTT GTA GAC ATC CCA CTG GC | [15] |
| qPCR bft – F | GGA TAA GCG TAC TAA AAT ACA GCT GGA T | This study |
| qPCR bft – R | CTG CGA ACT CAT CTC CCA GTA TAA A | This study |
| qPCR bft probe | CAG ACG GAC ATT CTC | This study |
F forward primer, R reverse primer
For qPCR reactions, customized primers and a TaqMan probe were designed using Primer Express software of Applied Biosystems (Table 1). The reactions were constructed as per the manufacturer’s protocol. qPCRs were performed using the Applied Biosystems 7900HT Sequence Detection System and SDS 2.3 software. The annealing temperature for all qPCRs was 60 °C, and the threshold for a positive result was set as fluorescent signal detection by 40 cycles. The results were analyzed using RQ Manager software. The mean detection values and standard errors (SEM) were calculated for the results of each assay.
Storage of fecal DNA extracts and ETBF-spiked stools
DNA extracted from mouse stool spiked with serial dilutions of ETBF cultures as described above was stored at 4 °C or −20 °C, with or without bovine serum albumin (BSA, final concentration 0.1 µg/µL). Two sets of stored DNA samples were tested by touchdown PCR as described above. In addition, aliquots of ETBF-negative stool or stool spiked with ETBF containing 1.6 × 109 CFU/g, 1.6 × 107 CFU/g, or 1.6 × 105 CFU/g were stored at −80 °C until DNA extraction and touchdown PCR testing. For the baseline data, DNA extracted from one set of spiked stools was tested by touchdown PCR on the day of sample preparation.
Statistics
A one-tailed paired Student’s t-test was used to compare the mean detection limit between samples augmented for 1- versus 2-day incubation in BHI and PYGB.
Results
Touchdown PCR and qPCR detection of ETBF
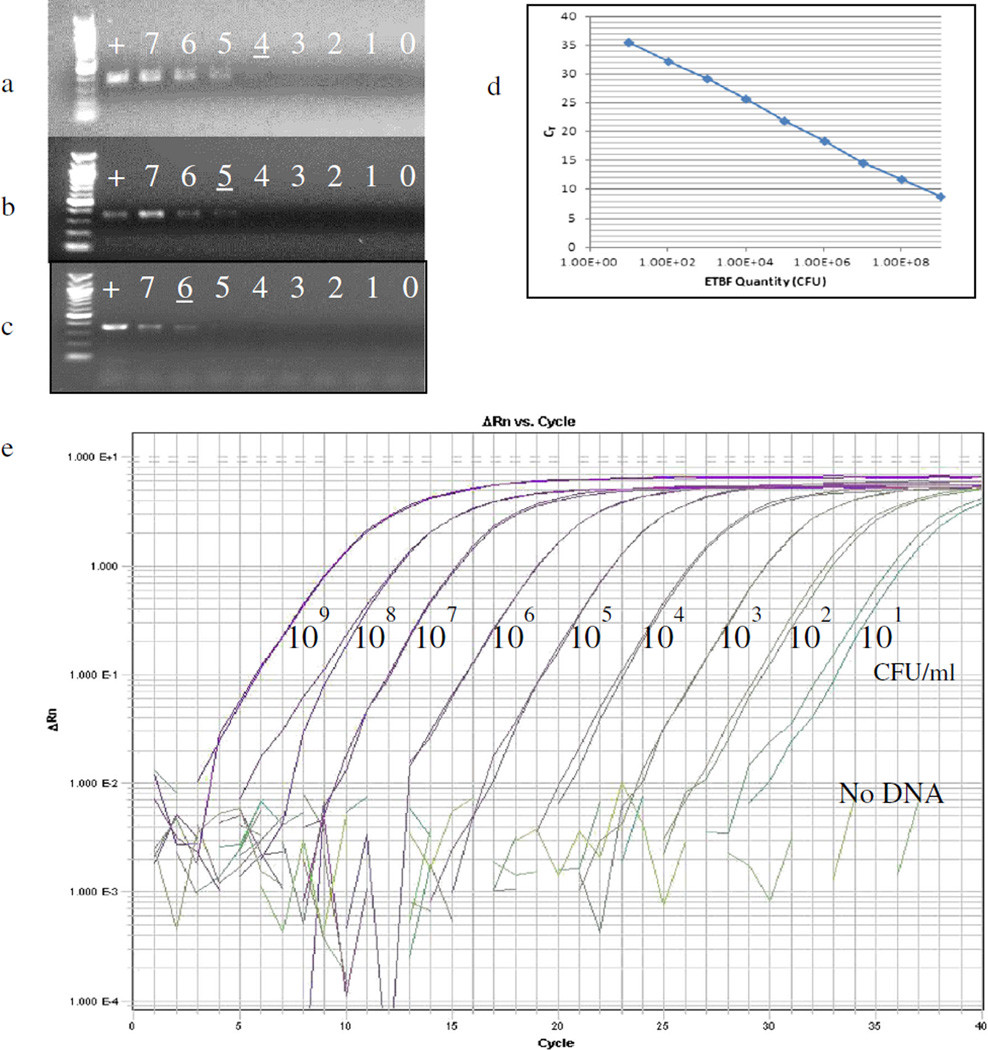
DNA was extracted from serial dilutions of a pure ETBF culture and tested by touchdown PCR, revealing a mean detection limit of 1.23 × 105 CFU/mL (Table 2). To determine if unrelated DNA alters the detection of bft in the sample, 1 ng of glioblastoma multiforme (GBM) DNA was added to the serial dilutions of ETBF DNA. Touchdown PCR of these samples revealed a similar ETBF detection limit of 2.91 × 105 CFU/mL. When serial dilutions of a pure ETBF culture were spiked into mouse stool, a mean detection limit of 1.55 × 106 CFU/g was obtained. Figure 1a shows the decreasing gradient of band intensity with serial ETBF culture dilutions. Figure 1b, c qualitatively show the CFU detection limit when ETBF is mixed with unrelated DNA or stool. Touchdown PCR testing of DNA extracted from ETBF-spiked stools from three individual mice demonstrated that the ETBF detection limit was within one log, indicating no marked variation in sensitivity from sample to sample (data not shown). In more limited experiments, we tested qPCR on a subset of samples tested by touchdown PCR as an alternative approach not requiring the added step of gel electrophoresis to determine results (Fig. 1d, e). In these experiments, qPCR demonstrated a detection limit of 1.33 × 104 CFU/g for ETBF-spiked mouse stool. Both touchdown PCR and qPCR primers were tested on unspiked stool from multiple mice and did not produce any product.
Table 2.
Detection limits of touchdown PCR and qPCR for bft
| Mean detection limit |
SEM, n | Individual experiments |
Log of mean |
Average of logs |
|
|---|---|---|---|---|---|
| Touchdown PCR: ETBF culture alone | 1.23×105 CFU/mL |
±9.60×104, n=4 | 4.6×104 | 4.66 | 4.55 |
| 4.1×105 | 5.61 | ||||
| 2.3×103 | 3.36 | ||||
| 3.55×104 | 4.55 | ||||
| Touchdown PCR: ETBF culture spiked into GBM DNA | 2.91×105 CFU/mL |
±1.45×105, n=3 | 4.6×105 | 5.66 | 4.88 |
| 4.1×105 | 5.61 | ||||
| 2.3×103 | 3.36 | ||||
| Touchdown PCR: ETBF-spiked stool | 1.55×106 CFU/g |
±1.52×106, n=3 | 4.6×106 | 6.66 | 5.19 |
| 2.3×104 | 4.36 | ||||
| 3.55×104 | 4.55 | ||||
| Touchdown PCR: ETBF-spiked stool augmented in PYGB | 2.93×102 CFU/g |
±63, n=2 | 2.3×102 | 2.36 | 2.46 |
| 3.55×102 | 2.55 | ||||
| qPCR: ETBF-spiked stool | 1.33×104 CFU/g |
n=2 | 2.3×104 | 4.36 | 3.96 |
| 3.55×103 | 3.55 | ||||
| qPCR: ETBF-spiked stool augmented in PYGB | 2.63×102 CFU/g |
±1.21×102, n=3 | 4.1×102 | 2.61 | 2.17 |
| 2.3×101 | 1.36 | ||||
| 3.55×102 | 2.55 |
bft B. fragilis toxin (gene), ETBF enterotoxigenic Bacteroides fragilis, GBM glioblastoma multiforme, PYGB Peptone Yeast Glucose with Bile broth media, CFU colony-forming units, SEM standard error of mean
Fig. 1.
bft-specific touchdown polymerase chain reaction (PCR) and qPCR assays. a–c Touchdown PCR. Representative images from one parallel experiment demonstrating touchdown PCR detection of bft in enterotoxigenic Bacteroides fragilis (ETBF) culture alone (a), ETBF culture spiked into glioblastoma multiforme (GBM) carrier DNA (b), and ETBF culture spiked into mouse stool (c). The numbered lanes refer to the log value in CFU/mL (a, b) or CFU/g of ETBF spiked into mouse stool (c). For example, the lane numbered “7” indicates that there were 107 CFU/mL or 107 CFU/g in that sample. “+” indicates the positive control lane, which contains pure ETBF DNA extract. The underlined numbers demonstrate the last sample where a band can be detected. d–e qPCR amplification of 10-fold dilutions of ETBF culture DNA extracts. d Standard curve generated from our bft-specific qPCR assay. e ΔRn demonstrating the magnitude of the signal generated by the qPCR conditions
We further tested whether augmenting bacterial growth in culture prior to touchdown PCR would increase assay sensitivity (termed “augmented touchdown PCR”). Preliminary comparisons between the less selective BHI broth and the Bacteroides spp.-promoting PYGB broth, as well as 1- versus 2-day of growth augmentation, were performed (Table 3). Although not significant, the use of PYGB and 2 days of growth augmentation appeared to improve the detection of small numbers of ETBF in mouse stool. Direct comparison of the touchdown PCR assay for ETBF-spiked stools with and without PYGB augmentation for 2 days revealed enhanced detection of bft qualitatively by gel electrophoresis and quantitatively (Table 2, Fig. 2), with a mean detection limit by augmented touchdown PCR of 2.93 × 102 CFU/g compared to 1.55 × 106 CFU/g without culture augmentation. The detection of ETBF in fecal samples was similarly enhanced by growth augmentation using qPCR (Table 2).
Table 3.
Touchdown PCR of enterotoxigenic Bacteroides fragilis (ETBF)-spiked mouse stool augmented in either Brain Heart Infusion (BHI) or Peptone Yeast Glucose with Bile (PYGB) broth for 1 or 2 days
| Detection limit after 1- day incubation (individual experiments), CFU/g |
Mean detection limit±SEM CFU/g |
Detection limit after 2-day incubation (individual experiments), CFU/g |
Mean detection limit±SEM CFU/g |
p-Value (1-day vs. 2-day) |
|
|---|---|---|---|---|---|
| BHI | >2.38×104 | >8.33×103±7.74×103 | 2.38×103 | ~831±775 | 0.20 |
| >1.18×103 | ≤11.8 | ||||
| 10 | 100 | ||||
| PYGB | 2.38×103 | 1.22×103±658 | ≤238 | ≤83.6±77 | 0.10 |
| 1.18×103 | ≤11.8 | ||||
| 100 | ≤1 |
CFU colony-forming units, SEM standard error of mean
Fig. 2.
Touchdown PCR demonstrating increased ETBF detection when ETBF-spiked samples are pre-incubated in Peptone Yeast Glucose with Bile (PYGB) broth for 2 days prior to PCR analysis. a In this representative experiment, the detection limit without broth amplification is 3.55 × 104 CFU/g stool. b The assay sensitivity is increased to 3.55 × 102 CFU/g after samples have been incubated for 2 days in PYGB broth, which promotes growth of Bacteroides spp. “+” indicates the positive control lane, which contains an ETBF culture spiked into mouse stool (109 CFU/g). The spiked positive control is also pre-incubated for 2 days in PYGB broth. The numbered lanes refer to the log value in ETBF CFU/g spiked into mouse stool (see also the caption for Fig. 1). The underlined numbers demonstrate the last sample where a band can be detected
Effect of storage and sample conditions
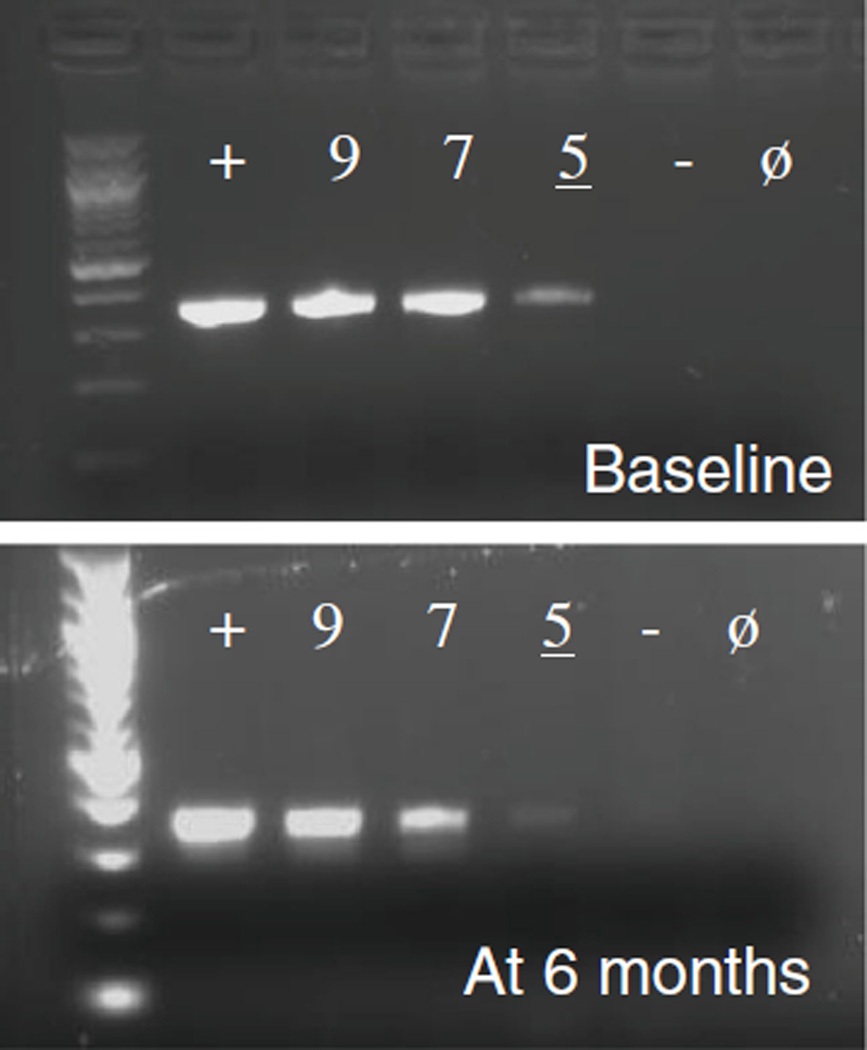
Touchdown PCR testing of ETBF-spiked mouse stool stored at −80 °C showed no change in the detection limit for bft (~105 CFU/g) after 6 months (Fig. 3). The stability of DNA extracts from ETBF-spiked fecal samples stored at 4 °C versus −20 °C, with and without BSA (0.1 µg/µL), was also examined weekly by touchdown PCR for up to 6 weeks and then at 13 weeks after extraction. Storage of fecal DNA extracts at −20 °C with BSA enhanced bft detection at 13 weeks (data not shown).
Fig. 3.
bft detection using touchdown PCR on ETBF-spiked mouse stool stored for 6 months at −80 °C. “+” indicates the positive control lane, which contains ETBF DNA extract. The numbered lanes refer to the log value in CFU/g of ETBF spiked into mouse stool (see also the caption for Fig. 1). “−” is ETBF-negative stool and “ø” is no DNA added
Discussion
Our results show that the fecal ETBF detection limit for both touchdown PCR (~106 CFU/g) and qPCR (~104 CFU/g) are comparable to the 104–105 CFU/g detection limit described in the first study of ETBF diagnostic development by Pantosti et al. in 1997 [11]. In that study, Pantosti et al. plated spiked stool samples on Bacteroides-promoting Bacteroides Bile Esculin (BBE) agar, which was then anaerobically incubated for 24 h. The resulting colonies were collected to create boiled lysates, which were tested by PCR for the bft gene. Later more sensitive assays included a protocol that could detect 102–103 CFU/g stool [12], but which used a nested PCR technique that is largely avoided by clinical microbiology laboratories, given high false-positivity rates and the suboptimal nature of the increased handling involved [16, 17; K. Carroll, personal communication]. A third method employed an immunomagnetic separation-PCR protocol that could detect approximately 50 CFU ETBF/g stool [13]. This study described the use of magnetic beads linked to a pair of monoclonal antibodies recognizing the lipopolysaccharide of B. fragilis. The beads were mixed with broth cultures of spiked stool with subsequent concentration of B. fragilis attached to the beads using immunomagnetic separation. Boiled lysates were made from the B. fragilis bound to the beads, which were then tested for bft using the inner primers of the nested PCR described by Shetab et al. [12]. Unfortunately, both monoclonal antibodies used in this assay have been lost (A. Weintraub, personal communication).
In conclusion, we show that growth augmentation in Bacteroides spp.-promoting PYGB media prior to touchdown PCR or qPCR testing enhances ETBF detection in spiked mouse stool, yielding a detection limit of ~102 CFU/g. This represents a significant improvement compared to the other approaches we tested and is comparable to available nested PCR techniques [12]. Collectively, our results suggest that culture augmentation followed by PCR testing (either touchdown PCR or qPCR) is a sensitive diagnostic approach for the detection of low-level fecal ETBF colonization. This technique also has high specificity for bft, as both touchdown PCR and qPCR primers were tested on unspiked stool from multiple mice and did not produce any product. Cost and laboratory resource considerations will likely determine which PCR approach to use. A similar strategy using culture augmentation of clinical stool samples followed by O157 antiserum or latex reagent testing is currently utilized in clinical practice for diagnosing O157 Shiga toxin-producing Escherichia coli [18]. Our storage and stability studies suggest that the detection of fecal bft is stable at −80 °C for at least 6 months, potentially facilitating the testing of fecal repositories for bft. Thus, growth augmentation to detect low-level ETBF colonization may facilitate future studies of the role of ETBF in human gastrointestinal illnesses, in particular, to understand the epidemiology of chronic asymptomatic ETBF colonization and its possible relationship to subclinical colon inflammation. This is a fertile area for investigation since experimental models suggest that ETBF has the capacity to induce chronic colitis and colon carcinogenesis [14, 19, 20].
Acknowledgments
This work was supported by T32 DK007632-23 (National Institutes of Health) and a Clinical Research Award from the American College of Gastroenterology to LAC and R01CA151393 and R01CA151325 (National Institutes of Health) to CLS.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J, MetaHIT Consortium. Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M’rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Namavar F, Theunissen EBM, Verweij-Van Vught AMJJ, Peerbooms PGH, Bal M, Hoitsma HFW, Maclaren DM. Epidemiology of the Bacteroides fragilis group in the colonic flora in 10 patients with colonic cancer. J Med Microbiol. 1989;29:171–176. doi: 10.1099/00222615-29-3-171. [DOI] [PubMed] [Google Scholar]
- 3.Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sack RB, Myers LL, Almeido-Hill J, Shoop DS, Bradbury WC, Reid R, Santosham M. Enterotoxigenic Bacteroides fragilis: epidemiologic studies of its role as a human diarrhoeal pathogen. J Diarrhoeal Dis Res. 1992;10:4–9. [PubMed] [Google Scholar]
- 5.Sears CL, Islam S, Saha A, Arjumand M, Alam NH, Faruque ASG, Salam MA, Shin J, Hecht D, Weintraub A, Sack RB, Qadri F. Association of enterotoxigenic Bacteroides fragilis infection with inflammatory diarrhea. Clin Infect Dis. 2008;47:797–803. doi: 10.1086/591130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prindiville TP, Sheikh RA, Cohen SH, Tang YJ, Cantrell MC, Silva J., Jr Bacteroides fragilis enterotoxin gene sequences in patients with inflammatory bowel disease. Emerg Infect Dis. 2000;6:171–174. doi: 10.3201/eid0602.000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basset C, Holton J, Bazeos A, Vaira D, Bloom S. Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig Dis Sci. 2004;49:1425–1432. doi: 10.1023/b:ddas.0000042241.13489.88. [DOI] [PubMed] [Google Scholar]
- 8.Toprak NU, Yagci A, Gulluoglu BM, Akin ML, Demirkalem P, Celenk T, Soyletir G. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect. 2006;12:782–786. doi: 10.1111/j.1469-0691.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- 9.Boleij A, Hechenbleikner EM, Goodwin AC, Badani R, Stein EM, Lazarev MG, Ellis B, Carroll KC, Albesiano E, Wick EC, Platz EA, Pardoll DM, Sears CL. The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin Infect Dis. 2015;60:208–215. doi: 10.1093/cid/ciu787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sears CL. Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin Microbiol Rev. 2009;22:349–369. doi: 10.1128/CMR.00053-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantosti A, Malpeli M, Wilks M, Menozzi MG, D’Ambrosio F. Detection of enterotoxigenic Bacteroides fragilis by PCR. J Clin Microbiol. 1997;35:2482–2486. doi: 10.1128/jcm.35.10.2482-2486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shetab R, Cohen SH, Prindiville T, Tang YJ, Cantrell M, Rahmani D, Silva J., Jr Detection of Bacteroides fragilis enterotoxin gene by PCR. J Clin Microbiol. 1998;36:1729–1732. doi: 10.1128/jcm.36.6.1729-1732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang G, Weintraub A. Rapid and sensitive assay for detection of enterotoxigenic Bacteroides fragilis. J Clin Microbiol. 1998;36:3545–3548. doi: 10.1128/jcm.36.12.3545-3548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhee KJ, Wu S, Wu X, Huso DL, Karim B, Franco AA, Rabizadeh S, Golub JE, Mathews LE, Shin J, Sartor RB, Golenbock D, Hamad AR, Gan CM, Housseau F, Sears CL. Induction of persistent colitis by a human commensal, enterotoxigenic Bacteroides fragilis, in wild-type C57BL/6 mice. Infect Immun. 2009;77:1708–1718. doi: 10.1128/IAI.00814-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato N, Liu CX, Kato H, Watanabe K, Tanaka Y, Yamamoto T, Suzuki K, Ueno K. A new subtype of the metalloprotease toxin gene and the incidence of the three bft subtypes among Bacteroides fragilis isolates in Japan. FEMS Microbiol Lett. 2000;182:171–176. doi: 10.1111/j.1574-6968.2000.tb08892.x. [DOI] [PubMed] [Google Scholar]
- 16.Apfalter P, Assadian O, Blasi F, Boman J, Gaydos CA, Kundi M, Makristathis A, Nehr M, Rotter ML, Hirschl AM. Reliability of nested PCR for detection of Chlamydia pneumoniae DNA in atheromas: results from a multicenter study applying standardized protocols. J Clin Microbiol. 2002;40:4428–4434. doi: 10.1128/JCM.40.12.4428-4434.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez IC, Le Guiner C, Ni W, Lyles J, Moullier P, Snyder RO. PCR-based detection of gene transfer vectors: application to gene doping surveillance. Anal Bioanal Chem. 2013;405:9641–9653. doi: 10.1007/s00216-013-7264-8. [DOI] [PubMed] [Google Scholar]
- 18.Gould LH, Bopp C, Strockbine N, Atkinson R, Baselski V, Body B, Carey R, Crandall C, Hurd S, Kaplan R, Neill M, Shea S, Somsel P, Tobin-D’Angelo M, Griffin PM, Gerner-Smidt P Centers for Disease Control and Prevention (CDC) Recommendations for diagnosis of shiga toxin-producing Escherichia coli infections by clinical laboratories. MMWR Recomm Rep. 2009;58:1–14. [PubMed] [Google Scholar]
- 19.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wick EC, Rabizadeh S, Albesiano E, Wu X, Wu S, Chan J, Rhee KJ, Ortega G, Huso DL, Pardoll D, Housseau F, Sears CL. Stat3 activation in murine colitis induced by enterotoxigenic Bacteroides fragilis. Inflamm Bowel Dis. 2014;20:821–834. doi: 10.1097/MIB.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]