Abstract
The signals and mechanisms that synchronize the timing of human parturition remain a mystery and a better understanding of these processes is essential to avert adverse pregnancy outcomes. Although our insights into human labor initiation have been informed by studies in animal models, the timing of parturition relative to fetal maturation varies among viviparous species, indicative of phylogenetically different clocks and alarms; but what is clear is that important common pathways must converge to control the birth process. For example, in all species, parturition involves the transition of the myometrium from a relaxed to a highly excitable state, where the muscle rhythmically and forcefully contracts, softening the cervical extracellular matrix to allow distensibility and dilatation and thus a shearing of the fetal membranes to facilitate their rupture. We review a number of theories promulgated to explain how a variety of different timing mechanisms, including fetal membrane cell senescence, circadian endocrine clocks, and inflammatory and mechanical factors, are coordinated as initiators and effectors of parturition. Many of these factors have been independently described with a focus on specific tissue compartments.
In this review, we put forth the core hypothesis that fetal membrane (amnion and chorion) senescence is the initiator of a coordinated, redundant signal cascade leading to parturition. Whether modified by oxidative stress or other factors, this process constitutes a counting device, i.e. a clock, that measures maturation of the fetal organ systems and the production of hormones and other soluble mediators (including alarmins) and that promotes inflammation and orchestrates an immune cascade to propagate signals across different uterine compartments. This mechanism in turn sensitizes decidual responsiveness and eventually promotes functional progesterone withdrawal in the myometrium, leading to increased myometrial cell contraction and the triggering of parturition. Linkage of these processes allows convergence and integration of the gestational clocks and alarms, prompting a timely and safe birth. In summary, we provide a comprehensive synthesis of the mediators that contribute to the timing of human labor. Integrating these concepts will provide a better understanding of human parturition and ultimately improve pregnancy outcomes.
Keywords: labor and delivery, corticosteroids, senescence, oxidative stress, NK cells, progesterone receptors, endoplasmic reticulum, unfolded proteins, p38MAPK, aging
Introduction
Parturition: evolutionary considerations
The appropriate timing of birth is a major determinant of pregnancy success and evolutionary fitness in viviparous species. Parturition, a key element in the reproductive cycle that is well matched to the species-specific reproductive strategy, is under strong selective pressure. There is wide diversity in the length of gestation and neonatal maturity at birth that is independent of size at birth. Some species (e.g. marsupials) have a relatively short gestation period and give birth to small and highly dependent (i.e. altricial) neonates, whereas others (e.g. sheep) experience longer gestations, giving birth to large, precocial infants able to stand and run independently within minutes after birth. With respect to maturity at birth (i.e. dependent/altricial or independent/precocial), human neonates are comparable with other altricial species even though primates are generally considered to be precocial at birth. This human trait, known as secondary altriciality, is thought to reflect the combined effects of bipedalism, with a narrow, funnel-like pelvic outlet and encephalization, which provides for a relatively large fetal head and accommodates a complex fetal brain. The combination of these unique hominid characteristics created an obstetric dilemma: how to deliver a large-headed infant through a small pelvic outlet. Strong selective pressure likely led to a birth timing system that optimizes the benefits of both encephalization and bipedalism. Moreover, the need to ensure successful pregnancy likely produced a redundancy of pathways to ensure reliable uterine emptying and expulsion of the fetus. In this context, the control of human birth timing is unique and it is not surprising that most animals are poor models for the physiology of human pregnancy and delivery. The parturition-triggering pathway that is most effectively activated and results in the birth of a viable neonate to a healthy mother, capable of nurturing the infant, is typically considered the ‘normal’ mechanism for that species. However, secondary pathways also evolve through natural selection, particularly in response to specific physiological or environmental conditions.
Herein, we develop the hypothesis that fetal membrane senescence is the initiator of a coordinated, redundant signal cascade leading to parturition. We propose that multiple inputs into an inflammatory load converge on common labor effectors such as progesterone withdrawal and increased sensitivity to uterotonins [e.g. oxytocin and prostaglandins (PGs)] to transform the uterus into a laboring phenotype. Each of these inputs constitutes a functional ‘clock’ with interdependent timing mechanisms. However, the extent to which any individual clock dominates over other physiological or pathophysiological signaling mechanisms that stem from infection or metabolic derangement is likely to explain the variability in human gestational length.
Birth usually occurs at a time in gestation referred to as ‘term’, when the fetus is physiologically prepared to maintain homeostasis as a neonate. A relatively wide range for human gestation (defined as 37 0/7 through 41 6/7 gestational weeks by the American College of Obstetrics and Gynecology (2013)) is recognized, when the probability for neonatal survival is optimal. Clearly, gestation must proceed for an amount of time needed for the fetus to develop. This review addresses the question of what mechanism controls the length of human gestation and how its various related timing mechanisms are synchronized. Even with a term delivery, several stereotypes support the concept that birth is a metaphysical event. Babies are very rarely born with intact membranes, a condition termed ‘en caul’, purportedly a good omen or a mark of greatness. In fact it is far more likely that membranes rupture before the onset of contractions in normal pregnancy, sometimes by many hours or even days. For women who present with contractions but do not go into active labor or for women who arrest in active labor, the artificial rupturing of fetal membranes is a common procedure to induce labor.
Biological clocks
The Merriam-Webster dictionary defines a biological clock as ‘an inherent timing mechanism in a living system that is inferred to exist in order to explain the timing or periodicity of various behaviors and physiological states and processes.’ A biological clock can function as either a counting device that records events (e.g. light–dark cycles or cell divisions) or as a responder that monitors the level of a specific signal (e.g. nutrient availability or level of circulating hormone). The system should respond after a specific number of events (analogous to a ticking, preset alarm clock) or at a specific signal vector (analogous to a sundial or a thermostat set to maintain a specific room temperature). In this context, pregnancy biological clocks could count recurring events and/or monitor a threshold of specific signals. Because fetal maturation is the core objective of gestation, it is reasonable to assume that the length of gestation is in part governed by a timing mechanism linked to the fetal developmental program. Such a system is exemplified in sheep, where birth timing is controlled by the activity of the fetal hypothalamic–pituitary–adrenal (HPA) axis. Increased activity of the fetal HPA axis induces a surge in cortisol production by the fetal adrenals to stimulate the functional maturation of the organ systems necessary for neonatal survival, in particular the lungs. Importantly, this cortisol surge concomitantly decreases production of progesterone in the placenta, leading to a systemic progesterone withdrawal that triggers parturition. In sheep, this fetal neuroendocrine clock thus determines the length of gestation and coordinates fetal maturation with parturition. Ablation of the fetal HPA axis in sheep abolishes parturition and fetal growth continues post-term until the size of the conceptus eventually overwhelms the maternal abdominal cavity, constricts the rumen and causes the pregnant ewe to die of starvation. Redundancy of parturition pathways appears to be lacking in sheep and because this system is highly efficient in neonatal loss as a result, aberrant birth timing is virtually absent in this species.
Parturition in the mouse is also triggered by a clock-like mechanism; however, this time-keeping device appears to be located in the maternal compartment. In rodents, the progesterone required to maintain pregnancy is supplied by the maternal corpus luteum, which persists for most of the pregnancy. Parturition is triggered by PG production in the maternal decidua (pregnancy endometrium) late in gestation in response to a presumed decidual clock. Decidual PGs induce luteolysis, causing a rapid fall in the level of progesterone and subsequently inducing parturition. Until recently, it had been assumed that the clock controlling decidual PG production in rodent parturition was independent of fetal development. However, it was recently reported that in mouse embryos lacking both SRC-1 and SRC-2 steroid receptor co-activators, parturition is severely delayed, but labor can be rescued by surfactant protein A (SP-A) and a glycerophospholipid (platelet-activating factor, PAF) derived from the lungs of wild-type mouse fetuses (Gao et al., 2015). Thus, an integrated embryonic component also appears to participate.
These examples of gestation clock mechanisms represent rather extreme cases: the first example exclusively residing in the sheep fetus, whereas in the second example predominantly residing in the rodent mother with new evidence of fetal lung contribution. Whether such similar systems control the length of human gestation is currently an area of intense research. The relatively wide range of human term gestation (37–42 gestational weeks) and the high incidence of pre- and post-term births in the human species (with neonatal risks associated with both conditions) suggest considerable slack and variance and ultimately the existence of multiple, redundant clock-and-monitoring systems. Additionally, the rupture of fetal membranes at term also exhibits distinct phenotypes. One-third of deliveries experience premature rupture of the membranes at term (PROM), whereas in many instances artificial rupture of the membranes is required prior to delivering the fetus even after prolonged labor. To date, many of the parturition phenotypes observed at term have not been fully characterized either biologically or mechanistically and thus our understanding of the pathways of parturition is limited.
Although the timing of parturition relative to fetal maturation varies among viviparous species, indicative of multiple gestation clock and parturition-triggering mechanisms, important common pathways and their associated hormonal controls exist in the birth process. In all species studied to date, parturition involves the transition of the myometrium from a relaxed to a highly excitable state in which the muscle rhythmically and forcefully contracts to become the engine for birth. Softening of the cervical extracellular matrix (ECM) allows the distensibility needed for dilation of the cervix, the gateway for birth and a weakening of the fetal membranes that facilitates their rupture. Another parturition feature common among viviparous species is inflammation within the gestational tissues, i.e. the cervix, decidua/fetal membranes and myometrium.
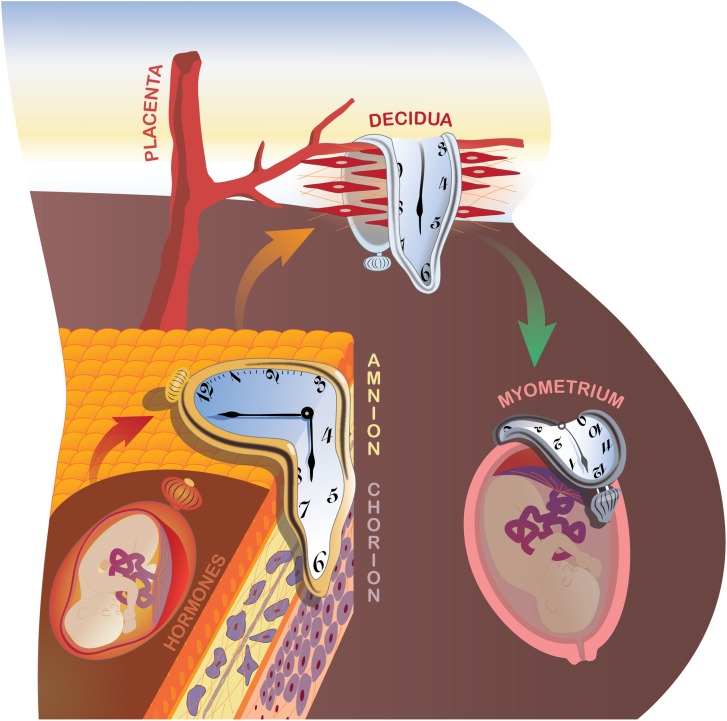
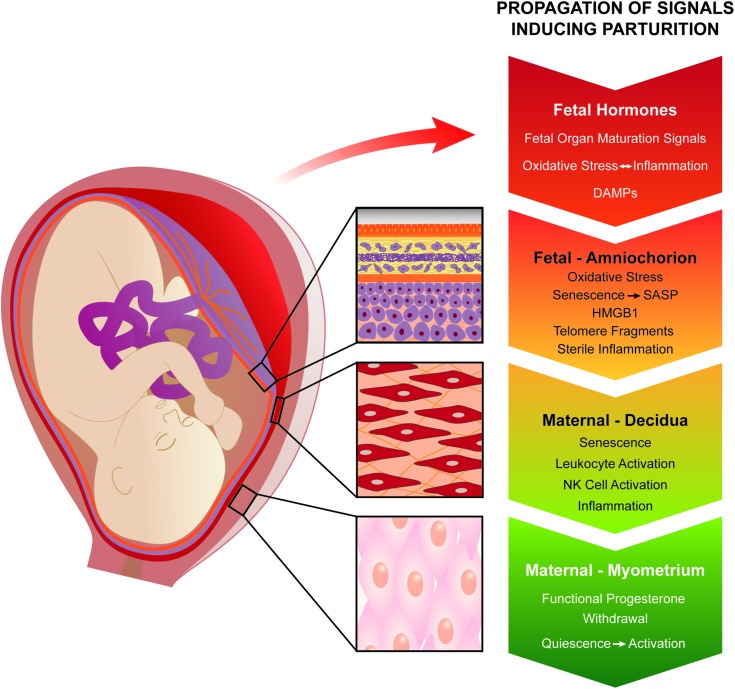
Inspired by the surrealistic masterpiece ‘The Persistence of Memory’ created by the late Salvador Dalí, we envision an integrated and potentially sequential cascade of local and systemic signals derived from multiple biological compartments, each with its own intrinsic clock or alarm characteristics (Fig. 1). To provide a comprehensive overview of the evidence, we address these inputs in separate sections. We start from a traditional view, as described above for sheep, where neuroendocrine signals derived from the fetus play a critical role in synchronizing the onset of parturition. However, our current working hypothesis, which is based on exciting new data that we summarize in this review, posits natural, physiological fetal membrane senescence as the prime instigator of normal labor.
Figure 1.
Proposed system of multiple biological clocks and alarms to optimize the duration of pregnancy. During pregnancy, at least four such timing mechanisms exist and these can operate in parallel or in series. Neuroendocrine fetal hormones and placental factors, particularly those modulating cortisol productions, have been classically invoked and are reviewed under ‘The endocrine clock’. The novel concept of natural fetal membrane cell senescence is developed under ‘Fetal membrane clock: a source of parturition signals’, where several testable, amnion-derived sterile inflammatory mediators are proposed. The decidual clock serves at the critical vascular feto–maternal interface (The decidual clock: inflammation and senescence). Ultimately, the myometrium becomes progesterone resistant and is activated to generate coordinated, forceful contractions, leading to fetal expulsion (Myometrial clock: progesterone and inflammation). Oxidative, nitrosative, folded-protein and ER stress pathways (Myometrial stretch inputs and activation of parturition) also contribute to the molecular regulation of labor onset. Finally, redundancy and synergy provide assurance against prolonged pregnancy duration, whereas premature activation of one or more pathways can trigger early delivery. Understanding the integrated actions of these systems will inform new clinical approaches to the control of labor onset.
The endocrine clock
The idea of a zeitgeber (i.e. time-keeper) or pacemaker, an external influence that synchronizes the setting of a biological clock, has been a popular concept in endocrinology for decades. The classical neuroendocrine axis zeitgeber has a short wavelength and is monochromatic; it which induces a phase-response shift in retinal ganglion cell signaling via the retino-hypothalamic tract to the suprachiasmatic nucleus, which in turn suppresses melatonin levels and entrains circadian (~24 hours) glucocorticoid rhythms (Copinschi et al. ,). Current molecular models of circadian clocks involve mechanisms whereby protein dimers of basic helix–loop–helix transcriptional enhancers (encoded by the Clock and Bmal1 genes, for example) form stimulatory complexes. Their nuclear phosphoprotein targets (derived from the Period and Cryptochrome genes (Hastings et al., 2007)) create an inhibitory feedback loop, thereby generating alternating metronomic signals. In an elegant conditional knockout model of pregnant mice, targeted deletion of Bmal1 circadian clock alleles in the maternal myometrium and bladder resulted in a circa 20% reduction in myometrial Bmal1 mRNA, characterized by early delivery in 18% and late delivery in 18% of litters. Thus, uterine clock genes may be directly involved in the timing of parturition (Ratajczak et al., 2012).
Shorter ultradian (circa 1–2 hours) and longer circannual (circa 1 year) patterns of hormone production also are recognized. Studies in mating and parturition have traditionally focused on circannual photoperiods in seasonal breeders, but in continuously reproductive species like humans emphasis has mostly focused on the oscillatory regulation of the hypothalamic–pituitary–ovarian function that leads to ovulation, fertilization and conception. Long periodicity effects have also been evaluated in human pregnancy. Onset of labor and spontaneous rupture of fetal membranes were reported to occur more commonly during a full moon (Stern et al., 1988) but a more recent, larger study failed to show a relationship between birth rate and lunar or atmospheric conditions (Morton-Pradhan et al., 2005). Seasonal patterns of pregnancy and birth exist across different populations and geographic regions, but these cannot be attributed to a biological clock per se. Instead, a high number of summer births is attributed to ‘Christmas effect’ conceptions that correspond with vacations and longer periods of darkness in the northern hemisphere of the Western world (Cesario, 2002).
The HPA axis
The HPA axis has its own circadian rhythms, with a glucocorticoid phase that changes the expression of Clock-related genes, including the Period gene in mouse heart, liver and kidney (So et al., 2009). By changing the expression of Clock genes, glucocorticoids influence responses to stressors, including the regulation of food intake (Damiola et al., 2000). Aberrant light–dark cycles have been shown to impair mood and learning in mice via melanopsin-expressing neurons. Increased glucocorticoid (corticosterone) concentrations in this model were suppressed by administering fluoxetine or desipramine, which are antidepressant drugs (LeGates et al., 2012). However, results from large clinical studies and a meta-analysis are mixed. In a Finnish population-based cohort of >845 000 singleton births, pregnant women exposed to selective serotonin reuptake inhibitors had a significantly reduced risk of preterm delivery (Malm et al., 2015). By contrast, a meta-analysis by Huybrechts et al. (2014) indicated that antidepressant medications during pregnancy were associated with a higher, not lower, risk of preterm birth, even when the diagnosis of depression was controlled. Future studies are needed to ascertain whether antidepressant medications pharmacologically modulate the onset of labor.
Following its identification in the 1980s, corticotrophin-releasing hormone (CRH) emerged as a crucial hypothalamic hormone regulator of human labor (Petraglia et al., 1987). As with oxytocin (discussed below), CRH is classically synthesized in the hypothalamus, but there is strong evidence that supports local placental production of CRH and its related urocortin peptides. Early experiments demonstrated that radiolabeled CRH can bind with high affinity and specificity to placental membranes, and just recently CRH receptor mRNA and proteins have been documented in human amnion, syncytiotrophoblast and term myometrial cells (Petraglia et al., 2010). In a recent study, 22 primigravid women in active labor were subjected to frequent plasma sampling to assess maternal CRH and IL-6 pulses. As the plasma half-lives of CRH and IL-6 in humans are 4 and 7 minutes, respectively, a 3-minute sampling interval was chosen to detect the two proteins (Papatheodorou et al., 2013). CRH pulses were identified with a frequency of ~2 per hour. These were paralleled by a slightly slower pattern of IL-6 pulses, wherein the cytokine peaks preceded each CRH peak by ~12 minutes. The authors interpreted the time-integrated data to indicate that an inflammatory stimulus, manifested as IL-6 peaks, drives CRH secretion (the role of inflammatory cytokines as parturition clocks is discussed in more detail below). Feedback regulation of CRH by cortisol in the intrauterine tissues is quite distinct from that of the adult HPA axis. In the former, a positive feedback loop drives the exponential production of glucocorticoids near term, synchronizing labor and fetal organ maturation (Robinson et al., 1988). Uterine contractions in the studied women (Behnia et al., 2015), measured as Montevideo units, had a periodicity of ~24 per hour, indicating substantially faster rhythm modulation by the myometrial clock. No significant time-ordered correlations between CRH pulses and myometrial contractility were observed.
Glucocorticosteroids and parturition
Glucocorticosteroid hormones are powerful synchronizers of biological clocks, and this class of hormones has long been thought to control the timing of parturition. In most mammalian species, increased concentrations of glucocorticoids are evident in the maternal and fetal circulations and amniotic fluid prior to the onset of labor (Li et al., 2014). As term approaches, the fetal membranes and placenta produce CRH and concomitantly synthesize less CRH binding proteins, robustly increasing bioavailable CRH (McLean et al., 1995; Golightly et al., 2011). High levels of CRH in turn up-regulate adrenocorticotropic hormone and cortisol production in the fetal pituitary and adrenal glands, respectively. However, unlike the adult HPA axis where feedback inhibition occurs, cortisol promotes placental CRH gene expression, thus creating a positive feedback loop and driving the exponential production of glucocorticoids (Robinson et al., 1988). Moreover, CRH appears to have a direct effect on fetal lung maturation (Muglia et al., 1999), simultaneously assuring that adequate pulmonary surfactant is present at the time of delivery.
Glucocorticoids predominantly exert their pleiotropic actions via intracellular glucocorticoid receptors (GRs) that function as ligand-activated transcription factors. Upon ligand binding, GRs dissociate from chaperone heat shock proteins (HSPs) and immunophilins, translocate into the nucleus and activate or repress target genes bearing glucocorticoid response elements. GR action can be directly modulated by heterodimerization with other transcription factors, including nuclear factor kappa B (NF-κB) (Wang et al., 2014) and the clock gene product itself (Nicolaides et al., 2014), which in turn influences immune responses. These effects may be ligand-dependent given that progesterone and the synthetic progestin, medroxyprogesterone acetate, repress cytokine-driven cyclooxygenase (COX) 2 expression via GR (Lei et al., 2012).
In most domestic animals, concentrations of glucocorticoids in maternal, fetal and amniotic fluid surge at the onset of labor. In contrast, corticosteroid concentrations in human pregnancy progressively increase during the third trimester of pregnancy without a dramatic peripartum endocrine surge. However, Li et al. (2014) provide a strong rationale for rapidly augmented local paracrine glucocorticoid action within the fetal membranes and subjacent myometrium. In human pregnancy, the fetus is protected from circulating maternal cortisol levels by the high level of expression of 11β hydroxysteroid dehydrogenase 2 (11β-HSD2), which is synthesized in the syncytiotrophoblast. This enzyme inactivates most of the maternal cortisol transferred across the fetal membranes by oxidizing it into inactive cortisone. However, with advancing gestation and effects of pro-inflammatory cytokines, maternal decidua and fetal amnion cells acquire the ability to locally regenerate cortisol from cortisone, via expression of the reductive isoenzyme 11β-HSD1 (Sun and Myatt, 2003), and hexose-6-phosphodehydrogenase. The latter enzyme produces nicotinamide adenine dinucleotide phosphate (NADPH), a critical co-factor that provides reducing equivalents for 11β-HSD1 activity. Thus, high concentrations of cortisol are generated within the fetal membranes, where they stimulate local production of PGs, senescence factors and other potent uterotonins. This model reveals how many labor mechanisms are preserved between ruminant and human parturition, even when the compartments the pathways lie in are divergent.
Finally, a number of targets down-stream of GR activation appear to increase susceptibility to senescence, including epigenetic programming effects, in what is referred to as the ‘glucocorticoid vulnerability hypothesis’ (Garrido, 2011). This concept is addressed in the next section.
Other hypothalamic–pituitary peptides
Oxytocin is a nine-amino acid peptide classically produced in association with neurophysin by hypothalamic magnocellular neurons whose axons project toward neurosecretory endings in the posterior pituitary. The same hormone is synthesized by chorionic trophoblasts in placental membranes (Chibbar et al., 1993; Blanks et al., 2003) and the decidua (Friebe-Hoffmann et al., 2007). Moreover, because umbilical artery concentrations are up to 10-fold higher than the levels in the umbilical vein (Malek et al., 1996), the fetus itself appears to be a source of oxytocin. Within the uterus, the primary targets of oxytocin are receptors in the myometrium (Arrowsmith and Wray, 2014), which mobilize intracellular Ca2+ transients and stimulate uterine contractility (Fuchs et al., 1982). Moreover, other local autocrine and/or paracrine actions have been described for this hormone, with oxytocin serving as a potent secretagogue for the release of both CRH and prostaglandin F2 alpha (PGF2α) from trophoblast cells (Fuchs et al., 1982; Petraglia et al., 1989). It has been reported that the frequency of spontaneous oxytocin pulses in women in labor (measured in ethylene diamine tetraacetic acid plasma to inhibit oxytocinase) is 4–5-fold faster (~14 per hour) than the pulse frequency prior to labor (~3 per hour). The zeitgeber for this clock has not yet been identified (Fuchs et al., 1991). Based on this physiological rhythm, it was hypothesized that pulsatile oxytocin infusion might be more efficacious in inducing labor than the continuous administration protocols that have been used in clinical practices over the past 50 years. However, in a well-powered study, Tribe et al. (2012) found no difference in the ratio (1.01, 95% confidence interval 0.81–1.26, P = 0.90) of cesarean to vaginal deliveries. Similar findings were reported by Cummiskey et al. (1989) in a randomized study of 94 women receiving continuous versus pulsed oxytocin infusion for labor augmentation. Although the total administered dose was ~28% less in the latter group, none of the clinical efficacy outcomes were different.
Pro-inflammatory cytokines, including interleukins, IL-1β and IL-6, and tumor necrosis factor alpha (TNF-α) have all been implicated in parturition (Romero et al., 2006) and also fluctuate in a circadian pattern with peak concentrations between midnight and early morning hours. Their actions partially explain the strong clustering of contractions (67%) that occur at night in human pregnancy (Moore et al., 1994; Olcese, 2012; Reiter et al., 2014). These appear in part to be due to the coordinated, nocturnal up-regulation of melatonin and oxytocin receptors in the myometrium (Sharkey et al., 2009), possibly driven by circadian cytokine release. A similar mechanism presumably underlies the tendency for inflammatory joint symptoms to flare in the early morning among patients with autoimmune disorders (Straub and Cutolo, 2007). Increasing evidence supports a role for cellular senescence in parturition (see below), and glucocorticoid exposure has been invoked as a major determinant of immunological aging (Bauer, 2008). Some of these effects appear to be mediated via elevated cortisol levels, altered GR signaling, telomere shortening and cell aging as observed in major depressive disorders (Wolkowitz et al., 2011). These are discussed in more detail in the following sections on fetal membranes and decidual clocks.
Eicosanoids and endothelins
In addition to their maturational effects on fetal physiology, including pulmonary, gut and neural systems, glucocorticoids can contribute to myometrial preparation for labor via increased PG production, through the up-regulation of COX-2 and down regulation of the catabolic 15-OH PG dehydrogenase (Challis et al., 2002). Cytosolic phospholipase A2, which liberates arachidonic acid from cell membrane phospholipids, and carbonyl reductase 1, an enzyme that can convert PGE2 to PGF2α, also are positively regulated by cortisol (Guo et al., 2014). That PGs serve as potent uterotonic mediators of labor is supported by evidence that increases in amniotic fluid PGE2 and PGF2α concentrations precede parturition (Romero et al., 1996), clinically administered PG preparations induce myometrial contractions at virtually any stage of pregnancy, and inhibitors of PG synthesis delay labor onset. Furthermore, some of the endocrine and paracrine factors reviewed in this section of the article (e.g. CRH, cortisol) have been shown to signal through local PG production within the decidua and/or fetal membranes. Other key myometrial contractile mediators have been identified. Endothelin-1 is a 21-amino acid peptide that is both a potent vasoconstrictor and a uterotonin (Carbillon et al., 2001). A single nucleotide polymorphism in the endothelin-1 gene was observed to contribute to a multilocus model of gene–gene interaction predisposing to pPROM (Romero et al., 2010), where elevated midtrimester amniotic fluid concentrations of ET-1 have been reported (Margarit et al., 2006, 2007).
In superfused amnion explants from 6 of 8 subjects in labor, but not from 10 women before labor, PGE2 and PGF2α release was noted to be pulsatile, with a periodicity of ~1 per hour (Reddi et al., 1990). The actions and regulation of myometrial PG receptors that ultimately transduce the effects of PGE2 and PGF2α on uterine smooth muscle are complex. EP3 and FP receptors appear to dominate the contractile effects of PGE2 and PGF2α, respectively. These receptors couple with Gαq/11 and Gαi proteins to activate phospholipase C, generate inositol triphosphate and mobilize intracellular Ca+2 (Sakamoto et al., 1995; Arulkumaran et al., 2012). The FP receptor also has been shown to modify multiple functions in the human myometrium and decidua, including responsiveness to oxytocin, cytokines and activation of matrix metalloproteinases (MMPs) to effect cervical remodeling and promote fetal membrane rupture (Makino et al., 2007). Thus, coordinated actions of eicosanoids and their receptors along with other uterotonins drive the parturition clock. To our knowledge, pulsatile release of endothelin-1 in the human uterus has not been reported, but microdialysis studies in bovine corpora lutea revealed tight correlations of circadian endothelin-1, oxytocin and PGF2α pulses in ovarian venous plasma (Shirasuna et al., 2007).
Summary
In this section of the review, we summarize the influences of what are regarded as many of the critical endocrine and paracrine factors that regulate the onset of labor. It is assumed, but still not rigorously established, that misexpression of combinations of these hormones and eicosanoids might also precipitate preterm labor. A few general principles are of note: (i) although central and systemic sources exist for many of these signaling molecules, local production of the identical hypothalamic–pituitary hormones, steroids and eicosanoids within the intrauterine membranes of the feto–maternal tissues allow paracrine and autocrine actions of these classical endocrine factors; (ii) pulsatile production and secretion of labor-activating factors have been demonstrated in the maternal circulation and often within organ explants in vitro, suggesting that rhythmic pacemaker functions are intrinsic characteristics of these reproductive tissues and finally (iii) complex, parallel and interactive signal transduction cascades provide mechanisms for the synchronization of several cooperating biological clocks that set the alarm for parturition. A more sophisticated understanding, and ultimately the simultaneous pharmacological manipulation, of multiple pathways are likely to be needed to establish successful strategies to reset the human labor clock and prevent preterm birth, which continues to be a vexing clinical problem.
Fetal membrane clock: a source of parturition signals
Fetal membrane: structure and function
The so-called fetal membranes comprise two concentric cellular layers: an internal amnion and an external chorion (Hay, 1981). The amnion is composed of a single inner layer of cuboidal epithelium, with a thickness of 50–500 μm, which surrounds the developing embryo and lines the uterine cavity. The outer chorion is a much thicker layer and comprises trophoblasts derived from the yolk sac and allantois, representing the remnant of chorion laeve. The trophoblast layer is directly connected to the maternal decidua and embedded within an ECM that is rich in type IV collagen (Bryant-Greenwood, 1998). The amnion is constantly bathed apically by amniotic liquor, preventing it from desiccation and providing it with a fluid-filled space that protects the growing fetus from mechanical ‘shocks’ that might cause fetal injury (Mossman, 1991). The chorion acts as a co-ordinating center for nutritional supply and metabolite exchange across the feto–maternal interface, providing immune protection, structural stability and a site for hormone production (e.g. chorionic gonadotropin, CRH, oxytocin and estrogens), which is critical for accommodation of fetal growth remodeling.
Fetal organ maturation signals and their impact on fetal membranes
As discussed above, the timing of parturition in most species is a result of coordinated signals arising from both the mother and fetus (Mendelson, 2009). Previously, it had been suggested that the fetus may provide a signal via pulmonary SP-A, secreted into the amniotic fluid and accumulating in increasing amounts during late gestation until myometrial contractility is activated (Condon et al., 2004). Increased fetal lung surfactants have been demonstrated to stimulate the release of PGs from the fetal membranes (Sun et al., 2006; Myatt and Sun, 2010). As the onset of labor is accompanied by increased PG levels in the fetal membranes, myometrium, amniotic fluid and maternal circulation, these analyses initially defined a mechanism whereby a fetal gestational clock can trigger a maternal parturition response. More recent analyses have proved unequivocally, at least in the mouse, that SP-A and PAF provide such pro-inflammatory fetal signals (Gao et al., 2015). As fetal lung production of SP-A and PAF leads to increasing concentrations in the amniotic fluid, maternal myometrial NF-κB activation and PGF2α levels follow. As a result of these gestationally regulated paracrine effects, local maternal expression of myometrial contraction-associated genes is increased. Furthermore, these analyses provide compelling evidence that a signal originating in the fetus can also modify the maternal endocrine axis by regulating ovarian luteolysis and evoking progesterone withdrawal in the pregnant mother as term approaches. In conclusion, at least one fetal paracrine clock, utilizing surfactant lipoproteins, regulates gestation by signaling via the fetal membranes and maternal myometrium when its lungs have developed the capacity for the transition from the aqueous environment of the amniotic cavity to aerobic environment outside.
Besides SP-A and PAF from the lung, fetal organ maturation signals such as endothelin-1 from kidneys, epidermal growth factor and transforming growth factor (TGF) from liver, steroid receptor co-activators 1 and 2, and a multitude of sensory input signals from the brain are also examples of organ maturation-related signals released into the amniotic fluid. Like SP-A, all these factors are known activators of inflammatory cascades and impact a fetal membrane response.
Fetal membrane aging
The above section described the signals generated by the maturing fetus and its likely impact on fetal membranes. In this section, we propose a novel mechanistic role played by fetal membranes to promote parturition. The concept described below is derived from our recent data on natural and physiological aging of fetal membranes and how the process associated with aging can generate a variety of biochemicals, constituting sterile inflammation to promote parturition (Behnia et al., 2015; Menon et al., 2016). Fetal membrane growth during gestation is tightly coupled to embryogenesis and fetal growth. The amnion layer surrounds the embryo and the fusion of amnion and chorion occurs between 14 and 16 weeks of gestation. This unit structure continues to expand by remodeling its ECM through processes that involve MMPs and their tissue-specific inhibitors (Vadillo-Ortega et al., 1995; Fortunato et al., 1997, 2003) , eventually obliterating the extra-embryonic celom and fusing with the chorion (Mossman, 1991). Cells of the fetal membranes continue to divide throughout pregnancy, although the rate of mitosis diminishes near term (Teasdale, 1980; Fox, 1997). In addition to cell division, development and maturation also progress throughout gestation. Membranes multitask throughout pregnancy, mediating fetal wellbeing by communicating between the fetus and the mother. As the fetus approaches maturity near term, physiological aging of membrane cells ensues and with it functional capacity declines. The status of the fetal membranes at this point resembles the end stages of an organismal life. Their dysfunction can be perceived as the final preparatory stages for the eventual delivery. Based on these developmental aspects, we postulate that fetal membranes function as an independent ‘Jeeva’ (organism or life form) to perform a specific function during gestation; i.e. to protect the fetus and aid in its growth. Continued progressive division of fetal membrane cells leads to a telomere-dependent cellular senescence that promotes an aging phenotype. The aging fetal membrane cells promote their final function by propagating signals that activate the decidua, resulting in functional progesterone withdrawal and the generation of inflammatory signals in myometrium, thus initiating contractions and ripening the cervix for fetal delivery (Fox and Faulk, 1981; Fox, 1997; Menon et al., 2012, 2013, 2014a,b; Menon, 2014; Bredeson et al., 2014; Behnia et al., 2015; Polettini et al., 2015b).
Telomeres and fetal membrane senescence at term
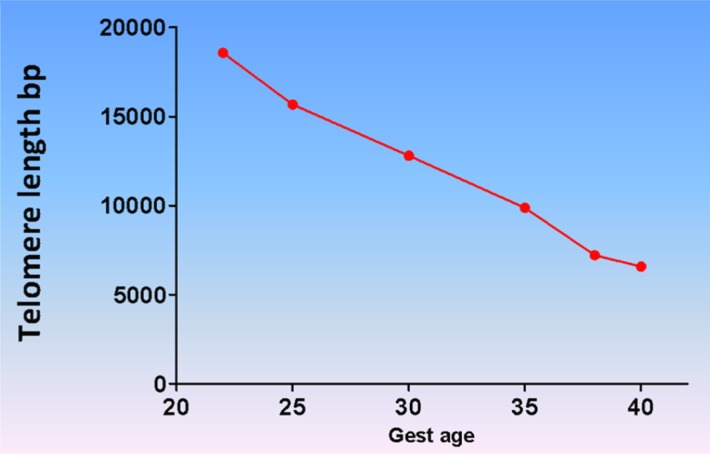
Replicative senescence, a telomere-dependent process, is a mechanism associated with aging and involves cell cycle arrest triggered by various endogenous and exogenous factors (Hayflick, 1974; Blackburn, 1991; Campisi, 1997; Blackburn, 2001; von and Martin-Ruiz, 2005; Campisi and d'Adda di, 2007; Coppe et al., 2010; Kuilman et al., 2010; Sanders and Newman, 2013). Telomeres are guanine nucleoside-rich caps at the ends of chromosomes, which function to protect chromosome integrity (Harley et al., 1992; von and Martin-Ruiz, 2005; Horn et al., 2010; Sanders and Newman, 2013). Telomere length is maintained in somatic cells by telomere terminal transferase (or ‘telomerase’), an enzyme that adds TTAGGG repeat sequences to the 3′ ends of deoxyribose nucleic acid (DNA) (Blackburn, 1997, 2001; Boccardi and Paolisso, 2014; Phillippe, 2014). Telomere length during embryogenesis peaks at the blastocyst stage (Liu et al., 2007) but its precise regulation and the role of telomerase activity in early pregnancy remain unclear. Gestational age-dependent reduction in telomere length of fetal leukocytes and fetal membrane cells was reported by Menon et al. (2012). A progressive diminution in telomere length from ~18 to ~7 kb was observed from 22 to 41 weeks of gestation (Menon et al., 2012) (Fig. 2). This decrease in fetal telomere length was rapid over a period of only 19 weeks. If the decrement in telomere length per cell division is assumed to be 50–100 bases (Zhang and Klotz, 2001; Liu et al., 2007; Ratsep et al., 2015), this rate of shortening corresponds to a cell-doubling rate of 1–2 days, typical of human cells in vitro (Kim, 1995). These values also are consistent with the so-called Hayflick limit of ~60 divisions (Hayflick, 1973a,b; Blackburn, 1991).
Figure 2.
Fetal telomere shortening during pregnancy. Fetal leukocyte DNA telomere analysis determined progressive reduction in telomere length as gestation progresses. The longest telomere length was seen at 22 weeks and the shortest at term. This is indicative of a telomere-dependent senescence process in the fetal compartment.
Multiple physiological factors contribute to telomere length loss, with a key mediator being oxidative stress (OS). Decreased telomere length was observed in term labor fetal membranes compared with term not-in-labor samples and also compared with membranes exposed to cigarette smoke extracts (CSEs) in vitro, and this was inversely correlated with amplifiable telomere fragments in the amniotic fluids (Polettini et al., 2015a). OS at term builds up in the intrauterine cavity as a result of increased fetal metabolic demands on mitochondria, reduced maternal supply of metabolic substrates, depleted antioxidants and increased stretching of placental and uterine tissues (Buhimschi et al., 2003; Myatt and Cui, 2004; Agarwal et al., 2005; Paamoni-Keren et al., 2007; Schulpis et al., 2007; Menon et al., 2014b). Guanine-rich repeats of 100–400 bases on the 3′ end of telomeres are particularly susceptible to OS because of the high density of guanine bases, which are oxidized through electron transfer (Saito et al., 1995; Stewart et al., 2003). Conversion of guanine into 8-oxoguanine (8-oxoG) creates lethal DNA lesions (Fortini et al., 2003), which are targets for base excision by 8-oxoG glycosylase (OGG1), a highly expressed DNA repair enzyme in human fetal membranes (Menon, 2014; Menon et al., 2014b). In-vitro analysis of normal term not-in-labor amnion epithelial cells exposed to CSE showed DNA damage and the formation 8-oxoG but with no increase in OGG1, indicating persistence of damage (Menon, 2014; Menon et al., 2014b). Exposure of primary amnion epithelial cells to CSE for up to 96 hours substantially reduced the telomere length compared with untreated controls. This experimental evidence shows that OS at term can result in accumulation of DNA damage and telomere fragmentation (Agarwal et al., 2005). Telomere reduction can be a marker for OS as well as aging. Therefore, the next set of experiments we performed using fetal membrane tissues provided evidence for senescence, a mechanism associated with aging (Menon et al., 2016).
Evidence for fetal membrane senescence
Masoro (1995) and Finch (1992) aided our understanding of fetal membrane aging by emphasizing that senescence of the amniochorion progresses slowly from as early as the 12th week of gestation, contributing to the decreased vitality of the fetal membranes and framing the time for parturition. A natural extension of this thinking is that premature senescence of these membranes might accelerate amniochorion aging and lead to preterm birth and other adverse pregnancy outcomes, like stillbirth or miscarriage (Polettini et al., 2015a,b).
Our group has systematically examined term human fetal membranes for signs of senescence consistent with the end stage of this tissue's life cycle. Several markers were observed to increase in specimens from laboring pregnancies compared with those sampled prior to labor (Behnia et al., 2015), including: (i) ultrastructural evidence of round mitochondria, swollen endoplasmic reticulum (ER), and contoured nuclei containing condensed chromatin; (ii) increased expression and activation of p38 mitogen-activated protein kinase (MAPK), a stress-responsive kinase; (iii) increased numbers of cells positive for senescence-associated beta-galactosidase (SA-β-gal); (iv) loss of lamin B1, a structural nuclear envelope protein. Additionally, an archetypal phenomenon known as the senescence-associated secretory phenotype (SASP) is characterized by the promulgation of pro-inflammatory cytokines, chemokines, growth factors and MMPs that manifest a form of sterile inflammation (Coppe et al., 2010; Rodier and Campisi, 2011; Velarde et al., 2013). Amniotic fluid samples from term labor have higher concentrations of pro-inflammatory cytokines and chemokines (e.g. IL-6, IL-8 and granulocyte monocyte colony-stimulating factor) compared with amniotic fluid from women from term not-in-labor (Behnia et al., 2015). Conversely, SASP markers related to pro-cell growth were lower in amniotic fluid from women during term labor. Although not designated as SASP markers (Behnia et al., 2015), changes in concentrations associated with many of these factors (cytokines, chemokines, growth factors, metalloproteinases, receptor agonizts and antagonists and PGs) are already documented in various feto–maternal compartments (Behnia et al., 2015). Their biological functions as pro-parturition factors have also been well documented in both human and animal model studies.
However, these data do not necessarily support senescence as a trigger for term labor because the association could simply reflect changes in response to parturition. Thus, using an in-vitro model of fetal membrane organ explant cultures and primary amnion epithelial cells from term not-in-labor tissue, we attempted to recapitulate these findings to establish causality. In such studies, we have shown that OS accelerates telomere shortening and senescence and produces overwhelming inflammation (SASP), recapitulating findings from clinical specimens.
How can senescent fetal membrane signal parturition?
Conventional theories of parturition initiation, as described above, include feto–maternal endocrine and immune changes within the intrauterine compartment (Casey et al., 1983; Smith et al., 2002; Romero et al., 2006; Gomez-Lopez et al., 2009, 2013; Mendelson, 2009; Shynlova et al., 2013; Vega-Sanchez et al., 2015). Herein, we propose that OS-induced senescence of fetal membranes may tilt the inflammatory threshold in the intrauterine cavity to generate signals required for parturition.
Besides SASP, senescent fetal membranes also release damage-associated molecular pattern (DAMP) markers. DAMPs are molecules that elicit an immune response to injured, necrotic or apoptotic endogenous factors (Farkas et al., 2007). Several DAMPs have been reported in laboring tissue, including high-mobility group box (HMGB) 1, HSP70, uric acid, single- and double-stranded DNA and RNA, S100 and hyaluronan (Buhimschi et al., 2004, 2009; Gravett et al., 2004; Lange and Vasquez, 2009; Romero et al., 2014b; Vega-Sanchez et al., 2015; Gomez-Lopez et al., 2016) DAMPs cause immune activation (Seong and Matzinger, 2004) and tissue injury in a feedback loop, additionally enhancing the inflammatory load of the uterine cavity. This review further highlights the mechanistic roles of DAMPs that can be propagated as signals enhancing the sterile inflammatory load in different uterine compartments. SASP and DAMPs reflect changes in fetal membrane physiology that contribute to inflammatory overload and may act as fetal signals that can be propagated to other compartments, where they cause tissue damage or increase inflammation. Below, we provide experimental evidence from our laboratory where we have shown two DAMPs, namely HMGB1 and cell-free fetal telomere fragments, that may function to enhance the overall inflammatory load, by providing a feed forward loop to enhance fetal cell senescence.
HMGB1 as a DAMP: role as a parturition signal
As mentioned above, one of the characteristic signs of senescence is loss of the nuclear envelope protein Lamin B1. Loss of Lamin B1 can cause nuclear contents to leak into the cytoplasm and extracellularly. One of the commonly externalized proteins is HMGB1, a 25-kD, non-histone chromatin-associated protein that binds double-stranded DNA (Andersson et al., 2002; Romero et al., 2012; Kang et al., 2013; Venereau et al., 2013; Janko et al., 2014) and stabilizes nucleosomes during DNA repair and recombination (Ticconi et al., 2007; Lange and Vasquez, 2009; Romero et al., 2011; Yanai et al., 2012). However, in response to OS and loss of Lamin B1, HMGB1 becomes acetylated and displaced into the cytoplasm, where it can then be secreted and function as a pro-inflammatory cytokine. HMGB1 is known to be expressed in human endometrium (Zicari et al., 2008; Nogueira-Machado et al., 2011), placenta (Wang et al., 2011), decidua, cervix (Oh et al., 2006), amnion epithelial cells and immune cells, and has been reported in cases of chorioamnionitis (Mercer and Lewis, 1997; Nogueira-Machado et al., 2011; Pisetsky et al., 2011; Romero et al., 2012). Higher concentrations of HMGB1 in the amniotic fluid of laboring (term and preterm) compared with non-laboring women suggest it has a role in parturition (Mercer and Lewis, 1997) and HMGB1 has been implicated recently as a mediator of sterile inflammation in preterm labor (Huang et al., 2010; Romero et al., 2014b). Animal models have shown that HMGB1 is a ligand for the receptor of advanced glycation end products (Buhimschi et al., 2009).
Bioinformatics analysis of differentially regulated SASP genes in human fetal membranes from term labor (in vivo) and after OS induction of amnion cells (in vitro) show that HMGB1 signaling is one of the top five canonical pathways. HMGB1 perpetuates a pro-inflammatory positive feedback loop through p38MAPK (a prosenescence marker in fetal membranes)-dependent and Toll-like receptor (TLR) 2-mediated pathway to enhance target cell senescence and tissue injury (Bredeson et al., 2014). HMGB1-induced production of inflammatory cytokines was blocked by the p38MAPK inhibitor SB203580. Taken together, these data support the concept that HMGB1, as a DAMP, induces a local inflammatory state within the fetal membranes. Its release from damaged fetal cells as an inflammatory cytokine can be considered a signal for parturition. A recent report showed that intrauterine administration of HMGB1 can cause preterm birth in animal models, which supports our concept (Gomez-Lopez et al., 2016).
Cell-free fetal telomere fragments as DAMPs: role as a parturition signal
As described above, fetal membrane senescence in humans is associated with OS and telomere attrition. The released telomere fragments are measurable in biological fluids such as amniotic fluid, where their concentrations are higher in term labor compared with term not-in-labor (Fig. 3). In-vitro telomere shortening in amnion cells occurs in response to OS. These free-floating DNA fragments are considered DAMPs because they can elicit pro-inflammatory responses. DNA as a DAMP is sensed directly or via specific binding proteins to instigate a pro-inflammatory response (Palmai-Pallag and Bachrati, 2014). This prompted us to test the role that shed telomere fragments play in enhancing senescence and inflammation using an in-vitro model of amnion epithelial cell cultures and in situ in pregnant CD1 mice.
Figure 3.
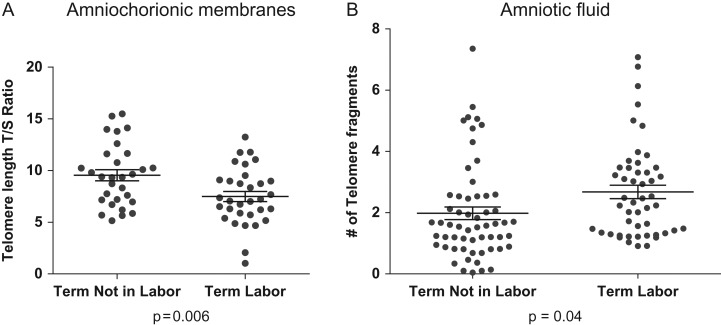
Telomere length changes in fetal membranes and telomere fragments in amniotic fluid at term. Telomere length reduction in fetal membranes in term laboring membranes compared with term not-in-labor membranes. (A) At term, the transition from not-in-labor to labor is associated with telomere length reduction in fetal membranes. (B) The decrease in fetal membrane telomere length is associated with an increase in telomere fragments in the amniotic fluid of women undergoing labor and delivery.
Normal term not-in-labor human amnion epithelial cells treated with tandem oligonucleotides [TTAGGG(2)] mimicking the 3′ ends of telomeres revealed additional OS-activated p38MAPK-induced senescence and increased cytokine and chemokine production compared with untreated cells (Polettini et al., 2015a). In pregnant CD1 mice, intrauterine administration of telomere oligonucleotides on day 14 of pregnancy elevated amniotic fluid IL-6 and IL-8 and caused OS, providing histological evidence of senescence in the amniotic sac compared with saline-injected mice. Senescence and inflammation were reduced to normal levels (saline-treated animals) after treatment with the p38MAPK inhibitor SB203580, confirming senescence-related mechanisms of inflammatory activation. Although the study did not test the initiation of parturition by telomere fragments, it does document that cell-free fetal telomere fragments can damage healthy fetal cells and induce a sterile inflammatory response. Phillippe theorizes that cell-free fetal DNA may function as a potential signal for parturition by enhancing inflammation through the TLR-9 pathway (Phillippe, 2014, 2015).
SASP and DAMPs produced by senescent fetal membranes act as signals for parturition
In summary, at least two DAMPs (HMGB1 and telomere fragments) activate positive feedback loops, cause senescence through stress-associated p38MAPK, increase SASP factors, and promote further deterioration of the fetal membranes. Moreover, ongoing studies indicate that HMGB1 induces the release of uric acid from fetal membrane cells (unpublished data), and that HMGB1 is also a DAMP that can increase inflammation (Foell et al., 2007; Mulla et al., 2011; Girard et al., 2014). In addition, recombinant HMGB1 causes a progesterone receptor switch (PR-A:PR-B) in favor-functional progesterone withdrawal in myometrial cells (see the section on the myometrial clock below), a process reversed by p38MAPK inhibitor SB203580. This suggests that HMGB1, which reaches the myometrium, can trigger parturition by activating p38MAPK also in the myometrium.
Other mediators of parturition linked to fetal membranes
Senescence of fetal membranes is one of the key events associated with human parturition, as we outlined here and reported in our publications (Menon et al., 2016). Either the result of senescence-dependent disturbances of the membrane structure or through a senescence-independent process, inflammatory mediators could be generated from fetal membranes. ECM fragments such as fetal fibronectin have also been shown to have immunogenic properties and induce inflammatory markers such as COX-2 and MMPs (Schaefer and Schaefer, 2010; Schaefer, 2010; Mogami et al., 2013). Therefore, aside from senescence-associated SASP and DAMPs, disturbances to extracellular proteins can also cause inflammatory changes in fetal compartments (Behnia et al., 2015). Their biological functions as pro-parturition factors have also been reviewed in both human and animal model studies (Romero et al., 1989, 2006; Kelly, 1996; Keelan et al., 2003; Lindstrom and Bennett, 2005; Elovitz, 2006; Lappas and Rice, 2007). We also acknowledge the latest reports by Mysorekar and colleagues and Aagaard and colleagues, where the sterility of the placenta and membranes has been challenged (Stout et al., 2013; Aagaard et al., 2014; Cao and Mysorekar, 2014). A change in the resident flora of the placenta and membranes as a result of inflammatory overload or senescence may contribute to parturition, likely by changing human leukocyte antigen-G expression at the feto–maternal interface, thus disturbing maternal tolerance of the semiallograft (Stout et al., 2013).
Summary
We propose that SASP and DAMPs released by senescent fetal membranes at term influence placental, decidual and myometrial cells in a sequential and progressive manner, propagating inflammatory signals. The signature and intensity of inflammation induced by SASP and DAMPs may vary based on intrauterine cell and tissue type and the initial signal strength received from the fetal cells. The strength of the signal generated by senescent fetal cells in turn may depend on the extent of senescence experienced by the fetal membrane cells.
The decidual clock: inflammation and senescence
The decidua has its own developmental time-course
Immediately subjacent to the fetal membranes lie the decidua. Its tissue is originally derived from the transformed maternal endometrium, but takes on unique biochemical functions upon fetal trophoblast invasion, and when leukocytes are recruited from the maternal circulation, the result is the creation of this complex organ.
The life of human decidua begins with perivascular pre-decidualization during the mid-luteal phase immediately prior to embryonic implantation and under circadian control (Muter et al., 2015) of established clock genes. The decidual stromal cell population includes subsets with characteristics of both fibroblasts and mesenchymal stems cells (Vacca et al., 2015). Endometrial stromal fibroblasts undergo dramatic differentiation to become the polygonal cells that comprise the majority of the early decidua. The stromal differentiation process includes change in cellular shape, expression of tight and gap junctions, and a redistribution of intermediate filaments (Oliveira et al., 2000). In the rodent, decidual cells become multinucleated. Several mechanisms are thought to be involved in this process, including neddylation of proteins (Liao et al., 2015). Compounds that have been known to induce decidualization in vitro include progesterone, cAMP and arachidonic acid (Tessier-Prigent et al., 1999). Decidual stromal cells express high levels of vimentin (Glasser and Julian, 1986) compared with the surrounding stroma, and in addition differentially express desmin (Glasser and Julian, 1986; Oliveira et al., 2000) prolactin (Jabbour and Critchley, 2001) and insulin-like growth factor binding protein-1 (Martina et al., 1997). Dysregulation of these molecules may lead to pregnancy failure (Rahkonen et al., 2010).
The decidua basalis, which interacts directly with invading extravillous trophoblast, is thought to be terminally differentiated following successful implantation (at about day 8 of gestation in the mouse) (Oliveira et al., 2000). In humans, the decidua covering the implanted embryo, called the ‘decidua capsularis’, eventually degenerate at about 16 weeks of gestation. The exact mechanisms involved are still under investigation (Genbacev et al., 2015); however, it is interesting to speculate that over-zealous degeneration of the decidua capsularis and related amniochorion might underlie so-called late miscarriages, and thus potentially represent a mechanism that bridges the gap between preterm birth and miscarriage. Decidual capsularis degeneration is followed by a fusion of the parietal decidua, which until this point had functioned as the lining in the rest of the uterine cavity. The decidua parietalis matures when chorionic cells interact with it and invade (Sindram-Trujillo et al., 2004; Genbacev et al., 2015). This is a time when the decidua structurally supports the fetal membranes by serving as a glue between the membranes and the myometrium.
Decidual senescence
As discussed above for fetal membrane cells, decidual stromal cells undergo spontaneous senescence, when critical cellular functions are inhibited (Liao et al., 2015). In the mouse, an examination of decidual and placental gene expression suggests that the two tissues express different patterns over time (Knox and Baker, 2008). Moreover, further analysis of the data (GSE11220, NCBI) suggests that there are distinct signatures generated by early-to-mid versus late gestation decidua. Late in gestation, newly or increased decidual expression of cathepsin and protein kinase B (Akt) (Hirota et al., 2010) family genes was found to be involved in the senescence process and as well as genes expressed as part of the SASP (Coppe et al., 2010), as described above. Disruption of key cellularathways such as those involving p53 (in mice) has been shown to lead to senescence, and furthermore, to preterm birth (Hirota et al., 2010). Aged mice experience increased reproductive failure, and this is related to an altered decidual physiology, which can be ameliorated by inhibition of NADPH oxidase 1 and enhanced activity of superoxide dismutase, which supports the idea that decidual stromal cells are susceptible to senescence via oxidative and nitrosative stress (Silva et al., 2015).
Differentiated endometrial stromal cells are not the only inhabitants of the decidua, nor are they the only focus of change during pregnancy. The decidua mediate inflammatory signals that activate parturition primarily by controlling the type and function of its resident immune cells. It is thought that a population of mesenchymal stem cells within the decidua exerts immune-modulating effects on extant inflammatory cells through expression of molecules such as indoleamine 2,3-dioxygenase, and through the support of differentiation and trafficking of M2 macrophages, tolerogenic dendritic cells, regulatory T cells, angiogenic neutrophils (Amsalem et al., 2014) and natural killer cells (Ratsep et al., 2015), with the help of molecules such as L-kynurenin, IL-33, IL-15, TGF-β (Vacca et al., 2015) and IL-10 (Liu et al., 2007; Prins et al., 2015). Early in gestation, decidual cells secrete factors such as growth-related oncogene alpha (GRO-α) (Engert et al., 2007), macrophage chemotactic protein-1 (Engert et al., 2007; Matta et al., 2007), C-X-C motif chemokine 5 (CXCL5 or ENA-78), IL-1, IL-8, Chemokine (C-C motif) Ligand 5 (CCL5) or RANTES, and Chemokine (C-C motif) Ligand 1 (CCL1) (Engert et al., 2007) among others that control the trafficking of peripheral immune cells into the decidua. In addition, it is thought that the decidua also control trafficking of incoming activated T cells (Nancy et al., 2012) and activated dendritic cells that drain the lymph nodes (Collins et al., 2009). Infection, however, can overcome these mechanisms [e.g. in the mouse (Constantin et al., 2007; Chaturvedi et al., 2015)], and other environmental stimuli can cause developmental dysregulation (Dixon et al., 2006; Brown et al., 2013), prematurely activating these pathways. At term, decidual leukocytes show an increasingly inflammatory phenotype (Galazka et al., 2009), including increased expression of TNF-·and IL-6 and reduced expression of immunoregulatory cytokines such as IL-4 and the IL-1 receptor antagonist (Castillo-Castrejon et al., 2014).
The decidua responds to signals from fetal membranes
Close approximation between decidua and the amniochorion has led to the hypothesis that, as the fetus grows larger, stretch might generate activation and senescence of decidual cells. Indeed it has been reported that stretch increases decidual cell expression of MMP1, IL-8 and GRO-α (Zhao et al., 2013), which are thought to play a part in the phenotype of senescent cells (Coppe et al., 2010). Infection can also generate the expression of senescence phenotype molecules such as vascular endothelial growth factor (VEGF) (Snegovskikh et al., 2009). Decidual cells themselves are highly responsive to inflammatory signals (Deb et al., 1999; Gomez-Chavez et al., 2015) such as the senescence/inflammatory cytokine IL-6 (Devi et al., 2015), and show an inherent up-regulation of molecules such as p38MAPK (Takanami-Ohnishi et al., 2001), which are part of the inflammatory cascade in several tissues (Emami et al., 2015; Li et al., 2015; Liu et al., 2015). These in turn may lead to an amplification of the inflammatory cascade and elaborate molecules that weaken fetal membranes (Kumar et al., 2014). Recently, it was reported that inflammatory signals generate senescence in human decidual cells, as in, for example, fetal membranes where IL-1· can induce expression of SA-β-gal in cultured decidual cells and further increase expression of p21 (Okabe et al., 2014).
Moreover, late gestation decidua express increased levels of FMS-like tyrosine kinase 1 (Knox and Baker, 2008), a receptor for the senescence proteins VEGF and placental growth factor (Coppe et al., 2010). This suggests that late gestation decidual cells in the mouse might be increased in their ability to respond to senescent signals generated by other tissues, such as fetal membranes. Leukocytes within the decidua are also likely to be able to respond to senescent signals over time and transmit them to other cells. For example, it has been reported that a population of natural killer cells in the decidua can express c-kit, the receptor for stem cell factor, another member of the SASP (Coppe et al., 2010). These interactions thus constitute a functional decidual clock for parturition.
Decidual transmission and generation of signals supportive of membrane rupture and uterine contractions
Within the decidua, there exist possible overlap and synergy between inflammation and senescence and between signals coming from and going to the membranes in the process of parturition. For example, inflammation can increase the expression of VEGF (Snegovskikh et al., 2009), which may be a driver of senescence. Because early human (6–9 weeks) decidual cells express receptor C-X-C chemokine receptor type 4 for the senescent protein SDF-1 (CXCL12) (Zhou et al., 2008), and binding of SDF-1 to these cells up-regulates MMP2 and MMP9 in these cells, it can be hypothesized that senescent signals received by decidual cells may induce the mechanism(s) leading to membrane rupture later in gestation.
The above-mentioned study of gene expression in mouse decidua suggests that near-term decidua show increased expression of genes thought to be part of SASP in other tissues, including fibronectin, osteoprotegerin, CCL2 and Colony Stimulating Factor 1. These molecules may then spatially signal ‘back’ to the fetal membranes and/or ‘forward’ to the uterus. There is strong evidence that decidual cell senescence leads to increased PG production (Hirota et al., 2010). For example, activated leukocytes within the decidua, including macrophages (Mackler et al., 2003), may produce PGs, which in turn promote uterine contractions. Moreover, dysregulation of this tissue through inflammation or senescence leads to lower expression of the progesterone receptor (PR) (Guzeloglu-Kayisli et al., 2015), which could contribute to a local but highly functional progesterone withdrawal. Progesterone is linked to unfolded protein response (UPR) in decidual cells (Yang et al., 2013), and one could speculate that functional withdrawal might lead to cellular stress in this tissue. In addition, it has been noted that decidual cells produce metalloproteinases in response to PGs (Ulug et al., 2001), and this may in turn enhance the breakdown of attached membranes.
It is unclear whether a genetic predisposition to decidual senescence exists, and further, it is unclear whether alteration in the regulation of decidual senescence leads to preterm birth or whether abnormal pregnancy outcome is an event that is remembered with the next pregnancy and contributes to recurrent preterm birth. Of note, chorio–decidual inflammation in one pregnancy has been linked with preterm birth in subsequent pregnancies (Hackney et al., 2014).
Summary
The decidua comprise multicellular tissue that is spatially and physiologically positioned to contribute to the termination of pregnancy. It receives senescence and inflammatory signals at a time appointed by the fetal membranes, augments these signals with molecules produced during its own developmental program, and transmits these signals to both fetal membranes and the uterus during the process of pregnancy termination.
Myometrial clock: progesterone and inflammation
Role of progesterone and PRs
Progesterone, as its name implies, is a ‘pro-gestation’ hormone that is essential for the establishment and maintenance of pregnancy (Corner and Allen, 2005). Progesterone maintains pregnancy by promoting myometrial relaxation, cervical closure, and decidual quiescence (Csapo, 1956; Siiteri et al., 1977). In all viviparous species studied thus far, withdrawal of progesterone induces parturition and is presumed to be the convergent point for the parturition-triggering mechanism and pregnancy zeitgeber (Young et al., 2010). Thus, progesterone withdrawal could be viewed as a zeitgeber responder, analogous to the alarm mechanism in an alarm clock or the feedback switch in a thermostat. Once progesterone withdrawal occurs, the tissue of the gravid uterus exhibits sterile, localized inflammation (Osman et al., 2003, 2006) and transforms from a quiescent to a laboring phenotype (i.e. myometrial contractions, cervical softening, and membrane weakening) to facilitate emptying. In the context of a pregnancy clock mechanism, key questions regarding the role of progesterone in the physiology of human parturition emerge: (1) What is the mechanism for progesterone withdrawal in human pregnancy and (2) what are the up-stream signals that induce progesterone withdrawal and how do they relate to a pregnancy clock mechanism?
Progesterone is produced by the placenta during pregnancy and secreted into the maternal circulation in large amounts. In contrast to most species (e.g. sheep, goat, rat, and mouse) in which parturition is triggered by a drop in maternal progesterone levels, progesterone production in women remains high throughout pregnancy and during parturition, decreasing only after the placental expulsion (Walsh et al., 1984). Thus, human parturition occurs without systemic progesterone withdrawal, arguing against progesterone withdrawal being a requirement to trigger parturition. However, treatment of women with PR antagonists/modulators, such as mifepristone and onapritsone, augments myometrial contractility and induces labor at all stages of pregnancy (Chwalisz and Garfield, 1994; Neilson, 2000; Hapangama and Neilson, 2009). This suggests that appropriate PR signaling is essential for pregnancy maintenance and that disruption of PR signaling alone is sufficient to trigger the full parturition cascade. It is therefore hypothesized that human parturition is triggered by functional rather than systemic progesterone withdrawal that involves a physiological control of PR signaling to desensitize target cells in the uterus to pro-gestational actions of progesterone (Avrech et al., 1991; Henshaw and Templeton, 1991; Ikuta et al., 1991; Carbonne et al., 1995; Cadepond et al., 1997). According to this model, up-stream gestation clocks/monitoring signals could induce labor by modulating PR signaling to cause functional progesterone withdrawal.
The human PRs exist as two major isoforms, PR-A and PR-B (Wei and Horwitz, 1985; Horwitz et al., 1986). The proteins in PR-A and PR-B are identical except that PR-B has an additional 165 N-terminal amino acids. As with other steroid hormone receptors, PR-A and PR-B primarily function as ligand-activated transcription factors. Progesterone responsiveness represents the net effect of PR-A and PR-B, each mediating distinct effects on specific gene targets (Mulac-Jericevic et al., 2000, 2003; Richer et al., 2002; McEwan, 2009). This genomic mode of PR-mediated progesterone action suggests that progesterone maintains pregnancy by promoting expression of genes encoding pro-gestational factors and/or by inhibiting expression of pro-labor genes. Because the promoter regions of most parturition-related genes lack classical progesterone response elements, it is thought that PRs function by interacting with other transcription factors or by modulating the abundance of specific microRNAs (miRNAs) that target pro-labor mRNAs and inhibit translation (Faivre et al., 2008; Renthal et al., 2010; Williams et al., 2012a,b).
Modulation of PR transcriptional activity in the uterine cell may be a mechanism for functional progesterone withdrawal. In some cells, including human myometrial cells, PR-A represses the transcriptional activity of PR-B and decreases net progesterone responsiveness (Tung et al., 1993; Vegeto et al., 1993; Pieber et al., 2001; Condon et al., 2006; Hardy et al., 2006; Merlino et al., 2007). These findings, coupled with the observation that PR-A levels in term myometrium increase in association with the onset of labor in women (Mesiano et al., 2002; Condon et al., 2006), led to the PR-A:PR-B hypothesis for functional progesterone withdrawal which posits that progesterone withdrawal is mediated by the increased abundance and transrepressive activity of PR-A which represses the transcriptional activity of PR-B. In this context PR-A abundance and transrepressive activity could serve as a master integrator of the gestation clock by mediating functional progesterone withdrawal. Other mechanisms for functional progesterone withdrawal also may exist, including increased expression of other transrepressive PR isoforms (Condon et al., 2006), altered expression of PR co-activators (Condon et al., 2003), metabolization of progesterone into less active forms within target cells (Andersson et al., 2008; Williams et al., 2012a), and neutralization of PR interaction with other transcription factors, especially NF-κB (Kalkhoven et al., 1996).
Progesterone withdrawal and inflammation
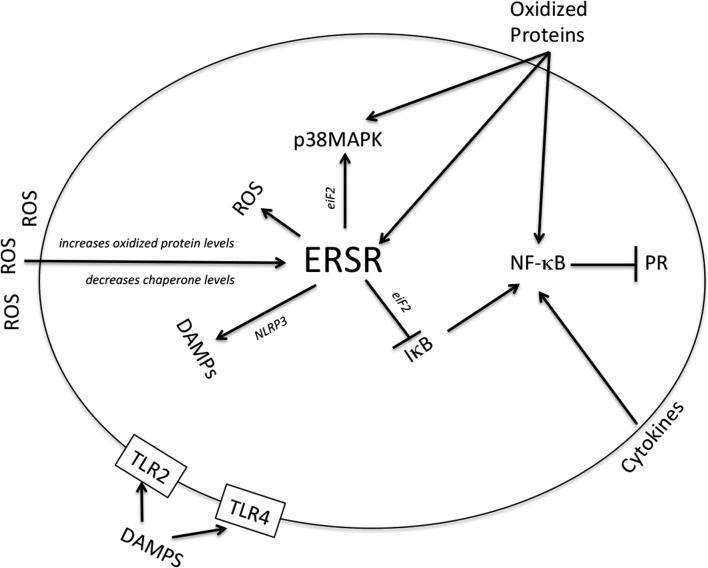
It is now generally accepted that parturition is an inflammatory process (Kelly, 1996; Romero et al., 2006, 2007, 2014a; Norman et al., 2007). Immediately prior to the onset of clinical labor, the myometrium, cervix, and decidua exhibit manifestations of inflammation in the form of edema, neutrophil infiltration, and expression of chemical mediators of inflammation, especially pro-inflammatory cytokines, chemokines, and PGs (Thomson et al., 1999; Osman et al., 2003, 2006). Other pro-inflammatory stimuli, such as mechanical distention of the myometrium and fetal membranes while the conceptus grows that induce inflammatory cytokine expression and tissue-level inflammation (Kendal-Wright, 2007; Shynlova et al., 2007, 2010; Hua et al., 2012) and fetal membrane senescence (see above), are associated with normal and preterm birth, especially in cases complicated by preterm premature rupture of the membranes (pPROM). We propose that the gravid uterus is sensitive to multiple inflammatory factors derived from developmental programs (e.g. fetal membrane senescence) and/or environmental influences (e.g. social stress or infection) that together constitute a net ‘inflammatory load,’ and that the parturition trigger is activated when the inflammatory load threshold is surpassed (Fig. 1). According to this paradigm, the rate of change in the inflammatory load and its trajectory toward reaching the parturition trigger threshold satisfy the properties for a zeitgeber.
Implicit in the inflammatory load zeitgeber hypothesis is the concept that uterine quiescence is maintained when inflammation is repressed. This concept is not novel. In the 1970s, Siiteri et al. proposed that progesterone promotes uterine quiescence by acting as an immunosuppressant agent in the gravid uterus (Siiteri et al., 1977). More recent data support this hypothesis by showing that progesterone, via its interaction with PR-B, inhibits the response of human myometrial cells to inflammatory stimuli (Hardy et al., 2006; Tan et al., 2012). Taken together, the data suggest that withdrawal of progesterone's PR-B–mediated anti-inflammatory activity in myometrial cells causes tissue-level inflammation, which prompts the uterus to move into a state of labor. Importantly, withdrawal of PR-B–mediated progesterone actions could be caused by the increased transrepressive activity of PR-A (Tan et al., 2012). According to this paradigm, functional progesterone withdrawal precedes inflammation. However, the fact that inflammation induces parturition suggests that inflammation may precede or supersede progesterone withdrawal. On the other hand, data showing that inflammatory cytokines and senescence factors such as HMGB1 increase PR-A abundance in human myometrial cells (see above) suggest that inflammation induces PR-A–mediated functional progesterone withdrawal and that this initiates a positive feedback tissue-level inflammatory state, leading to increased local cytokine production (especially PGs) that transform the uterine tissues into the laboring phenotype.
Positive feedback loops, although potentially disruptive, serve useful functions in biological systems by precipitating specific events. Cyclical biological systems typically rely on negative feedback servomechanisms. Mammalian reproduction in the female, with its characteristic temporal milestones like puberty, ovulation, and pregnancy, utilizes positive feedback loops. An example is the neuroendocrine control of ovulation, which is induced by a surge in gonadotropin secretion from the pituitary. The gonadotropin surge occurs because estradiol produced by developing ovarian follicles in response to gonadotropins switches from exerting negative feedback on to positive feedback stimulation of gonadotropin secretion. The estrogen–gonadotropin positive feedback loop is subsequently broken by ovulation (i.e. the terminal event precipitated by the positive feedback loop), which disrupts estradiol synthesis, thus breaking the positive feedback loop and restoring ovarian homeostasis and cyclicality. We propose that similar positive feedback hormonal loops likely operate to control parturition. For most of human pregnancy, progesterone acting via PR-B promotes uterine quiescence by repressing the responsiveness of gestational tissues to pro-inflammatory/pro-labor stimuli. However, the PR-B–mediated anti-inflammatory block to parturition is not absolute, and as pregnancy progresses, inflammatory load on the gestational tissues gradually increases until an inflammatory load threshold is reached, at which time the transrepressive activity of PR-A is activated. The counter-regulatory effect of PR-A abolishes the PR-B–mediated anti-inflammatory effects, leading to a net pro-inflammatory response and local production of cytokines and chemokines that further amplify the inflammatory state by promoting the infiltration of activated neutrophils and macrophages. The PGs produced by the inflamed tissues are potent stimulators of myometrial contraction and lead to cervical dilation and membrane weakening. PR-A–induced functional progesterone withdrawal may also potentiate inflammation in response to danger signals (e.g. intrauterine infection, fetal stress or maturation, and uterine wall over-distention) that induce labor. This reasoning would imply that a gestation clock impacts paracrine hormonal loops within the uterine tissues to either maintain a non-inflamed state or precipitate a positive feedback pro-inflammatory loop to induce parturition. Also implied is that induction of PR-A–mediated functional progesterone withdrawal by inflammation is a major integrator of signals derived from the gestation clock via the inflammatory load trajectory. In this context the pregnancy zeitgeber is analogous to a thermostat with inflammatory load represented by room temperature and activation of PR-A transrepression as the temporal switch mechanism.
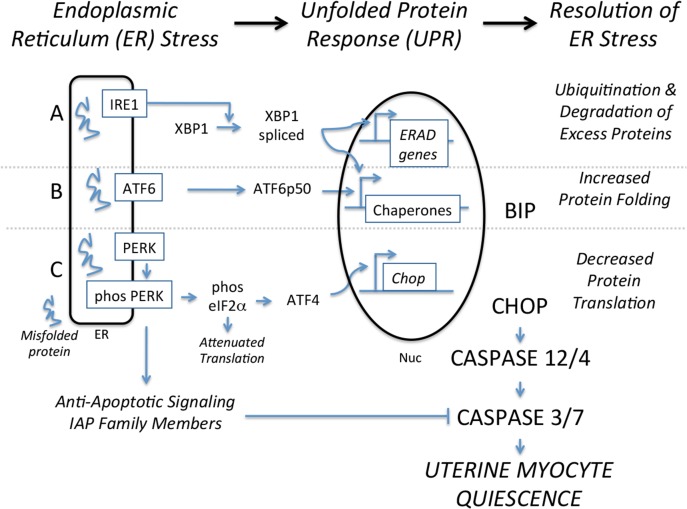
Responses to decidual and fetal membrane senescence
Multiple factors may contribute to the inflammatory load impacting the gravid uterus. One major factor described above may be senescence within the gestational tissues. We have found that stress-associated p38MAPK directly influences the PR-A:PR-B ratio by increasing the abundance of PR-A in myometrial cells likely via post-translational modifications (PTMs). It is now clear that PTM is a mechanism for fine tuning PR function to cell-type physiology by qualitatively and quantitatively affecting PR transcriptional activity and stability (Abdel-Hafiz and Horwitz, 2014). Phosphorylation of PR-B on serine-294 increases its transcriptional activity and promotes its ubiquitination and proteosomal degradation (Lange et al., 2000), which decrease PR-B abundance. In contrast, phosphorylation of PR-A on serine-294 results in its sumoylation, which increases its abundance by decreasing its degradation (Daniel et al., 2007). Importantly, sumoylation of PR-A is necessary for the transrepression of PR-B (Abdel-Hafiz et al., 2002). PR-A turnover is also decreased (leading to increased PR-A abundance) by MAPK signaling, especially mitogen-activated protein kinase kinase 1-induced p38MAPK activation (Khan et al., 2011). This effect may explain our finding that HMGB1, via p38MAPK activation, increases PR-A abundance in human myometrial cells and suggests that HMGB1 (and possibly other DAMPs) produced by the fetal membranes in response to senescence contribute to the inflammatory load and PR-A–mediated functional progesterone withdrawal thanks to increased PR-A abundance (and possibly increased transrepressive activity) as a result of p38MAPK-induced PTMs.
Summary
The contractile state of the gravid uterus is ultimately controlled by hormonal signaling networks that impact myometrial, cervical, and decidual cells. A key hormone in this process is progesterone, which for most of the duration of the pregnancy promotes uterine relaxation and quiescence. In all species examined to date, withdrawal of progesterone, whether systemic or functional, triggers the parturition process. In this context, the pregnancy zeitgeber likely impacts the progesterone withdrawal mechanism. Clinical and laboratory evidence suggests that inflammation within the uterine compartments also plays a central role in the parturition-triggering mechanism. We propose that net inflammatory load (i.e. the aggregate effect of multiple sources of pro-inflammatory stimuli) on the gravid myometrium gradually increases with advancing gestation until it reaches a threshold where it triggers progesterone withdrawal to induce parturition. In this context, the pregnancy clock, at least in part, involves the rate of change of the inflammatory load on the gravid uterus.
Myometrial stretch inputs and activation of parturition
Changes in the fetal membranes and myometrium in response to uterine wall distention caused by the growing conceptus also figure into the parturition-triggering signal scenario. During the third trimester, the uterine wall comprises the perimetrium (the outer-most layer), myometrium, decidua, and fetal membranes consisting of distinct layers of cells, and the ECM is subjected to increased distention as the rate of fetal/conceptus volume increases. At about the 20th week of gestation, the amnion adheres to the chorion and these two tissues fuse with the decidua to become an integral component of the uterus. The tensile strength and distensibility (i.e. elasticity) of this conglomerate is mainly attributed to the myometrium and amnion and associated ECM structures in these tissues (Strohl et al., 2010). Importantly, after the 28th week of gestation, the rate of fetal membrane growth slows as a result of the decreased proliferation of amnion epithelial cells (Schmidt, 1992) and decreased myometrial growth because of the transition of myometrial cell growth dynamics from hyperplasia to hypertrophy (Shynlova et al., 2006). Thus, with advancing gestation, a mismatch occurs between the rate of uterine wall growth and the growth of the conceptus, which translates into increased uterine wall distention. It is estimated that the amnion is distended up to 70% late in the third trimester (Millar et al., 2000). Through the process of mechanotransduction, physical forces generated by distention of amnion, chorion, decidua, and myometrial cells are transduced to changes in intracellular signals, gene transcription, ECM production and degradation, and cell adhesion (Ruoslahti, 1997; Langevin et al., 2005). Importantly, in-vitro studies show that a mechanotransduction response to distention of amnion and myometrial cells increases the production of inflammatory cytokines and ECM remodeling (Kendal-Wright, 2007; Hua et al., 2012; Adams Waldorf et al., 2015). In vivo, these changes would increase myometrial contractility, weakening the amniotic membrane. Although the relative contribution of this distention-induced signaling from the fetal membranes, decidua, and myometrium is not fully defined, it is clear that each tissue, and especially the amnion and myometrium, respond to physical distortion by altering their ECM structure and producing pro-inflammatory cytokines that could contribute to the triggering of parturition. With respect to the pregnancy clock, uterine wall distention is likely part of a feedback mechanism to ensure that the uterus is emptied before the conceptus exceeds the pelvic outlet capacity. A likely scenario is that a threshold of uterine wall distention exists, above which the induction of pro-labor and pro-inflammatory gene expression contributes to the activation of the parturition cascade.
Fetal membrane senescence as a potential regulator of myometrial contractility through modification of the uterine myocyte ERSR-UPR
The following discussion examines the potential links between the uterine myocyte ER stress response-unfolded protein response (ERSR-UPR) and accumulation or release of fetal membrane-related SASPs (such as reactive oxygen species, ROS) and the resulting advanced oxidation protein products (AOPPs), cytokines and other DAMPs associated with both premature and term rupture of fetal membranes. We postulate that these molecules function to alter the local uterine contractile response through modification of the ERSR-UPR. Our recent findings demonstrate that ERSR-UPR in the pregnant uterine myocyte has the capacity to generate a gestationally regulated tocolytic non-apoptotic caspase (CASP) 3- and 7-signaling cascade that accommodates uterine quiescence throughout gestation during transitional uterotonic episodes. Activation of CASP3/7 in the pregnant uterus occurs in the absence of cell death (Kyathanahalli et al., 2015). We propose that senescence of the fetal membranes acts as a biological clock that regulates the tocolytic action of non-apoptotic uterine myocyte CASP3/7, the uterine contractile potential and ultimately gestational length, through modification of the uterine myocyte ERSR-UPR.
Role of the ER
The ER is an organelle that serves an essential role in multiple cellular processes that are required for normal cell function and survival (Ma and Hendershot, 2004; Pizzo and Pozzan, 2007; Anelli and Sitia, 2008). The ER allows for the increased processing of newly made proteins needed for cellular adaptation to biochemical, hormonal and mechanical stimuli. The ER correctly folds and assembles newly made proteins prior to their transit to intracellular organelles and the cell surface. However, ER function requires a stable luminal environment. Accordingly, luminal perturbation during prolonged or excessive periods of cellular adaption can lead to an accumulation of misfolded and unfolded proteins. This is termed ‘ER stress’. During periods of ER stress, the UPR is activated. UPR alters transcription and translational programs to manage and adapt to the accumulation of misfolded or unfolded proteins in the ER lumen (as discussed in detail below). However, when this balance can no longer be maintained as a result of excessive and/or prolonged ER stress, CASP3/7-mediated cell death programs are activated to remove cells that have lost their ability to correctly fold proteins.
The unfolded protein response
The UPR encompasses a sophisticated integration of controls regulating gene expression at multiple levels, which can be described in three potential activation arms, as shown in Fig. 4 (Ron and Walter, 2007; Scheuner and Kaufman, 2008). Briefly, excess misfolded and unfolded proteins are (i) targeted for ubiquitin-mediated proteolytic degradation thereby resolving ER stress by decreasing the resident luminal protein load. In addition, (ii) elevated levels of chaperone proteins such as binding immunoglobulin protein allow for increased protein folding and export, which also permits the ER to regain homeostasis. Furthermore, (iii) attenuated protein translation decreases the protein levels in the ER lumen, thereby acting as another mechanism utilized by the ER to regain homeostasis during periods of ER stress. Although UPR manages the cellular adaptation to acute ER stress, chronic ER stress results in the activation of apoptosis, as discussed earlier, thereby preventing the propagation of damaged cells (Nakagawa et al., 2000; Morishima et al., 2002; Rao et al., 2002; Katayama et al., 2004; Hitomi et al., 2004a,b).
Figure 4.
The ERSR-UPR signaling cascade. IRE1, serine/threonine-protein kinase/endoribonuclease inositol-requiring enzyme 1; ATF6, activating transcription factor 6; PERK, protein kinase RNA-like endoplasmic reticulum kinase; ERSR, endoplasmic reticulum stress response; eIF2α, eukaryotic initiation factor 2 alpha; XBP1, X-box binding protein 1; BiP, binding immunoglobulin protein; CHOP, CCAAT-enhancer-binding protein homologous protein. This figure is adapted from in vivo cellular adaptation to ER stress: survival strategies with double-edged consequences (Tsang et al., 2010).
In this discussion, we examine evidence that senescence of the fetal membranes has the capacity to act as the biological clock driving the timing of labor by modulating gestational length through sensing ERSR-UPR.
OS regulates the ERSR-UPR
As outlined in the section on fetal membrane aging as a biological clock, increasing levels of ROS that have accumulated in the intrauterine cavity have the capacity to damage and accelerate fetal membrane senescence by damaging proteins, lipids and DNA in the cell, which in turn result in telomere reduction and accelerated cell cycle arrest, ultimately leading to the senescence of the fetal membranes associated with pPROM and at term.
In this section, we examine the capacity of OS to modulate ERSR-UPR and how ERSR-UPR may in turn regulate OS parameters (Fig. 5).
Figure 5.
Modulation of the uterine ERSR-UPR by senescence-associated secreted factors. ROS, reactive oxygen species; PR, progesterone receptor; DAMPs, damage associated molecular patterns.
The protein-folding process requires an oxidized environment, which is provided within the ER to promote appropriate disulfide bond formation (Chakravarthi et al., 2006). However, elevated ER stress can give rise to increased intracellular ROS production as a by-product of accelerated oxidative protein folding (Cullinan et al., 2003; Brookes and Darley-Usmar, 2004; Tu and Weissman, 2004). More importantly in this context, it has also been demonstrated that ROS and OS are up-stream regulators of ERSR-UPR (Yokouchi et al., 2008). Two mechanisms have been proposed to explain why ROS might cause ER stress: (i) the generation and accumulation of oxidatively modified abnormal proteins that become trapped in the ER lumen and (ii) ROS-induced functional perturbations of ER chaperones that inhibit appropriate protein folding (Brunet et al., 2000).
It has been demonstrated that accumulation of AOPPs in the serum as a result of OS act as reliable markers and potential mediators of ROS-induced protein damage (Kacerovsky et al., 2014; Menon, 2014; Menon et al., 2014a). Recently, ROS in circulation were shown to induce ER stress, which could be reversed by the addition of a ROS scavenger (Tang et al., 2015). These data indicate that exposure to oxidation products as a result of excessive OS is up-stream of, and regulates the induction of, an ER stress-induced signaling cascade. Furthermore, it has been demonstrated that lipid peroxidation products, produced in response to oxidation of phosphatidylcholine, accumulate in the ER, activate ERSR by directly modifying protein chaperones within the ER and inhibit protein-folding capacity (Takahashi et al., 1998; Carbone et al., 2005; Haberzettl and Hill, 2013; Levonen et al., 2014). Therefore, based on the known interactions between ROS, OS and ERSR-UPR, it is very likely that accumulation of systemic ROS-mediated oxidation protein products during fetal membrane senescence, such as elevated 8-oxoG in pPROM (Menon et al., 2014b), have the capacity to modulate local uterine myocyte ERSR-UPR-CASP, resulting in a modified uterine contractile responsiveness and altered gestational length.
NF-κB can be activated by ERSR-UPR
Many investigators have demonstrated that NF-κB coordinated transcription of immune response genes is tightly related with the onset of term and preterm birth through decreased local uterine myocyte PR transactivation (Condon et al., 2006; Lee et al., 2012). However, it has also been shown that stimulation of ERSR-UPR is critical to the activation of NF-κB (Jiang et al., 2003; Jiang and Wek, 2005) (Fig. 5). Phosphorylation of eukaryotic initiating factor 2 (eiF2), a major component of UPR, reduces global translation, allowing cells to conserve their resources to manage stress conditions and alleviate cellular injury by returning the ER to homeostasis. eiF2 phosphorylation can also lead to the activation of NF-κB by reducing levels of the endogenous NF-κB inhibitor I·B (Jiang et al., 2003; Jiang and Wek, 2005). It has been demonstrated that I·B is very sensitive to eiF2 phosphorylation, therefore reduced synthesis of I·B triggers translocation of active NF-κB to the nuclear fraction (Jiang et al., 2003; Jiang and Wek, 2005). These data suggest that changes in local uterine ERSR-UPR-CASP brought about by exposure to SASPs as a result of increased fetal membrane senescence may also have the capacity to regulate uterine contractility through myometrial NF-κB activation and impaired PR transactivation.
ERSR-UPR regulates p38MAPK action
eiF2 kinases can also function in conjunction with other kinases such as p38MAPK (Kato et al., 2003) to tailor gene expression programs under specific stress conditions (Fig. 5). C-Jun amino terminal kinase (c-JUN) and p38MAPK are described as stress-activated kinases and are both also triggered by ER stress and UPR signaling (Urano et al., 2000; Matsuzawa et al., 2002). For example, ER stress-activated p38MAPK greatly increases the emigration efficiency of transcription factor sXBP1 to the nucleus (Lee et al., 2011). sXBP1 is the master regulator of ER-mediated protein-folding capacity, increasing the expression of essential ER chaperone proteins (Lee et al., 2003). It has also been demonstrated that p38MAPK allows for PR isoform switching at term in the pregnant uterine myocyte. Therefore, again, at the level of the pregnant uterine myocyte, aberrant activation of the ERSR-UPR-CASP cascade by precociously increased SASPs associated with fetal membrane senescence, such as ROS and oxidized lipids and proteins, can shift the pregnant uterus to a more contractile phenotype through increased p38MAPK action.
Inflammatory mediators as regulators of the ERSR-UPR
Inflammation is frequently triggered by ERSR-UPR as a result of the accumulation of misfolded proteins. The nucleotide-binding oligomerization domain-like receptor (NLR) family, which mediates CASP1-dependent maturation of the highly pro-inflammatory cytokine IL-1, specifically the NLRP3 inflammasome, acts as an ER stress sensor (Menu et al., 2012). In resting cells, NLRP3 is associated with the ER membranes; when activated by ER stress, NLRP3 translocates to the mitochondria, leading to CASP2 and CASP1 activation, mitochondrial damage, DAMP production and IL-1· secretion (Bronner et al., 2015) (Fig. 5). However, although DAMP production has been found to be ER stress-dependent, and circulating DAMPs of fetal membrane origin can also affect local myometrial ERSR and uterine contractile responsiveness by binding to their receptors. For example, extracellular HMGB1 binds to the receptor for advanced glycation end products (RAGE) (Hori et al., 1995) and TLR2 and 4 (Park et al., 2006), stimulating NF-κB-induced transcription. Similarly, S100 proteins also bind and activate the RAGE and TLRs, producing pro-inflammatory effects in a manner similar to HMGB1 (Hofmann et al., 1999; Ryckman et al., 2003; Ehrchen et al., 2009). In related studies, activation of TLRs has also been found to up-regulate the protective adaptive arms of UPR (Woo et al., 2012). Indeed analysis of TLR2 and TLR4 knockout mice were each found to promote differential induction of ERSR and CASP3 activation (Messlik et al., 2009). These data suggest that senescence-associated factors such as DAMPs, which increase with gestational age, have the capacity to modulate local uterine inflammatory responses in an ERSR-UPR–dependent manner, acting as a fetal membrane-derived biological clock regulating the timing of labor.
Summary
In summary, multiple points of potential interaction exist among factors that are systemically secreted into the maternal circulation as a result of senescence of fetal membranes and ERSR-UPR (Fig. 5). Although these circulating factors are not classical endocrine mediators, their exchange could indeed alter uterine ERSR-UPR-mediated maintenance of normal gestational length. We speculate that fetal membrane SASPs, which are released as term approaches, act as a biological clock and drive the onset of myometrial contractions upon membrane rupture in an ERSR-UPR-dependent manner. In cases of pPROM, we speculate that modulation of uterine ERSR-UPR to promote precocious preterm uterine contractions may serve a protective role, promoting the successful delivery of a premature fetus to avoid potential fatal consequences to mother and fetus, associated with the retention of a prematurely ruptured fetal unit.
Cervix—a responder of feto–maternal clocks and alarms?
The clocks and alarms set and activated by circulating pregnancy hormones, senescing fetal membranes, decidua and myometrium propagate signals that initiate the process of parturition. The final process is ultimately effected by cervical effacement and dilatation, allowing a progressive and controlled delivery of the fetus (Stjernholm et al., 1996; Ekman-Ordeberg et al., 2003; Word et al., 2007; Myers et al., 2009). Cervical ripening represents a localized inflammatory process and its cellular and molecular mechanisms have been elucidated by several laboratories. The cervical microbiome and metabolome are increasingly recognized as contributors to the overall integrity of cervix during pregnancy and changes within the microbial environment are also associated with parturition (Danforth, 1983; Uldbjerg and Ulmsten, 1990; Norman et al., 1993; Hassan et al., 2010; Mahendroo, 2012; Prince et al., 2014; Ghartey et al., 2015; Sanders et al., 2015). Premature cervical ripening can accompany preterm parturition; however, the trigger for this process is still not clear (Nold et al., 2012; Parry and Elovitz, 2014). Precocious activation of cervical senescence may be a response to maternal risks for preterm delivery, or derive from an independent biological cervical alarm. The discussion of pregnancy clocks and alarms would not be complete without discussion of the cervix as it reflects an integral compartment of human parturition. The following section highlights the importance of cervical tissue biology. It is imperative that we better understand its mechanisms of activation, as interventions to prevent preterm birth using progesterone have had modest effects in improving neonatal health.
Although there is evidence suggesting that hormonal factors play a complex and possibly indirect role in cervical softening and ripening (Yellon et al., 2009; Akgul et al., 2012), controversy remains around the mechanism (Elovitz and Mrinalini, 2005) and efficacy of hormonal therapy to prevent or treat cervical insufficiency (Meis et al., 2003; Schuit et al., 2015; Norman et al., 2016). Differences among patient populations have been offered as an explanation, but the disparate findings imply that other factors may be important in this process (Norman et al., 2016). In keeping with the theoretical model of this review, it is important to note that the cervix is an organ that also undergoes its own developmental program, with remodeling occurring throughout pregnancy and accelerating towards the end of gestation (Dubicke et al., 2015). For example, collagen becomes progressively less densely structured due to reduced collagen gene expression, decreased crosslinking and increased collagen degradation. The glycosaminoglycan composition of the cervix also changes through pregnancy, with increased hyaluronan accumulation accompanied by a reduction in its average molecular weight at term (Akgul et al., 2012). In addition, the term cervix has an increased water content that supports softening. These events occur in parallel with functional changes in the smooth muscle, epithelial and fibroblast components (Larsen and Hwang, 2011). Data from women and animal models suggest there is a progressive increase in the expression of inflammatory cytokines, and the presence of macrophages (Mackler et al., 1999; Osman et al., 2006), that also contribute to ripening or decreased load-bearing capacity as pregnancy progresses. Recent gene array expression studies in rats suggest that cervical macrophages show up-and down-regulated genes that may relate to SASP responsiveness at term (Dobyns et al., 2015). The SASP factors, TNFα and PGE2, may also alter activity of hyaluronan by increasing its binding protein (Fujimoto et al., 2002). Moreover, it has been postulated that MMPs, themselves members of the SASP family of molecules, contribute to collagen disorganization that occurs near term (Larsen and Hwang, 2011). Finally, data from human tissues emphasize the increased inflammatory/SASP profile of fetal membranes overlying the cervix at term and the potential for regionalized interactions between the cervix and amniochorionic membrane-associated senescence signals (Lappas et al., 2008). Unpublished data from the Menon lab identified more senescence-associated factors (with >85% SA-β-gal positive cells and near total loss of lamin B1) in laboring fetal membranes at term overlying the cervix, compared to mid-uterine portions of the membranes, suggesting differential interactions between these compartments. It is likely that SASP factors can compromise cervical the homeostatic balance, co-ordinating the cervical program with the pace of the fetal membrane clock and tilting it to favor delivery. Thus, the cervix may represent a tissue with a unique capacity to both generate and receive senescent signals at parturition.
Conclusions
Pregnancy, an event that reveals and shapes the fundamental biology of mother and fetus, provides a critical window into future health. Advances in reproductive science (including cell and molecular biology), burgeoning ‘omics’ technologies, and increasing access to large data sets of well-phenotyped pregnancies are generating abundant information. Yet many critical questions remain about what risk factors lead to abnormal pregnancy. Many investigators believe that the understanding of preterm birth in humans is inextricably linked to the understanding of normal term birth. The current assessment of pregnancy complications falls short because of knowledge gaps in our understanding of normal parturition. One such example is preterm birth, the leading cause of neonatal death (Messlik et al., 2009). We propose that it is useful to pause and undertake a broad, system-wide analysis of existing data to formulate frameworks for interpretation and generation of stronger hypotheses. However, parturition is a complex and redundant process that involves multiple organ systems, and unique human labor characteristics are not experienced by other species, making the initiators and effectors of normal and pathological pregnancies challenging to elucidate. In addition, abnormal pregnancies arise abruptly rather than transition progressively from normal pregnancies. Our current understanding of biological mechanisms, pathways, and biomarkers of normal parturition have not led to effective interventions to reduce the risk of preterm births. This suggests either a lack of understanding of normal pregnancies or the independent activation of mechanisms of premature labor in abnormal pregnancies.
Integration of clocks and initiation of parturition
Current knowledge of fetal signaling associated with term parturition is restricted to fetal organ maturation signals and endocrine factors. Normal term parturition is driven by essential, redundant back-up processes, which must interact in a positive feedback loop, cascading and even accelerating toward delivery. Once started and the once the ‘tipping point’ is reached, the teleological objective is to expel the fetus from the uterus before placental function becomes irreversibly impaired and reduced oxygen flow causes fetal hypoxemia. Ultimately, the clock alarms with modulation of the degree of urgency. We propose a model that depicts the generation of fetally derived signals and their propagation (Fig. 6). OS at term and other factors from the fetus that are well documented can accelerate fetal membrane aging through p38MAPK-mediated senescence. Senescence and fetal membrane injury release sterile inflammatory agents that include, but are not limited to, SASP and DAMPs. These signals are diffused and propagate to other compartments, such as the decidua (causing decidual cell senescence and leukocyte and NK cell activation) and myometrium (causing p38MAPK-mediated functional progesterone withdrawal), transforming a quiescent myometrium into an active muscle, exhibiting labor-associated changes. We have reviewed the role of cellular organelles, such as the ER, which contribute to this overall process in response to OS or uterine stretch. In summary, we introduce a novel concept of fetal tissue aging, propagation of signals and involvement of multiple organ systems that can produce a sterile inflammatory overload, which leads to parturition.
Figure 6.
Propagation of fetal signals that will initiate parturition. In this review, we project fetal membrane senescence as one of the major contributors of initiation of human parturition. Natural and physiologic aging of fetal membranes mediated by telomere-dependent senescence and senescence-associated inflammation (contributed by senescence associated secretory phenotype [SASP] and damage associated molecular patterns [DAMP]) increases the overall inflammatory load in fetal membranes. These inflammatory signals get propagated to decidual layer, causing leukocyte and natural killer (NK) cell activation, causing inflammatory overload in the decidua. Fetal membrane and decidual inflammatory overload transition the quiescent myometrium to an active state of functional progesterone withdrawal.
Acknowledgements
The authors thank KIMEN Design4Research for creation of Figure 1 and 6.
Authors’ roles
The idea for this review was conceived and the manuscript was prepared through multiple discussions by all authors.
Funding
R.M. received funding from Innovation Catalyst grant from March of Dimes Prematurity Research Center, Ohio collaborative; National Institutes of Health (Grant # HD086354); and Faculty Development Fund, The University of Texas Medical Branch, Galveston, TX. E.B. received funding from University of Vermont College of Medicine, Department of Obstetrics, Gynecology and Reproductive Sciences and National Institutes of Health (Grant # P20 RR021905). J.C. received funding from the March of Dimes (21 FY2012-152) and National Institutes of Health (Grant # RO1H0065011). S.M. received funding from National Institutes of Health (Grant# HD069819).
Conflict of interest
The authors report no conflict of interest.
References
- Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med 2014;6:237ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Hafiz HA, Horwitz KB. Post-translational modifications of the progesterone receptors. J Steroid Biochem Mol Biol 2014;140:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Hafiz H, Takimoto GS, Tung L, Horwitz KB. The inhibitory function in human progesterone receptor N termini binds SUMO-1 protein to regulate autoinhibition and transrepression. J Biol Chem 2002;277:33950–33956. [DOI] [PubMed] [Google Scholar]
- ACOG Committee Opinion No 579: Definition of term pregnancy. Obstet Gynecol 2013;122:139–1140. [DOI] [PubMed] [Google Scholar]
- Adams Waldorf KM, Singh N, Mohan AR, Young RC, Ngo L, Das A, Tsai J, Bansal A, Paolella L, Herbert BR et al. Uterine overdistention induces preterm labor mediated by inflammation: observations in pregnant women and nonhuman primates. Am J Obstet Gynecol 2015;213:830.E1-830.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 2005;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgul Y, Holt R, Mummert M, Word A, Mahendroo M. Dynamic changes in cervical glycosaminoglycan composition during normal pregnancy and preterm birth. Endocrinology 2012;153:3493–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsalem H, Kwan M, Hazan A, Zhang J, Jones RL, Whittle W, Kingdom JC, Croy BA, Lye SJ, Dunk CE. Identification of a novel neutrophil population: proangiogenic granulocytes in second-trimester human decidua. J Immunol 2014;193:3070–3079. [DOI] [PubMed] [Google Scholar]
- Andersson U, Erlandsson-Harris H, Yang H, Tracey KJ. HMGB1 as a DNA-binding cytokine. J Leukoc Biol 2002;72:1084–1091. [PubMed] [Google Scholar]
- Andersson S, Minjarez D, Yost NP, Word RA. Estrogen and progesterone metabolism in the cervix during pregnancy and parturition. J Clin Endocrinol Metab 2008;93:2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anelli T, Sitia R. Protein quality control in the early secretory pathway. Embo J 2008;27:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowsmith S, Wray S. Oxytocin: its mechanism of action and receptor signalling in the myometrium. J Neuroendocrinol 2014;26:356–369. [DOI] [PubMed] [Google Scholar]
- Arulkumaran S, Kandola MK, Hoffman B, Hanyaloglu AC, Johnson MR, Bennett PR. The roles of prostaglandin EP 1 and 3 receptors in the control of human myometrial contractility. J Clin Endocrinol Metab 2012;97:489–498. [DOI] [PubMed] [Google Scholar]
- Avrech OM, Golan A, Weinraub Z, Bukovsky I, Caspi E. Mifepristone (RU486) alone or in combination with a prostaglandin analogue for termination of early pregnancy: a review. Fertil Steril 1991;56:385–393. [DOI] [PubMed] [Google Scholar]
- Bauer ME. Chronic stress and immunosenescence: a review. Neuroimmunomodulation 2008;15:241–250. [DOI] [PubMed] [Google Scholar]
- Behnia F, Taylor BD, Woodson M, Kacerovsky M, Hawkins H, Fortunato SJ, Saade GR, Menon R. Chorioamniotic Membrane Senescence: A Signal for Parturition. Am J Obstet Gynecol 2015;213:e1–e16. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Structure and function of telomeres. Nature 1991;350:569–573. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. The telomere and telomerase: nucleic acid-protein complexes acting in a telomere homeostasis system. A review. Biochemistry (Mosc) 1997;62:1196–1201. [PubMed] [Google Scholar]
- Blackburn EH. Switching and signaling at the telomere. Cell 2001;106:661–673. [DOI] [PubMed] [Google Scholar]
- Blanks AM, Vatish M, Allen MJ, Ladds G, de Wit NC, Slater DM, Thornton S. Paracrine oxytocin and estradiol demonstrate a spatial increase in human intrauterine tissues with labor. J Clin Endocrinol Metab 2003;88, 3392–3400. [DOI] [PubMed] [Google Scholar]
- Boccardi V, Paolisso G. Telomerase activation: a potential key modulator for human healthspan and longevity. Ageing Res Rev 2014;15:1–5. [DOI] [PubMed] [Google Scholar]
- Bredeson S, Papaconstantinou J, Deford JH, Kechichian T, Syed TA, Saade GR, Menon R. HMGB1 Promotes a p38MAPK Associated Non-Infectious Inflammatory Response Pathway in Human Fetal Membranes. PLoS One 2014;9:e113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner DN, Abuaita BH, Chen X, Fitzgerald KA, Nunez G, He Y, Yin XM, O'Riordan MX. Endoplasmic reticulum stress activates the inflammasome via NLRP3- and caspase-2-driven mitochondrial damage. Immunity 2015;43:451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes PS, Darley-Usmar VM. Role of calcium and superoxide dismutase in sensitizing mitochondria to peroxynitrite-induced permeability transition. Am J Physiol Heart Circ Physiol 2004;286:H39–H46. [DOI] [PubMed] [Google Scholar]
- Brown LY, Bonney EA, Raj RS, Nielsen B, Brown S. Generalized disturbance of DNA methylation in the uterine decidua in the CBA/J x DBA/2 mouse model of pregnancy failure. Biol Reprod 2013;89:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S, Thibault L, Lepage G, Seidman EG, Dube N, Levy E. Modulation of endoplasmic reticulum-bound cholesterol regulatory enzymes by iron/ascorbate-mediated lipid peroxidation. Free Radic Biol Med 2000;28:46–54. [DOI] [PubMed] [Google Scholar]
- Bryant-Greenwood GD. The extracellular matrix of the human fetal membranes: structure and function. Placenta 1998;19:1–11. [DOI] [PubMed] [Google Scholar]
- Buhimschi CS, Baumbusch MA, Dulay AT, Oliver EA, Lee S, Zhao G, Bhandari V, Ehrenkranz RA, Weiner CP, Madri JA et al. Characterization of RAGE, HMGB1, and S100beta in inflammation-induced preterm birth and fetal tissue injury. Am J Pathol 2009;175:958–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhimschi IA, Buhimschi CS, Christner R, Norwitz E, Weiner CP. Proteomic profiling and intra-amniotic infection. JAMA 2004;292:2338–2339. [DOI] [PubMed] [Google Scholar]
- Buhimschi IA, Buhimschi CS, Pupkin M, Weiner CP. Beneficial impact of term labor: nonenzymatic antioxidant reserve in the human fetus. Am J Obstet Gynecol 2003;189:181–188. [DOI] [PubMed] [Google Scholar]
- Cadepond F, Ulmann A, Baulieu EE. RU486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med 1997;48:129–156. [DOI] [PubMed] [Google Scholar]
- Campisi J. The biology of replicative senescence. Eur J Cancer 1997;33:703–709. [DOI] [PubMed] [Google Scholar]
- Campisi J, d'Adda di FF. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007;8:729–740. [DOI] [PubMed] [Google Scholar]
- Cao B, Mysorekar IU. Intracellular bacteria in placental basal plate localize to extravillous trophoblasts. Placenta 2014;35:139–142. [DOI] [PubMed] [Google Scholar]
- Carbillon L, Seince N, Uzan M. Myometrial maturation and labour. Ann Med 2001;33:571–578. [PubMed] [Google Scholar]
- Carbonne B, Brennand JE, Maria B, Cabrol D, Calder AA. Effects of gemeprost and mifepristone on the mechanical properties of the cervix prior to first trimester termination of pregnancy. Br J Obstet Gynaecol 1995;102:553–558. [DOI] [PubMed] [Google Scholar]
- Carbone DL, Doorn JA, Kiebler Z, Petersen DR. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem Res Toxicol 2005;18:1324–1331. [DOI] [PubMed] [Google Scholar]
- Casey ML, Winkel CA, Porter JC, MacDonald PC. Endocrine regulation of the initiation and maintenance of parturition. Clin Perinatol 1983;10:709–721. [PubMed] [Google Scholar]
- Castillo-Castrejon M, Meraz-Cruz N, Gomez-Lopez N, Flores-Pliego A, Beltran-Montoya J, Viveros-Alcaraz M, Vadillo-Ortega F. Choriodecidual cells from term human pregnancies show distinctive functional properties related to the induction of labor. Am J Reprod Immunol 2014;71:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesario SK. The ‘Christmas Effect’ and other biometeorologic influences on childbearing and the health of women. J Obstet Gynecol Neonatal Nurs 2002;31:526–535. [DOI] [PubMed] [Google Scholar]
- Chakravarthi S, Jessop CE, Bulleid NJ. The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep 2006;7:271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis JR, Sloboda DM, Alfaidy N, Lye SJ, Gibb W, Patel FA, Whittle WL, Newnham JP. Prostaglandins and mechanisms of preterm birth. Reproduction 2002;124:1–17. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Ertelt JM, Jiang TT, Kinder JM, Xin L, Owens KJ, Jones HN, Way SS. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. J Clin Invest 2015;125:1713–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chibbar R, Miller FD, Mitchell BF. Synthesis of oxytocin in amnion, chorion, and decidua may influence the timing of human parturition. J Clin Invest 1993;91:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chwalisz K, Garfield RE. Antiprogestins in the induction of labor. Ann NY Acad Sci 1994;734:387–413. [DOI] [PubMed] [Google Scholar]
- Collins MK, Tay CS, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J Clin Invest 2009;119:2062–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 2006;20:764–775. [DOI] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci USA 2004;101:4978–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA 2003;100:9518–9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin CM, Masopust D, Gourley T, Grayson J, Strickland OL, Ahmed R, Bonney EA. Normal establishment of virus-specific memory CD8 T cell pool following primary infection during pregnancy. J Immunol 2007;179:4383–4389. [DOI] [PubMed] [Google Scholar]
- Copinschi G, Turek FW, and van C. Endocrine rhythms, the sleep-wake cycle and biological clocks. In: Jameson JL and De Groot LJ (eds). Endocrinology: Adult and Pediatric. Saunders Elsevier, Philadelphia, PA, 2011, 201–202. [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 2010;5:99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner GW, Allen WM. Physiology of the corpus luteum. 1929. Am J Obstet Gynecol 2005;193, 1574. [DOI] [PubMed] [Google Scholar]
- Csapo A. Progesterone block. Am J Anat 1956;98:273–291. [DOI] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. NRF2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 2003;23:7198–7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummiskey KC, Gall SA, Dawood MY. Pulsatile administration of oxytocin for augmentation of labor. Obstet Gynecol 1989;74:869–872. [PubMed] [Google Scholar]
- Damiola F, Le MN, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 2000;14:2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth DN. The morphology of the human cervix. Clin Obstet Gynecol 1983;26:7–13. [DOI] [PubMed] [Google Scholar]
- Daniel AR, Faivre EJ, Lange CA. Phosphorylation-dependent antagonism of sumoylation derepresses progesterone receptor action in breast cancer cells. Mol Endocrinol 2007;21:2890–2906. [DOI] [PubMed] [Google Scholar]
- Deb S, Tessier C, Prigent-Tessier A, Barkai U, Ferguson-Gottschall S, Srivastava RK, Faliszek J, Gibori G. The expression of interleukin-6 (IL-6), IL-6 receptor, and gp130-kilodalton glycoprotein in the rat decidua and a decidual cell line: regulation by 17beta-estradiol and prolactin. Endocrinology 1999;140:4442–4450. [DOI] [PubMed] [Google Scholar]
- Devi YS, DeVine M, DeKuiper J, Ferguson S, Fazleabas AT. Inhibition of IL-6 signaling pathway by curcumin in uterine decidual cells. PLoS One 2015;10:e0125627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ME, Chien EK, Osol G, Callas PW, Bonney EA. Failure of decidual arteriolar remodeling in the CBA/J x DBA/2 murine model of recurrent pregnancy loss is linked to increased expression of tissue inhibitor of metalloproteinase 2 (TIMP-2). Am J Obstet Gynecol 2006;194:113–119. [DOI] [PubMed] [Google Scholar]
- Dobyns AE, Goyal R, Carpenter LG, Freeman TC, Longo LD, Yellon SM. Macrophage gene expression associated with remodeling of the prepartum rat cervix: microarray and pathway analyses. PLoS One 2015;10:e0119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubicke A, Ekman-Ordeberg G, Mazurek P, Miller L, Yellon SM. Density of stromal cells and macrophages associated with collagen remodeling in the human cervix in preterm and term birth. Reprod Sci 2015;23:595–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 2009;86:557–566. [DOI] [PubMed] [Google Scholar]
- Ekman-Ordeberg G, Stjernholm Y, Wang H, Stygar D, Sahlin L. Endocrine regulation of cervical ripening in humans--potential roles for gonadal steroids and insulin-like growth factor-I. Steroids 2003;68:837–847. [DOI] [PubMed] [Google Scholar]
- Elovitz MA. Anti-inflammatory interventions in pregnancy: now and the future. Semin Fetal Neonatal Med 2006;11:327–332. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Mrinalini C. Can medroxyprogesterone acetate alter Toll-like receptor expression in a mouse model of intrauterine inflammation. Am J Obstet Gynecol 2005;193:1149–1155. [DOI] [PubMed] [Google Scholar]
- Emami H, Vucic E, Subramanian S, Abdelbaky A, Fayad ZA, Du S, Roth E, Ballantyne CM, Mohler ER, Farkouh ME et al. The effect of BMS-582949, a P38 mitogen-activated protein kinase (P38 MAPK) inhibitor on arterial inflammation: a multicenter FDG-PET trial. Atherosclerosis 2015;240:490–496. [DOI] [PubMed] [Google Scholar]
- Engert S, Rieger L, Kapp M, Becker JC, Dietl J, Kammerer U. Profiling chemokines, cytokines and growth factors in human early pregnancy decidua by protein array. Am J Reprod Immunol 2007;58:129–137. [DOI] [PubMed] [Google Scholar]
- Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone receptor rapid signaling mediates serine 345 phosphorylation and tethering to specificity protein 1 transcription factors. Mol Endocrinol 2008;22:823–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas AM, Kilgore TM, Lotze MT. Detecting DNA: getting and begetting cancer. Curr Opin Investig Drugs 2007;8:981–986. [PubMed] [Google Scholar]
- Finch CE. Mechanisms in senescence: some thoughts in April 1990. Exp Gerontol 1992;27:7–16. [DOI] [PubMed] [Google Scholar]
- Foell D, Wittkowski H, Roth J. Mechanisms of disease: a ’DAMP’ view of inflammatory arthritis. Nat Clin Pract Rheumatol 2007;3:382–390. [DOI] [PubMed] [Google Scholar]
- Fortini P, Pascucci B, Parlanti E, D'Errico M, Simonelli V, Dogliotti E. 8-Oxoguanine DNA damage: at the crossroad of alternative repair pathways. Mutat Res 2003;531:127–139. [DOI] [PubMed] [Google Scholar]
- Fortunato SJ, LaFleur B, Menon R. Collagenase-3 (MMP-13) in fetal membranes and amniotic fluid during pregnancy. Am J Reprod Immunol 2003;49:120–125. [DOI] [PubMed] [Google Scholar]
- Fortunato SJ, Menon R, Lombardi SJ. Collagenolytic enzymes (gelatinases) and their inhibitors in human amniochorionic membrane. Am J Obstet Gynecol 1997;177:731–741. [DOI] [PubMed] [Google Scholar]
- Fox H. Aging of the placenta. Arch Dis Child Fetal Neonatal Ed 1997;77:F171–F175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox H, Faulk WP. The placenta as an experimental model. Clin Endocrinol Metab 1981;10:57–72. [DOI] [PubMed] [Google Scholar]
- Friebe-Hoffmann U, Baston DM, Hoffmann TK, Chiao JP, Rauk PN. The influence of interleukin-1beta on oxytocin signalling in primary cells of human decidua. Regul Pept 2007;142:78–85. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Fuchs F, Husslein P, Soloff MS, Fernstrom MJ. Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science 1982;215:1396–1398. [DOI] [PubMed] [Google Scholar]
- Fuchs AR, Romero R, Keefe D, Parra M, Oyarzun E, Behnke E. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol 1991;165:1515–1523. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Savani RC, Watari M, Day AJ, Strauss JF III. Induction of the hyaluronic acid-binding protein, tumor necrosis factor-stimulated gene-6, in cervical smooth muscle cells by tumor necrosis factor-alpha and prostaglandin E(2). Am J Pathol 2002;160:1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galazka K, Wicherek L, Pitynski K, Kijowski J, Zajac K, Bednarek W, Dutsch-Wicherek M, Rytlewski K, Kalinka J, Basta A et al. Changes in the subpopulation of CD25+ CD4+ and FOXP3+ regulatory T cells in decidua with respect to the progression of labor at term and the lack of analogical changes in the subpopulation of suppressive B7-H4 macrophages--a preliminary report. Am J Reprod Immunol 2009;61:136–146. [DOI] [PubMed] [Google Scholar]
- Gao L, Rabbitt EH, Condon JC, Renthal NE, Johnston JM, Mitsche MA, Chambon P, Xu J, O'Malley BW, Mendelson CR. Steroid receptor coactivators 1 and 2 mediate fetal-to-maternal signaling that initiates parturition. J Clin Invest 2015;125:2808–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido P. Aging and stress: past hypotheses, present approaches and perspectives. Aging Dis 2011;2:80–99. [PMC free article] [PubMed] [Google Scholar]
- Genbacev O, Vicovac L, Larocque N. The role of chorionic cytotrophoblasts in the smooth chorion fusion with parietal decidua. Placenta 2015;36:716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghartey J, Bastek JA, Brown AG, Anglim L, Elovitz MA. Women with preterm birth have a distinct cervicovaginal metabolome. Am J Obstet Gynecol 2015;212:776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard S, Heazell AE, Derricott H, Allan SM, Sibley CP, Abrahams VM, Jones RL. Circulating cytokines and alarmins associated with placental inflammation in high-risk pregnancies. Am J Reprod Immunol 2014;72:422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser SR, Julian J. Intermediate filament protein as a marker of uterine stromal cell decidualization. Biol Reprod 1986;35:463–474. [DOI] [PubMed] [Google Scholar]
- Golightly E, Jabbour HN, Norman JE. Endocrine immune interactions in human parturition. Mol Cell Endocrinol 2011;335:52–59. [DOI] [PubMed] [Google Scholar]
- Gomez-Chavez F, Castro-Leyva V, Espejel-Nunez A, Zamora-Mendoza RG, Rosas-Vargas H, Cancino-Diaz JC, Cancino-Diaz ME, Estrada-Gutierrez G, Rodriguez-Martinez S. Galectin-1 reduced the effect of LPS on the IL-6 production in decidual cells by inhibiting LPS on the stimulation of IkappaBzeta. J Reprod Immunol 2015;112:46–52. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, Vadillo-Ortega F. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol 2009;80:122–131. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Romero R, Plazyo O, Panaitescu B, Furcron AE, Miller D, Roumayah T, Flom E, Hassan SS. Intra-amniotic administration of HMGB1 induces spontaneous preterm labor and birth. Am J Reprod Immunol 2016;75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, Vadillo-Ortega F. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol 2013;69:212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, McCormack A, Lapidus JA, Hitti J, Eschenbach DA et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA 2004;292:462–469. [DOI] [PubMed] [Google Scholar]
- Guo C, Wang W, Liu C, Myatt L, Sun K. Induction of PGF2alpha synthesis by cortisol through GR dependent induction of CBR1 in human amnion fibroblasts. Endocrinology 2014;155:3017–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzeloglu-Kayisli O, Kayisli UA, Semerci N, Basar M, Buchwalder LF, Buhimschi CS, Buhimschi IA, Arcuri F, Larsen K, Huang JS et al. Mechanisms of chorioamnionitis-associated preterm birth: interleukin-1beta inhibits progesterone receptor expression in decidual cells. J Pathol 2015;237:423–434. [DOI] [PubMed] [Google Scholar]
- Haberzettl P, Hill BG. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol 2013;1:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney DN, Tirumala R, Salamone LJ, Miller RK, Katzman PJ. Do placental histologic findings of chorion-decidual hemorrhage or inflammation in spontaneous preterm birth influence outcomes in the subsequent pregnancy. Placenta 2014;35:58–63. [DOI] [PubMed] [Google Scholar]
- Hapangama D, Neilson JP. Mifepristone for induction of labour. Cochrane Database Syst Rev 2009;CD002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DB, Janowski BA, Corey DR, Mendelson CR. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol 2006;20:2724–2733. [DOI] [PubMed] [Google Scholar]
- Harley CB, Vaziri H, Counter CM, Allsopp RC. The telomere hypothesis of cellular aging. Exp Gerontol 1992;27:375–382. [DOI] [PubMed] [Google Scholar]
- Hassan SS, Romero R, Pineles B, Tarca AL, Montenegro D, Erez O, Mittal P, Kusanovic JP, Mazaki-Tovi S, Espinoza J et al. MicroRNA expression profiling of the human uterine cervix after term labor and delivery. Am J Obstet Gynecol 2010;202:80–88. [DOI] [PubMed] [Google Scholar]
- Hastings M, O'Neill JS, Maywood ES. Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol 2007;195:187–198. [DOI] [PubMed] [Google Scholar]
- Hay ED. Extracellular matrix. J Cell Biol 1981;91:205s–223s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayflick L. Aging human cells. Triangle 1973. a;12:141–147. [PubMed] [Google Scholar]
- Hayflick L. The biology of human aging. Am J Med Sci 1973. b;265:432–445. [DOI] [PubMed] [Google Scholar]
- Hayflick L. The strategy of senescence. Gerontologist 1974;14:37–45. [DOI] [PubMed] [Google Scholar]
- Henshaw RC, Templeton AA. Pre-operative cervical preparation before first trimester vacuum aspiration: a randomized controlled comparison between gemeprost and mifepristone (RU 486). Br J Obstet Gynaecol 1991;98:1025–1030. [DOI] [PubMed] [Google Scholar]
- Hirota Y, Daikoku T, Tranguch S, Xie H, Bradshaw HB, Dey SK. Uterine-specific p53 deficiency confers premature uterine senescence and promotes preterm birth in mice. J Clin Invest 2010;120:803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol 2004. a;165:347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Taniguchi M, Honda A, Imaizumi K, Tohyama M. Apoptosis induced by endoplasmic reticulum stress depends on activation of caspase-3 via caspase-12. Neurosci Lett 2004. b;357:127–130. [DOI] [PubMed] [Google Scholar]
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 1999;97:889–901. [DOI] [PubMed] [Google Scholar]
- Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 1995;270:25752–25761. [DOI] [PubMed] [Google Scholar]
- Horn T, Robertson BC, Gemmell NJ. The use of telomere length in ecology and evolutionary biology. Heredity (Edinb) 2010;105:497–506. [DOI] [PubMed] [Google Scholar]
- Horwitz KB, Wei LL, Francis MD. Structural analyses of progesterone receptors. J Steroid Biochem 1986;24:109–117. [DOI] [PubMed] [Google Scholar]
- Hua R, Pease JE, Sooranna SR, Viney JM, Nelson SM, Myatt L, Bennett PR, Johnson MR. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-kappaB activation. Endocrinology 2012;153:481–491. [DOI] [PubMed] [Google Scholar]
- Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine 2010;51:119–126. [DOI] [PubMed] [Google Scholar]
- Huybrechts KF, Sanghani RS, Avorn J, Urato AC. Preterm birth and antidepressant medication use during pregnancy: a systematic review and meta-analysis. PLoS One 2014;9:e92778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta Y, Matsuura K, Okamura H, Oyamada I, Usuku G. Effects of RU486 on the interstitial collagenase in the process of cervical ripening in the pregnant rat. Endocrinol Jpn 1991;5:491–496. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Critchley HO. Potential roles of decidual prolactin in early pregnancy. Reproduction 2001;121:197–205. [DOI] [PubMed] [Google Scholar]
- Janko C, Filipovic M, Munoz LE, Schorn C, Schett G, Ivanovic-Burmazovic I, Herrmann M. Redox modulation of HMGB1-related signaling. Antioxid Redox Signal 2014;20:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, Wek RC. Phosphorylation of the alpha-subunit of the eukaryotic initiation factor-2 (eIF2alpha) reduces protein synthesis and enhances apoptosis in response to proteasome inhibition. J Biol Chem 2005;280:14189–14202. [DOI] [PubMed] [Google Scholar]
- Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol 2003;23:5651–5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacerovsky M, Tothova L, Menon R, Vlkova B, Musilova I, Hornychova H, Prochazka M, Celec P. Amniotic fluid markers of oxidative stress in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2014;27:1–10. [DOI] [PubMed] [Google Scholar]
- Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem 1996;271:6217–6224. [DOI] [PubMed] [Google Scholar]
- Kang R, Zhang Q, Zeh HJ III, Lotze MT, Tang D. HMGB1 in cancer: good, bad, or both. Clin Cancer Res 2013;19:4046–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M. Induction of neuronal death by ER stress in Alzheimer's disease. J Chem Neuroanat 2004;28:67–78. [DOI] [PubMed] [Google Scholar]
- Kato T Jr, Delhase M, Hoffmann A, Karin M. CK2 is a C-terminal IkappaB kinase responsible for NF-kappaB activation during the UV response. Mol Cell 2003;12:829–839. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta 2003;24:S33–S46. [DOI] [PubMed] [Google Scholar]
- Kelly RW. Inflammatory mediators and parturition. Rev Reprod 1996;1:89–96. [DOI] [PubMed] [Google Scholar]
- Kendal-Wright CE. Stretching, mechanotransduction, and proinflammatory cytokines in the fetal membranes. Reprod Sci 2007;14:35–41. [DOI] [PubMed] [Google Scholar]
- Khan JA, Amazit L, Bellance C, Guiochon-Mantel A, Lombes M, Loosfelt H. p38 and p42/44 MAPKs differentially regulate progesterone receptor A and B isoform stabilization. Mol Endocrinol 2011;25:1710–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DK. Estimating doubling time of cells in vitro. In Vitro Cell Dev Biol Anim 1995;31:419–420. [DOI] [PubMed] [Google Scholar]
- Knox K, Baker JC. Genomic evolution of the placenta using co-option and duplication and divergence. Genome Res 2008;18:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev 2010;24:2463–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Moore RM, Nash A, Springel E, Mercer BM, Philipson E, Mansour JM, Moore JJ. Decidual GM-CSF is a critical common intermediate necessary for thrombin and TNF induced in-vitro fetal membrane weakening. Placenta 2014;35:1049–1056. [DOI] [PubMed] [Google Scholar]
- Kyathanahalli C, Organ K, Moreci RS, Anamthathmakula P, Hassan SS, Caritis SN, Jeyasuria P, Condon JC. Uterine endoplasmic reticulum stress-unfolded protein response regulation of gestational length is caspase-3 and -7-dependent. Proc Natl Acad Sci USA 2015;112:14090–14095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc Natl Acad Sci USA 2000;97:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange SS, Vasquez KM. HMGB1: the jack-of-all-trades protein is a master DNA repair mechanic. Mol Carcinog 2009;48:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin HM, Bouffard NA, Badger GJ, Iatridis JC, Howe AK. Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. Am J Physiol Cell Physiol 2005;288:C747–C756. [DOI] [PubMed] [Google Scholar]
- Lappas M, Odumetse TL, Riley C, Reti NG, Holdsworth-Carson SJ, Rice GE, Permezel M. Pre-labour fetal membranes overlying the cervix display alterations in inflammation and NF-kappaB signalling pathways. Placenta 2008;29:995–1002. [DOI] [PubMed] [Google Scholar]
- Lappas M, Rice GE. The role and regulation of the nuclear factor kappa B signalling pathway in human labour. Placenta 2007;28:543–556. [DOI] [PubMed] [Google Scholar]
- Larsen B, Hwang J. Progesterone interactions with the cervix: translational implications for term and preterm birth. Infect Dis Obstet Gynecol 2011;2011:353297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 2003;23:7448–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Sooranna SR, Terzidou V, Christian M, Brosens J, Huhtinen K, Poutanen M, Barton G, Johnson MR, Bennett PR. Interactions between inflammatory signals and the progesterone receptor in regulating gene expression in pregnant human uterine myocytes. J Cell Mol Med 2012;16:2487–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Sun C, Zhou Y, Gokalp D, Herrema H, Park SW, Davis RJ, Ozcan U. p38 MAPK-mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat Med 2011;17:1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature 2012;491:594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Feng XH, Deng WB, Ni H, Zhang ZR, Jia B, Yang XL, Wang TS, Liu JL, Su RW et al. Progesterone and DNA damage encourage uterine cell proliferation and decidualization through up-regulating ribonucleotide reductase 2 expression during early pregnancy in mice. J Biol Chem 2012;287:15174–15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levonen AL, Hill BG, Kansanen E, Zhang J, Darley-Usmar VM. Redox regulation of antioxidants, autophagy, and the response to stress: implications for electrophile therapeutics. Free Radic Biol Med 2014;71:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu Y, Chen HZ, Li FW, Wu JF, Zhang HK, He JP, Xing YZ, Chen Y, Wang WJ et al. Impeding the interaction between Nur77 and p38 reduces LPS-induced inflammation. Nat Chem Biol 2015;11:339–346. [DOI] [PubMed] [Google Scholar]
- Li XQ, Zhu P, Myatt L, Sun K. Roles of glucocorticoids in human parturition: a controversial fact. Placenta 2014;35:291–296. [DOI] [PubMed] [Google Scholar]
- Liao Y, Jiang Y, He H, Ni H, Tu Z, Zhang S, Wang B, Lou J, Quan S, Wang H. NEDD8-mediated neddylation is required for human endometrial stromal proliferation and decidualization. Hum Reprod 2015;30:1665–1676. [DOI] [PubMed] [Google Scholar]
- Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction 2005;130:569–581. [DOI] [PubMed] [Google Scholar]
- Liu L, Bailey SM, Okuka M, Munoz P, Li C, Zhou L, Wu C, Czerwiec E, Sandler L, Seyfang A et al. Telomere lengthening early in development. Nat Cell Biol 2007;9:1436–1441. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhu P, Tao Y, Shen C, Wang S, Zhao L, Wu H, Fan F, Lin C, Chen C et al. Cancer-promoting effect of capsaicin on DMBA/TPA-induced skin tumorigenesis by modulating inflammation, Erk and p38 in mice. Food Chem Toxicol 2015;81:1–8. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J Chem Neuroanat 2004;28:51–65. [DOI] [PubMed] [Google Scholar]
- Mackler AM, Ducsay TC, Ducsay CA, Yellon SM. Effects of endotoxin and macrophage-related cytokines on the contractile activity of the gravid murine uterus. Biol Reprod 2003;69:1165–1169. [DOI] [PubMed] [Google Scholar]
- Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod 1999;61:879–883. [DOI] [PubMed] [Google Scholar]
- Mahendroo M. Cervical remodeling in term and preterm birth: insights from an animal model. Reproduction 2012;143:429–438. [DOI] [PubMed] [Google Scholar]
- Makino S, Zaragoza DB, Mitchell BF, Robertson S, Olson DM. Prostaglandin F2alpha and its receptor as activators of human decidua. Semin Reprod Med 2007;25:60–68. [DOI] [PubMed] [Google Scholar]
- Malek A, Blann E, Mattison DR. Human placental transport of oxytocin. J Matern Fetal Med 1996;5:245–255. [DOI] [PubMed] [Google Scholar]
- Malm H, Sourander A, Gissler M, Gyllenberg D, Hinkka-Yli-Salomaki S, McKeague IW, Artama M, Brown AS. Pregnancy complications following prenatal exposure to SSRIs or maternal psychiatric disorders: results from population-based national register data. Am J Psychiatry 2015;72:1224–1232. [DOI] [PubMed] [Google Scholar]
- Margarit L, Griffiths AN, Tsapanos V, Tsakas S, Decavalas G. Amniotic fluid endothelin levels and the incidence of premature rupture of membranes. Int J Gynaecol Obstet 2006;93:18–21. [DOI] [PubMed] [Google Scholar]
- Margarit L, Griffiths AN, Tsapanos V, Tsakas S, Gumenos D, Decavalas G. Second trimester amniotic fluid endothelin-1 concentrations and subsequent development of intrauterine growth restriction. Eur J Obstet Gynecol Reprod Biol 2007;134:192–195. [DOI] [PubMed] [Google Scholar]
- Martina NA, Kim E, Chitkara U, Wathen NC, Chard T, Giudice LC. Gestational age-dependent expression of insulin-like growth factor-binding protein-1 (IGFBP-1) phosphoisoforms in human extraembryonic cavities, maternal serum, and decidua suggests decidua as the primary source of IGFBP-1 in these fluids during early pregnancy. J Clin Endocrinol Metab 1997;82:1894–1898. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Glucocorticoids and aging. Aging 1995;7:407–413. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid Redox Signal 2002;4:415–425. [DOI] [PubMed] [Google Scholar]
- Matta P, Lockwood CJ, Schatz F, Krikun G, Rahman M, Buchwalder L, Norwitz ER. Thrombin regulates monocyte chemoattractant protein-1 expression in human first trimester and term decidual cells. Am J Obstet Gynecol 2007;196:268. [DOI] [PubMed] [Google Scholar]
- McEwan IJ. Nuclear receptors: one big family. Methods Mol Biol 2009;505:3–18. [DOI] [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med 1995;1:460–463. [DOI] [PubMed] [Google Scholar]
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med 2003;348:2379–2385. [DOI] [PubMed] [Google Scholar]
- Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol 2009;23:947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R. Oxidative stress damage as a detrimental factor in preterm birth pathology. Front Immunol 2014;5:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY) 2016;8:216–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, Boldogh I, Hawkins HK, Woodson M, Polettini J, Syed TA, Fortunato SJ, Saade GR, Papaconstantinou J, Taylor RN. Histological evidence of oxidative stress and premature senescence in preterm premature rupture of the human fetal membranes recapitulated in vitro. Am J Pathol 2014. a;184:1740–1751. [DOI] [PubMed] [Google Scholar]
- Menon R, Boldogh I, Urrabaz-Garza R, Polettini J, Syed TA, Saade GR, Papaconstantinou J, Taylor RN. Senescence of primary amniotic cells via oxidative DNA damage. PLoS One 2013;8:e83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R, Polettini J, Syed TA, Saade GR, Boldogh I. Expression of 8-oxoguanine glycosylase in human fetal membranes. Am J Reprod Immunol 2014. b;72:75–84. [DOI] [PubMed] [Google Scholar]
- Menon R, Yu J, Basanta-Henry P, Brou L, Berga SL, Fortunato SJ, Taylor RN. Short fetal leukocyte telomere length and preterm prelabor rupture of the membranes. PLoS One 2012;7:e31136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menu P, Mayor A, Zhou R, Tardivel A, Ichijo H, Mori K, Tschopp J. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis 2012;3:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer BM, Lewis R. Preterm labor and preterm premature rupture of the membranes. Diagnosis and management. Infect Dis Clin North Am 1997;11:177–201. [DOI] [PubMed] [Google Scholar]
- Merlino AA, Welsh TN, Tan H, Yi LJ, Cannon V, Mercer BM, Mesiano S. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab 2007;92:1927–1933. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 2002;87:2924–2930. [DOI] [PubMed] [Google Scholar]
- Messlik A, Schmechel S, Kisling S, Bereswill S, Heimesaat MM, Fischer A, Gobel U, Haller D. Loss of Toll-like receptor 2 and 4 leads to differential induction of endoplasmic reticulum stress and proapoptotic responses in the intestinal epithelium under conditions of chronic inflammation. J Proteome Res 2009;8:4406–4417. [DOI] [PubMed] [Google Scholar]
- Millar LK, Stollberg J, DeBuque L, Bryant-Greenwood G. Fetal membrane distention: determination of the intrauterine surface area and distention of the fetal membranes preterm and at term. Am J Obstet Gynecol 2000;182:128–134. [DOI] [PubMed] [Google Scholar]
- Mogami H, Kishore AH, Shi H, Keller PW, Akgul Y, Word RA. Fetal fibronectin signaling induces matrix metalloproteases and cyclooxygenase-2 (COX-2) in amnion cells and preterm birth in mice. J Biol Chem 2013;288:1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TR, Iams JD, Creasy RK, Burau KD, Davidson AL. Diurnal and gestational patterns of uterine activity in normal human pregnancy. The Uterine Activity in Pregnancy Working Group. Obstet Gynecol 1994;83:517–523. [DOI] [PubMed] [Google Scholar]
- Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 2002;277:34287–34294. [DOI] [PubMed] [Google Scholar]
- Morton-Pradhan S, Bay RC, Coonrod DV. Birth rate and its correlation with the lunar cycle and specific atmospheric conditions. Am J Obstet Gynecol 2005;192:1970–1973. [DOI] [PubMed] [Google Scholar]
- Mossman HW. Classics revisited: comparative morphogenesis of the fetal membranes and accessory uterine structures. Placenta 1991;12:1–5. [DOI] [PubMed] [Google Scholar]
- Muglia LJ, Bae DS, Brown TT, Vogt SK, Alvarez JG, Sunday ME, Majzoub JA. Proliferation and differentiation defects during lung development in corticotropin-releasing hormone-deficient mice. Am J Respir Cell Mol Biol 1999;20:181–188. [DOI] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U SA 2003;100:9744–9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 2000;289:1751–1754. [DOI] [PubMed] [Google Scholar]
- Mulla MJ, Myrtolli K, Potter J, Boeras C, Kavathas PB, Sfakianaki AK, Tadesse S, Norwitz ER, Guller S, Abrahams VM. Uric acid induces trophoblast IL-1beta production via the inflammasome: implications for the pathogenesis of preeclampsia. Am J Reprod Immunol 2011;65:542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muter J, Lucas ES, Chan YW, Brighton PJ, Moore JD, Lacey L, Quenby S, Lam EW, Brosens JJ. The clock protein period 2 synchronizes mitotic expansion and decidual transformation of human endometrial stromal cells. FASEB J 2015;29:1603–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol 2004;122:369–382. [DOI] [PubMed] [Google Scholar]
- Myatt L, Sun K. Role of fetal membranes in signaling of fetal maturation and parturition. Int J Dev Biol 2010;54:545–553. [DOI] [PubMed] [Google Scholar]
- Myers K, Socrate S, Tzeranis D, House M. Changes in the biochemical constituents and morphologic appearance of the human cervical stroma during pregnancy. Eur J Obstet Gynecol Reprod Biol 2009;144:S82–S89. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 2000;403:98–103. [DOI] [PubMed] [Google Scholar]
- Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 2012;336:1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson JP. Mifepristone for induction of labour. Cochrane Database Syst Rev 2000;CD002865. [DOI] [PubMed] [Google Scholar]
- Nicolaides NC, Charmandari E, Chrousos GP, Kino T. Circadian endocrine rhythms: the hypothalamic-pituitary-adrenal axis and its actions. Ann NY Acad Sci 2014;1318:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira-Machado JA, Volpe CM, Veloso CA, Chaves MM. HMGB1, TLR and RAGE: a functional tripod that leads to diabetic inflammation. Expert Opin Ther Targets 2011;15:1023–1035. [DOI] [PubMed] [Google Scholar]
- Nold C, Anton L, Brown A, Elovitz M. Inflammation promotes a cytokine response and disrupts the cervical epithelial barrier: a possible mechanism of premature cervical remodeling and preterm birth. Am J Obstet Gynecol 2012;206:208–7. [DOI] [PubMed] [Google Scholar]
- Norman JE, Bollapragada S, Yuan M, Nelson SM. Inflammatory pathways in the mechanism of parturition. BMC Pregnancy Childbirth 2007;7:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman M, Ekman G, Malmstrom A. Prostaglandin E2-induced ripening of the human cervix involves changes in proteoglycan metabolism. Obstet Gynecol 1993;82:1013–1020. [PubMed] [Google Scholar]
- Norman JE, Marlow N, Messow CM, Shennan A, Bennett PR, Thornton S, Robson SC, McConnachie A, Petrou S, Sebire NJ et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet 2016;16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SY, Romero R, Shim SS, Park JS, Jun JK, Yoon BH. Fetal plasma cortisol and dehydroepiandrosterone sulfate concentrations in pregnancy and term parturition. J Matern Fetal Neonatal Med 2006;19:529–536. [DOI] [PubMed] [Google Scholar]
- Okabe H, Makino S, Kato K, Matsuoka K, Seki H, Takeda S. The effect of progesterone on genes involved in preterm labor. J Reprod Immunol 2014;104–105:80–91. [DOI] [PubMed] [Google Scholar]
- Olcese J. Circadian aspects of mammalian parturition: a review. Mol Cell Endocrinol 2012;349:62–67. [DOI] [PubMed] [Google Scholar]
- Oliveira SF, Greca CP, Abrahamsohn PA, Reis MG, Zorn TM. Organization of desmin-containing intermediate filaments during differentiation of mouse decidual cells. Histochem Cell Biol 2000;113:319–327. [DOI] [PubMed] [Google Scholar]
- Osman I, Young A, Jordan F, Greer IA, Norman JE. Leukocyte density and proinflammatory mediator expression in regional human fetal membranes and decidua before and during labor at term. J Soc Gynecol Investig 2006;13:97–103. [DOI] [PubMed] [Google Scholar]
- Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol Hum Reprod 2003;9:41–45. [DOI] [PubMed] [Google Scholar]
- Paamoni-Keren O, Silberstein T, Burg A, Raz I, Mazor M, Saphier O, Weintraub AY. Oxidative stress as determined by glutathione (GSH) concentrations in venous cord blood in elective cesarean delivery versus uncomplicated vaginal delivery. Arch Gynecol Obstet 2007;276:43–46. [DOI] [PubMed] [Google Scholar]
- Palmai-Pallag T, Bachrati CZ. Inflammation-induced DNA damage and damage-induced inflammation: a vicious cycle. Microbes Infect 2014;16:822–832. [DOI] [PubMed] [Google Scholar]
- Papatheodorou DC, Karagiannidis LK, Paltoglou G, Margeli A, Kaparos G, Valsamakis G, Chrousos GP, Creatsas G, Mastorakos G. Pulsatile interleukin-6 leads CRH secretion and is associated with myometrial contractility during the active phase of term human labor. J Clin Endocrinol Metab 2013;98:4105–4112. [DOI] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A et al. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol 2006;290:C917–C924. [DOI] [PubMed] [Google Scholar]
- Parry S, Elovitz MA. Pros and cons of maternal cervical length screening to identify women at risk of spontaneous preterm delivery. Clin Obstet Gynecol 2014;57:537–546. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Imperatore A, Challis JR. Neuroendocrine mechanisms in pregnancy and parturition. Endocr Rev 2010;31:783–816. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Sawchenko PE, Rivier J, Vale W. Evidence for local stimulation of ACTH secretion by corticotropin-releasing factor in human placenta. Nature 1987;328:717–719. [DOI] [PubMed] [Google Scholar]
- Petraglia F, Sutton S, Vale W. Neurotransmitters and peptides modulate the release of immunoreactive corticotropin-releasing factor from cultured human placental cells. Am J Obstet Gynecol 1989;160:247–251. [DOI] [PubMed] [Google Scholar]
- Phillippe M. Cell-free fetal DNA--a trigger for parturition. N Engl J Med 2014;370:2534–2536. [DOI] [PubMed] [Google Scholar]
- Phillippe M. Cell-free fetal DNA, telomeres, and the spontaneous onset of parturition. Reprod Sci 2015;22:1186–1201. [DOI] [PubMed] [Google Scholar]
- Pieber D, Allport VC, Bennett PR. Progesterone receptor isoform A inhibits isoform B-mediated transactivation in human amnion. Eur J Pharmacol 2001;427:7–11. [DOI] [PubMed] [Google Scholar]
- Pisetsky DS, Gauley J, Ullal AJ. HMGB1 and microparticles as mediators of the immune response to cell death. Antioxid Redox Signal 2011;15:2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol 2007;17:511–517. [DOI] [PubMed] [Google Scholar]
- Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere fragment induced amnion cell senescence: a contributor to parturition. PLoS One 2015. a;10:e0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polettini J, Dutta EH, Behnia F, Saade GR, Torloni MR, Menon R. Aging of intrauterine tissues in spontaneous preterm birth and preterm premature rupture of the membranes: A systematic review of the literature. Placenta 2015. b;36:969–973. [DOI] [PubMed] [Google Scholar]
- Prince AL, Antony KM, Chu DM, Aagaard KM. The microbiome, parturition, and timing of birth: more questions than answers. J Reprod Immunol 2014;104–105:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins JR, Zhang B, Schjenken JE, Guerin LR, Barry SC, Robertson SA. Unstable Foxp3+ regulatory T cells and altered dendritic cells are associated with lipopolysaccharide-induced fetal loss in pregnant IL10-deficient mice. Biol Reprod 2015;93:95. [DOI] [PubMed] [Google Scholar]
- Rahkonen L, Rutanen EM, Nuutila M, Sainio S, Saisto T, Paavonen J. Elevated levels of decidual insulin-like growth factor binding protein-1 in cervical fluid in early and mid-pregnancy are associated with an increased risk of spontaneous preterm delivery. BJOG 2010;117:701–710. [DOI] [PubMed] [Google Scholar]
- Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem 2002;277:21836–21842. [DOI] [PubMed] [Google Scholar]
- Ratajczak CK, Asada M, Allen GC, McMahon DG, Muglia LM, Smith D, Bhattacharyya S, Muglia LJ. Generation of myometrium-specific Bmal1 knockout mice for parturition analysis. Reprod Fertil Dev 2012;24:759–767. [DOI] [PubMed] [Google Scholar]
- Ratsep MT, Felker AM, Kay VR, Tolusso L, Hofmann AP, Croy BA. Uterine natural killer cells: supervisors of vasculature construction in early decidua basalis. Reproduction 2015;149:R91–102. [DOI] [PubMed] [Google Scholar]
- Reddi K, Deppe WM, Norman RJ. Increased and intermittent prostaglandin release from amnion detected by a new superfusion technique for full thickness fetal membrane. Prostaglandins 1990;39:601–610. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Korkmaz A, Rosales-Corral SA. Melatonin and stable circadian rhythms optimize maternal, placental and fetal physiology. Hum Reprod Update 2014;20:293–307. [DOI] [PubMed] [Google Scholar]
- Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci USA 2010;107:20828–20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 2002;277:5209–5218. [DOI] [PubMed] [Google Scholar]
- Robinson BG, Emanuel RL, Frim DM, Majzoub JA. Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc Natl Acad Sci USA 1988;85:5244–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 2011;192:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160:1117–1123. [DOI] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Alpay SZ, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med 2011;24:1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaiworapongsa T, Savasan ZA, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, Hassan SS. Clinical chorioamnionitis is characterized by changes in the expression of the alarmin HMGB1 and one of its receptors, sRAGE. J Matern Fetal Neonatal Med 2012;25:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007;25:21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006;11:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Friel LA, Velez Edwards DR, Kusanovic JP, Hassan SS, Mazaki-Tovi S, Vaisbuch E, Kim CJ, Erez O, Chaiworapongsa T et al. A genetic association study of maternal and fetal candidate genes that predispose to preterm prelabor rupture of membranes (PROM). Am J Obstet Gynecol 2010;203:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 2014. a;1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014. b;72:458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Munoz H, Gomez R, Parra M, Polanco M, Valverde V, Hasbun J, Garrido J, Ghezzi F, Mazor M et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids 1996;54:187–191. [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007;8:519–529. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Stretching is good for a cell. Science (New York, NY) 1997;276:1345–1346. [DOI] [PubMed] [Google Scholar]
- Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J Immunol 2003;170:3233–3242. [DOI] [PubMed] [Google Scholar]
- Saito I, Takayama M, Nakamura T, Sugiyama H, Komeda Y, Iwasaki M. The most electron-donating sites in duplex DNA: guanine-guanine stacking rule. Nucleic Acids Symp Ser 1995;191–192. [PubMed] [Google Scholar]
- Sakamoto K, Kamimura M, Kurozumi S, Ito S. Prostaglandin F2 alpha receptor. J Lipid Mediat Cell Signal 1995;12:405–411. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Burris HH, Just AC, Motta V, Svensson K, Mercado-Garcia A, Pantic I, Schwartz J, Tellez-Rojo MM, Wright RO et al. microRNA expression in the cervix during pregnancy is associated with length of gestation. Epigenetics 2015;10:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither. Epidemiol Rev 2013;35:112–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L. Extracellular matrix molecules: endogenous danger signals as new drug targets in kidney diseases. Curr Opin Pharmacol 2010;10:185–190. [DOI] [PubMed] [Google Scholar]
- Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res 2010;339:237–246. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev 2008;29:317–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. The amniotic fluid compartment: the fetal habitat. Adv Anat Embryol Cell Biol 1992;127:1–100. [DOI] [PubMed] [Google Scholar]
- Schuit E, Stock S, Rode L, Rouse DJ, Lim AC, Norman JE, Nassar AH, Serra V, Combs CA, Vayssiere C et al. Effectiveness of progestogens to improve perinatal outcome in twin pregnancies: an individual participant data meta-analysis. BJOG 2015;122:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulpis KH, Lazaropoulou C, Vlachos GD, Partsinevelos GA, Michalakakou K, Gavrili S, Gounaris A, Antsaklis A, Papassotiriou I. Maternal-neonatal 8-hydroxy-deoxyguanosine serum concentrations as an index of DNA oxidation in association with the mode of labour and delivery. Acta Obstet Gynecol Scand 2007;86:320–326. [DOI] [PubMed] [Google Scholar]
- Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 2004;4:469–478. [DOI] [PubMed] [Google Scholar]
- Sharkey JT, Puttaramu R, Word RA, Olcese J. Melatonin synergizes with oxytocin to enhance contractility of human myometrial smooth muscle cells. J Clin Endocrinol Metab 2009;94:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasuna K, Shimizu T, Hayashi KG, Nagai K, Matsui M, Miyamoto A. Positive association, in local release, of luteal oxytocin with endothelin 1 and prostaglandin F2alpha during spontaneous luteolysis in the cow: a possible intermediatory role for luteolytic cascade within the corpus luteum. Biol Reprod 2007;76:965–970. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Dorogin A, Lye SJ. Stretch-induced uterine myocyte differentiation during rat pregnancy: involvement of caspase activation. Biol Reprod 2010;82:1248–1255. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci 2013;20:154–167. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Oldenhof A, Dorogin A, Xu Q, Mu J, Nashman N, Lye SJ. Myometrial apoptosis: activation of the caspase cascade in the pregnant rat myometrium at midgestation. Biol Reprod 2006;74:839–849. [DOI] [PubMed] [Google Scholar]
- Shynlova O, Williams SJ, Draper H, White BG, Macphee DJ, Lye SJ. Uterine stretch regulates temporal and spatial expression of fibronectin protein and its alpha 5 integrin receptor in myometrium of unilaterally pregnant rats. Biol Reprod 2007;77:880–888. [DOI] [PubMed] [Google Scholar]
- Siiteri PK, Febres F, Clemens LE, Chang RJ, Gondos B, Stites D. Progesterone and maintenance of pregnancy: is progesterone nature's immunosuppressant. Ann NY Acad Sci 1977;286:384–397. [DOI] [PubMed] [Google Scholar]
- Silva E, Soares AI, Costa F, Castro JP, Matos L, Almeida H. Antioxidant supplementation modulates age-related placental bed morphology and reproductive outcome in mice. Biol Reprod 2015;15:127746. [DOI] [PubMed] [Google Scholar]
- Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, Claas FH. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol 2004;62:125–137. [DOI] [PubMed] [Google Scholar]
- Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept 2002;108:159–164. [DOI] [PubMed] [Google Scholar]
- Snegovskikh VV, Schatz F, Arcuri F, Toti P, Kayisli UA, Murk W, Guoyang L, Lockwood CJ, Norwitz ER. Intra-amniotic infection upregulates decidual cell vascular endothelial growth factor (VEGF) and neuropilin-1 and -2 expression: implications for infection-related preterm birth. Reprod Sci 2009;16:767–780. [DOI] [PubMed] [Google Scholar]
- So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci USA 2009;106:17582–17587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EW, Glazer GL, Sanduleak N. Influence of the full and new moon on onset of labor and spontaneous rupture of membranes. J Nurse Midwifery 1988;33:57–61. [DOI] [PubMed] [Google Scholar]
- Stewart SA, Ben-Porath I, Carey VJ, O'Connor BF, Hahn WC, Weinberg RA. Erosion of the telomeric single-strand overhang at replicative senescence. Nat Genet 2003;33:492–496. [DOI] [PubMed] [Google Scholar]
- Stjernholm Y, Sahlin L, Akerberg S, Elinder A, Eriksson HA, Malmstrom A, Ekman G. Cervical ripening in humans: potential roles of estrogen, progesterone, and insulin-like growth factor-I. Am J Obstet Gynecol 1996;174:1065–1071. [DOI] [PubMed] [Google Scholar]
- Stout MJ, Conlon B, Landeau M, Lee I, Bower C, Zhao Q, Roehl KA, Nelson DM, Macones GA, Mysorekar IU. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol 2013;208:226–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Cutolo M. Circadian rhythms in rheumatoid arthritis: implications for pathophysiology and therapeutic management. Arthritis Rheum 2007;56:399–408. [DOI] [PubMed] [Google Scholar]
- Strohl A, Kumar D, Novince R, Shaniuk P, Smith J, Bryant K, Moore RM, Novak J, Stetzer B, Mercer BM et al. Decreased adherence and spontaneous separation of fetal membrane layers--amnion and choriodecidua--a possible part of the normal weakening process. Placenta 2010;31:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Brockman D, Campos B, Pitzer B, Myatt L. Induction of surfactant protein A expression by cortisol facilitates prostaglandin synthesis in human chorionic trophoblasts. J Clin Endocrinol Metab 2006;91:4988–4994. [DOI] [PubMed] [Google Scholar]
- Sun K, Myatt L. Enhancement of glucocorticoid-induced 11beta-hydroxysteroid dehydrogenase type 1 expression by proinflammatory cytokines in cultured human amnion fibroblasts. Endocrinology 2003;144:5568–5577. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Odani N, Tomokiyo K, Furuta K, Suzuki M, Ichikawa A, Negishi M. Localization of a cyclopentenone prostaglandin to the endoplasmic reticulum and induction of BiP mRNA. Biochem J 1998;335:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanami-Ohnishi Y, Asada S, Tsunoda H, Fukamizu A, Goto K, Yoshikawa H, Kubo T, Sudo T, Kimura S, Kasuya Y. Possible involvement of p38 mitogen-activated protein kinase in decidual function in parturition. Biochem Biophys Res Commun 2001;288:1155–1161. [DOI] [PubMed] [Google Scholar]
- Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab 2012;97:E719–E730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Rong G, Bu Y, Zhang S, Zhang M, Zhang J, Liang X. Advanced oxidation protein products induce hypertrophy and epithelial-to-mesenchymal transition in human proximal tubular cells through induction of endoplasmic reticulum stres. 2015;35:816–828. [DOI] [PubMed] [Google Scholar]
- Teasdale F. Gestational changes in the functional structure of the human placenta in relation to fetal growth: a morphometric study. Am J Obstet Gynecol 1980;137:560–568. [DOI] [PubMed] [Google Scholar]
- Tessier-Prigent A, Willems R, Lagarde M, Garrone R, Cohen H. Arachidonic acid induces differentiation of uterine stromal to decidual cells. Eur J Cell Biol 1999;78:398–406. [DOI] [PubMed] [Google Scholar]
- Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, Greer IA, Norman JE. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod 1999;14:229–236. [PubMed] [Google Scholar]
- Ticconi C, Zicari A, Belmonte A, Realacci M, Rao C, Piccione E. Pregnancy-promoting actions of HCG in human myometrium and fetal membranes. Placenta 2007;28:S137–S143. [DOI] [PubMed] [Google Scholar]
- Tribe RM, Crawshaw SE, Seed P, Shennan AH, Baker PN. Pulsatile versus continuous administration of oxytocin for induction and augmentation of labor: two randomized controlled trials. Am J Obstet Gynecol 2012;206:230–238. [DOI] [PubMed] [Google Scholar]
- Tsang KY(1), Chan D, Bateman JF, Cheah KS. In vivo cellular adaptation to ER stress: survival strategies with double-edged consequences. J Cell Sci 2010;123:2145–2154. [DOI] [PubMed] [Google Scholar]
- Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol 2004;164:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung L, Mohamed MK, Hoeffler JP, Takimoto GS, Horwitz KB. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol Endocrinol 1993;7:1256–1265. [DOI] [PubMed] [Google Scholar]
- Uldbjerg N, Ulmsten U. The physiology of cervical ripening and cervical dilatation and the effect of abortifacient drugs. Baillieres Clin Obstet Gynaecol 1990;4:263–282. [DOI] [PubMed] [Google Scholar]
- Ulug U, Goldman S, Ben-Shlomo I, Shalev E. Matrix metalloproteinase (MMP)-2 and MMP-9 and their inhibitor, TIMP-1, in human term decidua and fetal membranes: the effect of prostaglandin F(2alpha) and indomethacin. Mol Hum Reprod 2001;7:1187–1193. [DOI] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 2000;287:664–666. [DOI] [PubMed] [Google Scholar]
- Vacca P, Montaldo E, Vitale C, Croxatto D, Moretta L, Mingari MC. MSC and innate immune cell interactions: a lesson from human decidua. Immunol Lett 2015;15:00074–00077. [DOI] [PubMed] [Google Scholar]
- Vadillo-Ortega F, Gonzalez-Avila G, Furth EE, Lei H, Muschel RJ, Stetler-Stevenson WG, Strauss JF III. 92-kd type IV collagenase (matrix metalloproteinase-9) activity in human amniochorion increases with labor. Am J Pathol 1995;146:148–156. [PMC free article] [PubMed] [Google Scholar]
- Vega-Sanchez R, Arenas-Hernandez M, Vazquez-Perez JA, Moreno-Valencia Y, Gomez-Lopez N. Evaluation of reference genes for expression studies in leukocytes from term human pregnancy. Placenta 2015;36:240–245. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol 1993;7:1244–1255. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Demaria M, Campisi J. Senescent cells and their secretory phenotype as targets for cancer therapy. Interdiscip Top Gerontol 2013;38:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venereau E, Schiraldi M, Uguccioni M, Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol 2013;55:76–82. [DOI] [PubMed] [Google Scholar]
- von ZT, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med 2005;5:197–203. [DOI] [PubMed] [Google Scholar]
- Walsh SW, Stanczyk FZ, Novy MJ. Daily hormonal changes in the maternal, fetal, and amniotic fluid compartments before parturition in a primate species. J Clin Endocrinol Metab 1984;58:629–639. [DOI] [PubMed] [Google Scholar]
- Wang B, Koga K, Osuga Y, Hirata T, Saito A, Yoshino O, Hirota Y, Harada M, Takemura Y, Fujii T et al. High mobility group box 1 (HMGB1) levels in the placenta and in serum in preeclampsia. Am J Reprod Immunol 2011;66:143–148. [DOI] [PubMed] [Google Scholar]
- Wang B, Parobchak N, Rosen M, Roche N, Rosen T. Negative effects of progesterone receptor isoform-A on human placental activity of the noncanonical NF-kappaB signaling. J Clin Endocrinol Metab 2014;99:E320–E328. [DOI] [PubMed] [Google Scholar]
- Wei LL, Horwitz KB. The structure of progesterone receptors. Steroids 1985;46:677–695. [DOI] [PubMed] [Google Scholar]
- Williams KC, Renthal NE, Condon JC, Gerard RD, Mendelson CR. MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor. Proc Natl Acad Sci USA 2012. a;109:7529–7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Renthal NE, Gerard RD, Mendelson CR. The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol Endocrinol 2012. b;107:1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci 2011;13:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Kutzler L, Kimball SR, Tabas I. Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat Cell Biol 2012;14:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med 2007;25:69–79. [DOI] [PubMed] [Google Scholar]
- Yanai H, Ban T, Taniguchi T. High-mobility group box family of proteins: ligand and sensor for innate immunity. Trends Immunol 2012;33:633–640. [DOI] [PubMed] [Google Scholar]
- Yang Y, Jin Y, Martyn AC, Lin P, Song Y, Chen F, Hu L, Cui C, Li X, Li Q et al. Expression pattern implicates a potential role for luman recruitment factor in the process of implantation in uteri and development of preimplantation embryos in mice. J Reprod Dev 2013;59:245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon SM, Burns AE, See JL, Lechuga TJ, Kirby MA. Progesterone withdrawal promotes remodeling processes in the nonpregnant mouse cervix. Biol Reprod 2009;81:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokouchi M, Hiramatsu N, Hayakawa K, Okamura M, Du S, Kasai A, Takano Y, Shitamura A, Shimada T, Yao J et al. Involvement of selective reactive oxygen species upstream of proapoptotic branches of unfolded protein response. J Biol Chem 2008;283:4252–4260. [DOI] [PubMed] [Google Scholar]
- Young IR, Renfree MB, Mesiano S, Shaw G, Jenkin G, Smith R. The comparative physiology of parturition in mammals: hormones and parturition in mammals In: Norris D and Lopez K (eds). Hormones and Reproduction in Vertebrates 2010, Academic Press, London. [Google Scholar]
- Zhang WW, Klotz LH. Telomerase assay in renal cancer. Methods Mol Med 2001;53:55–67. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Koga K, Osuga Y, Izumi G, Takamura M, Harada M, Hirata T, Hirota Y, Yoshino O, Inoue S et al. Cyclic stretch augments production of neutrophil chemokines and matrix metalloproteinases-1 (MMP-1) from human decidual cells, and the production was reduced by progesterone. Am J Reprod Immunol 2013;69:454–462. [DOI] [PubMed] [Google Scholar]
- Zhou WH, Du MR, Dong L, Yu J, Li DJ. Chemokine CXCL12 promotes the cross-talk between trophoblasts and decidual stromal cells in human first-trimester pregnancy. Hum Reprod 2008;23:2669–2679. [DOI] [PubMed] [Google Scholar]
- Zicari A, Centonze C, Realacci M, Buchetti B, Pietropolli A, Ticconi C. Estradiol 17-beta and progesterone modulate inducible nitric oxide synthase and high mobility group box 1 expression in human endometrium. Reprod Sci 2008;15:559–566. [DOI] [PubMed] [Google Scholar]