Abstract
The reaction of [Fe(TMP)(OClO3)], where TMP is the dianion of tetramesitylporphyrin, with a combination of a strong π-acceptor ligand and a π-donating imidazole can lead to the preparation of mixed-ligand complexes [Fe(Porph)(4-CNPy)(L)]+ where L is imidazole itself or 1-acetylimidazole and 4-cyanopyridine is the strong π acceptor ligand. The stability of the new mixed-ligand pair is the presumed result of synergic bonding between the two axial ligands. The molecular structure and other characterization of the new mixed axial ligand complex, [Fe(TMP)(4-CNPy)(HIm)]ClO4 is described. The axial ligands have a relative perpendicular arrangement with Fe–N(imidazole) = 1.945 Å and Fe–N(pyridine) = 2.021 Å The average equatorial Fe–Np distance is 1.963 Å, which is consistent with the S4-ruffled TMP core. Despite the relative perpendicular arrangement of axial ligands, the EPR spectrum of the complex is a rhombic signal and not a large gmax signal. The EPR g-values are g1 = 3.05, g2 = 2.07, and g3 = 1.22. A quadrupole doublet was seen in the Mössbauer spectrum with an isomer shift of 0.197 mm/s and quadrupole splitting of 1.935 mm/s. Two crystalline forms of [Fe(TMP)(4-CNPy)(HIm)]ClO4 have been characterized; the two forms differ only in the solvent content of the lattice. Crystal data for form A: a = 15.432 (12) Å, b = 20.696 (2) Å, c = 19.970 (5) Å, and β = 99.256 (14)°, monoclinic, space group P21/n, V = 6295 (2) Å3, Z = 4, formula FeCl3O4N8C69H69, 8397 observed data, R1 = 0.086, wR2 = 0.210, refinement on F 2. Crystal data for form B: a = 15.267 (3) Å, b = 20.377 (6) Å, c = 19.670 (4) Å, and β = 98.14 (1)°, monoclinic, space group P21/n, V = 6058 (4) Å3, Z = 4, formula C65.25H60.5Cl1.5FeN8O4, 5464 observed data, R1 = 0.096, wR2 = 0.112, refinement on F.
Introduction
The electronic structures of the iron porphyrinates are rich and diverse. Such diversity includes, but is not limited to, a variety of oxidation states. Such richness is also mirrored the diversity of biological functions ranging from catalysis, electron transfer, to oxygen transfer and utilization [1].
For the bis-ligated iron(III) porphyrinates with two equivalent ligands, the effects of the axial ligand orientation are substantial and well understood. The absolute axial ligand orientation has been defined as the dihedral angle between the ligand plane and the plane defined by the donor atom, the iron and the porphyrinato nitrogen atom closest to the ligand (sometimes the Fe–Np bond direction is used instead). This angle is frequently called ϕ. The relative ligand orientation is defined as the dihedral angle between the two axial ligand planes. Most such dihedral angles are close to either close to 0° or 90°; these limiting forms are termed as the (relative) parallel or perpendicular orientation, respectively.
Changes in the absolute orientation of the two ligands can lead to the differing spin states of the iron with high-spin, intermediate-spin and low-spin species resulting that are clearly related to the absolute ligand orientations [2, 3]. In addition for low-spin species, the relative orientation of the two axial ligands has substantial effects on the energies of the three lowest energy d orbitals [4, 5, 6, 7, 8, 9, 10, 11].
We report in this paper strategies for the isolation and characterization of mixed axial ligand complexes of iron(III) porphyrinates, which to our knowledge have not been previously reported. We have used appropriately chosen imidazole and pyridine ligand pairs to accomplish this. In addition to their novelty, such mixed-ligand species are interesting because one can study both the orientation effects (particular orientation of the axial imidazole and pyridine with respect to the heme axes, and the relative orientation of the two ligand planes with respect to each other) and the crystal field effects (splitting of the d orbitals) of the axial ligands.
The major complex reported herein is [(imidazole)(4-cyanopyridine)(tetremesitylpoprhinato)-iron(III)] perchlorate, [Fe(TMP)(4-CNPy)(HIm)][ClO4]. [12] Two crystalline forms of this complex have been isolated that differ only in solvent content. Their preparation and characterization are described here.
Experimental Section
General Information
All reactions were performed under an argon atmosphere with Schlenkware and cannula techniques. All solvents were distilled under argon prior to use. Dichloromethane and hexane were distilled from CaH2 and sodium/benzophenone, respectively. 4-Cyanopyridine was recrystallized from diethyl ether and imidazole was recrystallized from dichloromethane. Tetramesitylporphyrin was prepared by a modified version of the procedure published by Lindsey et al.[13] and iron was inserted into H2TMP [12] by standard techniques [14]. [Fe(TMP)OClO3] was prepared as previously described. [6] Caution! These perchlorate salts can detonate spontaneously and should be handled only in small quantities; other safety precautions are also warranted. UV-vis spectra were recorded on a Perkin-Elmer Lambda 6 spectrophotometer. Mössbauer samples were prepared from ground single-crystal samples as mulls in Apiezon L grease, as previously described [7]. Mössbauer measurements were made on a constant acceleration spectrometer. The spectra were fitted with Lorentzian line shapes. Isomer shifts are quoted relative to metallic iron at room temperature.
Synthesis of [Fe(TMP)(4-CNPy)(HIm)][ClO 4 ]
At least two crystalline forms of [Fe-(TMP)(4-CNPy)(HIm)]ClO4 have been prepared by slight variation in crystallization procedures. Crystal form B was synthesized as follows: [Fe(TMP)OClO3] (30 mg, 0.032 mmol) and 4-CNPy (20 mg, 0.192 mmol) were placed in an argon-purged, 15 × 1.5 cm test tube. Dichloromethane (2 mL) was added and the solution was stirred for 3 min. Imidazole (2.4 mg, 1.35 mmol) was added and stirred for an additional 3 min. The solution was then layered with 6 mL of hexane. Crystal form A was subsequently prepared in the following manner: [Fe(TMP)OClO3] (120 mg, 0.127 mmol) and 4-CNPy (79.5 mg, 0.763 mmol) were placed in a 25-mL Schlenk flask. Dichloromethane (~8 mL) was added and the solution was stirred for 10 minutes. The UV-vis (CH2Cl2) spectrum (410, 534, 571.5 (sh) nm) is that of [Fe(TMP)(4-CNPy)2][ClO4]. [6] A dichloromethane solution of HIm (0.127 mmol) was added to the solution with syringe and stirred for an additional 20 minutes. A new UV-vis spectrum results (CH2Cl2) λmax: 414 (Soret), 546, 580 (sh) nm. The reaction mixture was transferred to four 15 × 1.5 cm test tubes and layered with 15 mL of hexane. X-ray quality crystals formed after 4 days.
X-ray Structure Determinations
Two black, crystalline forms of [Fe(TMP)(4-CNPy)-(HIm)]ClO4 were examined on an Enraf-Nonius FAST area detector diffractometer at 127 K with graphite-monochromated MoKα radiation. Unit cell determination and data collection procedures with the area detector have been described previously [15]. A summary of cell constants and refinement results is given in Table 1; complete details are given in Table S1. Crystal form B was the first form investigated, but form A also confirms the preparation of the same mixed-ligand species. Slight variations in data collection instrument settings were used owing to differing crystal quality. Both data sets were corrected for Lorentz-polarization and absorption effects [16]. The structures were solved by Patterson methods with the shelxs program [18]. During the course of structure solution and refinement, the solvent content of form B was found to be a single, partially occupied, methylene chloride molecule, located near an inversion center. This leads to the idealized formula [Fe(TMP)(4-CNPy)(HIm)]ClO4·1/4CH2Cl2 for form B. Similarly, the formula for form A was established during structure solution as [Fe(TMP)(4-CNPy)(HIm)]ClO4·CH2Cl2·1/2C6H14.
Table 1.
Brief Crystallographic Data and Data Collection Parameters for the two forms of [Fe(TMP)(4-CNPy)(HIm)]ClO4.
| Molecule | [Fe(TMP)(4-CNPy)(HIm)]ClO4
CH2Cl2·0.5C6H14 Form A |
[Fe(TMP)(4-CNPy)(HIm)]ClO4
0.25CH2Cl2 Form B |
|---|---|---|
| formula | C69H69Cl3FeN8O4 | C65.25H60.5Cl1.5FeN8O4 |
| FW, amu | 1236.57 | 1129.78 |
| a, Å | 15.4318 (12) | 15.267 (3) |
| b, Å | 20.696 (2) | 20.377 (6) |
| c, Å | 19.970 (5) | 19.670 (4) |
| β, deg | 99.256 (14) | 98.14 (1) |
| V, Å3 | 6295 (2) | 6058 (4) |
| space group | P2 1 /n | P2 1 /n |
| Z | 4 | 4 |
| Dc, g/cm3 | 1.30 | 1.24 |
| μ, mm−1 | 0.420 | 0.365 |
| radiation, MoKα, | 0.71073 Å | 0.71073 Å |
| temperature, K | 127(2) | 127(2) |
| unique data | 16241 | 13051 |
| unique observed data [I > 2σ(I)] | 8397 | 5464 |
| refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F |
| final R indices [I > 2σ(I)] | R1 = 0.086, ωR2 = 0.210 | R1 = 0.096, ωR2 = 0.112 |
| final R indices (all data) | R1 = 0.168, ωR2 = 0.262 |
Least-squares refinement of the structural model for form B was carried out with a traditional refinement on F using the “observed” data, while that for form A was on F2 using all the unique, measured data including the reflections with negative intensities. In both structures, all nonhydrogen atoms were refined with atomic displacement factors. Hydrogen atoms were included as fixed, idealized contributors. The structures were then refined to convergence with the discrepancy indices listed in Table 1. Since the crystal and data quality of form A proved to be superior to that of form B and the structures are essentially identical, we report more details for form A herein. Complete sets of atomic coordinates, atomic displacement factors, bond distances, and bond angle tabulations for both A and B forms are given in the Supplementary Information.
Results
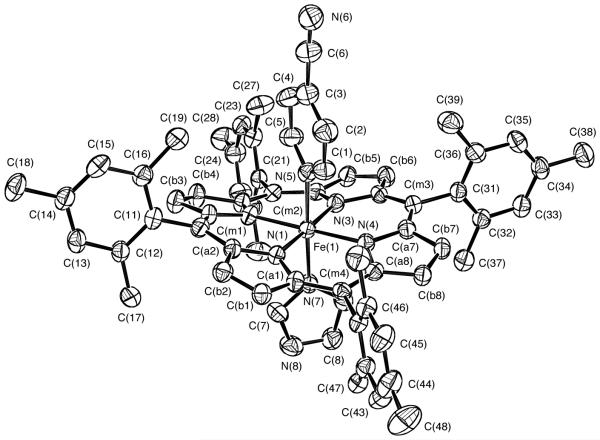
We have prepared the mixed axial ligand complex, [Fe(TMP)(4-CNPy)(HIm)]ClO4, as a crystalline solid and have characterized it by single-crystal X-ray structure determinations and by Mössbauer and EPR spectroscopies. [Fe(TMP)(4-CNPy)(HIm)]ClO4 has been obtained in two crystalline forms which we call form A and form B; both have been characterized by single-crystal X-ray structure determinations. As we will later discuss, the two forms differ only in solvent content of the lattice. Hence we report details for form A only and summary information for form B. The molecular structure of form A is shown in Figure 1 which also illustrates the atom labeling scheme used for all tables. The ortep diagram shows the interesting features of the molecule, viz., that there are two nonequivalent ligands and that the two planar axial ligands have a relative perpendicular orientation. The actual dihedral angle between the two axial ligands is 85.4°. (In form B, this angle is 84.9°.) The projection of the imidazole ligand plane onto the porphyrin plane makes an angle of 42° with the closest Fe–Np vector; the corresponding value for the pyridine ligand is 38.2°. These angles are frequently denoted by the symbol ϕ.
Figure 1.
ORTEP diagram of [Fe(TMP)(4-CNPy)(HIm)]ClO4(Crystal form A). Thermal ellipsoids are drawn at the 50% probability level. Porphyrin hydrogen atoms have been omitted for clarity.
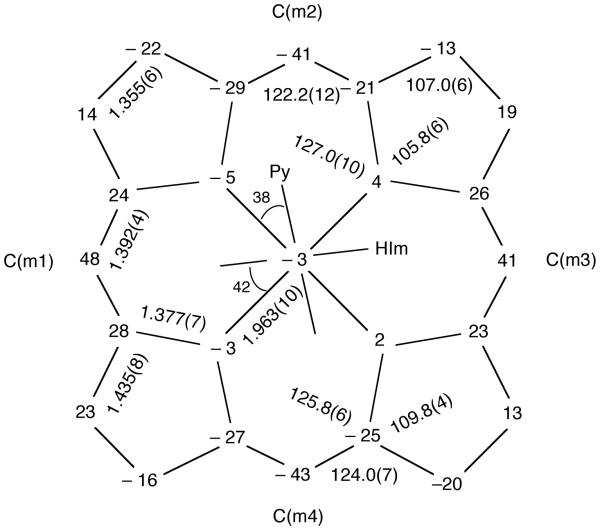
Consistent with observations of [FeIII(TMP)L2]+ derivatives having axial ligands with relative perpendicular orientations, the porphinato core exhibits an S4-ruffling, which is illustrated in the formal diagram of Figure 2. This figure displays the perpendicular displacements of each atom (in units of 0.01Å) from the mean plane of the core. Also consistent with the ruffled core are the relatively short equatorial Fe–Np bond distances which average to 1.963 (10) Å. The axial (FeIII-N) distances are 2.021 (4) Å to the pyridine ligand and 1.945 (4) Å to the imidazole ligand. (For form B, the axial (FeIII-N) distances are 2.026 (9) Å and 1.933 (8) Å, respectively). The axial N–Fe–N angle is 177.6 (2)°; the Nax–Fe–Np angles range from 88.4 (2) to 92.4 (2)°. Individual values of the bond distances and bond angles in form A are given in the Supporting Information and in the CIF file. Averaged values of the chemically equivalent bond distances and angles in the core are entered on Figure 2. The dihedral angles between the peripheral mesityl groups and the porphinato core are reasonably close to perpendicular (77.0, 88.5, 89.5, and 84.1°). Equivalent drawings for form B are given in Figures S1 and S2 of Supplementary Information.
Figure 2.
Formal diagram of the porphinato core in [Fe(TMP)(4-CNPy)(HIm)]ClO4 for form A displaying displacement of each unique atom from the 24-atom mean plane. All displacements are given in units of 0.01 Å. Negative values of atom displacements are towards the imidazole ligand. Also entered on the diagram are the averaged values of all bond distances and angles of the core. The orientations of the two planar ligands with respect to core atoms are also displayed.
A second example of a mixed-ligand system, [Fe(TMP)(4-CNPy)(1-AcIm)]ClO4, has also been obtained as a solid-state species but is less definitively characterized than the unsubstituted imidazole derivative. A combination of relatively poor crystal specimens and solvent disorder severely limit the quality of the structure determination [19]. However, the structural results are reasonably interpreted in terms of a mixed-ligand species with all structural features similar to those observed for the two forms of [Fe(TMP)(4-CNPy)(HIm)]ClO4.
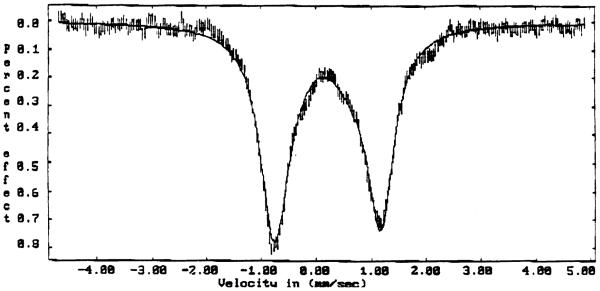
Mössbauer spectroscopic measurements have been made on form A of [Fe(TMP)(4-CNPy)-(1-AcIm)]ClO4 and on [Fe(TMP)(4-CNPy)(1-AcIm)]ClO4. The Mössbauer spectrum of polycrystalline form A taken at 170 K is shown in Figure 3. Form A has a quadrupole doublet with a splitting (ΔEq) of 1.935 (8) mm/s and an isomer shift (δ) of 0.197 (7) mm/s. The Mössbauer parameters for [Fe(TMP)(4-CNPy)(1-AcIm)]ClO4 are 1.82 (4) mm/s and an isomer shift of 0.18 (3). See Figure S3 of the Supporting Information.
Figure 3.
Mössbauer spectrum of [Fe(TMP)(4-CNPy)(HIm)]ClO4 (Crystal form A) at 170 K and in a field of 2.2 kG perpendicular to the γ beam. The solid line is a Lorentzian fit with parameters given in the text with a quadrupole splitting ΔEq = 1.935 mm/s and an isomer shift δ of 0.197 mm/s.
Discussion
Although there are a number of mixed-ligand species of the general form [FeIII(Porph)(L)(X)], where X is an anionic ligand and L a neutral nitrogen donor, there are to our knowledge no reports of the preparation of species of the general form [FeIII(Porph)(L)(L′)]+ where L and L′ are different neutral donors. The reaction of iron(III) porphyrinates with neutral axial nitrogen donors, L, has been long known to lead to six-coordinate complexes, [FeIII(Porph)L2]ClO4:
| (1) |
For any given ligand L, the first binding constant, K1, is generally much smaller than the second (K2) such that typically only logβ2 (K1K2) values can be measured [20, 21]. For neutral nitrogen donors the magnitude of the binding constant β2 is known to vary over a number of orders of magnitude and is also generally related to ligand pKa [20, 21]. In general, the differing values of binding constants suggest that for any arbitrarily chosen pair of potential ligands, one member of the ligand pair will have a much higher affinity for the iron(III) center that the other and the reaction will lead to the preparation of one of the possible bis-symmetrically ligated complexes. In particular, the binding constants for 4-cyanopyridine with iron(III) porphyrinates are known to be quite small, on the order of 108 smaller than imidazoles in DMF [21] and a 4-cyanopyridine ligand might thus be expected to be readily supplanted by almost any other neutral nitrogen donor.
However, our prior synthetic and structural work and that of others shows that there are large differences in the bonding characteristics of neutral nitrogen ligands ranging from strong π-donating to σ-donating to strong π-accepting character. Moreover, these differences have real effects on the electronic structure of the iron(III) center as revealed by EPR and Mössbauer spectroscopy in this work. Our strategy in the investigation of possible mixed-ligand species was to make use of the (expected) synergic bonding characteristics of differing axial ligands. In this study we used a π-accepting (4-cyanopyridine) and a σ- and π-donating (imidazole) combination. We first allowed up to two 4-CNPy ligands to bind to the iron(III) porphyrinate by using a modestly high concentration of that ligand in concentrated solution, followed by adding only one equivalent of the imidazole, which we hoped would thus only replace one of the 4-CNPy ligands. Clearly this strategy worked to produce the mixed-ligand complex.
The crystal structure determinations of the two forms of [Fe(TMP)(4-CNPy)(HIm)]ClO4 and the preliminary results for [Fe(TMP)(4-CNPy)(1-AcIm)]ClO4 clearly demonstrate that ferric porphyrinates with mixed, neutral nitrogen ligands can be prepared as solid-state species. Indeed, the relative perpendicular orientation of the mixed axial ligands is that expected for such a synergic π-bonding ligand pair where the π-accepting ligand interacts with the filled dxz orbital and the π-donating ligand with the orthogonal, half-filled dyz orbital.
UV-vis spectra and solution EPR spectra, taken under conditions as similar to our preparative reaction conditions as possible, provides evidence that a mixed-ligand species exists to a significant extent in solution. The spectra of solutions of [Fe(TMP)(OClO3)] containing, respectively, 10 equivalents of 4-CNPy, 10 equivalents of 4-CNPy and 1 equivalent of imidazole, or 10 equivalents of imidazole per equivalent of iron(III) are shown in Figure 4. The figure clearly shows that the spectrum of the solution containing both 4-CNPy and imidazole is not simply the sum of the two end species, suggesting the presence of a significant concentration of the mixed-ligand species in solution. The fact that any mixed-ligand complex forms at all in solution is remarkable given that the overall equilibrium constant (β2) for 4-CNPy or imidazole with [Fe(TMP)(OClO3)] differs by up to eight orders of magnitude.
Figure 4.
Electronic spectra of a 9.6× 10−6 M (300-500 nm) or 1.28 × 10−3 M (480-800 nm) CHCl3 solutions of [Fe(TMP)(OClO3)] containing, respectively, 10 equivalents of 4-CNPy(· · ·), 10 equivalents of imidazole (—), or 10 equivalents of 4-CNPy and 1 equivalent of imidazole (- - -), per equivalent of iron(III).
The structural features of [Fe(TMP)(4-CNPy)(HIm)]ClO4 are those generally expected for a low-spin iron(III) porphyrinate. The average Fe–Np distance of 1.963 (10) Å (form A) or 1.964 (12) Å (form B) are within the range of values observed for derivatives with significantly S4-ruffled cores and the magnitude of the ruffling is within the range observed previously for FeIIITMP derivatives [22].
We tabulate, for comparison, selected structural parameters for the known low-spin derivatives with at least one imidazole or pyridine as axial ligand in Table 2. The absolute orientation of the pyridine ligand (ϕ = 38.2°) is within the range (24–45°) apparently required to form low-spin pyridine complexes. The absolute orientation of imidazole ligands in observed in low-spin complexes encompasses the entire possible range of 0–45° and thus no particular absolute orientation is needed to form a low-spin imidazole complex (even with an S4-ruffled core). Thus there appears to be no steric constraints on either the absolute or relative imidazole orientation in order to form the low-spin complex [Fe(TMP)(4-CNPy)(HIm)]ClO4. We therefore believe that the relative perpendicular orientation of the imidazole and pyridine ligands in low-spin [Fe(TMP)(4-CNPy)(HIm)]ClO4 are the result of bonding considerations and are not required by steric considerations. The relative perpendicular orientation is in agreement with the bonding requirements of the π-acceptor ligand which requires a filled orbital (dxz) and the π-donor ligand which interacts with the orthogonal half-filled orbital (dyz).
Table 2.
Summary of Structural Parameters and EPR for Low-Spin Six-Coordinate Iron(III) Imidazole and Pyridine Derivatives.
| Complex | Fe–Npa | Fe–Naxa,b | ϕ c | relative orient.c,d |
porphyrin core conformation |
EPR Type |
Ref. |
|---|---|---|---|---|---|---|---|
| [Fe(TMP)(1,2-Me2HIm)2]ClO4 | 1.937 (12) | 2.004 (5) 2.004 (5) |
44 44 |
89 | S4-ruffled | NRe | 23 |
| [Fe(TPP)(2-MeHIm)2]ClO4 | 1.970 (4) | 2.010 (4) 2.015 (4) |
33 32 |
89 | S4-ruffled | gmax | 24 |
| [Fe(T2,6Cl2PP)(1-VinIm)2]ClO4f | 1.972 (6) | 1.968 (4) 1.976 (4) |
5 14, 20 |
6 76 |
S4-ruffled | rhombic gmax |
4 |
| [Fe(TPP)(1-MeIm)2]ClO4 | 1.982 (11) | 1.970 (3) 1.978 (3) |
32 22 |
10 | S4-ruffled | rhombic | 25 |
| [Fe(TMP)(1-MeIm)2]ClO4h | 1.987(1) 1.988(20) |
1.965 (3) 1.975 (3) |
42 23 |
0g 0g | planar | rhombic | 6 |
| [Fe(TPP)(HIm)2]Cl | 1.989 (8) | 1.957 (5) 1.991 (5) |
39 18 |
57 | S4-ruffled | NRe | 26 |
| [Fe(Proto IX)(1-MeIm)2] | 1.990 (16) | 1.966 (5) 1.988 (5) |
16 3 |
13 | S4-ruffled | NRe | 27 |
| [Fe(TPP)(tMU)2]SbF6 | 1.992 (5) | 1.983 (4) | 22 | 0g | planar | rhombic | 8 |
| [Fe(TPP)(cMU)2]SbF6h | 1.996 (10) | 1.967 (7) 1.979 (7) |
29 15 |
0g
0g |
planar planar |
rhombic | 8 |
| [Fe(TPP)(HIm)2]Cl.H2Oh | 1.993 (7) | 1.964 (3) 1.977 (3) |
41 6 |
0g
0g |
planar | rhombic | 28 |
| [Fe(TPP)(CuIm)2]+ | 2.00 (3) | 1.98 (1) | 7 | 9 | S4-ruffled | NRe | 29 |
| [Fe(TPP)(4-CNPy)2]ClO4 | 1.952 (7) | 2.008 (4) 1.997 (4) |
35 36 |
89 | S4-ruffled | axial | 9 |
| [Fe(TMP)(4-CNPy)2]ClO4 | 1.961 (6) | 2.001 (5) 2.021 (6) |
44 43 |
90 | S4-ruffled | axial | 10 |
| [Fe(TMP)(3-EtPy)2]ClO4 | 1.964 (4) | 1.989 (4) 2.002 (4) |
44 44 |
90 | S4-ruffled | gmax | 10 |
| [Fe(TMP)(3-ClPy)2]ClO4 | 1.968 (7) | 2.006 (7) 2.018 (7) |
42 29 |
77 | S4-ruffled | gmax | 10 |
| [Fe(TMP)(4-NMe2 Py)2]ClO4 | 1.964 (10) | 1.978 (4) 1.989 (4) |
42 37 |
79 | S4-ruffled | gmax | 6 |
| [Fe(OEP)(4-NMe2 Py)2]ClO4 | 2.002 (4) | 1.995 (3) | 36 | 0g | planar | rhombic | 6 |
| [Fe(TPP)(Py)2]ClO4 | 1.982 (6) | 2.001 (5) 2.005 (5) |
38 34 |
86 | S4-ruffled | gmax | 30 |
| tri-[Fe(OEP)(3-ClPy)2]ClO4 i | 1.995 (6) | 2.031 (2) | 41 | 0g | planar | NRe | 2 |
| [Fe(TMP)(4-CNPy)(HIm)]ClO4 j | 1.963 (10) | 1.954 (4) (Im) 2.021 (4) (Py) |
42 38 |
85 | S4-ruffled | rhombic | this work |
| [Fe(TMP)(4-CNPy)(HIm)]ClO4k | 1.964 (8) | 1.933 (8) (Im) 2.026 (9) (Py) |
44 41 |
85 | S4-ruffled | NRe | this work |
| [Fe(OEP)(1-MeIm)2]+ | 2.004 (2) | 1.975 (2) | 20 | 0g | planar | NR | 31 |
| paral-[Fe(TMP)(5-MeHIm)2]ClO4 | 1.983 (4) | 1.978 (6) 1.961 (5) |
20 10 |
30 | S4-ruffled | rhombic | 11 |
| paral-[Fe(TMP)(5-MeHIm)2]ClO4 | 1.981 (5) | 1.980 (5) 1.985 (5) |
12 14 |
26 | planar | rhombic | 11 |
| perp-[Fe(TMP)(5-MeHIm)2]ClO4 | 1.981 (7) | 1.957 (6) 1.973 (6) |
30 40 |
76 | S4-ruffled | gmax | 11 |
| [Fe(TPP)(5-MeHIm)2]Cl | 2.008 (2) | 1.975 (2) | 3.1 | 0 | planar | rhombic | 32 |
| [Fe(TPP)(5-MeHIm)2]Cl | 2.002 (2) | 1.987 (3) | 4.6 | 0 | planar | rhombic | 32 |
| paral-[Fe(OMTPP)(1-MeIm)2]Cl | 1.990 (2) | 1.975 (2) 2.016 (2) |
13 6 |
19 | S4-saddled | rhombic | 33 |
| perp-[Fe(OMTPP)(1-MeIm)2]Cl | 1.969 (7) | 1.982 (10) 1.982 (10) |
29 61 |
90 | S4-saddled | gmax | 33 |
| [Fe(OETPP)(1-MeIm)2]Cl | 1.970 (7) | 1.976 (3) 1.978 (3) |
10 17 |
73 | S4-saddled | gmax | 33 |
| [Fe(OETPP)(2-MeHIm)2]+ | 1.974 (9) | 2.09 (2) 2.09 (2) |
14 14 |
90 | S4-saddled | gmax | 34 |
| [Fe(OETPP)(4-Me2 Py) 2]Cl | 1.951 (5) | 1.984 (5) 2.015 (6) |
9 29 |
70 | S4-saddled | gmax | 34 |
| [Fe(OEP)(2-MeHIm)2]Cl | 1.974 (4) | 1.998 (2) 2.012 (2) |
40.8 43.4 |
87.6 | S4-ruffled | NR | 35 |
| [Fe(TiPrP)(BzHIm)2]+ | 1.915 (5) | 2.070 (5) | 45 44 |
90 | S4-ruffled | axial | 36 |
| [Fe(TiPrP)(HIm)2]+ | 1.938 (3) | 1.993 (3) | 45 45 |
~90 | S4-ruffled | axial | 36 |
| [Fe(TpivPP)(NO2)(HIm)] | 1.970 (4) | 2.037 (10) (Im) 1.949 (10) (NO2) |
16 37 |
69 | S4-ruffled | rhombic | 37 |
| [Fe(TPP)(CN)(Py)] | 1.970 (14) | 2.075 (3) (Py) | 40 | - | S4-ruffled | NR | 38 |
| [Fe(TPP)(NCS)(Py)] | 1.988 (9) | 2.082 (3) (Py) 1.942 (4) (NCS) |
39 | – | S4-ruffled | NR | 39 |
| [Fe(TPP)(N3)(Py)] | 1.989 (6) | 2.089 (6) (Py) 1.925 (7) (N3) |
40 | 10 | S4-ruffled | NR | 40 |
Values in Å.
All independent axial distances are listed.
Values in degrees.
Dihedral angle between pair of axial ligands.
Not Reported
Disordered structure with relative parallel and perpendicular imidazoles.
Value of 0° required by symmetry.
Two independent molecules.
Triclinic form at 100K.
Form A.
Form B
Axial bond distance comparisons are also consistent with the idea that the relative ligand orientation is controlled by bonding. The axial Fe-N(imidazole) distance of 1.945 (4) Å is shorter than that observed in any bis-imidazole iron(III) derivative (Table 2), while the axial Fe-N(pyridine) distance of 2.021 (4) is as long as or longer than the Fe-N(pyridine) distances observed in the bis-pyridine derivatives. The short Fe-N(imidazole) distance is most consistent with very strong π-bonding between the iron(III) and imidazole. The Fe–N(imidazole) distance is also much shorter than the 2.068 (4) Å distance found for the five-coordinate high-spin complex [Fe(OEP)(2-MeHIm)]ClO4 where strong axial bonding might be expected [42].
Although we have found and characterized two different crystalline forms of [Fe(TMP)(4-CNPy)(HIm)]ClO4, it is to be noted that the crystal structure, as well as the molecular structure, of both forms are quite similar. The cell packing diagrams for form A and form B (see Figure 5) show that the packing of [Fe(TMP)(4-CNPy)(HIm)]+ cations is essentially identical even though the solvent content in the two lattices clearly requires different cell volumes. Thus the two forms of [Fe(TMP)(4-CNPy)(HIm)]ClO4 represent an additional set of cases of lattice packing dominated by the large metalloporphyrin species [43, 44, 45, 46].
Figure 5.
Steroscopic packing diagrams of the forms of [Fe(TMP)(4-CNPy)(HIm)]ClO4. Top shows form A and the bottom shows form B.
The Mössbauer spectrum of polycrystalline form A taken at 170 K is shown in Figure 3. The spectrum illustrates a doublet with a quadrupole splitting of ΔEq = 1.935 (9) mm/s and an isomer shift δ of 0.189 (7) mm/s. The isomer shift is similar to values reported for other low-spin ferric hemes [5, 6, 10, 47]. Quadrupole splitting values less than ~ 1.75 mm/s are typical for low-spin iron(III) porphyrinates with axial ligands in the perpendicular orientation and a “pure” (dxy)2(dxz,dyz)3 ground state [5, 6, 10], while values greater than ~ 2.00 mm/s are typical for species with relative parallel orientations. [5, 6] With a quadrupole splitting value for form A in the middle (between) of the two limiting set of values, this is consistent with an interesting and possibly distinctive ground state. A final system with two strong π-accepting ligands ([Fe(TPP)(4-CNPy)2]ClO4) has a quadrupole splitting value of 0.65 mm/s and an axial EPR spectrum [9].
EPR spectra of low-spin bis-ligated iron(III) porphyrinates have been shown to be particularly informative about the electronic structure. The relative energies of the three d-orbitals lowest in energy can be determined from the EPR g-values utilizing the Taylor formulation [48, 49]. Moreover, the EPR spectral type provides additional information. The observed type of EPR spectrum for all complexes listed in Table 2 are also given in the table when the spectrum has been measured. When the two planar axial ligands have a relative parallel orientation, rhombic spectra, with three distinct g-values, are observed. This is the result of a modest energy difference between the dxy and dxz orbitals since both axial ligands interact with only one of the two. However, when the two axial ligands planes have a dihedral angle close to 90°, i.e., a relative perpendicular orientation, a spectral type called gmax is observed. In this case, the two d orbitals interact more or less equivalently with the perpendicularly aligned ligands, leading to a very small energy gap between the dxy and dxz orbitals. A third type, with two strong π-acceptor ligands such as isocyanides, leads to an axial spectrum being observed. This is the result of the dxy orbital becoming the highest energy orbital of the three t2g orbitals as the energies of two dπ orbitals are lowered because of the interaction with the π-acceptor ligands. We know of no case where a relative orienation of the two planar ligands leads to a rhombic EPR spectrum.
What type of EPR spectrum will [Fe(TMP)(4-CNPy)(HIm)]ClO4 display? From the data of Table 2, two distinct possibilities can be envisioned. The perpendicular orientation of the two ligands in [Fe(TMP)(4-CNPy)(HIm)]ClO4 suggests that a strong gmax spectrum would be expected. However, the expected synergic bonding of the two ligands, one π accepting and one π donating, leads to a different prediction. The axial ligands and the metal dxy and dxz orbitals are 90° apart. Thus, one ligand (the π acceptor) will interact with the filled dxz orbital and the other ligand (the π donor) will interact with the singly occupied dyz orbital to give the two orbitals differing energies and an expected rhombic EPR spectrum. Single-crystal EPR spectral measurements for [Fe(TMP)(4-CNPy)(HIm)]ClO4 reveal a rhombic spectrum with g values of 3.05, 2.07, and 1.22. This clearly shows that the dxz and dyz orbitals are separated in energy.
With the available g-values for [Fe(TMP)(4-CNPy)(HIm)]ClO4 the Taylor formalism [48] can now be used to evaluate the relative energies of the three lowest d orbitals. These values, expressed in terms of the energy of spin orbit coupling constant λ are shown on the right hand side of Figure 6. For comparison, the energies of a bis-ligated 1-methylimidazole complex is shown at the left [6]. Although the energy difference between the dxz and dyz orbitals is slightly smaller in the mixed-ligand complex, the two orbitals are clearly still well-separated.
Figure 6.
Diagram showing the relative energies of the three lowest d-orbitals for [Fe(TMP)(4-CNPy)(HIm)]ClO4 (right) and a comparison complex (left).
The axial bonding to iron was thus controlled using 4-CNPy as a strong π acceptor (poor base) with low affinity for the ferric porphyrinate, while HIm was used as a strong π donor (good base) with high affinity for the ferric porphyrinate. Thus the ligand pair chosen to obtain a synergic effect or a “push-pull” system was found to be a viable choice for a mixed axial ligand system.
Supplementary Material
Summary.
The reaction of [Fe(TMP)(OClO3)] with 6 equivalents of 4-CNPy followed by the addition of 1 equivalent of imidazole yields the mixed-ligand complex [Fe(TMP)(4-CNPy)(HIm)]ClO4. The two distinct ligands are found to have a relative perpendicular orientation that is consistent with a push-pull synergic effect of the strongly π-accepting ligand 4-CNPy and the π and σ donating imidazole ligand. This is also consistent with the Mössbauer and EPR spectra that show a classical (dxy)2(dxz,dyz)3 ground state.
Acknowledgments
We thank the National Institutes of Health for support of this research under Grant GM-38401. Funds for the purchase of the FAST area detector diffractometer was provided through NIH Grant RR-06709.
Footnotes
Supporting Information Available. ORTEP and formal diagrams for form B (Figures S1 and S2) and the Mössbauer spectrum for [Fe(TMP)(4-CNPy)(1-AcIm)]ClO4 (Figure S3). Crystallographic details for both forms A and B (Tables S1–11) are given in the supplementary material. This material is available free of charge via the Internet at http://www.worldscinet.-com/jpp/jpp.shtml. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under number CCDC 1432125. Copies can be obtained on request, free of charge, via www.ccdc.cam.ac.uk/data_request/cif or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 1223-336-033 or deposit@ccdc.cam.ac.uk).
References and Notes
- 1.Kadish KM, Smith K, Guilard R, editors. Handbook of Porphyrin Science. World Scientific; Singapore and Hackensack, NJ: 2012. [Google Scholar]
- 2.Scheidt WR, Geiger DK, Haller KJ. J. Am. Chem. Soc. 1982;104:495–499. [Google Scholar]
- 3.Scheidt WR, Geiger DK, Hayes RG, Lang G. J. Am. Chem. Soc. 1983;105:2625–2632. [Google Scholar]
- 4.Hatano K, Safo MK, Walker FA, Scheidt WR. Inorg. Chem. 1991;30:1643–1650. [Google Scholar]
- 5.Walker FA, Huynh BH, Scheidt WR, Osvath SR. J. Am. Chem. Soc. 1986;108:5288–5297. [Google Scholar]
- 6.Safo MK, Gupta GP, Walker FA, Scheidt WR. J. Am. Chem. Soc. 1991;113:5497–5510. [Google Scholar]
- 7.Scheidt WR, Osvath SR, Lee YJ, Reed CA, Schaevitz B, Gupta GP. Inorg. Chem. 1989;28:1591–1595. [Google Scholar]
- 8.Quinn R, Valentine JS, Byrn MP, Strouse CE. J. Am. Chem. Soc. 1987;109:3301–3308. [Google Scholar]
- 9.Safo MK, Walker FA, Raitsimring AM, Walters WP, Dolata DP, Debrunner PG, Scheidt WR. J. Am. Chem. Soc. 1994;116:7760–7770. [Google Scholar]
- 10.Safo MK, Gupta GP, Walker FA, Watson CT, Simonis U, Scheidt WR. J. Am. Chem. Soc. 1992;114:7066–7075. [Google Scholar]
- 11.Munro OQ, Serth-Guzzo JA, Turowska-Tyrk I, Mohanrao K, Shokhireva TKh, Walker FA, Debrunner PG, Scheidt WR. J. Am. Chem. Soc. 1999;121:11144–11155. [Google Scholar]
- 12. Abbreviations used: Porphyrin Ligands: TMP, 5,10,15,20-tetramesitylporphyrin dianion; TPP, tetraphenylporphyrin dianion; OEP, octaethylporphyrin dianion; OETPP, octaethyltetraphenylporphyrin dianion; TCl2PP, meso-tetra(2,6-dichlorophenyl)porphyrin dianion; Proto, protoporphyrin IX dianion; OMTPP, octamethyltetraphenylporphyrin dianion; TpivPP, picket fence porphyrin dianiom; TiPrP tetra(i-propyl)porphyrin dianion. Other Ligands: HIm, imidazole; 4-CNPy, 4-cyanopyridine; 1-AcIm, 1-acetylimidazole; 1-MeIm, 1-methylimidazole; 2-MeHIm, 2-methylimidazole; 3-ClPy, 3-chloropyridine; 4-NMe2Py, 4-dimethylaminopyridine; Py, pyridine; 5-MeHIm, 5-methylimidazole; BzHIm, 1-benzimidazole; Nax, axial nitrogen donor; Np, porphinato nitrogen.
- 13.Wagner RW, Lawrence DS, Lindsey JS. Tetrahedron Lett. 1987;28:3069. [Google Scholar]; Lindsey JS, Wagner RW. J. Org. Chem. 1988;54:828–836. [Google Scholar]
- 14.Adler AD, Longo FR, Kampas F, Kim J. J. Inorg. Nucl. Chem. 1970;32:2443–2448. [Google Scholar]
- 15.Scheidt WR, Turowska-Tyrk I. Inorg. Chem. 1994;33:1314–1318. [Google Scholar]
- 16. The process is based on an adaptation of the DIFABS [17] logic to area detector geometry by Karaulov: Karaulov AI. School of Chemistry and Applied Chemistry, University of Wales, College of Cardiff, Cardiff CF1 3TB, UK, personal communication.
- 17.Walker NP, Stuart D. Acta Crystallogr. 1983;A39:158–166. [Google Scholar]
- 18.Sheldrick GM. Acta Crystallogr. 2008;A64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 19.Hughes C. Unpublished data.
- 20.Walker FA, Lo M-W, Ree MT. J. Am. Chem. Soc. 1976;98(18):5552–5560. doi: 10.1021/ja00434a024. [DOI] [PubMed] [Google Scholar]
- 21.Nesset MJM, Shokhirev NV, Enemark PD, Jacobson SE, Walker FA. Inorg. Chem. 1995;35:5188–5200. [Google Scholar]
- 22.Scheidt WR. Stereochemical Systematics for Porphyrins and Metalloporphyrins. In: Kadish KM, Smith K, Guilard R, editors. Handbook of Porphyrin Science. Vol. 24. World Scientific; Singapore and Hackensack, NJ: 2012. pp. 1–180. Coordination Chemistry and Materials. [Google Scholar]
- 23.Munro OQ, Marques HM, Debrunner PG, Mohanrao K, Scheidt WR. J. Am. Chem. Soc. 1995;117:935–954. [Google Scholar]
- 24.Scheidt WR, Kirner JF, Hoard JL, Reed CA. J. Am. Chem. Soc. 1987;109:1963–1968. [Google Scholar]
- 25.Higgins TB, Safo MK, Scheidt WR. Inorg. Chim. Acta. 1990;178:261–267. [Google Scholar]
- 26.Collins DM, Countryman R, Hoard JL. J. Am. Chem. Soc. 1972;94:2066–2072. doi: 10.1021/ja00761a045. [DOI] [PubMed] [Google Scholar]
- 27.Little RG, Dymock KR, Ibers JA. J. Am. Chem. Soc. 1975;97:4532–4539. doi: 10.1021/ja00849a014. [DOI] [PubMed] [Google Scholar]
- 28.Scheidt WR, Osvath SR, Lee YJ. J. Am. Chem. Soc. 1987;109:1958–1963. [Google Scholar]
- 29.Koch CA, Reed CA, Brewer GA, Rath NP, Scheidt WR, Gupta G, Lang G. J. Am. Chem. Soc. 1989;111:7645–7648. [Google Scholar]
- 30.Inniss D, Soltis SM, Strouse CE. J. Am. Chem. Soc. 1988;110:5644–5650. [Google Scholar]
- 31.Mylrajan M, Andersson LA, Sun J, Loehr TM, Thomas CA, Sullivan EP, Jr, Thomson MA, Long KM, Anderson OP, Strauss SH. Inorg. Chem. 1995;34:3953–3963. [Google Scholar]
- 32.Silver J, Marsh PJ, Symons MCR, Svistunenko DA, Frampton CS, Fern GR. Inorg. Chem. 2000l;39:2874–2881. doi: 10.1021/ic990848s. [DOI] [PubMed] [Google Scholar]
- 33.Yatsunyk LA, Carducci MD, Walker FA. J. Am. Chem. Soc. 2003;125:15986–16005. doi: 10.1021/ja036398r. [DOI] [PubMed] [Google Scholar]
- 34.Ogura H, Yatsunyk L, Medforth CJ, Smith KM, Barkigia KM, Renner MW, Melamed D, Walker FA. J. Am. Chem. Soc. 2001;123:6564–6578. doi: 10.1021/ja004053s. [DOI] [PubMed] [Google Scholar]
- 35.Hu C, Noll BC, Schulz CE, Scheidt WR. Inorg. Chem. 2006;45:9721–9728. doi: 10.1021/ic061014u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ikezakia A, Nakamura M. Dalton Trans. 2011;40:3455–3458. doi: 10.1039/c1dt10042d. [DOI] [PubMed] [Google Scholar]
- 37.Nasri H, Wang Y, Huynh BH, Walker FA, Scheidt WR. Inorg. Chem. 1991;30:1483–1489. [Google Scholar]
- 38.Scheidt WR, Lee YJ, Luangdilok W, Haller KJ, Anzai K, Hatano K. Inorg. Chem. 1983;22:1516–1522. [Google Scholar]
- 39.Scheidt WR, Lee YJ, Geiger DK, Taylor K, Hatano K. J. Am. Chem. Soc. 1982;104:3367–3374. [Google Scholar]
- 40.Adams KM, Rasmussen PG, Scheidt WR, Hatano K. Inorg. Chem. 1979;18:1892–1899. [Google Scholar]
- 41.Scheidt WR, Hatano K. Acta Crystallogr., Sect. C. 1991;C47:2201–2203. doi: 10.1107/s0108270191003220. [DOI] [PubMed] [Google Scholar]
- 42.Scheidt WR, Geiger DK, Lee YJ, Reed CA, Lang G. J. Am. Chem. Soc. 1985;107:5693–5699. [Google Scholar]
- 43.Scheidt WR, Lee YJ. Recent Advances in the Stereochemistry of Metallotetrapyrroles. Struct. Bonding (Berlin) 1987;64:1–70. [Google Scholar]
- 44.Byrne MP, Curtis CJ, Khan SI, Sawin PA, Tsurumi R, Strouse CE. J. Am. Chem. Soc. 1990;112:1865–1874. [Google Scholar]
- 45.Byrne MP, Strouse CE. J. Am. Chem. Soc. 1991;113:2501–2508. [Google Scholar]
- 46.Byrne MP, Curtis CJ, Goldberg I, Hsiou Y, Khan SI, Sawin PA, Tendick SK, Strouse CE. J. Am. Chem. Soc. 1991;113:6549–6557. [Google Scholar]
- 47.Debrunner PG. In: Iron Porphyrins, Part III. Lever ABP, Gray HB, editors. VCH; New York NY: 1989. pp. 139–227. [Google Scholar]
- 48.Taylor CPS. Biochim. Biophys. Acta. 1977;491:137–148. doi: 10.1016/0005-2795(77)90049-6. [DOI] [PubMed] [Google Scholar]
- 49.Shelnutt JA, Song X-Z, Ma J-G, Jia S-L, Jentzen W, Medforth CJ. Chem. Soc. Rev. 1998;27:31–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.