Abstract
BACKGROUND
Important roles for G-protein-coupled receptors (GPCRs) have been identified in the maternal physiological adaptations to pregnancy and in the pathogenesis of preeclampsia. On this basis, GPCRs are potential therapeutic targets for preeclampsia.
OBJECTIVES AND RATIONALE
In this review, vasopressin and apelin are initially considered in this context before the focus on the hormone relaxin and its cognate receptor, the relaxin/insulin-like family peptide receptor 1 (RXFP1). Based on both compelling scientific rationale and a promising safety profile, the relaxin ligand–receptor system is comprehensively evaluated as a potential therapeutic endpoint in preeclampsia.
SEARCH METHODS
The published literature relating to the topic was searched through January 2016 using PubMed.
OUTCOMES
Relaxin is a peptide hormone secreted by the corpus luteum; it circulates in the luteal phase and during pregnancy. Activation of RXFP1 is vasodilatory; thus, relaxin supplementation is expected to at least partly restore the fundamental vasodilatory changes of normal pregnancy, thereby alleviating maternal organ hypoperfusion, which is a major pathogenic manifestation of severe preeclampsia. Specifically, by exploiting its pleiotropic hemodynamic attributes in preeclampsia, relaxin administration is predicted to (i) reverse robust arterial myogenic constriction; (ii) blunt systemic and renal vasoconstriction in response to activation of the angiotensin II receptor, type 1; (iii) mollify the action of endogenous vasoconstrictors on uterine spiral arteries with failed remodeling and retained smooth muscle; (iv) increase arterial compliance; (v) enhance insulin-mediated glucose disposal by promoting skeletal muscle vasodilation and (vi) mobilize and activate bone marrow-derived angiogenic progenitor cells, thereby repairing injured endothelium and improving maternal vascularity in organs such as breast, uterus, pancreas, skin and fat. By exploiting its pleiotropic molecular attributes in preeclampsia, relaxin supplementation is expected to (i) enhance endothelial nitric oxide synthesis and bioactivity, as well as directly reduce vascular smooth muscle cytosolic calcium, thus promoting vasodilation; (ii) improve the local angiogenic balance by augmenting arterial vascular endothelial and placental growth factor (VEGF and PLGF) activities; (iii) ameliorate vascular inflammation; (iv) enhance placental peroxisome proliferator-activated receptor gamma, coactivator 1 alpha (PCG1α) expression, and hence, peroxisome proliferator-activated receptor gamma (PPAR-γ) activity and (v) confer cytotrophoblast and endothelial cytoprotection. Insofar as impaired endometrial maturation (decidualization) predisposes to the development of preeclampsia, relaxin administration in the late secretory phase and during early pregnancy would be anticipated to improve decidualization, and hence trophoblast invasion and spiral artery remodeling, thereby reducing the risk of preeclampsia. Relaxin has a favorable safety profile both in the non-pregnant condition and during pregnancy.
WIDER IMPLICATIONS
There is a strong scientific rationale for RXFP1 activation in severe preeclampsia by administration of relaxin, relaxin analogs or small molecule mimetics, in order to mollify the disease pathogenesis for safe prolongation of pregnancy, thus allowing time for more complete fetal maturation, which is a primary therapeutic endpoint in treating the disease. In light of recent data implicating deficient or defective decidualization as a potential etiological factor in preeclampsia and the capacity of relaxin to promote endometrial maturation, the prophylactic application of relaxin to reduce the risk of preeclampsia is a plausible therapeutic approach to consider. Finally, given its pleiotropic and beneficial attributes particularly in the cardiovascular system, relaxin, although traditionally considered as a ‘pregnancy’ hormone, is likely to prove salutary for several disease indications in the non-pregnant population.
Keywords: arginine vasopressin, apelin, endothelium, hemodynamics, insulin resistance, cell death, nitric oxide, angiogenic growth factors, PPAR, placenta
Introduction
Severe preeclampsia can be a volatile, dangerous, and potentially life-threatening syndrome. The disease afflicts 3–5% of pregnancies, typically manifesting during the third trimester, and is a major cause of maternal, fetal and neonatal, morbidity and mortality (Duley, 2003; Backes et al., 2011; Ghulmiyyah and Sibai, 2012). Globally, ~7 million women annually are afflicted by preeclampsia, which is diagnosed by new onset hypertension after 20 gestational weeks accompanied by one or more of the following features: proteinuria, thrombocytopenia, renal or hepatic insufficiency, pulmonary edema, cerebral or visual symptoms (Hypertension in pregnancy, 2013; Kuklina et al., 2009). In developed countries, preeclampsia often leads to iatrogenic premature delivery that can cause major morbidity in the neonate immediately after delivery and long term. In the developing world where (timely) access to health care may be lacking, preeclampsia can result in maternal, fetal and neonatal death (Duley, 2003; Backes et al., 2011; Ghulmiyyah and Sibai, 2012). Of equal or perhaps even of greater concern is that women and children who survive preeclampsia are at greater risk for developing future adverse cardiovascular events (Roberts et al., 2003; Sibai et al., 2005; Anderson, 2007; Bellamy et al., 2007; Gluckman et al., 2008; Vikse et al., 2008; Williams, 2011; Davis et al., 2012). Whether preeclampsia itself inflicts long-term, residual vascular damage in the mother is unclear (Epstein, 1964; Levine et al., 2009), but if so, it is not inconceivable that amelioration of the disease during pregnancy could mitigate the increased risk of remote cardiovascular disease. Hence, effective treatments for preeclampsia are sought not only to alleviate morbidity, mortality and iatrogenic premature delivery in the perinatal period, but because they may also lower remote cardiovascular disease risk in affected women and their children. In addition, low birth weight is a frequent neonatal outcome of preeclampsia (Rich-Edwards et al., 2015) and is independently associated with elevated occurrence of hypertension and ischemic heart disease (among other pathologies) during adulthood (Barker et al., 1989, 1990). Disappointingly, despite first being recognized over 2000 years ago, therapies for preeclampsia that counteract the pathogenic mechanisms are currently lacking. Rather, ‘palliative’ measures are imposed to manage blood pressure and prevent seizures, and delivery remains the only known cure. Nor are there prophylactic strategies available, although low dose aspirin may have a small protective effect in women at elevated risk of developing preeclampsia (Henderson et al., 2014).
Development of therapeutics specific for preeclampsia has been hampered by several factors. First, an insufficient understanding of the pathophysiology, and consequently, a lack of known pathogenic targets has precluded their development. However, our understanding of pathophysiology has improved over the past decade, insofar as circulating molecules of placental origin and maternal constitutional factors that contribute or predispose to disease manifestations have been identified (Roberts et al., 2003; Young et al., 2010; Redman et al., 2012). Second, preeclampsia appears to be a uniquely human affliction, which has reduced enthusiasm for underwriting research proposals designed to test potential therapeutics in animal models of the disease (Conrad, 1990; Granger et al., 2014). Third, confined vision and short-range goals, low risk tolerance and funding pay lines have conspired to assign low priority to grant applications in pursuit of novel therapeutic candidates. Last, the pharmaceutical industry has traditionally exercised risk aversion for developing therapeutics in pregnancy-related diseases.
Despite these handicaps, several potential therapeutic targets including the G-protein-coupled receptor (GPCR) relaxin/insulin-like family peptide receptor, RXFP1, and its cognate ligand, relaxin, were showcased in a first-ever symposium titled ‘Novel Therapies for Preeclampsia’ at the International Society for the Study of Hypertension in Pregnancy Meeting October 2014. The main goal of this review is to extend the (necessarily abridged) relaxin presentation at this meeting, particularly in the context of recent findings in the field, with the goal of comprehensively evaluating the scientific rationale for considering relaxin or relaxin mimetics as potential therapeutics in preeclampsia. To this end, cardiovascular function in uncomplicated pregnancy and preeclampsia will first be discussed. Next, several candidate GPCRs will be reviewed as potential drug targets in the disease. Last, a comprehensive appraisal of RXFP1, arguably one of the most promising therapeutic targets in preeclampsia, will be presented.
Methods
PubMed searches through January 2016 were conducted using the keywords: adrenomedullin-2/intermedin and amylin, ligands and receptors; apelin and apelin receptor, arginine vasopressin and vasopressin receptor, vasopressinase, copeptin, and oxytocin in the context of pregnancy, preeclampsia and placenta. The relaxin receptor and relaxin, as well as relaxin/insulin-like family peptides Insl 3–6 were also searched, each as standalone keywords or phrases. Not all of the topics yielded sufficient literature to be included. This work critically evaluates the apelin, vasopressin and relaxin ligand–receptor systems as potential therapeutic targets in preeclampsia.
Cardiovascular function in normal pregnancy and preeclampsia
As summarized in Table I, normal pregnancy is associated with profound vasodilation of the maternal renal and systemic circulations. Reduced renal and systemic vascular resistances lead to marked increases in renal blood flow, glomerular filtration rate and cardiac output (Conrad, 2011). These physiological changes commence during the luteal phase of the menstrual cycle, becoming more pronounced in the first trimester and thereafter (Jeyabalan and Conrad, 2010). Two major events conspire to increase cardiac output during normal pregnancy: a decline in systemic vascular resistance or cardiac afterload (to which renal vasodilation majorly contributes) and a concurrent increase of venous return to the heart or cardiac preload; the latter at least partly results from mobilization of the large reservoir of blood located in the splanchnic circulation. This mobilization is made possible by reduced hepatic vascular resistance, mainly regulated in the sinusoids of the microvasculature, as reflected by augmentation of portal venous and hepatic artery blood flow during normal pregnancy (Clapp et al., 2000; Nakai et al., 2002; Vollmar and Menger, 2009; Mandic-Markovic et al., 2014). Increased splanchnic venous tone is another likely mechanism contributing to mobilization of blood from the splanchnic circulation. Particularly pronounced in the second half of pregnancy, plasma volume expands dramatically, further augmenting cardiac preload (Dechend et al., 2015). Finally, uterine blood flow also increases markedly, especially in the latter half of gestation during the rapid growth phase of the fetus and placenta (Dickey and Hower, 1995).
Table I.
Pathophysiological features of preeclampsia.
| Normal pregnancy | Preeclampsia |
|---|---|
| Systemic and renal vasodilation | Systemic and renal vasoconstriction |
| ↓ Sensitivity to vasoconstrictors | ↑ Sensitivity to vasoconstrictors |
| ↑ Arterial compliance | ↓ Arterial compliance |
| ↓ Myogenic constriction | ↑ Myogenic constriction |
| ↑ Uterine blood flow | ↓ Uterine blood flow |
| ↑ Circulating bone marrow-derived angiogenic progenitor cells | ↓ Circulating bone marrow-derived angiogenic progenitor cells |
| Robust endothelial function | Endothelial dysfunction |
| ↑ Insulin resistance | ↑↑ Insulin resistance |
↑ increased, ↓ decreased: normal pregnancy relative to the non-pregnant condition or preeclampsia relative to normal pregnancy. See text for further details and references.
There are additional changes in the maternal circulation consistent with, or contributing to, the profound vasodilatory state (Table I). These include attenuated pressor responses to vasoconstrictors such as angiotensin II and norepinephrine (Gant et al., 1973; Nisell et al., 1985; Conrad and Colpoys, 1986; Danielson and Conrad, 1995), inhibited arterial myogenic constriction (Gandley et al., 2001; Matsubara et al., 2006), robust endothelial function (Sladek et al., 1997) and increased global arterial compliance, the latter being required to maintain ventricular–arterial coupling in the face of the dramatic decline in systemic vascular resistance (Poppas et al., 1997; Debrah et al., 2006). Another intriguing pregnancy adaptation is an increased number of circulating maternal bone marrow-derived angiogenic progenitor cells (Gammill et al., 2007). Although the role of these angiogenic progenitor cells in pregnancy remains unexplored, we hypothesized that they abet maternal angiogenesis and vasculogenesis in organs such as breast, uterus, adipose, skin and pancreas, further contributing to reduced systemic vascular resistance and increased global arterial compliance (Segal et al., 2012), among other physiological roles.
During active disease, the aforementioned maternal adaptations to normal pregnancy are impaired in women with preeclampsia (Table I). The typical vasodilatory responses are either reduced or completely absent resembling the non-pregnant state, and in many cases, profound vasoconstriction ensues. Thus, systemic and renal vascular resistances are elevated relative to normal pregnancy with corresponding reductions in cardiac output, renal blood flow and glomerular filtration rate (Conrad and Davison, 2014; Hibbard et al., 2015). In one study, evidence for increased hepatic vascular resistance was reported in preeclampsia with or without HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome (Oosterhof et al., 1994), while in another, reduced hepatic arterial and portal venous blood flow was observed in preeclampsia, but only when accompanied by HELLP (Kawabata et al., 2006). Thus, changes in the hepatic microcirculation likely impede mobilization of blood from the splanchnic reservoir; this deficit, in concert with the well-known reduction in plasma volume during preeclampsia (Dechend et al., 2015), further compromises cardiac preload and output. Last, uterine blood flow is frequently depressed in preeclampsia contributing to fetal growth restriction (Velauthar et al., 2014).
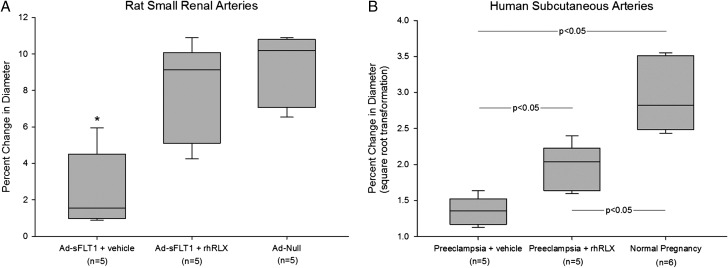
Relative to normal pregnancy, in preeclampsia the pressor responses to vasoconstrictors such as angiotensin II and norepinephrine are enhanced (Gant et al., 1973; Nisell et al., 1985), global arterial compliance is reduced (Hibbard et al., 2015), endothelial structure and function are compromised (Davidge et al., 2015), and there are fewer circulating maternal, bone marrow-derived angiogenic progenitor cells (Gammill et al., 2007). Consistent with the last observation is reduced capillary density of the nail beds in preeclamptic women (Hasan et al., 2002). A major pathogenic mechanism leading to endothelial dysfunction is believed to be the angiogenic imbalance created by excess production and release into the maternal circulation of soluble vascular endothelial growth factor receptor-1 (sVEGF-R1) among other factors mainly derived from the stressed placenta (Maynard et al., 2003; Venkatesha et al., 2006). Compared to the metabolic adaptations observed in normal pregnancy, there is further impairment of glucose disposal and insulin resistance in women with preeclampsia (Table I) (Catalano, 2010). A contributing factor is likely to be pathological compromise of insulin-mediated increases in skeletal muscle blood flow, an event mediated through endothelial-derived nitric oxide (Steinberg et al., 1994). Finally, based on a limited number of experiments, myogenic constriction of subcutaneous arteries isolated from women with preeclampsia is robust rather than inhibited as in normal pregnancy, thus resembling the non-pregnant condition (Debrah, Novak and Conrad, unpublished preliminary data; Fig. 1; Table I). In essence, endothelial dysfunction can explain many of the disease manifestations of preeclampsia including hypertension, proteinuria and excessive edema.
Figure 1.
Normal pregnancy phenotype of inhibited myogenic constriction is restored (A) or party restored (B) by incubation of arteries from pathological pregnancies with recombinant human relaxin (rhRLX; 30 ng/ml) for 3 hours in vitro. Myogenic constriction is expressed as the percentage change of internal diameter in response to a step increase of intraluminal pressure from 60 to 80 mmHg after reaching a new steady state (~5 minutes). A larger percent change in diameter corresponds with inhibition of myogenic constriction. Box depicts median, first and third quartiles, and whiskers indicate the highest and lowest data points. (A) Small renal arteries were harvested from the sVEGF-R1 (or sFLT1) rat model of preeclampsia (Maynard et al., 2003) (kidneys kindly provided by SA Karumanchi, Harvard Medical School) under IACUC approval from the Magee-Womens Research Institute, University of Pittsburgh. Ad-sFLT1, adenovirus containing the sFLT1 construct; Ad-Null, adenovirus without the sFLT1 construct. N = number of rats. P < 0.001 by one way-ANOVA. *P < 0.05 vs Ad-sFLT1 + rhRLX and Ad-Null by Student Newman Keuls (SNK) post hoc test. (B) Small subcutaneous arteries were dissected from adipose tissue obtained from the Caesarean section site under IRB approval from the Magee-Womens Research Institute, University of Pittsburgh. N = number of subjects. In order to pass the Normality and Equal Variance Tests, the data were subjected to square root transformation. P < 0.001 by one way-ANOVA. P-values in the figure were derived from the SNK post hoc test. (Debrah, Novak and Conrad, preliminary unpublished data). See text for abbreviations, further details and references.
Potential role of GPCRs as therapeutic targets in preeclampsia
Because a large proportion of the GPCR target pharmaceuticals on the market today actually represent only a small number of the GPCRs that could be exploited for medicinal use, the scope of this review will encompass these seven-transmembrane-domain receptors. GPCRs and their ligands with cardiovascular actions have been evaluated in normal pregnancy and preeclampsia. McGuane and Conrad recently reviewed several GPCRs in the context of possible drug development for preeclampsia including calcitonin receptor-like receptor/RAMP1, calcitonin receptor-like receptor/RAMP2, angiotensin 1 and 2 receptors, and the MAS receptor (McGuane and Conrad, 2012). Endothelin-1 and its corresponding GPCR, the endothelin A receptor, were discussed by Granger and colleagues at the 2014 ISSHP Meeting symposium on novel therapies for preeclampsia. The reader is referred to a recent review of the therapeutic potential of endothelin A (ETA) receptor antagonists by this research group (Sasser et al., 2015). Thus, these GPCRs and their cognate ligands will not be further considered in this review, rather two others recently emerging receptors in the preeclampsia field will be critically evaluated, namely, the arginine vasopressin (AVP) and apelin (APJ) receptors. Although other GPCRs and cognate ligands might be scrutinized, including adrenomedullin-2/intermedin, amylin and other members of the relaxin/insulin-like family peptides Insl 3–6, there is little or no information to date describing their potential involvement in preeclampsia. The concept of RXFP1 as a potential therapeutic target was previously advanced (Davison et al., 2004; Dechend and Luft, 2008; Unemori et al., 2009; McGuane and Conrad, 2012). However, the scientific rationale for relaxin or relaxin mimetics as potential therapeutics in preeclampsia has not been comprehensively laid out especially in the context of recent advances made in relaxin research. Moreover, irrespective of the outcome of the Phase 3B Clinical Trial of recombinant human relaxin (Serelaxin) in acute heart failure initiated 2014 (ClinicalTrials.gov Identifier: NCT02064868), the timing is right to set the stage for preeclampsia as one of the next disease targets for relaxin. To this end, compelling scientific rationale enters prominently in the decision-making by the pharmaceutical industry to embark upon clinical trials of relaxin in preeclampsia, and by clinicians and their patients to participate in these trials.
Arginine vasopressin receptors
Arginine vasopressin is a 9-amino acid peptide produced by the hypothalamus and released from the posterior pituitary in response to increases in plasma osmolality or reductions in blood pressure or volume (Valtin and Schafer, 1995). The sensitivity of the former mechanism is exquisite requiring only 1 to 2 mOsm/Kg H2O elevation in plasma osmolality, while the latter is only triggered by relatively large ~10% declines, but the gain is considerably higher, yielding marked increases in plasma AVP. AVP then acts on kidney tubules through the V2 receptor to promote water reabsorption, and on blood vessels through the V1 receptor to cause vasoconstriction, thereby primarily restoring water homeostasis but also blood volume and pressure. Obviously, thirst is the other essential component of this homeostatic system.
A reduction in the osmotic threshold for both release of AVP and stimulation of thirst transpires in the late luteal phase continuing during early pregnancy (Conrad and Karumanchi, 2013). Relaxin may be the mediator of these osmoregulatory changes in pregnancy (Weisinger et al., 1993; Danielson et al., 1999; Novak et al., 2001). Thus, AVP circulates in concentrations comparable to the non-pregnant condition, but at a plasma osmolality of ~10 mOsm/Kg H2O lower; otherwise, pregnant women concentrate and dilute their urine appropriately around this lower set point of plasma osmolality (Conrad and Karumanchi, 2013). Rarely, pregnant women may experience transient diabetes insipidus secondary to inappropriately high circulating vasopressinase emanating from the placenta (Barron et al., 1984; Durr et al., 1987). This syndrome can occur in the setting of hepatic dysfunction associated with acute fatty liver, HELLP syndrome and preeclampsia, because vasopressinase is normally cleared by the liver (Durr and Lindheimer, 1996).
The pre-pro-vasopressin precursor in the hypothalamus consists of a signal peptide, AVP, neurophysin II and copeptin. Like neurophysin II, copeptin may be involved in the intracellular processing of AVP, but otherwise this 39 amino acid peptide (~5 kDa) has no known function. Neurophysin II and copeptin are released from the posterior pituitary in a 1:1 molar ratio with AVP (Morgenthaler et al., 2006). There are technical challenges to measuring plasma AVP including the necessity for radioimmunoassay (RIA) due to its small molecular weight (1084 Da) and low circulating concentration (1–10 pM), and the importance for prior extraction of plasma before RIA (Robertson et al., 1973). In addition, AVP binds to platelets with high avidity (Preibisz et al., 1983), its immunoreactivity decreases rapidly ex vivo even at −20°C (Robertson et al., 1973), and the hormone has a short half-life in vivo particularly in pregnancy due to circulating leucyl/cystinyl aminopeptidases (vasopressinase or oxytocinase) emanating from the placenta. Because copeptin is released in equimolar quantities from the posterior pituitary, it was evaluated as a possible surrogate for AVP. Copeptin circulates in sufficiently high concentrations to be assayed by sensitive sandwich immunoassay and is stable ex vivo (although it also has a short half-life in vivo). Thus, Morgenthaler et al. (2006) investigated copeptin as a surrogate measurement for AVP showing an excellent correlation with AVP in normal healthy subjects and patients with sepsis. Balanescu et al. (2011) subsequently demonstrated strong correlations between copeptin and AVP in healthy volunteers subjected to water load and hypertonic saline challenges.
A potential pathogenic role of arginine vasopressin in preeclampsia was proposed based on elevated circulating concentrations of copeptin. Most (Zulfikaroglu et al., 2011; Santillan et al., 2014; Yeung et al., 2014; Tuten et al., 2015), but not all (Birdir et al., 2015) groups reported elevated circulating copeptin levels before and/or during the clinical manifestations of the disease. In one study, the increase in copeptin concentrations measured at 16 weeks of gestation was specific for preeclampsia, not being observed in women who developed gestational hypertension (without proteinuria), gestational diabetes mellitus or preterm birth in the absence of preeclampsia (Yeung et al., 2014). Santillan et al. (2014) reported that increases in copeptin early in gestation were predictive of preeclampsia, and chronic administration of AVP to pregnant, but not non-pregnant mice significantly increased systolic blood pressure by 5–10 mmHg and induced proteinuria, glomerular endotheliosis and fetal growth restriction, all hallmarks of preeclampsia, thus providing proof of principle that AVP may be a pathogenic agent in the disease.
Although the potential role for AVP in the pathogenesis of preeclampsia is intriguing, there are perhaps a few cautionary points that should be made. First, although Morgenthaler et al. (2006) meticulously validated the copeptin sandwich immunoassay for plasma from non-pregnant subjects, apparently copeptin immunoassays have not been validated using plasma from normal pregnant and preeclamptic women as matrices in the assay. Second, as discussed above, Morgenthathler and coworkers also demonstrated a significant correlation between copeptin and AVP in healthy non-pregnant subjects and patients with sepsis, and this correlation was subsequently corroborated by Balanescu and colleagues. But, so far, this relationship has apparently not been established for normal pregnancy and preeclampsia. Elevated copeptin in preeclamptic women could conceivably be secondary to other reasons such as decreased hepatic or renal clearance. In an attempt to rule out renal clearance as a confounder, Santillan et al. (2014) showed that plasma cystatin C was comparable in the women who developed preeclampsia or experienced a normal pregnancy outcome. But there is considerable controversy about whether cystatin C is a reliable marker of renal function in pregnancy (Saxena et al., 2012), perhaps in part due to placental production (Kristensen et al., 2007). Because copeptin is only ~5 kDa, it likely undergoes glomerular filtration, which is frequently compromised in preeclampsia.
The measurement of AVP per se is really critical to the hypothesis. Indeed, many investigators have successfully measured AVP in non-pregnant subjects (Robertson et al., 1973; Balanescu et al., 2011; El-Farhan et al., 2013), and by taking appropriate steps to inactivate vasopressinase, AVP measurements in pregnancy have also been reliable (Davison et al., 1984). Inconsistent with the hypothesis is that, to date, not a single publication has demonstrated elevated AVP or reduced plasma osmolality in preeclampsia relative to normal pregnancy (MacGillivray et al., 1976; Pedersen et al., 1985; van der Post et al., 1993; Gordge et al., 1994; Eguchi et al., 1996), except for the occasional report of severe hyponatremia in which AVP was not measured, but presumed to be elevated due to volume contraction and consequent non-osmotic release (Wilson and Shutt, 2007).
Nevertheless, the mouse model developed by Santillan et al. (2014) is promising particularly because the preeclampsia-like changes elicited by AVP administration were restricted to pregnancy and not observed in the non-pregnant animals. However, measurements of arterial pressure by tail-cuff should be confirmed using telemetry-based approaches in conscious, unstressed mice, and based on studies in conscious rats and dogs (Gellai et al., 1984; Brown et al., 1986; Conrad et al., 1993), chronic infusion of AVP even at low doses is likely to exert profound changes in renal and osmoregulatory function, which were not examined in the study by Santillan and coworkers. Thus, the value of this animal model may be obfuscated by markedly altered physiology created by the AVP infusion and not typically observed in preeclampsia. Moreover, a clear mechanism linking AVP to preeclampsia symptoms was not established. Thus, although provocative, this animal model requires further study.
Previously, it was hypothesized that vasopressinase might be the link between AVP and preeclampsia (Krege and Katz, 1990). That is, hydrolysis of AVP at the Cys–Tyr bond by vasopressinase precludes interaction with the V2 receptor by breaking the ring structure, while preserving the N terminus required for V1 activity. Earlier studies in rats, dogs and humans demonstrate that AVP is vasodilatory in the setting of V1 blockade, illustrating the opposing vascular actions of these two receptor subtypes (Glanzer et al., 1982; Liard, 1986; Walker, 1986; Naitoh et al., 1993; Cooke et al., 2001). Thus, it is not inconceivable that unopposed V1 activation by ‘vasopressinase altered vasopressin’ could promote vasoconstriction, platelet aggregation and endothelial dysfunction. One argument in favor of this hypothesis is that transient diabetes insipidus of pregnancy believed to be secondary to excessive placental production of vasopressinase can occur in the setting of preeclampsia, but this is an association, and as such, does not discern cause from consequence. Because vasopressinase is cleared by the liver and hepatic function can be compromised in preeclampsia, then preeclampsia is the likely cause of elevated vasopressinase and diabetes insipidus, not vice versa, although a feed forward mechanism remains a possibility. If circulating vasopressinase is elevated before the onset of disease manifestations, then a causal relationship might be inferred. However, vasopressinase was comparable in the first and second trimesters between women who developed preeclampsia or experienced a normal pregnancy outcome, and it was actually decreased during the third trimester in preeclamptic women (Santillan et al., 2014). Finally, mice do not have placental vasopressinase (Pham et al., 2009), and as such, one cannot invoke ‘vasopressinase altered vasopressin’ activation of V1 receptors in the mouse study by Santillan et al. (2014).
In conclusion, although both intriguing and provocative, whether AVP or a degradation product has a pathogenic role in preeclampsia requires further investigation. As a logical extension of this hypothesis, perhaps oxytocin should be considered in the context of its own receptor on vascular smooth muscle or even in relation to the V1 receptor despite ~30X less affinity than AVP (Holmes et al., 2003). Circulating oxytocin concentrations increase progressively during normal human pregnancy and manifest episodic peaks during labor and lactation (Dawood et al., 1979, 1981; Risberg et al., 2009), the latter perhaps affording a pathogenic link to postpartum preeclampsia. However, like AVP, circulating oxytocin does not appear to be elevated in preeclampsia either (Risberg et al., 2009). Finally, because copeptin has no known function other than a potential role in processing of the AVP pre-pro-hormone before secretion, perhaps this peptide warrants investigation rather than or in addition to AVP and oxytocin.
Apelin receptor
The 77-amino acid pre-pro-apelin dimer is expressed in several tissue- and cell-types including heart and endothelium, where it is subsequently cleaved by endopeptidases into apelin-36, -17, -13 and after post-translational modification, pyroglumatamate (pyr1)apelin-13. These peptides are agonists of the apelin G protein-coupled-receptor, APJ (Pitkin et al., 2010). APJ also has widespread tissue and cell distribution including endothelium and vascular smooth muscle (Barnes et al., 2010). In many endothelium-intact blood vessels, apelin stimulates NO production leading to vascular smooth muscle relaxation and vasodilation, whereas in endothelium-denuded vessels, vasoconstriction ensues. Apelin inhibits angiotensin II-induced elevated cytosolic calcium and vasoconstriction contributing to its overall vasodilatory action (Hus-Citharel et al., 2008). Apelin is expressed in the supraoptic and paraventricular nuclei of the hypothalamus where it decreases AVP release. Water loading and fluid restriction lower and raise plasma AVP, respectively; the same maneuvers raise and lower plasma apelin suggesting a counter-regulatory role for apelin in water homeostasis (Pitkin et al., 2010). Apelin is also an adipokine that may abet insulin action (Boucher et al., 2005).
Using immunohistochemistry, Cobellis et al. (2007) reported increased expression of apelin and APJ in many cellular compartments of the placenta from women with preeclampsia. However, subsequent studies failed to corroborate these findings. In one study, apelin and APJ mRNA and protein were reduced in preeclamptic placentas as determined by RT-PCR and western blot/immunohistochemistry, respectively (Inuzuka et al., 2013), and in another, placental apelin was assessed by radioimmunoassay and also found to be decreased (Yamaleyeva et al., 2015). In the same report, increased ACE2 expression was observed in the preeclamptic placenta, which could explain reduced apelin, because apelin is metabolized by ACE2; also, (pyr1)apelin-13 was demonstrated to be the major isoform in placenta (Yamaleyeva et al., 2015).
The reports on circulating apelin in preeclampsia are not entirely consistent either, although the majority report an increase. Bortoff et al. (2012) observed a decrease in circulating apelin in preeclamptic women relative to normal pregnancy, while three groups reported increases in circulating apelin during early and late onset, as well as mild and severe, preeclampsia (Simsek et al., 2012; Inuzuka et al., 2013; Kucur et al., 2014). The higher circulating levels may serve a compensatory role to counteract systemic vasoconstriction in preeclampsia. On the surface, lower expression of apelin in preeclamptic placentas appears to be incongruous with higher concentrations measured in the blood, but clearly, other tissues may contribute to circulating levels and differences in metabolic clearance could also come into play. On balance, more investigations of apelin and its receptor in preeclampsia are needed, but in light of its vasodilatory and other potentially beneficial properties, the apelin/APJ receptor system may be a potential therapeutic target in preeclampsia.
Relaxin/insulin-like family peptide receptor 1
Role of relaxin in the cardiovascular adaptations to pregnancy
In 1926, relaxin was discovered by Frederick Hisaw to be a factor circulating in the blood of late pregnant rabbits and guinea pigs. When the blood was injected into virgin guinea pigs during early post-estrus, it produced relaxation of the pubic ligament (Hisaw, 1926; Sherwood, 1994). Relaxin was later identified as a ~6 kDa peptide, and more recently the relaxin receptor was revealed to be a leucine-rich repeat GPCR (relaxin/insulin-like family peptide receptor, RXFP1) (Hsu et al., 2002; Bathgate et al., 2013). The hormone emanates from the corpus luteum circulating during the luteal phase and during pregnancy in many species (Sherwood, 1994). In women, relaxin reaches serum concentrations of ~0.1 ng/ml on Days 7–12 post luteinizing hormone-surge in a non-conceptive cycle, and in a conceptive cycle, relaxin rises to ~1 ng/ml during the first trimester, falling to intermediate values thereafter for the duration of pregnancy (Szlachter et al., 1982; Stewart et al., 1990). Relaxin has traditionally been considered to be a reproductive hormone modifying the reproductive tract in some, but not all species (Sherwood, 1994). Later, relaxin was found to exert actions in the circulation (Hisaw et al., 1967; St-Louis and Massicotte, 1985; Massicotte et al., 1989; Bani-Sacchi et al., 1995; Danielson et al., 1999), and within the last decade or two, has become firmly ensconced in the cardiovascular field.
The role of relaxin in the maternal systemic and renal adaptations to pregnancy was extensively explored in the conscious gravid rat model, because this species undergoes gestational circulatory changes similar to women (Conrad and Davison, 2014). Specifically, neutralization or elimination of circulating relaxin with specific antibodies or by ovariectomy, respectively, inhibited the increases of cardiac output, global arterial compliance, renal blood flow and glomerular filtration rate, as well as the decreases in systemic and renal vascular resistances, and in plasma osmolality during midterm pregnancy (Novak et al., 2001; Debrah et al., 2006). Small renal arteries harvested from gravid rats with neutralized or absent circulating relaxin demonstrated robust myogenic constriction similar to the non-pregnant condition rather than the inhibited myogenic constriction typical of normal pregnancy (Novak et al., 2001). Interestingly, the relaxin-neutralizing antibodies only inhibited ~50% of the gestational increase in cardiac output and global arterial compliance, and reduction in systemic vascular resistance during late gestation suggesting a role for other vasodilator(s) possibly of placental origin (Debrah, Shroff and Conrad, unpublished data). However, additional experiments are needed to explain these observations from late stage rat pregnancy.
Whether relaxin (or other corpus luteal factors) plays a similar role in the cardiovascular and renal circulations during human pregnancy is currently under investigation. To this end, a multitude of cardiovascular and renal parameters are being assessed in women who conceive with and without assisted reproductive technologies (ART). On the one hand, women conceiving through oocyte donation lack a corpus luteum, and hence, circulating relaxin, as a consequence of ovarian failure or hypothalamic-pituitary suppression, the latter utilized as part of the ART procedure (Johnson et al., 1991). Similarly, many women who undergo autologous frozen embryo transfer frequently do so in a medicated cycle involving hypothalamic-pituitary suppression. On the other hand, women undergoing ovarian stimulation, IVF and fresh embryo transfer have multiple corpora lutea, and thus, elevated levels of circulating relaxin (Mushayandebvu et al., 1998). Relative to spontaneous conceptions, we anticipate subdued changes in the maternal circulation (‘hypodynamic’) of women conceiving by ART with absent corpus luteum and circulating relaxin, particularly during early pregnancy, consistent with the rat studies (Conrad and Baker, 2013). In support of this concept is a small pilot study conducted throughout the first trimester of women with ovarian failure who conceived using donor oocytes; in these women there was a subdued gestational increase in 24-h endogenous creatinine clearance (an estimation of glomerular filtration rate or GFR) and an attenuated decline in plasma osmolality (Smith et al., 2006b). On the other hand, we expect that, relative to spontaneous conceptions, women conceiving through ART with multiple corpora lutea and elevated circulating relaxin will have an exaggerated or ‘hyperdynamic’ circulation (Conrad and Baker, 2013).
After 8–10 weeks of gestation when the placenta becomes sufficiently mature and functional, we may find that vasodilators of placental origin are sufficient to rescue maternal vasodilation in the gravid women without a corpus luteum (Conrad and Baker, 2013). Nevertheless, there is evidence to suggest that women who develop early-onset preeclampsia with fetal growth restriction (<34 gestational weeks) or normotensive fetal growth restriction manifest a hypodynamic circulation during early pregnancy with relatively reduced cardiac output and increased systemic vascular resistance relative to women who experience a normal pregnancy (Vasapollo et al., 2004; Salas et al., 2006; De Paco et al., 2008; Khaw et al., 2008). Thus, if women conceiving through ART who lack a corpus luteum and circulating relaxin reveal a hypodynamic circulation during early pregnancy as predicted, then by analogy, they could be at increased risk of developing fetal growth restriction with or without early-onset preeclampsia. In contrast, women who develop late-onset preeclampsia or gestational hypertension may manifest a hyperdynamic circulation during early pregnancy with relatively increased cardiac output and reduced systemic vascular resistance compared to women who experience a normal pregnancy (Easterling et al., 1990; Bosio et al., 1999; De Paco et al., 2008; Khaw et al., 2008). If women conceiving by ART who have multiple corpora lutea and elevated circulating relaxin present with a hyperdynamic circulation during early pregnancy as hypothesized, then again by analogy, they could be at increased risk of developing late-onset preeclampsia or gestational hypertension. Notably, emerging evidence suggests that ART is indeed associated with increased risk for preeclampsia and delivery of small for gestational age infants (Conrad and Baker, 2013). However, it is unknown whether the postulated hypodynamic or hyperdynamic circulation in early pregnancy might contribute to the eventual development of adverse obstetrical outcomes later in gestation reflect a more fundamental pathology or both, and in the case of ART, other factors such as the preexisting infertility or the added immunological challenge of donor oocytes are likely to also play a role (Salha et al., 1999; Jaques et al., 2010).
Relaxin administration mimics the circulatory changes of pregnancy
As summarized in Tables II and III, relaxin administration to rats and humans reproduced many of the circulatory changes observed in normal pregnancy. Essentially, relaxin is a vasodilator in both the renal and systemic circulation of conscious rats and humans (Danielson et al., 1999; Danielson and Conrad, 2003; Conrad et al., 2004; Bogzil et al., 2005; Smith, et al., 2006a; Dschietzig et al., 2009; Khanna et al., 2009; Conrad, 2011; Ponikowski et al., 2014; Voors et al., 2014), although the influence of relaxin on cardiac output in humans is controversial, but has only been tested in the setting of heart failure and not in normal human volunteers (Dschietzig et al., 2009; Ponikowski et al., 2014). Relaxin infusion in conscious rats rapidly increased renal blood flow and glomerular filtration rate, which was sustained upon long-term administration (Danielson et al., 1999; Danielson and Conrad, 2003). Comparable findings were observed in women, with the exception that increased glomerular filtration was observed only after a longer duration of administration (Smith et al., 2006a; Dschietzig et al., 2009; Khanna et al., 2009; Weiss et al., 2009; Conrad and Davison, 2014). Finally, relaxin administration to conscious rats blunted the systemic and renal pressor responses to angiotensin II (Danielson et al., 1999; Debrah et al., 2005), and increased global arterial compliance (Conrad et al., 2004).
Table II.
Relaxin administered to conscious, non-pregnant rats and humans recapitulates many aspects of the maternal systemic circulatory adaptations of pregnancy.
| Circulatory adaptation | Pregnancy | Relaxin | ||
|---|---|---|---|---|
| Rat | Human | Rat | Human | |
| Cardiac output | ↑ | ↑ | ↑ | +/−a |
| Systemic vascular resistance | ↓ | ↓ | ↓ | ↓ |
| Portal venous blood flow | ? | ↑ | ↑ | ? |
| Arterial compliance | ↑ | ↑ | ↑ | ? |
| Angiotensin II pressor response | ↓ | ↓ | ↓ | ? |
| Myogenic constriction | ↓ | ↓ | ↓ | ↓ |
| Circulating bone marrow-derived angiogenic progenitor cell number and/or activity | ↑ (mice)b | ↑ | ↑ (mice) | ? |
Relaxin administered to non-pregnant females and males. ?, not tested, ↑ increased, ↓ decreased: pregnancy relative to the non-pregnant state or relaxin administration in non-pregnant rats and humans relative to vehicle infusion. See text for further details and references.
aThe influence of relaxin on cardiac output in humans is disputed, but has only been tested in the setting of heart failure and not in normal human volunteers (see text for citations).
bSegal, Sautina, Li and Conrad, unpublished.
Table III.
Relaxin administered to conscious, non-pregnant rats and humans recapitulates many aspects of the maternal renal circulatory and osmoregulatory changes of pregnancy.
| Circulatory or osmoregulatory adaptation | Pregnancy | Relaxin | ||
|---|---|---|---|---|
| Rat | Human | Rat | Human | |
| Glomerular filtration rate | ↑ | ↑ | ↑ | +/− ↑a |
| Renal plasma flow | ↑ | ↑ | ↑ | ↑ |
| Renal vascular resistance | ↓ | ↓ | ↓ | ↓ |
| Angiotensin II-induced renal vasoconstriction | ↓ | ? | ↓ | ? |
| Plasma osmolality | ↓ | ↓ | ↓ | ? |
Relaxin administered to non-pregnant females and males. ?, not tested, ↑ increased, ↓ decreased: pregnancy relative to the non-pregnant state or relaxin administration in non-pregnant rats and humans relative to vehicle infusion. See text for further details and references.
aChronic, not acute relaxin administration.
Early morphological findings of hepatic sinusoids obtained from rats administered relaxin were indicative of reduced hepatic vascular resistance and increased hepatic blood flow consistent with the mobilization of splanchnic blood to augment venous return, as observed in normal human pregnancy (Bani et al., 2001). More recently, functional evidence supports this concept, revealing that relaxin reduced portal venous pressure and increased portal venous blood flow in a rat model of portal hypertension through two mechanisms: augmentation of NO production by hepatic sinusoidal endothelial cells that, in turn, inhibited hepatic stellate cell contraction, and downregulation of hepatic stellate cell contractile filament expression (Fallowfield et al., 2014).
Small renal arteries isolated from rats and mice administered relaxin were more compliant and demonstrated inhibition of myogenic constriction ex vivo (Novak et al., 2002; Conrad et al., 2004; Debrah et al., 2011; McGuane et al., 2011a). Incubation of small renal arteries from rats and mice, as well as subcutaneous arteries from humans, with the hormone also inhibited myogenic constriction (McGuane et al., 2011a). Relaxin elicited a rapid relaxation response in pre-constricted small renal arteries from rats and mice, as well as gluteal and subcutaneous arteries, but not pulmonary resistance arteries from humans (Fisher et al., 2002; McGuane et al., 2011b), and in hamster, cremaster muscle arterioles in situ (Willcox et al., 2013). Moreover, relaxin potentiated endothelium-dependent vasorelaxation by bradykinin in mesenteric arteries isolated from male rats treated with the hormone in vivo (Leo et al., 2014). Relaxin was shown to increase uterine blood flow velocity in conscious, non-pregnant rats and augment uterine artery compliance during pregnancy in rats (Vodstrcil et al., 2012). Finally, when administered to mice, relaxin increased circulating bone marrow-derived angiogenic progenitor cells and their incorporation into the vasculature of subcutaneous matrigel plug implants (Segal et al., 2012). The hormone also increased nitric oxide production by isolated human CD34+ cells and stimulated their migration (Segal et al., 2012).
Rationale for the potential therapeutic use of relaxin in preeclampsia: exploiting the beneficial hemodynamic attributes of relaxin
During the overt, clinical phase of disease, circulating concentrations of immunoreactive relaxin in preeclampsia appear to be comparable to normal pregnancy (Szlachter et al., 1982). However, recent evidence suggests that women with serum levels in the lowest 10th centile during the first trimester are at increased risk of developing late, but not early-onset preeclampsia (Jeyabalan et al., 2009; Post Uiterweer et al., 2014). Whether the bioactivity of circulating relaxin or RXFP1 signaling is impaired in preeclampsia has not been evaluated. Nor is it known whether relaxin locally produced by tissues such as arteries, placenta and decidua may be reduced. (The reader is referred to several excellent reviews on local relaxin-ligand expression in uterine and other tissues: Kong et al., 2010; Bathgate et al., 2013; Anand-Ivell and Ivell, 2014). Nevertheless, whether or not circulating or locally produced relaxin is deficient, thereby potentially contributing to disease pathogenesis, administration of the hormone to reach supra-physiological concentrations may prove salutary as a therapeutic strategy. This may be particularly apropos for pregnant women conceiving by ART who lack a corpus luteum, and hence, any circulating relaxin, and who are at increased risk of developing preeclampsia.
As described above, in many respects, the circulatory pattern in preeclampsia at least during the overt phase of the disease is the antithesis of normal pregnancy. Because relaxin administration can reproduce many of the circulatory changes of normal pregnancy, and indeed, mediates these changes in gravid rats and possibly in pregnant women, too, it is logical to investigate whether relaxin administration to preeclamptic women could restore, or at least partially so, circulatory changes to normal pregnancy levels (Table IV). As a consequence of its pleiotropic hemodynamic properties outlined above, relaxin treatment would be expected to relieve one of the fundamental pathologies of preeclampsia, maternal organ vasoconstriction. Presuming that myocardial function is not unduly compromised, relaxin would be anticipated to improve cardiac output due to a concurrent reduction in systemic vascular resistance and augmentation of venous return, as well as ameliorate systemic and renal vasoconstriction, thereby improving maternal organ perfusion. A reciprocal rise in cardiac output should buffer the decline in arterial pressure as a consequence of the beneficial reduction in systemic vascular resistance, thereby preserving uterine blood flow, which may not have an efficient autoregulatory capacity. If unacceptably high arterial pressure persists despite relaxin treatment, routine anti-hypertensive therapy could be implemented. Finally, relaxin may be further beneficial by increasing uterine blood flow in preeclampsia (Vodstrcil et al., 2012; Gooi et al., 2013).
Table IV.
Expected improvements of cardiovascular and renal function in preeclampsia during recombinant human relaxin (rhRLX) administration.
| Preeclampsia | Preeclampsia + rhRLX |
|---|---|
| Systemic and renal vasoconstriction | Systemic and renal vasodilation |
| ↑ Sensitivity to vasoconstrictors | ↓ Sensitivity to vasoconstrictors |
| ↓ Arterial compliance | ↑ Arterial compliance |
| ↑ Myogenic constriction | ↓ Myogenic constriction |
| ↓ Uterine blood flow | ↑ Uterine blood flow |
| ↓ Circulating bone marrow-derived angiogenic progenitor cells and activity | ↑ Circulating bone marrow-derived angiogenic progenitor cells and activity |
| Endothelial dysfunction | Improved endothelial function |
| Vascular inflammation | Reduced vascular inflammation |
| Insulin resistance | Improved insulin resistance |
| Cytotrophoblast apoptosis and necrosis | Reduced cytotrophoblast apoptosis and necrosis |
↑ increased, ↓ decreased: preeclampsia relative to normal pregnancy; preeclampsia + rhRLX vs preeclampsia. See text for further details and references.
Relaxin administration would be expected to reverse robust myogenic constriction (Debrah, Novak and Conrad, unpublished preliminary data; Fig. 1), as well as blunt systemic and renal pressor responses to activation of the angiotensin II receptor, type 1 (AGTR1) by circulating and locally derived angiotensin II, and AGTR1 autoantibodies (Wallukat et al., 1999), thereby reducing arterial tone. In the same vein, relaxin should blunt the response to endogenous vasoconstrictors in uterine spiral arteries with failed remodeling and retained smooth muscle, thereby improving uteroplacental blood flow. In addition to possible improvements of vessel wall geometry and composition given a sufficient duration of relaxin infusion, vasodilation or reduction in arterial tone would be anticipated to improve arterial compliance in preeclampsia (Conrad and Shroff, 2011). Through promoting skeletal muscle vasodilation, thus enhancing insulin-mediated glucose disposal, relaxin should ameliorate insulin resistance in preeclampsia (Bonner et al., 2013). Finally, by mobilization and activation of bone marrow-derived angiogenic progenitor cells, the hormone would be expected to repair injured endothelium and improve maternal vascularity in organs such as breast, uterus, pancreas, skin and fat. Formation of new or remodeling of existing blood vessels by relaxin would also contribute to the decline in systemic vascular resistance and increase in global arterial compliance. Table IV summarizes some of the theoretical rationale based on relaxin's salutary physiological attributes for underwriting preclinical and clinical investigation of the hormone as a potential therapeutic in preeclampsia, by granting agencies and pharmaceutical companies.
Rationale for the potential therapeutic use of relaxin in preeclampsia: exploiting the beneficial molecular attributes of relaxin
As a consequence of its pleiotropic molecular properties, relaxin treatment would be expected to improve many aspects of maternal circulatory pathology in preeclampsia. Of course, these pleiotropic molecular properties ultimately underlie the beneficial hemodynamic actions of relaxin as discussed above. Many of the vasodilatory effects of relaxin appear to be mediated through endothelium-derived NO (Conrad, 2010). The rapid vasodilatory response (within minute(s)) observed in small renal arteries isolated from rats and mice, as well as human subcutaneous arteries is mediated through Gαi/o protein coupling to PI3 kinase, protein kinase B, and endothelial NO synthase, but not VEGF receptor transactivation or increased endothelial cell calcium (McGuane et al., 2011b; Sarwar et al., 2015). The sustained vasodilatory response (hour(s) to day(s)) involves upregulation of arterial gelatinase activities by relaxin that, in turn, hydrolyze big ET (endothelin) to ET1−32, thereby activating the endothelial ETB receptor-NO vasodilatory pathway (Jeyabalan et al., 2003). Though a logical extension of these findings, whether relaxin also upregulates the endothelial ETB receptor is disputed (Dschietzig et al., 2003; Kerchner et al., 2005; Sarwar et al., 2015). By increasing endothelial NO production, relaxin could contribute to therapeutic vasodilation in preeclampsia. Interestingly, the sustained vasodilatory pathway critically depends on arterial-derived VEGF and PLGF, but the underlying mechanisms are presently unknown (McGuane et al., 2011a). One possibility is that relaxin increases arterial VEGF and PLGF activities, and thus, could mitigate the detrimental effects of circulating sVEGF-R1 on endothelial cell health in preeclampsia by improving local, arterial angiogenic balance and nitric oxide production. Another interesting, but untested possibility is that RXFP1 may heterodimerize with the AGTR2 receptor in the endothelium (by analogy to myofibroblasts (Chow et al., 2014)), thereby promoting NO production and vasodilation. Indeed, pharmacological blockade or genetic deletion of the AGTR2 receptor prevented the physiological decline of arterial pressure during early gestation in mice (Chen et al., 2007; Carey and Rose, 2011).
Recent evidence has suggested that relaxin abrogates angiotensin II-induced increases of intracellular calcium in cultured rat mesangial cells independently of NO or AGTR2 (Carvalho et al., 2012). Reductions in both basal and angiotensin II-induced cytosolic calcium by relaxin have also been observed in human mesangial cells (Costers and Conrad, unpublished). Theoretically, this action might contribute to the gestational increases of glomerular filtration in normal pregnancy by relaxing mesangial cells and increasing surface area for filtration. If these findings in mesangial cells carry over to vascular smooth muscle, then vasodilation would result. Interestingly, relaxin elicits comparable systemic vasodilation in normotensive control rats with normal endothelial function and in rats with genetic hypertension (spontaneously hypertensive rats) or angiotensin II-induced hypertension with some degree of endothelial dysfunction (Debrah et al., 2005). Thus, despite endothelial dysfunction, relaxin would be expected to exert a vasodilatory response in preeclampsia by directly acting on vascular smooth muscle to lower intracellular calcium.
Relaxin was recently shown to confer anti-inflammatory properties, which may ultimately prove therapeutic in preeclampsia (Dschietzig et al., 2012; Brecht et al. ). Specifically, relaxin mitigated TNF-α-induced increases of vascular cell adhesion molecule-1 (VCAM-1), monocyte chemotactic protein-1 (MCP-1) and platelet endothelial cell adhesion molecule-1 (PECAM-1) expression in cultured human umbilical vein endothelial cells (HUVEC). Relaxin also reduced a TNF-α-mediated increase in chemokine receptor type-1 (CCR-1) secretion by THP-1 cells, a human monocyte leukemia cell line, and suppressed THP-1 adhesion to a monolayer of HUVEC (Brecht et al. ). Moreover, the hormone improved acetylcholine-induced relaxation in rat aortic rings that were incubated with TNF-α (Dschietzig et al., 2012). The mechanisms underlying this beneficial action were several-fold: enhanced eNOS activity, but not expression through increased phosphorylation of Ser1177 and Ser633 and reduced phosphorylation of Thr495, inhibited arginase II expression and increased superoxide dismutase-1 (SOD-1) that, in turn, mitigated oxidative and nitrosative stress (Dschietzig et al., 2012). Because circulating TNF-α and adhesion molecules are increased in preeclampsia and endothelium-dependent relaxation is impaired (Conrad and Benyo, 1997), relaxin administration may be beneficial in preeclampsia by reducing vascular inflammation. Moreover, in the setting of chronic angiotensin II-induced hypertension, relaxin decreased arterial pressure coincident with reductions in renal oxidative stress and circulating dimethylarginine that resulted in increased NO bioavailability (Sasser et al., 2014). Because a prominent pathological feature of preeclampsia is enhanced vascular AGTR1 activity, oxidative stress and circulating ADMA (Fickling et al., 1993; Hubel, 1999), relaxin administration would be anticipated to mitigate this pathology. Thus, there is good reason to consider relaxin administration in the context of improving endothelial health and ameliorating vascular inflammation.
Interestingly, relaxin was reported to activate PPAR-γ transcriptional activity in a ligand-independent fashion through increasing expression of PPAR-γ coactivator 1α (PCG1α) (Singh et al., 2015). Deficiency of placental PPAR-γ activity has been implicated in the pathogenesis of preeclampsia (McCarthy et al., 2013). Antagonism of PPAR-γ in pregnant rats produced a syndrome resembling preeclampsia (McCarthy et al., 2011a) and PPAR-γ activation by rosiglitazone in a rat model of preeclampsia ameliorated disease symptoms (McCarthy et al., 2011b). Thus, relaxin may also be therapeutic in preeclampsia from the standpoint of enhancing placental PCG1α expression, and hence, PPAR-γ transcriptional activity.
Rationale for the potential therapeutic use of relaxin in preeclampsia: exploiting the cell survival properties of relaxin
Placental cells, cytotrophoblast (CTB), undergo accelerated apoptosis and cell death in preeclampsia (Al-Nasiry et al., 2006; Heazell and Crocker, 2008). Relaxin administration would be expected to exert cytoprotective action in CTB (and maternal endothelium) because the hormone activates the well-known cell survival pathway, PI3 kinase/Akt (Luo et al., 2003; McGuane et al., 2011b; Cudmore et al., 2012). Moreover, relaxin is likely to increase NO production in CTB, which is also cytoprotective (Dash et al., 2003). Relaxin was shown to be anti-apoptotic in epithelial and stromal cells of the mouse vagina and cervix, as well as in prostate cancer cells and rat neonatal cardiomyocytes (Feng et al., 2007; Moore et al., 2007; Yao et al., 2008), and more recently in the CTB cell line, HTR-8/SVneo (Campo et al., 2013; Lodhi et al., 2013). Importantly, both adult endothelial and CTB cells express the major relaxin receptor, RXFP1 ((Maruo et al., 2007), Conrad, unpublished). In addition to mitigating apoptosis, relaxin may also promote CTB invasion (Maruo et al., 2007; Post Uiterweer et al., 2012), which is impaired in preeclampsia resulting in deficient remodeling of uterine spiral arteries, and consequently, compromise of blood flow to the placenta.
Rationale for the potential prophylactic use of relaxin in preeclampsia
The origin of preeclampsia is widely believed to involve defective trophoblast invasion, and hence, impaired placentation. Because these deficits transpire in early pregnancy, molecular pathology of delivered placentas may not be informative. Recent DNA microarray analyses and bioinformatics identified differentially expressed genes between early placentas (chorionic villous samples; ~11.5 gestational weeks) from women who developed preeclampsia 5–6 months later and those who experienced an uncomplicated pregnancy (Founds et al., 2009; Rabaglino et al., 2015). These differentially expressed genes were evaluated in the context of DNA microarray databases in the public domain that were derived from endometrial biopsies obtained during the menstrual cycle and early pregnancy in healthy subjects. The bioinformatics revealed provocative, but compelling evidence for insufficient or defective endometrial maturation (decidualization) including reduced uterine natural killer cell number and/or activity in the late secretory phase and during early pregnancy in the women who developed preeclampsia (Rabaglino et al., 2015). This work provided the heretofore missing, but essential prospective data supporting the hypothesis that the genesis of impaired trophoblast invasion and placentation in preeclampsia could reside in a poorly developed endometrium.
Interestingly, expression of endometrial relaxin increases upon decidualization, and relaxin itself induces decidualization. The combination of methoxyprogesterone acetate and relaxin produced a synergistic increase in prolactin and insulin growth factor binding protein-1 (IGFBP-1) secretion from human endometrial stromal cells, two classic biomarkers of decidualization (Lane et al., 1994). Moreover, relaxin increased transcription of prolactin and IGFBP-1 in human decidual and endometrial stromal cells (Tang et al., 2005). The hormone also stimulated VEGF secretion in cultured human endometrial cells (Unemori et al., 1999), and in cultured glandular epithelial and stromal cells isolated from secretory phase endometrium (Palejwala et al., 2002). Consistent with an in vivo study described below, relaxin stimulated glycodelin mRNA and protein in cultured human glandular epithelial cells, another biomarker of decidualization (Tseng et al., 1999). Relaxin synergized with phosphodiesterase inhibition to promote decidualization of human endometrial stromal cells as reflected by increased prolactin and IGFBP-1 secretion (Bartsch et al., 2004). The hormone was also reported to increase its own receptor, RXFP1, and IGFBP-1 in human endometrial-decidual cells (Mazella et al., 2004). Finally, relaxin was shown to induce decidualization of cultured endometrial stromal cells, mediated through IL-11 production and action (Dimitriadis et al., 2005).
Progesterone-dependent expression of relaxin protein was detected by immunohistochemistry in human secretory endometrium (Yki-Jarvinen et al., 1985). Again using immunohistochemistry, relaxin expression was marked in glandular epithelium, endometrial stromal cells and decidua beginning in the mid-secretory endometrium and extending through pregnancy (Bryant-Greenwood et al., 1993). Serum concentrations of relaxin and glycodelin secreted by the corpus luteum and endometrium, respectively, coincided during the late luteal phase and early pregnancy suggesting that relaxin stimulates glycodelin secretion (Stewart et al., 1997). The relaxin receptor, RXFP1, increased expression and ligand binding activity starting with the early secretory phase in the human endometrium (Bond et al., 2004). Finally, when administered to ovariectomized, steroid replaced rhesus monkeys, relaxin increased uterine weight and angiogenesis, as well as lymphocyte number, all hallmarks of decidualization (Goldsmith et al., 2004).
If the antecedents of preeclampsia reside in poorly decidualized endometrium and relaxin has the potential to promote decidualization, then conceivably relaxin could be administered in the late secretory phase and during early pregnancy to improve endometrial maturation, thereby reducing the risk of developing preeclampsia. Thus, women with a history of severe preeclampsia or who have comorbidities that increase the risk of preeclampsia such as obesity, chronic hypertension, diabetes, autoimmune disease or polycystic ovary syndrome might be treated empirically with supplemental relaxin administration to improve endometrial maturation. Indeed, these comorbidities themselves may impair endometrial maturation in the secretory phase (Schulte et al., 2015). Alternatively, women who manifest impaired endometrial maturation might be identified by an abnormal molecular signature on a diagnostic endometrial biopsy or in uterine secretions during the late secretory phase or by circulating biomarkers indicative of inadequate decidualization. The idea that relaxin might be administered in the late secretory phase and early pregnancy to improve endometrial function (and possibly maternal circulatory adaptations as well), in order to reduce the risk of preeclampsia and perhaps of other placental syndromes is a new concept that requires future testing.
Relaxin safety profile
Based on its reported matrix-degrading attributes and a favorable Phase II trial, a larger randomized, double-blinded, placebo-controlled trial was undertaken to investigate subcutaneous administration of recombinant human relaxin for 24 weeks in patients with stable and diffuse, moderate to severe scleroderma (Khanna et al., 2009). Primary and secondary outcomes related to improvement in disease pathology were not met. Of notable concern was an overall decline of ~10 ml/min from baseline in predicted creatinine clearance after abrupt cessation of therapy, which was not observed in the placebo-controlled cohort. There were also serious adverse renal events consisting of doubling of serum creatinine, renal crisis or severe rebound hypertension in 6 of 169 subjects after abrupt cessation of relaxin. Thus, treatment was tapered in the remaining 62 patients of this study with only 1 serious adverse event. It is possible that these serious adverse events were related to the long-term duration of the relaxin exposure or to the underlying pathology (scleroderma). Nevertheless, the experience strongly suggested that cessation of relaxin therapy should be gradual especially after prolonged administration, and arterial pressure and renal function should be frequently monitored during tapering.
There is extensive experience with short-term intravenous infusion (<48 h) of recombinant human relaxin in heart failure (Dschietzig et al., 2009; Teerlink et al., 2009; Teerlink et al., 2013). Although hypotension was more frequent in relaxin cohorts, this complication was mitigated by selecting patients with systolic blood pressure >125 mmHg and by closely monitoring blood pressure and adjusting the infusion rate or stopping the infusion as needed using pre-specified guidelines. Otherwise, adverse events were comparable between the relaxin and placebo groups, and relaxin appeared to be well tolerated and safe. Intriguingly, survival at 180 days was significantly improved in the relaxin-treated group.
In a double-blind, placebo-controlled trial, recombinant human relaxin was infused for 24-h in women >40 gestational weeks scheduled for labor induction (Weiss et al., 2009). Contrary to the investigators’ hypothesis, relaxin did not accelerate cervical ripening, reduce the time to delivery or elevate the incidence of spontaneous labor or the onset of active labor, relative to placebo. However, consistent with the cardiorenal actions of relaxin as described above, the hormone decreased serum creatinine and blood urea nitrogen, increased predicted creatinine clearance, and produced a small decrease in systolic blood pressure. No adverse side effects were reported.
Theoretically, a potentially deleterious consequence of relaxin administration could arise in the setting of pathological oxidative stress as in preeclampsia, where more NO produced in response to the hormone could react with superoxide to form the potentially damaging free radical, peroxynitrite (Pacher et al., 2007). However, several investigators have found that relaxin reduces oxidative stress in the setting of pathological challenges, albeit outside of preeclampsia (Dschietzig et al., 2012; Sasser et al., 2014). To date, relaxin has been administered as a recombinant protein, and as such, it has the theoretical potential to elicit antibody formation some of which could be neutralizing. Like oestrogen and other growth factors, relaxin may promote some tumors such as prostate and breast (Feng et al., 2007; Binder et al., 2014). The relatively short time course of administration that we envision for preeclampsia therapy would seem to present minimal risk in this regard, although preeclamptic woman with known cancer or at high risk would clearly be excluded. Finally, the consequences of fetal exposure are unknown, although cord blood relaxin is normally a fraction of maternal circulating concentrations, and in rhesus monkeys, there was little transfer from the maternal to the fetal circulation (Weiss et al., 1978; Lucas et al., 1989; Cossum et al., 1991; Johnson et al., 1992). Nevertheless, relaxin cord blood levels at delivery and neonatal/pediatric monitoring would be important endpoints of a clinical trial.
To summarize, relaxin is a naturally occurring molecule that circulates in the luteal phase and during pregnancy, and is expressed locally by many tissues including blood vessels (Novak et al., 2006). Based on the limited number of clinical trials described above, the safety profile of relaxin has so far been favorable particularly when administered short-term, and thus, does not contraindicate its use as a potential therapeutic in preeclampsia. Blood pressure and renal function should be frequently monitored during and after the infusion, which should be tapered and not abruptly stopped. Importantly, relaxin is not expected to accelerate cervical ripening or induce labor. Thus, short-term relaxin administration to women with severe preeclampsia, in order to achieve supra-physiological circulating levels of ≤10 ng/ml would be expected to ameliorate the maternal disease sufficiently to permit safe prolongation of pregnancy allowing for further fetal maturation.
Conclusions
One approach to drug development is designing pharmaceuticals targeting individual inciting factors. A potential downside is that elimination of only one of several inciting factors that circulate in preeclampsia might be insufficient for some women. For example, in preeclampsia specifically targeting sVEGF-R1 may be inadequate due to other, unopposed pathologic factors, for example, soluble endoglin or AGTR1 activation by autoantibodies. Another potential drawback is that any given pharmaceutical may have untoward side effects, for example, administration of VEGF (previously identified as vascular permeability factor) to neutralize sVEGF-R1 may result in excessive capillary leakage, and treatment with PLGF may exacerbate activation of leukocytes, which are already activated in preeclampsia (Clauss et al., 1996). Likewise, administration of an antibody to bind sVEGF-R1 is likely to antagonize the physiological cellular actions of membrane VEGF-R1, a potentially undesirable consequence.
We propose a nontraditional, but logical approach to developing therapeutics for preeclampsia, that is, the administration of pregnancy hormone(s) that underpin the circulatory adaptations of uncomplicated pregnancy, in order to at least partly restore the maternal circulation to normal in preeclampsia. Mainly based on preclinical (animal) studies, we identified circulating relaxin as a potent vasodilator in the renal and systemic circulations during normal pregnancy. Relaxin exerts pleiotropic molecular and physiological actions, through the GPCR, RXFP1, activating many cell signaling pathways, several of which are likely to be beneficial to women suffering from preeclampsia. Thus, rather than targeting one pathogenic factor, our approach would enable the activation of several potentially salutary signaling pathways and physiological actions simultaneously through the administration of one therapeutic. In other words, given the complex pathogenesis of preeclampsia most likely involving more than one inciting factor and multiple organ dysfunction, a therapeutic with the potential to combat several of these at once would seem to be advantageous. Moreover, relaxin is a naturally occurring protein, which normally circulates in the luteal phase and during pregnancy, and as such, modest elevation of circulating levels by supplementation for days to weeks(s), in order to quell disease pathogenesis and safely prolong gestation is less likely than exogenous compounds to have untoward side effects. As detailed above, relaxin has an encouraging safety profile both outside of and within pregnancy. In particular, when administered to gravid women near term in a clinical trial of cervical ripening, the only effects noted were small decreases in arterial pressure and serum creatinine, both consistent with its known physiological actions. Importantly, the hormone did not promote cervical ripening (Weiss et al., 2009). Again, based on its pleiotropic actions, relaxin may see clinical utility soon in the setting of acute heart failure, another complex disease with multiple pathogenic mechanisms (Teerlink et al., 2013).
For preeclampsia, there would seem to be reasonable justification for evaluating preventative, therapeutic and curative strategies that are plausible, having emerged from sound basic research. In this regard, the strategy proposed herein, we believe, offers a plausible therapeutic approach to preeclampsia that warrants testing. Like insulin, relaxin is currently cumbersome to administer, but there are several laboratories pursuing the development of small molecule, orally bioavailable, RXFP1 mimetics, and these are likely to be a reality soon (e.g. Xiao et al., 2013). Although we hope that statins prove to be effective in treating preeclampsia or as a prophylaxis (Costantine and Cleary, 2013), clinical trials are just beginning and we may not know for some time whether they will be successful or have unforeseen side effects precluding or limiting their use in pregnancy. Even if trials are successful, as pertains to the non-pregnant population, statins may not be tolerated by all women (Matteucci and Giampietro, 2013). Thus, it would seem prudent to have more therapeutic candidates for preeclampsia including relaxin in the pipeline beginning with their preclinical testing, and to have more than one therapeutic option, if at all possible, for the disease.
Acknowledgements
The author gratefully acknowledges fruitful collaborations with many colleagues, in particular, John M. Davison MD, Lee A Danielson PhD, Arun Jeyabalan MD, Jonathan T. McGuane PhD, Jacqueline Novak PhD, Laura J. Parry PhD, Mark S. Segal MD, PhD and Sanjeev G. Shroff PhD. The author also thanks Elizabeth Currin for excellent clerical support in preparing the manuscript and Melissa D. Lingis PhD for preparation of the figures.
Author's role
The author conducted the literature searches, drafted the manuscript, responded to requests for revisions and prepared the final version.
Funding
Work in the author's laboratory was underwritten by National Institutes of Health Grants (K11 HD00662, RO1 HL038076, KO4 HD01098, RO1 HD030325, RO1 DK063321, RO1 HL056410, PO1 HL067937, R21 HL093605, PO1 HD065647); American Heart Association (Flinn Newly Independent Investigator Award), American Heart Association (no. 0855090E); 8th Mallinckrodt Scholar Award and the Preeclampsia Foundation.
Conflict of Interest
The author is an inventor or co-inventor of use patents for relaxin, and has served as a paid or unpaid consultant to Connetics, BAS Medical, Corthera, Novartis and other companies concerning the use of relaxin.
References
- Al-Nasiry S, Spitz B, Hanssens M, Luyten C, Pijnenborg R. Differential effects of inducers of syncytialization and apoptosis on BeWo and JEG-3 choriocarcinoma cells. Hum Reprod 2006;21:193–201. [DOI] [PubMed] [Google Scholar]
- Anand-Ivell R, Ivell R. Regulation of the reproductive cycle and early pregnancy by relaxin family peptides. Mol Cell Endocrinol 2014;382:472–479. [DOI] [PubMed] [Google Scholar]
- Anderson CM. Preeclampsia: exposing future cardiovascular risk in mothers and their children. J Obstet Gynecol Neonatal Nurs 2007;36:3–8. [DOI] [PubMed] [Google Scholar]
- Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal preeclampsia and neonatal outcomes. J Pregnancy 2011;2011:214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanescu S, Kopp P, Gaskill MB, Morgenthaler NG, Schindler C, Rutishauser J. Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar states. J Clin Endocrinol Metab 2011;96:1046–1052. [DOI] [PubMed] [Google Scholar]
- Bani D, Nistri S, Quattrone S, Bigazzi M, Bani Sacchi T. The vasorelaxant hormone relaxin induces changes in liver sinusoid microcirculation: a morphologic study in the rat. J Endocrinol 2001;171:541–549. [DOI] [PubMed] [Google Scholar]
- Bani-Sacchi T, Bigazzi M, Bani D, Mannaioni PF, Masini E. Relaxin-induced increased coronary flow through stimulation of nitric oxide production. Br J Pharmacol 1995;116:1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. Br Med J 1990;301:259–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, Osmond C, Winter PD, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 1989;334:577–580. [DOI] [PubMed] [Google Scholar]
- Barnes G, Japp AG, Newby DE. Translational promise of the apelin – APJ system. Heart 2010;96:1011–1016. [DOI] [PubMed] [Google Scholar]
- Barron WM, Cohen LH, Ulland LA, Lassiter WE, Fulghum EM, Emmanouel D, Robertson G, Lindheimer MD. Transient vasopressin-resistant diabetes insipidus of pregnancy. N Engl J Med 1984;310:442–444. [DOI] [PubMed] [Google Scholar]
- Bartsch O, Bartlick B, Ivell R. Phosphodiesterase 4 inhibition synergizes with relaxin signaling to promote decidualization of human endometrial stromal cells. J Clin Endocrinol Metab 2004;89:324–334. [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Halls ML, van der Westhuizen ET, Callander GE, Kocan M, Summers RJ. Relaxin family peptides and their receptors. Physiol Rev 2013;93:405–480. [DOI] [PubMed] [Google Scholar]
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Br Med J 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder C, Chuang E, Habla C, Bleckmann A, Schulz M, Bathgate R, Einspanier A. Relaxins enhance growth of spontaneous murine breast cancers as well as metastatic colonization of the brain. Clin Exp Metastasis 2014;31:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdir C, Janssen K, Stanescu AD, Enekwe A, Kasimir-Bauer S, Gellhaus A, Kimmig R, Koninger A. Maternal serum copeptin, MR-proANP and procalcitonin levels at 11–13 weeks gestation in the prediction of preeclampsia. Arch Gynecol Obstet 2015;292:1033–1042. [DOI] [PubMed] [Google Scholar]
- Bogzil AH, Eardley R, Ashton N. Relaxin-induced changes in renal sodium excretion in the anesthetized male rat. Am J Physiol Regul Integr Comp Physiol 2005;288:R322–R328. [DOI] [PubMed] [Google Scholar]
- Bond CP, Parry LJ, Samuel CS, Gehring HM, Lederman FL, Rogers PA, Summers RJ. Increased expression of the relaxin receptor (LGR7) in human endometrium during the secretory phase of the menstrual cycle. J Clin Endocrinol Metab 2004;89:3477–3485. [DOI] [PubMed] [Google Scholar]
- Bonner JS, Lantier L, Hocking KM, Kang L, Owolabi M, James FD, Bracy DP, Brophy CM, Wasserman DH. Relaxin treatment reverses insulin resistance in mice fed a high-fat diet. Diabetes 2013;62:3251–3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoff KD, Qiu C, Runyon S, Williams MA, Maitra R. Decreased maternal plasma apelin concentrations in preeclampsia. Hypertens Pregnancy 2012;31:398–404. [DOI] [PubMed] [Google Scholar]
- Bosio PM, McKenna PJ, Conroy R, O'Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol 1999;6:978–984. [DOI] [PubMed] [Google Scholar]
- Boucher J, Masri B, Daviaud D, Gesta S, Guigne C, Mazzucotelli A, Castan-Laurell I, Tack I, Knibiehler B, Carpene C et al. . Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005;146:1764–1771. [DOI] [PubMed] [Google Scholar]
- Brecht A, Bartsch C, Baumann G, Stangl K, Dschietzig T. Relaxin inhibits early steps in vascular inflammation. Regul Pept 2011;166:76–82. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Lohmeier TE, Carroll RG, Meydrech EF. Cardiovascular and renal responses to chronic vasopressin infusion. Am J Physiol 1986;250:H584–H594. [DOI] [PubMed] [Google Scholar]
- Bryant-Greenwood GD, Rutanen EM, Partanen S, Coelho TK, Yamamoto SY. Sequential appearance of relaxin, prolactin and IGFBP-1 during growth and differentiation of the human endometrium. Mol Cell Endocrinol 1993;95:23–29. [DOI] [PubMed] [Google Scholar]
- Campo B, Post Uiterweer ED, Conrad KP. Developing novel therapeutics for preeclampsia. Reprod Sci 2013;20:259A. [Google Scholar]
- Carey LC, Rose JC. The midgestational maternal blood pressure decline is absent in mice lacking expression of the angiotensin II AT2 receptor. J Renin Angiotensin Aldosterone Syst 2011;12:29–35. [DOI] [PubMed] [Google Scholar]
- Carvalho LN, Cristovam PC, Passos CS, Boim MA. Mesangial cells cultured from pregnant rats display reduced reactivity to angiotensin II: the role of relaxin, nitric oxide and AT2 receptor. Cell Physiol Biochem 2012;30:1456–1464. [DOI] [PubMed] [Google Scholar]
- Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction 2010;140:365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Merrill DC, Rose JC. The importance of angiotensin II subtype receptors for blood pressure control during mouse pregnancy. Reprod Sci 2007;14:694–704. [DOI] [PubMed] [Google Scholar]
- Chow BS, Kocan M, Bosnyak S, Sarwar M, Wigg B, Jones ES, Widdop RE, Summers RJ, Bathgate RA, Hewitson TD et al. . Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int 2014;86:75–85. [DOI] [PubMed] [Google Scholar]
- Clapp JF III, Stepanchak W, Tomaselli J, Kortan M, Faneslow S. Portal vein blood flow-effects of pregnancy, gravity, and exercise. Am J Obstet Gynecol 2000;183:167–172. [DOI] [PubMed] [Google Scholar]
- Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem 1996;271:17629–17634. [DOI] [PubMed] [Google Scholar]
- Cobellis L, De Falco M, Mastrogiacomo A, Giraldi D, Dattilo D, Scaffa C, Colacurci N, De Luca A. Modulation of apelin and APJ receptor in normal and preeclampsia-complicated placentas. Histol Histopathol 2007;22:1–8. [DOI] [PubMed] [Google Scholar]
- Conrad KP. Animal models of preeclampsia: do they exist. Fetal Med Rev 1990;2:67–89. [Google Scholar]
- Conrad KP. Unveiling the vasodilatory actions and mechanisms of relaxin. Hypertension 2010;56:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP. Maternal vasodilation in pregnancy: the emerging role of relaxin. Am J Physiol Regul Integr Comp Physiol 2011;301:R267–R275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Baker VL. Corpus luteal contribution to maternal pregnancy physiology and outcomes in assisted reproductive technologies. Am J Physiol Regul Integr Comp Physiol 2013;304:R69–R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol 1997;37:240–249. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Colpoys MC. Evidence against the hypothesis that prostaglandins are the vasodepressor agents of pregnancy. Serial studies in chronically instrumented, conscious rats. J Clin Invest 1986;77:236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Davison JM. The renal circulation in normal pregnancy and preeclampsia: is there a place for relaxin. Am J Physiol Renal Physiol 2014;306:F1121–F1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KP, Debrah DO, Novak J, Danielson LA, Shroff SG. Relaxin modifies systemic arterial resistance and compliance in conscious, nonpregnant rats. Endocrinology 2004;145:3289–3296. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Gellai M, North WG, Valtin H. Influence of oxytocin on renal hemodynamics and sodium excretion. Ann NY Acad Sci 1993;689:346–362. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Karumanchi SA. Renal physiology and disease in pregnancy In: Alpern RJ, Caplan MJ, Moe OW (eds). Seldin and Giebisch's The Kidney. San Diego: Academic Press, 2013, 2689–2761. [Google Scholar]
- Conrad KP, Shroff SG. Effects of relaxin on arterial dilation, remodeling, and mechanical properties. Curr Hypertens Rep 2011;13:409–420. [DOI] [PubMed] [Google Scholar]
- Cooke CR, Wall BM, Huch KM, Mangold T. Cardiovascular effects of vasopressin following V(1) receptor blockade compared to effects of nitroglycerin. Am J Physiol Regul Integr Comp Physiol 2001;281:R887–R893. [DOI] [PubMed] [Google Scholar]
- Cossum PA, Hill DE, Bailey JR, Anderson JH, Slikker W Jr. Transplacental passage of a human relaxin administered to rhesus monkeys. J Endocrinol 1991;130:339–345. [DOI] [PubMed] [Google Scholar]
- Costantine MM, Cleary K. Pravastatin for the prevention of preeclampsia in high-risk pregnant women. Obstet Gynecol 2013:121:349–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudmore MJ, Ahmad S, Sissaoui S, Ramma W, Ma B, Fujisawa T, Al-Ani B, Wang K, Cai M, Crispi F et al. . Loss of Akt activity increases circulating soluble endoglin release in preeclampsia: identification of inter-dependency between Akt-1 and heme oxygenase-1. Eur Heart J 2012;33:1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson LA, Conrad KP. Acute blockade of nitric oxide synthase inhibits renal vasodilation and hyperfiltration during pregnancy in chronically instrumented conscious rats. J Clin Invest 1995;96:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson LA, Conrad KP. Time course and dose response of relaxin-mediated renal vasodilation, hyperfiltration, and changes in plasma osmolality in conscious rats. J Appl Physiol 2003;95:1509–1514. [DOI] [PubMed] [Google Scholar]
- Danielson LA, Sherwood OD, Conrad KP. Relaxin is a potent renal vasodilator in conscious rats. J Clin Invest 1999;103:525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PR, Cartwright JE, Baker PN, Johnstone AP, Whitley GS. Nitric oxide protects human extravillous trophoblast cells from apoptosis by a cyclic GMP-dependent mechanism and independently of caspase 3 nitrosylation. Exp Cell Res 2003;287:314–324. [DOI] [PubMed] [Google Scholar]
- Davidge ST, de Groot CJM, Taylor RN. Endothelial cell dysfunction In: Taylor RN, Rpberts JM, Cunningham FG, Linheimer MD (eds). Chesley's Hypertensive Disorders of Pregnancy. San Diego, CA: Elsevier-Academic Press, 2015, 181–207. [Google Scholar]
- Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, Adwani S, Wilkinson AR, McCormick K, Sargent I et al. . Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics 2012;129:e1552–e1561. [DOI] [PubMed] [Google Scholar]
- Davison JM, Gilmore EA, Durr J, Robertson GL, Lindheimer MD. Altered osmotic thresholds for vasopressin secretion and thirst in human pregnancy. Am J Physiol 1984;246:F105–F109. [DOI] [PubMed] [Google Scholar]
- Davison JM, Homuth V, Jeyabalan A, Conrad KP, Karumanchi SA, Quaggin S, Dechend R, Luft FC. New aspects in the pathophysiology of preeclampsia. J Am Soc Nephrol 2004;15:2440–2448. [DOI] [PubMed] [Google Scholar]
- Dawood MY, Khan-Dawood FS, Wahi RS, Fuchs F. Oxytocin release and plasma anterior pituitary and gonadal hormones in women during lactation. J Clin Endocrinol Metab 1981;52:678–683. [DOI] [PubMed] [Google Scholar]
- Dawood MY, Ylikorkala O, Trivedi D, Fuchs F. Oxytocin in maternal circulation and amniotic fluid during pregnancy. J Clin Endocrinol Metab 1979;49:429–434. [DOI] [PubMed] [Google Scholar]
- Debrah DO, Conrad KP, Jeyabalan A, Danielson LA, Shroff SG. Relaxin increases cardiac output and reduces systemic arterial load in hypertensive rats. Hypertension 2005;46:745–750. [DOI] [PubMed] [Google Scholar]
- Debrah DO, Debrah JE, Haney JL, McGuane JT, Sacks MS, Conrad KP, Shroff SG. Relaxin regulates vascular wall remodeling and passive mechanical properties in mice. J Appl Physiol 2011;111:260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debrah DO, Novak J, Matthews JE, Ramirez RJ, Shroff SG, Conrad KP. Relaxin is essential for systemic vasodilation and increased global arterial compliance during early pregnancy in conscious rats. Endocrinology 2006;147:5126–5131. [DOI] [PubMed] [Google Scholar]
- Dechend R, Lamarca B, Taylor RN. The renin-angiotensin system, its autoantibodies, and body fluid volume in preeclampsia In: Taylor RN, Roberts JM, Cunningham FG, Lindheimer MD (eds). Chesley's Hypertensive Disorders in Pregnancy. San Diego, CA: Elsevier-Academic Press, 2015, 315–334. [Google Scholar]
- Dechend R, Luft FC. Are we getting closer to a Nobel prize for unraveling preeclampsia. Curr Cardiol Rep 2008;10:440–447. [DOI] [PubMed] [Google Scholar]
- Dickey RP, Hower JF. Ultrasonographic features of uterine blood flow during the first 16 weeks of pregnancy. Hum Reprod 1995;10:2448–2452. [DOI] [PubMed] [Google Scholar]
- Dimitriadis E, Stoikos C, Baca M, Fairlie WD, McCoubrie JE, Salamonsen LA. Relaxin and prostaglandin E(2) regulate interleukin 11 during human endometrial stromal cell decidualization. J Clin Endocrinol Metab 2005;90:3458–3465. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Bartsch C, Richter C, Laule M, Baumann G, Stangl K. Relaxin, a pregnancy hormone, is a functional endothelin-1 antagonist: attenuation of endothelin-1-mediated vasoconstriction by stimulation of endothelin type-B receptor expression via ERK-1/2 and nuclear factor-kappaB. Circ Res 2003;92:32–40. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Brecht A, Bartsch C, Baumann G, Stangl K, Alexiou K. Relaxin improves TNF-alpha-induced endothelial dysfunction: the role of glucocorticoid receptor and phosphatidylinositol 3-kinase signalling. Cardiovasc Res 2012;95:97–107. [DOI] [PubMed] [Google Scholar]
- Dschietzig T, Teichman S, Unemori E, Wood S, Boehmer J, Richter C, Baumann G, Stangl K. Intravenous recombinant human relaxin in compensated heart failure: a safety, tolerability, and pharmacodynamic trial. J Card Fail 2009;15:182–190. [DOI] [PubMed] [Google Scholar]
- Duley L. Pre-eclampsia and the hypertensive disorders of pregnancy. Br Med Bull 2003;67:161–176. [DOI] [PubMed] [Google Scholar]
- Durr JA, Hoggard JG, Hunt JM, Schrier RW. Diabetes insipidus in pregnancy associated with abnormally high circulating vasopressinase activity. N Engl J Med 1987;316:1070–1074. [DOI] [PubMed] [Google Scholar]
- Durr JA, Lindheimer MD. Diagnosis and management of diabetes insipidus during pregnancy. Endocr Pract 1996;2:353–361. [DOI] [PubMed] [Google Scholar]
- Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol 1990;76:1061–1069. [PubMed] [Google Scholar]
- Eguchi K, Oguni N, Sawai T, Yonezawa M. Comparison of plasma concentrations of arginine vasopressin (AVP) and atrial natriuretic peptide (ANP) in normal and preeclamptic pregnancies. J Perinat Med 1996;24:437–443. [DOI] [PubMed] [Google Scholar]
- El-Farhan N, Hampton D, Penney M. Measurement of arginine vasopressin. Methods Mol Biol 2013;1065:129–139. [DOI] [PubMed] [Google Scholar]
- Epstein FH. Late vascular effects of toxemia of pregnancy. N Engl J Med 1964;271:391–395. [DOI] [PubMed] [Google Scholar]
- Fallowfield JA, Hayden AL, Snowdon VK, Aucott RL, Stutchfield BM, Mole DJ, Pellicoro A, Gordon-Walker TT, Henke A, Schrader J et al. . Relaxin modulates human and rat hepatic myofibroblast function and ameliorates portal hypertension in vivo. Hepatology 2014;59:1492–1504. [DOI] [PubMed] [Google Scholar]
- Feng S, Agoulnik IU, Bogatcheva NV, Kamat AA, Kwabi-Addo B, Li R, Ayala G, Ittmann MM, Agoulnik AI. Relaxin promotes prostate cancer progression. Clin Cancer Res 2007;13:1695–1702. [DOI] [PubMed] [Google Scholar]
- Fickling SA, Williams D, Vallance P, Nussey SS, Whitley GS. Plasma concentrations of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and pre-eclampsia. Lancet 1993;342:242–243. [DOI] [PubMed] [Google Scholar]
- Fisher C, MacLean M, Morecroft I, Seed A, Johnston F, Hillier C, McMurray J. Is the pregnancy hormone relaxin also a vasodilator peptide secreted by the heart. Circulation 2002;106:292–295. [DOI] [PubMed] [Google Scholar]
- Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta 2009;30:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammill HS, Lin C, Hubel CA. Endothelial progenitor cells and preeclampsia. Front Biosci 2007;12:2383–2394. [DOI] [PubMed] [Google Scholar]
- Gandley RE, Conrad KP, McLaughlin MK. Endothelin and nitric oxide mediate reduced myogenic reactivity of small renal arteries from pregnant rats. Am J Physiol 2001;280:R1–R7. [DOI] [PubMed] [Google Scholar]
- Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest 1973;52:2682–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellai M, Silverstein JH, Hwang JC, LaRochelle FT Jr, Valtin H. Influence of vasopressin on renal hemodynamics in conscious Brattleboro rats. Am J Physiol 1984;246:F819–F827. [DOI] [PubMed] [Google Scholar]
- Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol 2012;36:56–59. [DOI] [PubMed] [Google Scholar]
- Glanzer K, Prussing B, Dusing R, Kramer HJ. Hemodynamic and hormonal responses to 8-arginine-vasopressin in healthy man: effects of indomethacin. Klin Wochenschr 1982;60:1234–1239. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith LT, Weiss G, Palejwala S, Plant TM, Wojtczuk A, Lambert WC, Ammur N, Heller D, Skurnick JH, Edwards D et al. . Relaxin regulation of endometrial structure and function in the rhesus monkey. Proc Natl Acad Sci USA 2004;101:4685–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooi JH, Richardson ML, Jelinic M, Girling JE, Wlodek ME, Tare M, Parry LJ. Enhanced uterine artery stiffness in aged pregnant relaxin mutant mice is reversed with exogenous relaxin treatment. Biol Reprod 2013;89:18. [DOI] [PubMed] [Google Scholar]
- Gordge MP, Robson SC, Williams DJ, Payne NN, Neild GH. Serum vasopressinase and platelet responses to arginine vasopressin in normal pregnancy, pregnancy-induced hypertension and pre-eclampsia. Platelets 1994;5:90–95. [DOI] [PubMed] [Google Scholar]
- Granger GP, George EM, Roberts JM. Animal models for investigating the pathophysiological mechanisms of preeclampsia In: Taylor RN, Cunningham FG, Roberts JM, Lindheimer MD (eds). Chesley's Hypertensive Disorders in Pregnancy. San Diego, CA: Academic Press Elsevier, 2014, 209–220 [Google Scholar]
- Hasan KM, Manyonda IT, Ng FS, Singer DR, Antonios TF. Skin capillary density changes in normal pregnancy and pre-eclampsia. J Hypertens 2002;20:2439–2443. [DOI] [PubMed] [Google Scholar]
- Heazell AE, Crocker IP. Live and let die - regulation of villous trophoblast apoptosis in normal and abnormal pregnancies. Placenta 2008;29:772–783. [DOI] [PubMed] [Google Scholar]
- Henderson JT, O'Connor E, Whitlock EP. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia. Ann Intern Med 2014;161:613–614. [DOI] [PubMed] [Google Scholar]
- Hibbard JU, Shroff SG, Cunningham FG. Cardiovascular Alterations in Normal and Preeclamptic Pregnancy, 4th edn. San Diego, CA: Academic Press Elsevier, 2015. [Google Scholar]
- Hibbard JU, Shroff SG, Cunningham FG. Cardiovascular alterations in normal and preeclamptic pregnancy In: Taylor RN, Rpberts JM, Cunningham FG, Linheimer MD (eds). Chesley's Hypertensive Disorders of Pregnancy. San Diego, CA: Elsevier-Academic Press, 2015, 291–313. [Google Scholar]
- Hisaw FL. Experimental relaxation of the public ligament of the guinea pig. Proc Exp Biol Med 1926;23:661–663. [Google Scholar]
- Hisaw FL, Hisaw FL Jr, Dawson AB. Effects of relaxin on the endothelium of endometrial blood vessels in monkeys (Macaca mulatta). Endocrinology 1967;81:375–385. [DOI] [PubMed] [Google Scholar]
- Holmes CL, Landry DW, Granton JT. Science review: vasopressin and the cardiovascular system part 1 – receptor physiology. Crit Care 2003;7:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SY, Nakabayashi K, Nishi S, Kumagai J, Kudo M, Sherwood OD, Hsueh AJ. Activation of orphan receptors by the hormone relaxin. Science 2002;295:671–674. [DOI] [PubMed] [Google Scholar]
- Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 1999;222:222–235. [DOI] [PubMed] [Google Scholar]
- Hus-Citharel A, Bouby N, Frugiere A, Bodineau L, Gasc JM, Llorens-Cortes C. Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int 2008;74:486–494. [DOI] [PubMed] [Google Scholar]
- Hypertension in pregnancy Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- Inuzuka H, Nishizawa H, Inagaki A, Suzuki M, Ota S, Miyamura H, Miyazaki J, Sekiya T, Kurahashi H, Udagawa Y. Decreased expression of apelin in placentas from severe pre-eclampsia patients. Hypertens Pregnancy 2013;32:410–421. [DOI] [PubMed] [Google Scholar]
- Jaques AM, Amor DJ, Baker HW, Healy DL, Ukoumunne OC, Breheny S, Garrett C, Halliday JL. Adverse obstetric and perinatal outcomes in subfertile women conceiving without assisted reproductive technologies. Fertil Steril 2010;94:2674–2679. [DOI] [PubMed] [Google Scholar]
- Jeyabalan A, Conrad KP. Renal physiology and pathophysiology in pregnancy In: Schrier RW. (ed). Renal and Electrolyte Disorders. Philadelphia: Lippincott Williams & Wilkins, 2010, 462–518. [Google Scholar]
- Jeyabalan A, Novak J, Danielson LA, Kerchner LJ, Opett SL, Conrad KP. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, reduced myogenic reactivity of small arteries. Circ Res 2003;93:1249–1257. [DOI] [PubMed] [Google Scholar]
- Jeyabalan A, Stewart DR, McGonigal SC, Powers RW, Conrad KP. Low relaxin concentrations in the first trimester are associated with increased risk of developing preeclampsia [abstract]. Reproductive Sci 2009;16:101A. [Google Scholar]
- Johnson MR, Abbas A, Nicolaides KH, Lightman SL. Distribution of relaxin between human maternal and fetal circulations and amniotic fluid. J Endocrinol 1992;134:313–317. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Abdalla H, Allman AC, Wren ME, Kirkland A, Lightman SL. Relaxin levels in ovum donation pregnancies. Fertil Steril 1991;56:59–61. [PubMed] [Google Scholar]
- Kawabata I, Nakai A, Takeshita T. Prediction of HELLP syndrome with assessment of maternal dual hepatic blood supply by using Doppler ultrasound. Arch Gynecol Obstet 2006;274:303–309. [DOI] [PubMed] [Google Scholar]
- Kerchner LJ, Novak J, Hanley-Yanez K, Doty KD, Danielson LA, Conrad KP. Evidence against the hypothesis that endothelial endothelin B receptor expression is regulated by relaxin and pregnancy. Endocrinology 2005;146:2791–2797. [DOI] [PubMed] [Google Scholar]
- Khanna D, Clements PJ, Furst DE, Korn JH, Ellman M, Rothfield N, Wigley FM, Moreland LW, Silver R, Kim YH et al. . Recombinant human relaxin in the treatment of systemic sclerosis with diffuse cutaneous involvement: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2009;60:1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw A, Kametas NA, Turan OM, Bamfo JE, Nicolaides KH. Maternal cardiac function and uterine artery Doppler at 11-14 weeks in the prediction of pre-eclampsia in nulliparous women. BJOG 2008;115:369–376. [DOI] [PubMed] [Google Scholar]
- Kong RC, Shilling PJ, Lobb DK, Gooley PR, Bathgate RA. Membrane receptors: structure and function of the relaxin family peptide receptors. Mol Cell Endocrinol 2010;320:1–15. [DOI] [PubMed] [Google Scholar]
- Krege JH, Katz VL. A proposed relationship between vasopressinase altered vasopressin and preeclampsia. Med Hypotheses 1990;31:283–287. [DOI] [PubMed] [Google Scholar]
- Kristensen K, Larsson I, Hansson SR. Increased cystatin C expression in the pre-eclamptic placenta. Mol Hum Reprod 2007;13:189–195. [DOI] [PubMed] [Google Scholar]
- Kucur M, Tuten A, Oncul M, Acikgoz AS, Yuksel MA, Imamoglu M, Balci Ekmekci O, Yilmaz N, Madazli R. Maternal serum apelin and YKL-40 levels in early and late-onset pre-eclampsia. Hypertens Pregnancy 2014;33:467–475. [DOI] [PubMed] [Google Scholar]
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 2009;113:1299–1306. [DOI] [PubMed] [Google Scholar]
- Lane B, Oxberry W, Mazella J, Tseng L. Decidualization of human endometrial stromal cells in vitro: effects of progestin and relaxin on the ultrastructure and production of decidual secretory proteins. Hum Reprod 1994;9:259–266. [DOI] [PubMed] [Google Scholar]
- Leo CH, Jelinic M, Parkington HC, Tare M, Parry LJ. Acute intravenous injection of serelaxin (recombinant human relaxin-2) causes rapid and sustained bradykinin-mediated vasorelaxation. J Am Heart Assoc 2014;3:e000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJ, Vatten LJ, Horowitz GL, Qian C, Romundstad PR, Yu KF, Hollenberg AN, Hellevik AI, Asvold BO, Karumanchi SA. Pre-eclampsia, soluble fms-like tyrosine kinase 1, and the risk of reduced thyroid function: nested case-control and population based study. BMJ 2009;339:b4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liard JF. Cardiovascular effects of vasopressin: some recent aspects. J Cardiovasc Pharmacol 1986;8:S61–S65. [DOI] [PubMed] [Google Scholar]
- Lodhi RS, Nakabayashi K, Suzuki K, Yamada AY, Hazama R, Ebina Y, Yamada H. Relaxin has anti-apoptotic effects on human trophoblast-derived HTR-8/SV neo cells. Gynecol Endocrinol 2013;29:1051–1054. [DOI] [PubMed] [Google Scholar]
- Lucas C, Bald LN, Martin MC, Jaffe RB, Drolet DW, Mora-Worms M, Bennett G, Chen AB, Johnston PD. An enzyme-linked immunosorbent assay to study human relaxin in human pregnancy and in pregnant rhesus monkeys. J Endocrinol 1989;120:449–457. [DOI] [PubMed] [Google Scholar]
- Luo HR, Hattori H, Hossain MA, Hester L, Huang Y, Lee-Kwon W, Donowitz M, Nagata E, Snyder SH. Akt as a mediator of cell death. Proc Natl Acad Sci USA 2003;100:11712–11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray I, Campbell D, Pirani BB. Changes preceding the development of preeclamptic toxemia. Isr J Med Sci 1976;12:504–507. [PubMed] [Google Scholar]
- Mandic-Markovic VD, Mikovic ZM, Djukic MK, Vasiljevic MD, Jankovic G. Doppler parameters of the maternal hepatic artery blood flow in normal pregnancy: maternal hepatic artery blood flow in normal pregnancy. Eur J Obstet Gynecol Reprod Biol 2014;181:275–279. [DOI] [PubMed] [Google Scholar]
- Maruo N, Nakabayashi K, Wakahashi S, Yata A, Maruo T. Effects of recombinant H2 relaxin on the expression of matrix metalloproteinases and tissue inhibitor metalloproteinase in cultured early placental extravillous trophoblasts. Endocrine 2007;32:303–310. [DOI] [PubMed] [Google Scholar]
- Massicotte G, Parent A, St-Louis J. Blunted responses to vasoconstrictors in mesenteric vasculature but not in portal vein of spontaneously hypertensive rats treated with relaxin. Proc Soc Exp Biol Med 1989;190:254–259. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Abe E, Matsubara Y, Kameda K, Ito M. Circulating endothelial progenitor cells during normal pregnancy and pre-eclampsia. Am J Reprod Immunol 2006;56:79–85. [DOI] [PubMed] [Google Scholar]
- Matteucci E, Giampietro O. Statin intolerance: why and what to do - with a focus on diabetic people. Curr Med Chem 2013;20:1397–1408. [DOI] [PubMed] [Google Scholar]
- Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE et al. . Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003;111:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazella J, Tang M, Tseng L. Disparate effects of relaxin and TGFbeta1: relaxin increases, but TGFbeta1 inhibits, the relaxin receptor and the production of IGFBP-1 in human endometrial stromal/decidual cells. Hum Reprod 2004;19:1513–1518. [DOI] [PubMed] [Google Scholar]
- McCarthy FP, Delany AC, Kenny LC, Walsh SK. PPAR-gamma -- a possible drug target for complicated pregnancies. Br J Pharmacol 2013;168:1074–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy FP, Drewlo S, English FA, Kingdom J, Johns EJ, Kenny LC, Walsh SK. Evidence implicating peroxisome proliferator-activated receptor-gamma in the pathogenesis of preeclampsia. Hypertension 2011. a;58:882–887. [DOI] [PubMed] [Google Scholar]
- McCarthy FP, Drewlo S, Kingdom J, Johns EJ, Walsh SK, Kenny LC. Peroxisome proliferator-activated receptor-gamma as a potential therapeutic target in the treatment of preeclampsia. Hypertension 2011. b;58:280–286. [DOI] [PubMed] [Google Scholar]
- McGuane J, Conrad K. GPCRs as potential therapeutic targets in preeclampsia. Drug Discov Today Dis Models 2012;9:e119–e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuane JT, Danielson LA, Debrah JE, Rubin JP, Novak J, Conrad KP. Angiogenic growth factors are new and essential players in the sustained relaxin vasodilatory pathway in rodents and humans. Hypertension 2011. a;57:1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuane JT, Debrah JE, Sautina L, Jarajapu YP, Novak J, Rubin JP, Grant MB, Segal M, Conrad KP. Relaxin induces rapid dilation of rodent small renal and human subcutaneous arteries via PI3 kinase and nitric oxide. Endocrinology 2011. b;152:2786–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore XL, Tan SL, Lo CY, Fang L, Su YD, Gao XM, Woodcock EA, Summers RJ, Tregear GW, Bathgate RA et al. . Relaxin antagonizes hypertrophy and apoptosis in neonatal rat cardiomyocytes. Endocrinology 2007;148:1582–1589. [DOI] [PubMed] [Google Scholar]
- Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006;52:112–119. [DOI] [PubMed] [Google Scholar]
- Mushayandebvu TI, Goldsmith LT, Von Hagen S, Santoro N, Thurston D, Weiss G. Elevated maternal serum relaxin concentrations throughout pregnancy in singleton gestations after superovulation. Obstet Gynecol 1998;92:17–20. [DOI] [PubMed] [Google Scholar]
- Naitoh M, Suzuki H, Murakami M, Matsumoto A, Ichihara A, Nakamoto H, Yamamura Y, Saruta T. Arginine vasopressin produces renal vasodilation via V2 receptors in conscious dogs. Am J Physiol 1993;265:R934–R942. [DOI] [PubMed] [Google Scholar]
- Nakai A, Sekiya I, Oya A, Koshino T, Araki T. Assessment of the hepatic arterial and portal venous blood flows during pregnancy with Doppler ultrasonography. Arch Gynecol Obstet 2002;266:25–29. [DOI] [PubMed] [Google Scholar]
- Nisell H, Hjemdahl P, Linde B. Cardiovascular responses to circulating catecholamines in normal pregnancy and in pregnancy-induced hypertension. Clin Physiol 1985;5:479–493. [DOI] [PubMed] [Google Scholar]
- Novak J, Danielson LA, Kerchner LJ, Sherwood OD, Ramirez RJ, Moalli PA, Conrad KP. Relaxin is essential for renal vasodilation during pregnancy in conscious rats. J Clin Invest 2001;107:1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J, Parry LJ, Matthews JE, Kerchner LJ, Indovina K, Hanley-Yanez K, Doty KD, Debrah DO, Shroff SG, Conrad KP. Evidence for local relaxin ligand-receptor expression and function in arteries. FASEB J 2006;20:2352–2362. [DOI] [PubMed] [Google Scholar]
- Novak J, Ramirez RJ, Gandley RE, Sherwood OD, Conrad KP. Myogenic reactivity is reduced in small renal arteries isolated from relaxin-treated rats. Am J Physiol 2002;283:R349–R355. [DOI] [PubMed] [Google Scholar]
- Oosterhof H, Voorhoeve PG, Aarnoudse JG. Enhancement of hepatic artery resistance to blood flow in preeclampsia in presence or absence of HELLP syndrome (hemolysis, elevated liver enzymes, and low platelets). Am J Obstet Gynecol 1994;171:526–530. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 2007;87:315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paco C, Kametas N, Rencoret G, Strobl I, Nicolaides KH. Maternal cardiac output between 11 and 13 weeks of gestation in the prediction of preeclampsia and small for gestational age. Obstet Gynecol 2008;111:292–300. [DOI] [PubMed] [Google Scholar]
- Palejwala S, Tseng L, Wojtczuk A, Weiss G, Goldsmith LT. Relaxin gene and protein expression and its regulation of procollagenase and vascular endothelial growth factor in human endometrial cells. Biol Reprod 2002;66:1743–1748. [DOI] [PubMed] [Google Scholar]
- Pedersen EB, Johannesen P, Rasmussen AB, Danielsen H. The osmoregulatory system and the renin-angiotensin-aldosterone system in pre-eclampsia and normotensive pregnancy. Scand J Clin Lab Invest 1985;45:627–633. [DOI] [PubMed] [Google Scholar]
- Pham V, Burns P, Albiston AL, Yeatman HR, Ng L, Diwakarla S, Chai SY. Reproduction and maternal behavior in insulin-regulated aminopeptidase (IRAP) knockout mice. Peptides 2009;30:1861–1865. [DOI] [PubMed] [Google Scholar]
- Pitkin SL, Maguire JJ, Bonner TI, Davenport AP. International Union of Basic and Clinical Pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and function. Pharmacol Rev 2010;62:331–342. [DOI] [PubMed] [Google Scholar]
- Ponikowski P, Mitrovic V, Ruda M, Fernandez A, Voors AA, Vishnevsky A, Cotter G, Milo O, Laessing U, Zhang Y et al. . A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur Heart J 2014;35:431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppas A, Shroff SG, Korcarz CE, Hibbard JU, Berger DS, Lindheimer MD, Lang RM. Serial assessment of the cardiovascular system in normal pregnancy. Role of arterial compliance and pulsatile arterial load. Circulation 1997;95:2407–2415. [DOI] [PubMed] [Google Scholar]
- van der Post JA, Konijnenberg A, Boer K, Schaap MC, van Boxtel CE, Sturk A, Boer GJ, Swaab DF. Preeclampsia is not associated with altered platelet vasopressin binding and cytosolic Ca++ concentration. Am J Obstet Gynecol 1993;169:1169–1178. [DOI] [PubMed] [Google Scholar]
- Post Uiterweer ED, Herrera D, McGuane JT, Parry LJ, Conrad KP. Relaxin stimulates trophoblast invasion in vitro. Reproductive Sci 2012;19:387A. [Google Scholar]
- Post Uiterweer ED, Koster MP, Jeyabalan A, Kuc S, Siljee J, Conrad KP, Franx A. First trimester serum relaxin concentration and prediction of early and late onset preeclampsia. Reprod Sci 2014;21:394A. [Google Scholar]
- Preibisz JJ, Sealey JE, Laragh JH, Cody RJ, Weksler BB. Plasma and platelet vasopressin in essential hypertension and congestive heart failure. Hypertension 1983;5:I129–138. [DOI] [PubMed] [Google Scholar]
- Rabaglino MB, Post Uiterweer ED, Jeyabalan A, Hogge WA, Conrad KP. Bioinformatics approach reveals evidence for impaired endometrial maturation before and during early pregnancy in women who developed preeclampsia. Hypertension 2015;65:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW, Tannetta DS, Dragovic RA, Gardiner C, Southcombe JH, Collett GP, Sargent IL. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia. Placenta 2012;33:S48–S54. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Ness RB, Roberts JM. Epidemiology of pregnancy-related hypertension In: Taylor RN, Roberts JM, Cunningham GF, Lindheimer MD (eds). Chesley's Hypertensive Disorders in Pregnancy. San Diego, CA: Elsevier/Academic Press, 2015, 37–56. [Google Scholar]
- Risberg A, Olsson K, Lyrenas S, Sjoquist M. Plasma vasopressin, oxytocin, estradiol, and progesterone related to water and sodium excretion in normal pregnancy and gestational hypertension. Acta Obstet Gynecol Scand 2009;88:639–646. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension 2003;41:437–445. [DOI] [PubMed] [Google Scholar]
- Robertson GL, Mahr EA, Athar S, Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest 1973;52:2340–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas SP, Marshall G, Gutierrez BL, Rosso P. Time course of maternal plasma volume and hormonal changes in women with preeclampsia or fetal growth restriction. Hypertension 2006;47:203–208. [DOI] [PubMed] [Google Scholar]
- Salha O, Sharma V, Dada T, Nugent D, Rutherford AJ, Tomlinson AJ, Philips S, Allgar V, Walker JJ. The influence of donated gametes on the incidence of hypertensive disorders of pregnancy. Hum Reprod 1999;14:2268–2273. [DOI] [PubMed] [Google Scholar]
- Santillan MK, Santillan DA, Scroggins SM, Min JY, Sandgren JA, Pearson NA, Leslie KK, Hunter SK, Zamba GK, Gibson-Corley KN et al. . Vasopressin in preeclampsia: a novel very early human pregnancy biomarker and clinically relevant mouse model. Hypertension 2014;64:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar M, Samuel CS, Bathgate RA, Stewart DR, Summers RJ. Serelaxin-mediated signal transduction in human vascular cells: bell-shaped concentration-response curves reflect differential coupling to G proteins. Br J Pharmacol 2015;172:1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser JM, Cunningham MW Jr, Baylis C. Serelaxin reduces oxidative stress and asymmetric dimethylarginine in angiotensin II-induced hypertension. Am J Physiol Renal Physiol 2014;307:F1355–F1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasser JM, Murphy SR, Granger JP. Emerging drugs for preeclampsia - the endothelium as a target. Expert Opin Emerg Drugs 2015;20:527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena AR, Ananth Karumanchi S, Fan SL, Horowitz GL, Hollenberg NK, Graves SW, Seely EW. Correlation of cystatin-C with glomerular filtration rate by inulin clearance in pregnancy. Hypertens Pregnancy 2012;31:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte MM, Tsai JH, Moley KH. Obesity and PCOS: the effect of metabolic derangements on endometrial receptivity at the time of implantation. Reprod Sci 2015;22: 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal MS, Sautina L, Li S, Diao Y, Agoulnik AI, Kielczewski J, McGuane JT, Grant MB, Conrad KP. Relaxin increases human endothelial progenitor cell NO and migration and vasculogenesis in mice. Blood 2012;119:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood OD. Relaxin In: Knobil E, Neill JD, Greenwald GS, Markert CL, Pfaff DW (eds). The Physiology of Reproduction. New York: Raven Press, 1994, 861–1008. [Google Scholar]
- Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 2005;365:785–799. [DOI] [PubMed] [Google Scholar]
- Simsek Y, Celik O, Yilmaz E, Karaer A, Dogan C, Aydin S, Ozer A. Serum levels of apelin, salusin-alpha and salusin-beta in normal pregnancy and preeclampsia. J Matern Fetal Neonatal Med 2012;25:1705–1708. [DOI] [PubMed] [Google Scholar]
- Singh S, Simpson RL, Bennett RG. Relaxin activates peroxisome proliferator-activated receptor gamma (PPARgamma) through a pathway involving PPARgamma coactivator 1alpha (PGC1alpha). J Biol Chem 2015;290:950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek SM, Magness RR, Conrad KP. Nitric oxide and pregnancy. Am J Physiol 1997;272:R441–R463. [DOI] [PubMed] [Google Scholar]
- Smith MC, Danielson LA, Conrad KP, Davison JM. Influence of recombinant human relaxin on renal hemodynamics in healthy volunteers. J Am Soc Nephrol 2006. a;17:3192–3197. [DOI] [PubMed] [Google Scholar]
- Smith MC, Murdoch AP, Danielson LA, Conrad KP, Davison JM. Relaxin has a role in establishing a renal response in pregnancy. Fertil Steril 2006. b;86:253–255. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 1994;94:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DR, Celniker AC, Taylor CA Jr, Cragun JR, Overstreet JW, Lasley BL. Relaxin in the peri-implantation period. J Clin Endocrinol Metab 1990;70:1771–1773. [DOI] [PubMed] [Google Scholar]
- Stewart DR, Erikson MS, Erikson ME, Nakajima ST, Overstreet JW, Lasley BL, Amento EP, Seppala M. The role of relaxin in glycodelin secretion. J Clin Endocrinol Metab 1997;82:839–846. [DOI] [PubMed] [Google Scholar]
- St-Louis J, Massicotte G. Chronic decrease of blood pressure by rat relaxin in spontaneously hypertensive rats. Life Sci 1985;37:1351–1357. [DOI] [PubMed] [Google Scholar]
- Szlachter BN, Quagliarello J, Jewelewicz R, Osathanondh R, Spellacy WN, Weiss G. Relaxin in normal and pathogenic pregnancies. Obstet Gynecol 1982;59:167–170. [PubMed] [Google Scholar]
- Tang M, Mazella J, Zhu HH, Tseng L. Ligand activated relaxin receptor increases the transcription of IGFBP-1 and prolactin in human decidual and endometrial stromal cells. Mol Hum Reprod 2005;11:237–243. [DOI] [PubMed] [Google Scholar]
- Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF Jr et al. . Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 2013;381:29–39. [DOI] [PubMed] [Google Scholar]
- Teerlink JR, Metra M, Felker GM, Ponikowski P, Voors AA, Weatherley BD, Marmor A, Katz A, Grzybowski J, Unemori E et al. . Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet 2009;373:1429–1439. [DOI] [PubMed] [Google Scholar]
- Tseng L, Zhu HH, Mazella J, Koistinen H, Seppala M. Relaxin stimulates glycodelin mRNA and protein concentrations in human endometrial glandular epithelial cells. Mol Hum Reprod 1999;5:372–375. [DOI] [PubMed] [Google Scholar]
- Tuten A, Oncul M, Kucur M, Imamoglu M, Ekmekci OB, Acikgoz AS, Cebe FS, Yesilbas C, Madazli R. Maternal serum copeptin concentrations in early- and late-onset pre-eclampsia. Taiwan J Obstet Gynecol 2015;54:350–354. [DOI] [PubMed] [Google Scholar]
- Unemori E, Sibai B, Teichman SL. Scientific rationale and design of a phase I safety study of relaxin in women with severe preeclampsia. Ann NY Acad Sci 2009;1160:381–384. [DOI] [PubMed] [Google Scholar]
- Unemori EN, Erikson ME, Rocco SE, Sutherland KM, Parsell DA, Mak J, Grove BH. Relaxin stimulates expression of vascular endothelial growth factor in normal human endometrial cells in vitro and is associated with menometrorrhagia in women. Hum Reprod 1999;14:800–806. [DOI] [PubMed] [Google Scholar]
- Valtin H, Schafer J. Renal Function, 3rd edn. Boston: Little, Brown and Company, 1995, 98–101. [Google Scholar]
- Vasapollo B, Valensise H, Novelli GP, Altomare F, Galante A, Arduini D. Abnormal maternal cardiac function precedes the clinical manifestation of fetal growth restriction. Ultrasound Obstet Gynecol 2004;24:23–29. [DOI] [PubMed] [Google Scholar]
- Velauthar L, Plana MN, Kalidindi M, Zamora J, Thilaganathan B, Illanes SE, Khan KS, Aquilina J, Thangaratinam S. First-trimester uterine artery Doppler and adverse pregnancy outcome: a meta-analysis involving 55,974 women. Ultrasound Obstet Gynecol 2014;43:500–507. [DOI] [PubMed] [Google Scholar]
- Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA et al. . Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med 2006;12:642–649. [DOI] [PubMed] [Google Scholar]
- Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med 2008;359:800–809. [DOI] [PubMed] [Google Scholar]
- Vodstrcil LA, Tare M, Novak J, Dragomir N, Ramirez RJ, Wlodek ME, Conrad KP, Parry LJ. Relaxin mediates uterine artery compliance during pregnancy and increases uterine blood flow. FASEB J 2012;26: 4035––4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev 2009;89:1269–1339. [DOI] [PubMed] [Google Scholar]
- Voors AA, Dahlke M, Meyer S, Stepinska J, Gottlieb SS, Jones A, Zhang Y, Laurent D, Slart RH, Navis GJ. Renal hemodynamic effects of serelaxin in patients with chronic heart failure: a randomized, placebo-controlled study. Circ Heart Fail 2014;7:994–1002. [DOI] [PubMed] [Google Scholar]
- Walker BR. Evidence for a vasodilatory effect of vasopressin in the conscious rat. Am J Physiol 1986;251:H34–H39. [DOI] [PubMed] [Google Scholar]
- Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D et al. . Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest 1999;103:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisinger RS, Burns P, Eddie LW, Wintour EM. Relaxin alters the plasma osmolality-arginine vasopressin relationship in the rat. J Endocrinol 1993;137:505–510. [DOI] [PubMed] [Google Scholar]
- Weiss G, O'Byrne EM, Hochman J, Steinetz BG, Goldsmith L, Flitcraft JG. Distribution of relaxin in women during pregnancy. Obstet Gynecol 1978;52:569–570. [PubMed] [Google Scholar]
- Weiss G, Teichman S, Stewart D, Nader D, Wood S, Unemori E. A randomized, double-blind, placebo-controlled trial of relaxin for cervical ripening in post-delivery date pregnancies. Ann NY Acad Sci 2009;1160:385–386. [DOI] [PubMed] [Google Scholar]
- Willcox JM, Summerlee AJ, Murrant CL. Relaxin induces rapid, transient vasodilation in the microcirculation of hamster skeletal muscle. J Endocrinol 2013;218:179–191. [DOI] [PubMed] [Google Scholar]
- Williams D. Long-term complications of preeclampsia. Semin Nephrol 2011;31:111–122. [DOI] [PubMed] [Google Scholar]
- Wilson HJ, Shutt LE.. Syndrome of inappropriate ADH secretion in a woman with preeclampsia. Int J Obstet Anesth 2007;16:360–362. [DOI] [PubMed] [Google Scholar]
- Xiao J, Huang Z, Chen CZ, Agoulnik IU, Southall N, Hu X, Jones RE, Ferrer M, Zheng W, Agoulnik AI et al. . Identification and optimization of small-molecule agonists of the human relaxin hormone receptor RXFP1. Nat Commun 2013;4:1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaleyeva LM, Chappell MC, Brosnihan KB, Anton L, Caudell DL, Shi S, McGee C, Pirro N, Gallagher PE, Taylor RN et al. . Downregulation of apelin in the human placental chorionic villi from preeclamptic pregnancies. Am J Physiol Endocrinol Metab 2015;309:E852–E860. [DOI] [PubMed] [Google Scholar]
- Yao L, Agoulnik AI, Cooke PS, Meling DD, Sherwood OD. Relaxin acts on stromal cells to promote epithelial and stromal proliferation and inhibit apoptosis in the mouse cervix and vagina. Endocrinology 2008;149:2072–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EH, Liu A, Mills JL, Zhang C, Mannisto T, Lu Z, Tsai MY, Mendola P. Increased levels of copeptin before clinical diagnosis of preelcampsia. Hypertension 2014;64:1362–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Jarvinen H, Wahlstrom T, Seppala M. Human endometrium contains relaxin that is progesterone-dependent. Acta Obstet Gynecol Scand 1985;64:663–665. [DOI] [PubMed] [Google Scholar]
- Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol 2010;5:173–192. [DOI] [PubMed] [Google Scholar]
- Zulfikaroglu E, Islimye M, Tonguc EA, Payasli A, Isman F, Var T, Danisman N. Circulating levels of copeptin, a novel biomarker in pre-eclampsia. J Obstet Gynaecol Res 2011;37:1198–1202. [DOI] [PubMed] [Google Scholar]