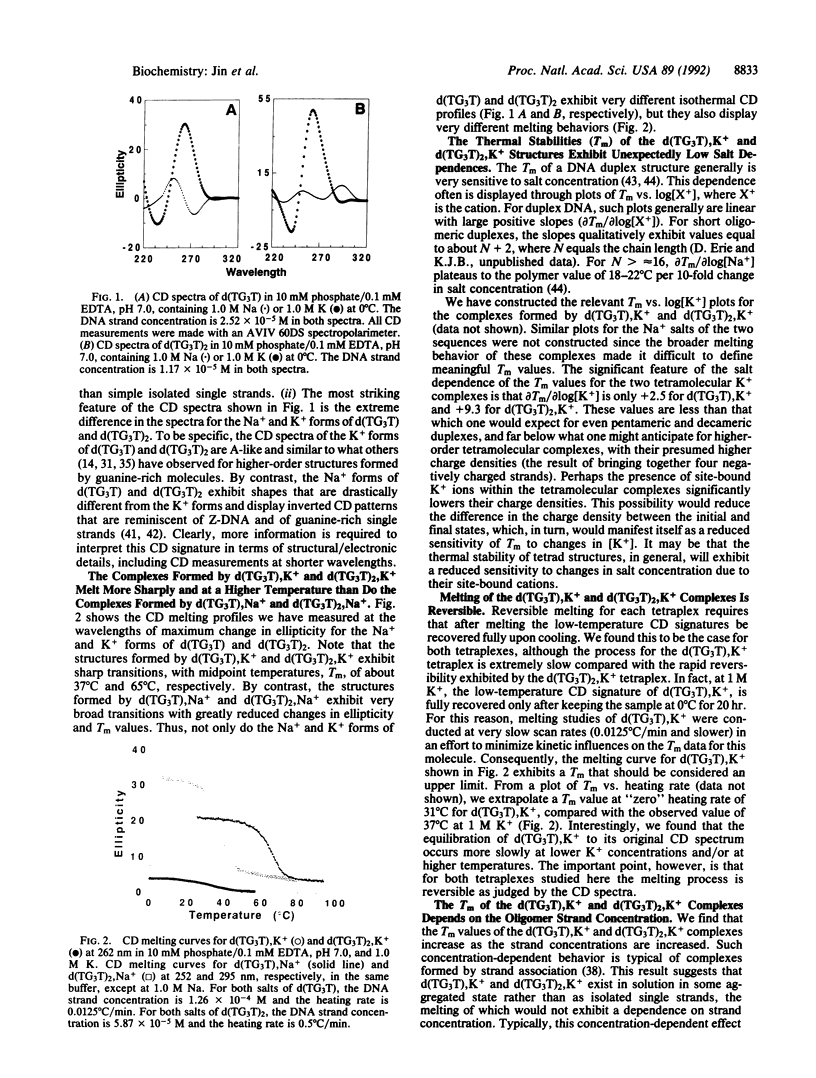
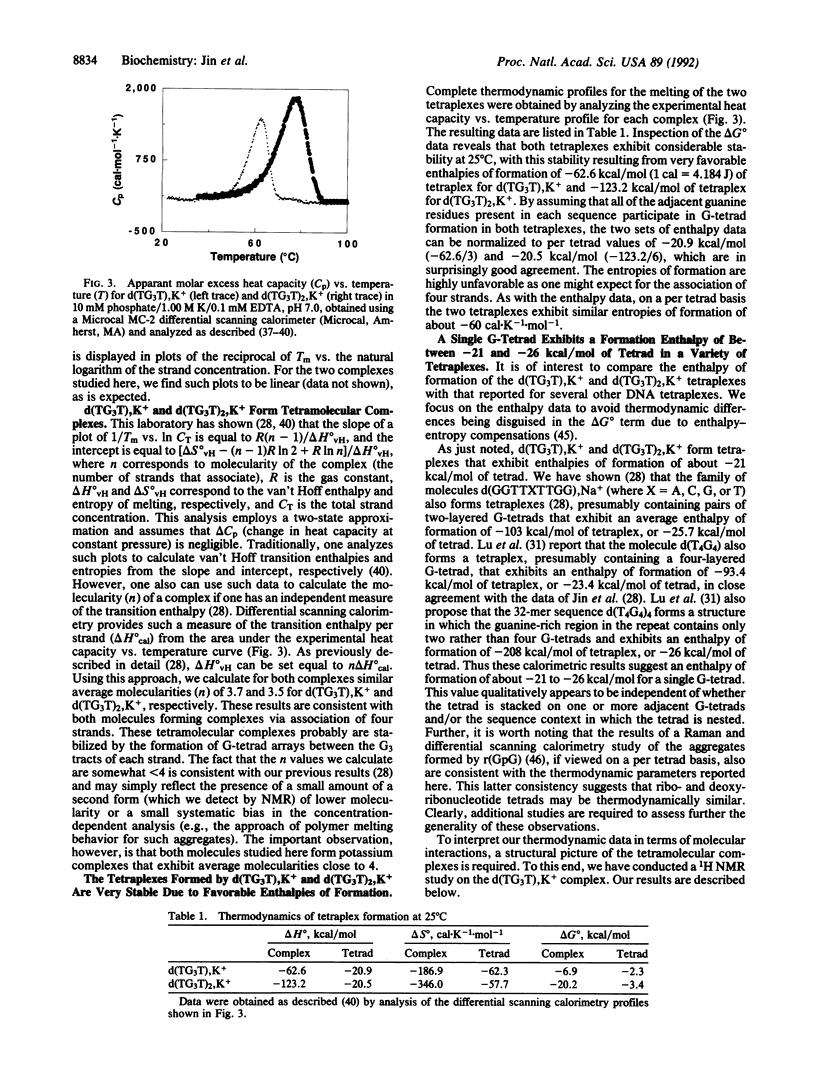
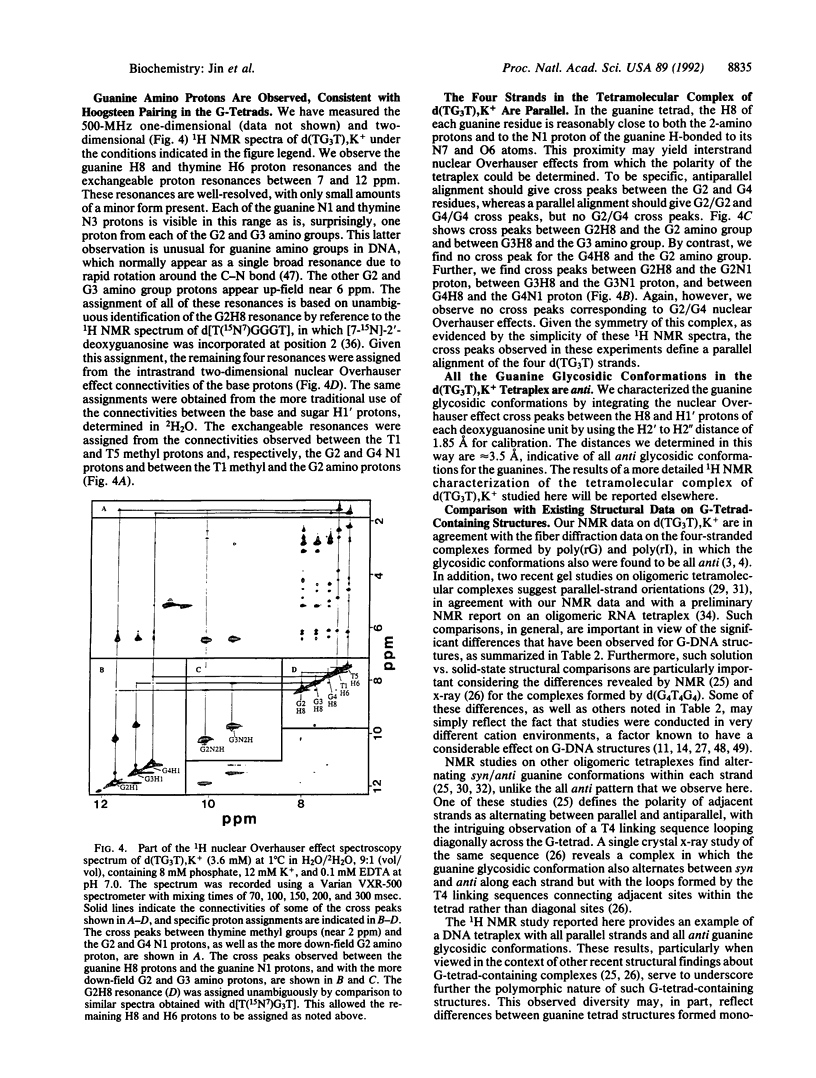
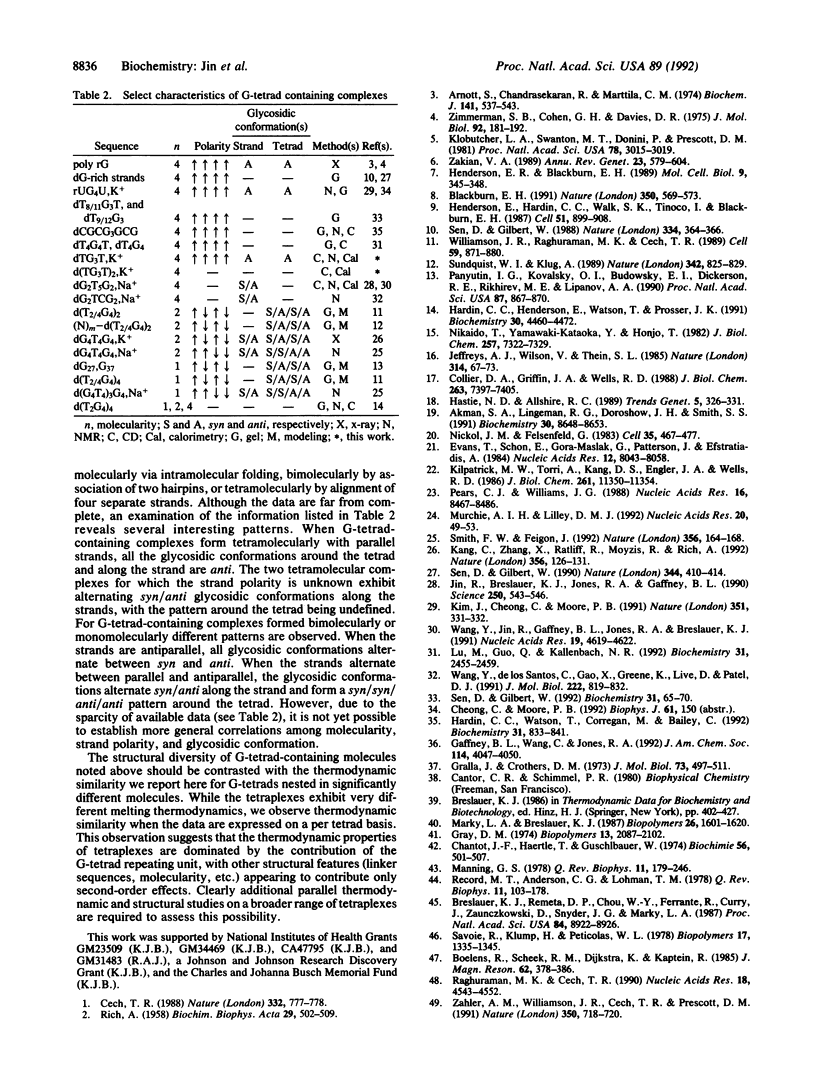
Abstract
We report a combined thermodynamic and structural characterization of a DNA tetraplex. Using spectroscopic and calorimetric techniques, we demonstrate that d(TG3T) and d(TG3T2G3T), in the presence of K+, form stable tetramolecular complexes. From differential scanning calorimetry measurements, we obtain the following thermodynamic profiles for formation of each tetraplex at 25 degrees C: delta G degrees = -6.9 kcal/mol of tetraplex (or -2.3 kcal/mol of tetrad; 1 cal = 4.184 J), delta H degrees = -62.6 kcal/mol of tetraplex (or -20.9 kcal/mol of tetrad), and delta S degrees = -186.9 cal.K-1.mol-1 of tetraplex (or -62.3 cal.K-1.mol-1 of tetrad) for the d(TG3T) tetraplex; and delta G degrees = -20.2 kcal/mol of tetraplex (or -3.4 kcal/mol of tetrad), delta H degrees = -123.2 kcal/mol of tetraplex (or -20.5 kcal/mol of tetrad), and delta S degrees = -346.0 cal.K-1.mol-1 of tetraplex (or -57.7 cal.K-1.mol-1 of tetrad) for the d(TG3T2G3T) tetraplex. These data demonstrate that at 25 degrees C a G-tetrad can exhibit considerable stability, comparable to or even exceeding that of most Watson-Crick nearest-neighbor interactions, with this stability resulting from a very favorable enthalpy of formation. Temperature-dependent CD measurements reveal that the melting temperatures of both tetraplexes exhibit unusually low salt dependences. This unexpected behavior may reflect a diminished charge density due to bound K+ ions. For each complex, the Na+ and K+ forms exhibit drastically different isothermal and temperature-dependent CD profiles, with the K+ forms of each tetraplex melting more sharply and at a higher temperature than the Na+ forms. Using one- and two-dimensional NMR techniques, we show that the strands in the tetramolecular complex of d(TG3T), K+ are all parallel and that the guanine glycosidic conformations are all anti.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akman S. A., Lingeman R. G., Doroshow J. H., Smith S. S. Quadruplex DNA formation in a region of the tRNA gene supF associated with hydrogen peroxide mediated mutations. Biochemistry. 1991 Sep 3;30(35):8648–8653. doi: 10.1021/bi00099a022. [DOI] [PubMed] [Google Scholar]
- Arnott S., Chandrasekaran R., Marttila C. M. Structures for polyinosinic acid and polyguanylic acid. Biochem J. 1974 Aug;141(2):537–543. doi: 10.1042/bj1410537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Breslauer K. J., Remeta D. P., Chou W. Y., Ferrante R., Curry J., Zaunczkowski D., Snyder J. G., Marky L. A. Enthalpy-entropy compensations in drug-DNA binding studies. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8922–8926. doi: 10.1073/pnas.84.24.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R. G-strings at chromosome ends. Nature. 1988 Apr 28;332(6167):777–778. doi: 10.1038/332777a0. [DOI] [PubMed] [Google Scholar]
- Chantot J. F., Haertle T., Guschlbauer W. Nucleoside conformations. XIII. Circular dichroïsm of guanosine gels and the conformation of GpG and poly (G). Biochimie. 1974;56(4):501–507. doi: 10.1016/s0300-9084(74)80065-9. [DOI] [PubMed] [Google Scholar]
- Collier D. A., Griffin J. A., Wells R. D. Non-B right-handed DNA conformations of homopurine.homopyrimidine sequences in the murine immunoglobulin C alpha switch region. J Biol Chem. 1988 May 25;263(15):7397–7405. [PubMed] [Google Scholar]
- Evans T., Schon E., Gora-Maslak G., Patterson J., Efstratiadis A. S1-hypersensitive sites in eukaryotic promoter regions. Nucleic Acids Res. 1984 Nov 12;12(21):8043–8058. doi: 10.1093/nar/12.21.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Gray D. M. A circular dichroism study of poly dG, poly dC, and poly dG:dC. Biopolymers. 1974;13(10):2087–2102. doi: 10.1002/bip.1974.360131011. [DOI] [PubMed] [Google Scholar]
- Hardin C. C., Henderson E., Watson T., Prosser J. K. Monovalent cation induced structural transitions in telomeric DNAs: G-DNA folding intermediates. Biochemistry. 1991 May 7;30(18):4460–4472. doi: 10.1021/bi00232a013. [DOI] [PubMed] [Google Scholar]
- Hardin C. C., Watson T., Corregan M., Bailey C. Cation-dependent transition between the quadruplex and Watson-Crick hairpin forms of d(CGCG3GCG). Biochemistry. 1992 Jan 28;31(3):833–841. doi: 10.1021/bi00118a028. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Allshire R. C. Human telomeres: fusion and interstitial sites. Trends Genet. 1989 Oct;5(10):326–331. doi: 10.1016/0168-9525(89)90137-6. [DOI] [PubMed] [Google Scholar]
- Henderson E. R., Blackburn E. H. An overhanging 3' terminus is a conserved feature of telomeres. Mol Cell Biol. 1989 Jan;9(1):345–348. doi: 10.1128/mcb.9.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson E., Hardin C. C., Walk S. K., Tinoco I., Jr, Blackburn E. H. Telomeric DNA oligonucleotides form novel intramolecular structures containing guanine-guanine base pairs. Cell. 1987 Dec 24;51(6):899–908. doi: 10.1016/0092-8674(87)90577-0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Jin R. Z., Breslauer K. J., Jones R. A., Gaffney B. L. Tetraplex formation of a guanine-containing nonameric DNA fragment. Science. 1990 Oct 26;250(4980):543–546. doi: 10.1126/science.2237404. [DOI] [PubMed] [Google Scholar]
- Kang C., Zhang X., Ratliff R., Moyzis R., Rich A. Crystal structure of four-stranded Oxytricha telomeric DNA. Nature. 1992 Mar 12;356(6365):126–131. doi: 10.1038/356126a0. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M. W., Torri A., Kang D. S., Engler J. A., Wells R. D. Unusual DNA structures in the adenovirus genome. J Biol Chem. 1986 Aug 25;261(24):11350–11354. [PubMed] [Google Scholar]
- Kim J., Cheong C., Moore P. B. Tetramerization of an RNA oligonucleotide containing a GGGG sequence. Nature. 1991 May 23;351(6324):331–332. doi: 10.1038/351331a0. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Swanton M. T., Donini P., Prescott D. M. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3' terminus. Proc Natl Acad Sci U S A. 1981 May;78(5):3015–3019. doi: 10.1073/pnas.78.5.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Guo Q., Kallenbach N. R. Structure and stability of sodium and potassium complexes of dT4G4 and dT4G4T. Biochemistry. 1992 Mar 10;31(9):2455–2459. doi: 10.1021/bi00124a003. [DOI] [PubMed] [Google Scholar]
- Manning G. S. The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotides. Q Rev Biophys. 1978 May;11(2):179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]
- Marky L. A., Breslauer K. J. Calculating thermodynamic data for transitions of any molecularity from equilibrium melting curves. Biopolymers. 1987 Sep;26(9):1601–1620. doi: 10.1002/bip.360260911. [DOI] [PubMed] [Google Scholar]
- Murchie A. I., Lilley D. M. Retinoblastoma susceptibility genes contain 5' sequences with a high propensity to form guanine-tetrad structures. Nucleic Acids Res. 1992 Jan 11;20(1):49–53. doi: 10.1093/nar/20.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickol J. M., Felsenfeld G. DNA conformation at the 5' end of the chicken adult beta-globin gene. Cell. 1983 Dec;35(2 Pt 1):467–477. doi: 10.1016/0092-8674(83)90180-0. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Yamawaki-Kataoka Y., Honjo T. Nucleotide sequences of switch regions of immunoglobulin C epsilon and C gamma genes and their comparison. J Biol Chem. 1982 Jul 10;257(13):7322–7329. [PubMed] [Google Scholar]
- Panyutin I. G., Kovalsky O. I., Budowsky E. I., Dickerson R. E., Rikhirev M. E., Lipanov A. A. G-DNA: a twice-folded DNA structure adopted by single-stranded oligo(dG) and its implications for telomeres. Proc Natl Acad Sci U S A. 1990 Feb;87(3):867–870. doi: 10.1073/pnas.87.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears C. J., Williams J. G. Multiple copies of a G-rich element upstream of a cAMP-inducible Dictyostelium gene are necessary but not sufficient for efficient gene expression. Nucleic Acids Res. 1988 Sep 12;16(17):8467–8486. doi: 10.1093/nar/16.17.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICH A. The molecular structure of polyinosinic acid. Biochim Biophys Acta. 1958 Sep;29(3):502–509. doi: 10.1016/0006-3002(58)90005-2. [DOI] [PubMed] [Google Scholar]
- Raghuraman M. K., Cech T. R. Effect of monovalent cation-induced telomeric DNA structure on the binding of Oxytricha telomeric protein. Nucleic Acids Res. 1990 Aug 11;18(15):4543–4552. doi: 10.1093/nar/18.15.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M. T., Jr, Anderson C. F., Lohman T. M. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Q Rev Biophys. 1978 May;11(2):103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature. 1990 Mar 29;344(6265):410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Formation of parallel four-stranded complexes by guanine-rich motifs in DNA and its implications for meiosis. Nature. 1988 Jul 28;334(6180):364–366. doi: 10.1038/334364a0. [DOI] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Novel DNA superstructures formed by telomere-like oligomers. Biochemistry. 1992 Jan 14;31(1):65–70. doi: 10.1021/bi00116a011. [DOI] [PubMed] [Google Scholar]
- Smith F. W., Feigon J. Quadruplex structure of Oxytricha telomeric DNA oligonucleotides. Nature. 1992 Mar 12;356(6365):164–168. doi: 10.1038/356164a0. [DOI] [PubMed] [Google Scholar]
- Sundquist W. I., Klug A. Telomeric DNA dimerizes by formation of guanine tetrads between hairpin loops. Nature. 1989 Dec 14;342(6251):825–829. doi: 10.1038/342825a0. [DOI] [PubMed] [Google Scholar]
- Wang Y., Jin R., Gaffney B., Jones R. A., Breslauer K. J. Characterization by 1H NMR of glycosidic conformations in the tetramolecular complex formed by d(GGTTTTTGG). Nucleic Acids Res. 1991 Sep 11;19(17):4619–4622. doi: 10.1093/nar/19.17.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., de los Santos C., Gao X. O., Greene K., Live D., Patel D. J. Multinuclear nuclear magnetic resonance studies of Na cation-stabilized complex formed by d(G-G-T-T-T-T-C-G-G) in solution. Implications for G-tetrad structures. J Mol Biol. 1991 Dec 5;222(3):819–832. doi: 10.1016/0022-2836(91)90513-6. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Raghuraman M. K., Cech T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989 Dec 1;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Williamson J. R., Cech T. R., Prescott D. M. Inhibition of telomerase by G-quartet DNA structures. Nature. 1991 Apr 25;350(6320):718–720. doi: 10.1038/350718a0. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Cohen G. H., Davies D. R. X-ray fiber diffraction and model-building study of polyguanylic acid and polyinosinic acid. J Mol Biol. 1975 Feb 25;92(2):181–192. doi: 10.1016/0022-2836(75)90222-3. [DOI] [PubMed] [Google Scholar]





