Abstract
The mechanisms that modulate and limit the signaling output of adult stem cell niches remain poorly understood. To gain further insights into how these microenvironments are regulated in vivo, we performed a candidate gene screen designed to identify factors that restrict BMP signal production to the cap cells that comprise the germline stem cell (GSC) niche of Drosophila ovaries. Through these efforts, we found that disruption of Wnt4 and components of the canonical Wnt pathway results in a complex germ cell phenotype marked by an expansion of GSC-like cells, pre-cystoblasts and cystoblasts in young females. This phenotype correlates with an increase of decapentaplegic (dpp) mRNA levels within escort cells and varying levels of BMP responsiveness in the germline. Further genetic experiments show that Wnt4, which exhibits graded expression in somatic cells of germaria, activates the Wnt pathway in posteriorly positioned escort cells. The activation of the Wnt pathway appears to be limited by the BMP pathway itself, as loss of Mad in escort cells results in the expansion of Wnt pathway activation. Wnt pathway activity changes within germaria during the course of aging, coincident with changes in dpp production. These data suggest that mutual antagonism between the BMP and Wnt pathways in somatic cells helps to regulate germ cell differentiation.
Keywords: Niche, Stem Cells, Wnt, BMP, Germline
Introduction
Stem cells support tissue homeostasis by providing a continuous source of new cells that replace cells lost to damage, disease or normal turnover. Specialized microenvironments called niches govern the self-renewing activity of stem cells by producing a variety of factors that keep stem cells in an undifferentiated state (Losick et al., 2011). Stem cell daughters that remain within the niche remain stem cells, whereas daughters displaced away from the niche typically undergo differentiation. The size and signaling output of niches has a direct effect on stem cell number and activity. Therefore, changes in niche activity must be tightly regulated since inappropriate increases or decreases in niche function may contribute to tumor formation and age-related declines in tissue homeostasis, respectively.
The Drosophila ovary has provided a valuable platform for studying the mechanisms that influence niche formation and function in vivo. The germline stem cell (GSC) niche, comprised of terminal filament (TF) cells and cap cells, supports two to three GSCs. Anterior escort cells (ECs) that lie immediately adjacent to the cap cells may also assist with GSC maintenance (Rojas-Rios et al., 2012). Additional escort cells help support the early differentiation of germline cysts (Kirilly et al., 2011; Morris and Spradling, 2011).
The niche produces two bone morphogenetic protein (BMP) family members: Decapentaplegic (Dpp) and Glass Bottom Boat (Gbb) (Song et al., 2004; Xie and Spradling, 1998). These ligands initiate a canonical BMP signal transduction cascade by binding to a heterodimeric receptor on the surface of GSCs, resulting in the phosphorylation of Mothers against Dpp (Mad). pMad then binds to its partner Medea and translocates into the GSC nucleus. pMad and Medea directly repress the transcription of differentiation genes, including bag of marbles (bam) and induce the expression of canonical BMP targets such as Daughters against dpp (Dad) (Chen and McKearin, 2003a; Chen and McKearin, 2003b; Song et al., 2004).
In wild-type germaria, BMP signaling rapidly decreases in GSC daughters, known as cystoblasts (CBs), as they exit the cap cell niche. Recent efforts have sought to define and characterize mechanisms that establish this sharp gradient of BMP responsiveness between GSCs and their differentiating daughters. Intrinsic and extrinsic mechanisms, involving down-regulation of BMP pathway components, ensure that cystoblasts displaced away from the niche experience less BMP signaling (Harris et al., 2011; Liu et al., 2010; Schulz et al., 2002; Xia et al., 2010). However, mis-expression of dpp, but not gbb, results in the formation of GSC-like tumors (Song et al., 2004; Xie and Spradling, 1998), suggesting that despite the down-regulation of pathway components (Harris et al., 2011; Xia et al., 2010), germ cells remain competent to respond to BMP ligands on some level, even as they move posteriorly through the germarium.
Besides BMP ligands, the cap cells and escort cells of the Drosophila germarium express a variety of other signaling molecules including Unpaired (Upd), Hedgehog (Hh) and Wingless (Wg) (Decotto and Spradling, 2005; Forbes et al., 1996a; Forbes et al., 1996b; Lopez-Onieva et al., 2008; Sahai-Hernandez and Nystul, 2013; Wang et al., 2008). Recent results show that the expression of Wg, a member of the Wnt family of signaling molecules, in escort cells regulates the activity of follicle stem cells (Sahai-Hernandez and Nystul, 2013). In addition to Wg, the Drosophila genome contains a number of other genes encoding Wnt ligand family members including Wnt2, Wnt4, Wnt6 and Wnt10, which act either through a canonical pathway, involving β-catenin dependent transcriptional regulation, or a non-canonical pathway (Coudreuse and Korswagen, 2007; Logan and Nusse, 2004). Besides Wg, disruption of Wnt4 also results in a number of morphological defects in the ovary (Cohen et al., 2002). These phenotypes are likely caused by defects in the apical movement of somatic cells in the developing gonad, marked by the disruption of the normal expression and distribution of FAK (Cohen et al., 2002). More recently, Wnt4 has also been suggested to play of role in the regulation of the germline stem cell niche (Hamada-Kawaguchi et al., 2014; Wang et al., 2015).
Here we provide evidence that disruption of Wnt4 and downstream components of the canonical Wnt signaling pathway in escort cells results in an expansion of BMP responsiveness in the germline and a subsequent increase in the number of GSCs, pre-cystoblasts and cystoblasts. In addition, we find loss of Wnt pathway components is accompanied by an increase of dpp mRNA levels specifically within escort cells. Further genetic experiments show that Wnt4 tends to induce activation of the Wnt pathway in escort cells and early follicle cells of the germaria. Signaling within somatic cells of germaria appears to change during the course of aging. In older flies, dpp expression within the cap cell niche decreases. This coincides with a switch in Wnt pathway activation from the posterior escort cells to the terminal filament and cap cells. These results provide new insights into how cell-cell communication between specific somatic cell populations helps to modulate niche signaling within the Drosophila germarium.
Results
The canonical Wnt pathway non-autonomously promotes stem cell differentiation
In order to identify factors that act in escort cells to limit niche signaling and promote the differentiation of germ cells, we carried out a candidate gene RNAi screen. Targeted genes included various chromatin factors and signaling molecules. We conducted the screen by crossing available UAS-gene specific RNAi lines with the c587-gal4 driver, which, in adult germaria, drives expression in the escort cells and early follicle cells (Song et al., 2007). Ovaries from the resulting females were stained for Vasa, to visualize the germline, and Hts, an adducin-like protein that localizes to an endoplasmic-like organelle called the fusome. In GSCs and cystoblasts, the fusome (also referred to as the spectrosome in single cells) usually appears round (de Cuevas and Spradling, 1998). This structure subsequentially becomes branched within germline cysts progressing through their incomplete mitotic divisions. A substantial increase in the number of single cells with round fusomes indicates defects in germline differentiation. Through this initial small-scale screen, we found that knockdown of Wnt4 using the c587-gal4 driver resulted in an increased number of GSC-like cells with round fusomes, albeit with a low penetrance (15%, n=120 germaria) (Fig. 1B). To confirm the RNAi phenotype, we next stained ovaries carrying two loss-of-function Wnt4 alleles: Wnt4C1, which contains a 3 bp deletion that removes a highly conserved glutamate residue at position 299 and Wnt4EMS23, which exhibits a premature stop codon at position 343 mutants (Cohen et al., 2002). Both of these alleles cause partial lethality and female sterility and are believed to be amorphic or strongly hypomorphic (Cohen et al., 2002). The initial characterization of these alleles showed that Wnt4 mutant ovarioles were disorganized (Cohen et al., 2002). Delays in the migration of cells that form the muscle sheet surrounding the ovarioles during development likely contribute this phenotype (Cohen et al., 2002). In addition to these phenotypes, we noted that germaria from Wnt4C1/Df(2L)BSC291, Wnt4EMS23/Df(2L)BSC291 and from transheterozygous Wnt4C1/Wnt4EMS23 females also displayed an increased number of single cells with round fusomes (Fig. 1C,J). Wnt4C1/Df(2L)BSC291 and Wnt4C1/Wnt4EMS23 germaria contained large germ cell tumors (containing more than 15 single germ cells with round fusomes) at 82% (n=103) and 85% (n=47) penetrance compared to Wnt4EMS23/Df(2L)BSC291, which displayed large GSC/cystoblast-like tumors in 25% (n=77) of the germaria examined.
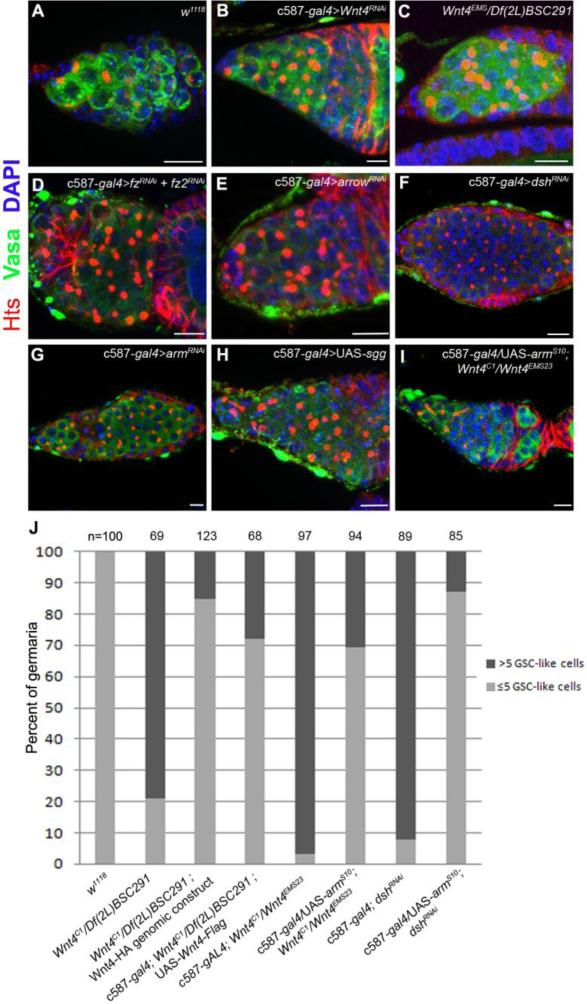
Fig. 1. Disruption of Wnt4 and canonical Wnt pathway components in escort cells results in the formation of GSC-like tumors.
(A-I) Germaria stained for Vasa (green), Hts (red) and DNA (blue). (A) Control germaria contain two to three GSCs. (B) Germaria from c587-gal4/+; UAS-Wnt4RNAi/+ and from Wnt4EMS/Df(2L)BSC291 displayed an increase of GSC-like cells with round fusomes. RNAi knockdown of (D) the fz and fz2 receptors, (E) the co-receptor arrow, (F) dsh or (G) arm, using the escort cell/early follicle cell c587-gal4 driver, results in the accumulation of GSC-like cells. (H) Over-expression of sgg in escort cells and early follicle cells also results in an expansion of undifferentiated germ cells. (I) Overexpression of a constitutively activated form of arm (armS10) suppresses the Wnt4C1/ Wnt4EMS23 phenotype. (J) A graph illustrating the percentage of germaria (Y-axis) that contained more (black bars) or less (light gray) than 5 round fusomes per germarium in the indicated genetic backgrounds (X axis). Note that the GSC-like tumor phenotypes observed after disruption of the canonical pathway are rescued by a genomic Wnt4 HA-tagged construct, as well as by expression of a Wnt4 cDNA or by expression of armS10 driven by c587-gal4. (Scale bars, 10 μm)
The Wnt4 mutants display a reduction of the number of egg chambers compared to wild type (Cohen et al., 2002). The GSC/cystoblast-like tumor phenotype, the reduction of the number of egg chambers and female sterility of these alleles were rescued by a genomic HA-Wnt4 tagged construct (Fig. 1J, S1). In addition, expression of a Wnt4 cDNA driven by c587-gal4 also partially rescued these phenotypes, supporting the model that Wnt4 is required in the ECs and follicle cells to control the number of GSCs (Fig. 1J).
Previous results showed that wingless (wg) regulates the activity and differentiation of follicle cells within the germarium, but does not appear to greatly influence the earliest steps of germline development (Forbes et al., 1996b; Sahai-Hernandez and Nystul, 2013; Song and Xie, 2003). We also found that knocking down wg, using UAS-RNAi lines in combination with the c587-gal4 driver does not result in an appreciable germ cell phenotype, consistent with the aforementioned studies. Furthermore, RNAi knock-down of the other Drosophila Wnt ligands using the same driver did not result in germ cell phenotypes. However, we cannot exclude the possibility that other Wnt ligands help to influence GSC differentiation, as the loss-of-function achieved through RNAi knock-down may be incomplete in our hands.
Given the phenotypes of Wnt4 mutants, we next sought to determine whether loss-of-function of other canonical Wnt pathway components in escort cells and follicle cells results in an expansion of the number of single cells with round fusomes within germaria. RNAi knockdown of either frizzled (fz) and frizzled 2 (fz2) alone did not result in any obvious defects in the germarium, but knock-down of both (Fig. 1D) resulted in an increased number of GSC-like cells in 65% of the germaria examined (n=103). These results suggest that these receptors have redundant functions within somatic cells of the germarium, consistent with previous results that showed Fz and Fz2 are fully redundant for Wg-dependent signaling (Bhanot et al., 1999; Chen and Struhl, 1999). Knock-down of arrow (also named LRP5/6 co-receptor) and dishevelled (dsh) also resulted in the formation of ectopic GSC-like cells in 45% and 100% (n=100) of germaria respectively (Fig. 1E,F). The knockdown of the Drosophila β-catenin homolog armadillo (arm) led to a similar phenotype (100%; n=200) (Fig 1G). Moreover, overexpression of the kinase Shaggy (Sgg), which targets β-catenin for degradation, also resulted in a tumor phenotype in 94% of the germaria examined (Fig. 1H). In addition, we observed cyst formation defects when we knockdown dsh or overexpress sgg using the c587-gal4 driver, consistent with the role of the Wnt pathway in controlling follicle cell activity (Sahai-Hernandez and Nystul, 2013).
To further test whether the canonical Wnt pathway regulates germ cell differentiation, we overexpressed a constitutively active form of arm (UAS-armS10), using the c587-gal4 driver in the Wnt4 mutant background. This mutant form of Arm escapes from negative regulation by Sgg, and thus accumulates even in the absence of Wnt signal (Pai et al., 1997). When driven in the escort cells and early follicle cells, armS10 partially rescued the Wnt4C1/Wnt4EMS23 tumor phenotype (Fig. 1I,J), but did not fully rescue the sterility of these transheterozygotes. In addition, expressing armS10 rescued the tumor phenotype observed in the c587-gal4>UAS-dshRNAi flies (Fig. 1J). These results indicate that a canonical β-catenin dependent pathway functions in escort cells to promote germ cell differentiation downstream of the GSC.
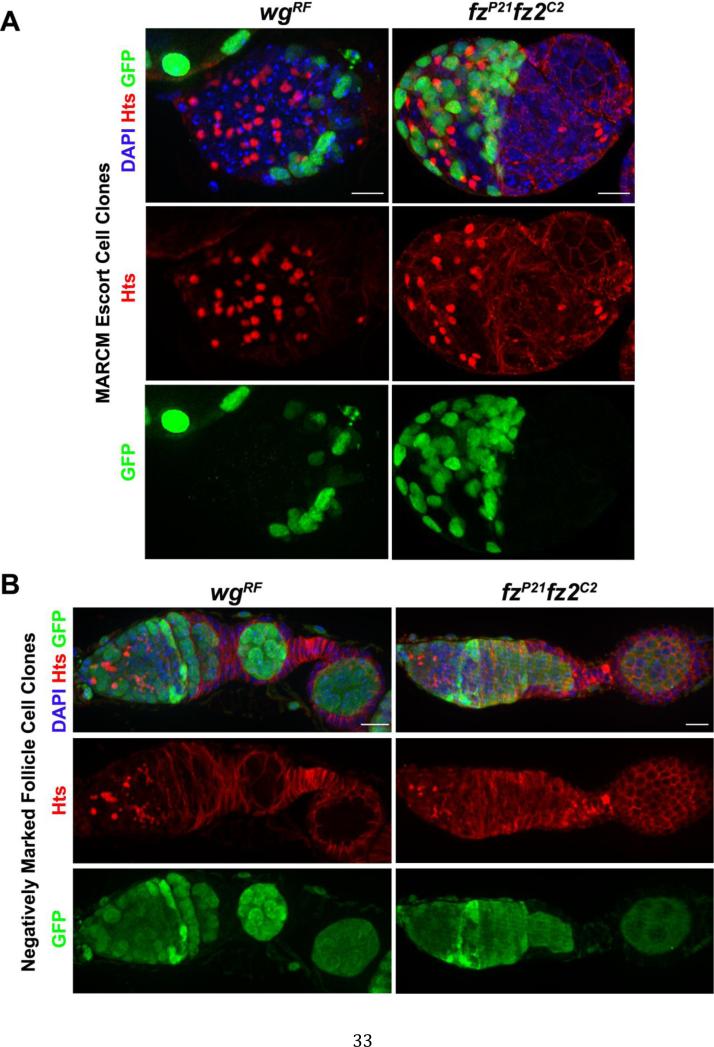
Next, we induced homozygous mutant clones to further corroborate that disruption of the Wnt pathway in somatic cells of the germarium results in expansion of undifferentiated germline cells. This analysis also allowed us to further map whether the Wnt pathway acted in the escort cells or follicle cells to promote cyst development. Because of its availability, we first generated positively marked mosaic analysis with a repressible cell marker (MARCM) clones using the wgRF deficiency, which deletes a segment of genomic DNA that harbors many genes including Wnt4, wg, Wnt10 and Wnt6. Escort cell clones of this deficiency resulted in the formation of germ cell tumors, marked by single cells with round fusomes (Fig. 2A). Next, we generated double homozygous clones of the fzp21 and fz2C2 mutations. Consistent with the phenotypes seen using RNAi knock-down, loss of fz and fz2 in the majority of escort cells resulted in the expansion of GSC-like cells, marked by the presence of round fusomes (Fig. 2A). Of note, wgRF and fzp21 and fz2C2double mutant negatively marked follicle cell clones did not result in an expanded number of undifferentiated germ cells (Fig. 2B). These results were consistent with our RNAi knock-down data and suggest that loss of Wnt signaling in escort cells delays normal germ cell development.
Fig. 2. Wnt pathway mutant escort cell clones are associated with undifferentiated germ cells.
(A) The MARCM system was used to generate wgRF or double fzp21 fz2C2 mutant clones marked by GFP. Both samples were stained for GFP (green), Hts (red) and DNA (blue). Loss of Wnt pathway components in escort cells resulted in the appearance of many single cells with round fusomes (B) Negatively marked wgRF or double fzp21 fz2C2 follicle cell clones stained for GFP (green), Hts (red) and DNA (blue). Loss of Wnt pathway components within follicle cells did not appear to perturb early germ cell differentiation. (Scale bars, 10 μm).
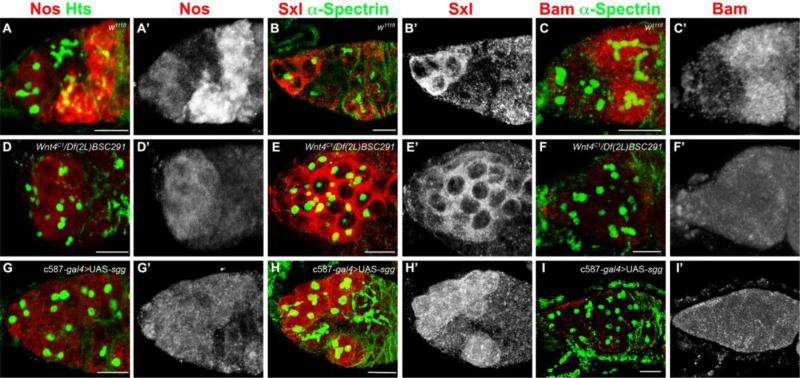
To characterize the developmental state of the GSC-like cells in Wnt pathway mutants, we examined the expression of three molecular markers which are used to distinguish GSCs from their daughter cells: Nanos (Nos), Sxl (Sex-Lethal) and Bag-of-Marbles (Bam). GSCs and 16-cell cysts express Nos, a RNA binding protein that represses translation and helps to maintain GSCs (Forbes and Lehmann, 1998; Kobayashi et al., 1996; Wang and Lin, 2004). All the GSC-like cells in Wnt4C1/Wnt4EMS23 and c587-gal4>UAS-sgg germline tumors express Nos at a level comparable to wild-type GSCs (n=80, Fig. 3A,D,G). We next analyzed the expression of Sxl and Bam, two differentiation-promoting factors. Wild-type GSCs, cystoblasts (CBs) and two cell cysts express Sxl, while cystoblasts and early differentiating cysts express Bam. In Wnt4C1/Wnt4EMS23 and c587-gal4>UAS-sgg ovaries, the expression of Sxl expanded to all the single cells with round fusomes (Fig. 3E,H). By contrast, Bam remained largely undetected in Wnt4C1/Wnt4EMS23 and c587-gal4>UAS-sgg germaria (only 35% and 12% of the tumorous germaria displayed Bam expression in subsets of germ cells) (Fig. 3F,I). These observations suggest that the majority of single cells that arise upon Wnt pathway disruption remain in an undifferentiated GSC, pre-cystoblast-like or cystoblast-like state.
Fig. 3. Changes in germline markers upon disruption of the Wnt pathway in escort cells.
Germaria of (A) control, (D) Wnt4C1/Df(2L)BSC291 and (G) c587-gal4/+; UAS-sgg/+ stained for Hts (green) and Nos (red). (B) Control, (E) Wnt4C1/Df(2L)BSC291 and (H) c587-gal4/+; UAS-sgg/+ germaria stained for α-Spectrin (green) and Sxl (red). (C) Control, (F) Wnt4C1/Df(2L)BSC291 and (I) c587-gal4/+; UAS-sgg/+ stained for α–Spectrin (green) and Bam (red). The GSC-like cells that accumulate upon Wnt pathway disruption express Nos and Sxl, but not the differentiation factor Bam. (Scale bars, 10 μm).
Wnt pathway functions in adulthood to promote germ cell differentiation
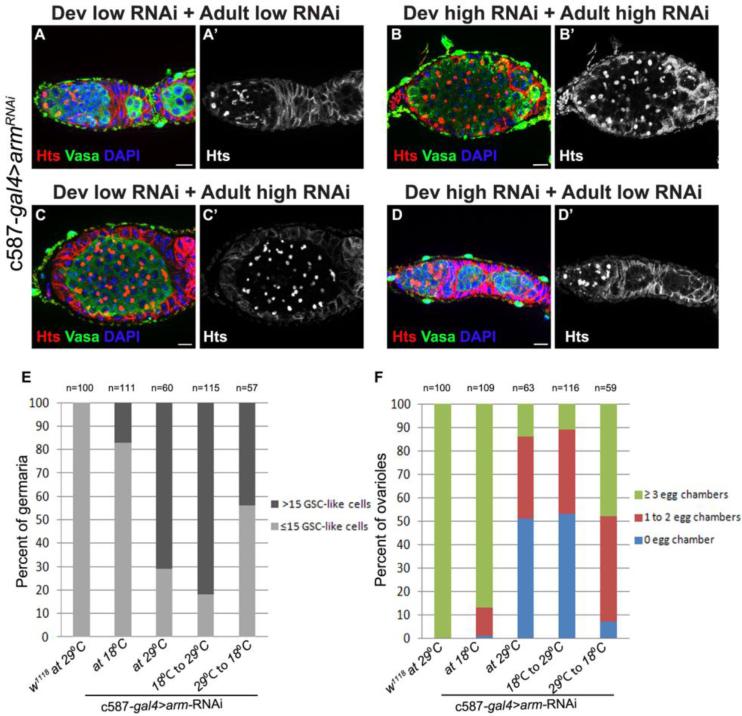
To assess the extent to which the Wnt pathway functions during development or in adulthood, we performed a series of temporally controlled knock-down experiments. We previously noted that the c587-gal4 driver was less efficient at lower temperatures (Eliazer et al., 2011). We crossed the c587-gal4 driver to a line carrying UAS-armRNAi. The majority of progeny from this cross did not exhibit a discernable phenotype when maintained at 18°C during development and in adulthood (Fig. 4A,E). In contrast, when female progeny resulting from this cross where kept at 29°C both during development and in adulthood, over 80% of their germaria displayed a striking expansion of single undifferentiated germ cells with round fusomes and a substantial decrease in the number of developing egg chambers (Figure 4B,E). Next, we set c587-gal4>UAS-armRNAi crosses either at 18°C, shifting the resulting progeny up to 29°C upon eclosion, or at 29°C, shifting the females down to 18°C when they emerged as adults. These temperature shift experiments showed that knocking down arm expression specifically in escort cells during adulthood resulted in the formation of germ cell tumors, accompanied by a reduction in the number of developing egg chambers (Fig. 4C,E,F). Similarly, restoring arm expression after developmental knock-down resulted in more phenotypically normal germaria, marked by a reduction in the size of the germline tumors and an increase in the number of developing egg chambers (Fig. 4D,E,F). These results indicate that the Wnt pathway functions in adult escort cells to promote the differentiation of germ cells. These findings also suggest that reducing Wnt pathway signal transduction in c587-gal4 positive cells during larval and pupal development also results in an expansion of undifferentiated germ cells. However, loss of Wnt4, dsh or arm does not cause escort cells to adopt a cap cell-like fate (Fig. S2).
Fig. 4. The canonical Wnt pathway functions during adulthood to promote germ cell differentiation.
(A–D) Germaria from c587-Gal4/+; UAS-arm-RNAi/+ females raised at (A) 18°C, (B) 29°C, (C) shifted from 18°C to 29°C upon eclosion or (D) shifted from 29°C to 18°C upon eclosion stained for Vasa (green), Hts (red) and DNA (blue). Knockdown of arm in adulthood led to the formation of tumors containing more than 15 round fusomes (B and C) while knockdown of arm only during development led to the formation of smaller tumors (D). (E) A graph showing the percent of germaria (Y axis) that contain more (black bars) or less (light gray) than 15 round fusomes per germarium in c587-gal4/+; UAS-armRNAi/+ females. (F) Graph showing the percentage of ovarioles that contain the indicated numbers of maturing egg chambers under each of the conditions. (Scale bars, 10 μm).
The canonical Wnt pathway limits Dpp signaling within the germarium
Previous studies have shown that expansion of dpp expression, changes in escort cell morphology and mutations in differentiation factors can all block normal germ cell development (Kirilly et al., 2011; McKearin and Spradling, 1990; Xie and Spradling, 1998). Given the loss of Bam expression and the cell autonomous function of the Wnt pathway in escort cells, we tested whether disruption of Wnt4 and downstream pathway components resulted in escort cell morphological defects, ectopic expression of dpp or both. In germaria from young females, loss of dsh and arm or over-expression of sgg in escort cells resulted in an expansion of single germ cells with round fusomes despite the clear presence of some escort cell extensions as revealed by the mCD8::GFP reporter (Fig. S3). However, we do observe a loss of escort cell extensions over time upon loss of Wnt pathway activity. These observations suggest that the disruption of escort cell extensions by itself does not initiate the observed Wnt pathway mutant phenotypes. However changes in escort cell morphology likely do contribute to the complexity and severity of the terminal phenotype within the germaria of older females.
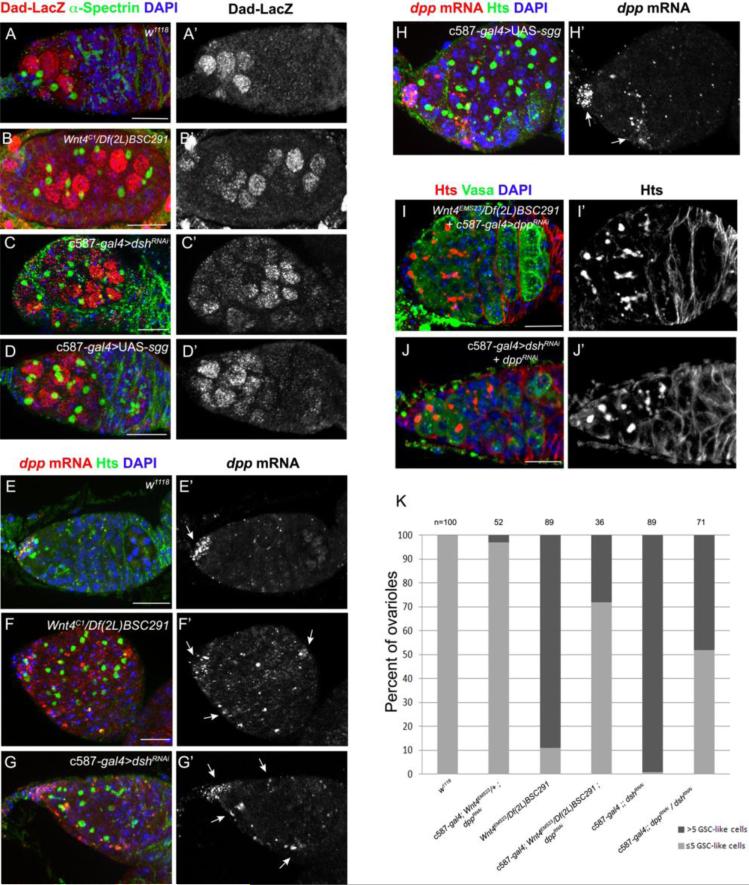
Next we tested whether disruption of the Wnt pathway in escort cells resulted in BMP pathway activation in germ cells outside of the cap cell niche. The Dad-LacZ enhancer trap has been extensively used as a reporter of BMP signaling in the germline (Song et al., 2004). This reporter is normally limited to the GSCs and newly formed cystoblasts (Fig. 5A). In Wnt4 mutants and c587-gal4>UAS-dshRNAi and UAS-sgg ovaries, the Dad-LacZ expression domain expands throughout the germarium (Fig. 5B-D).
Fig. 5. Canonical Wnt pathway limits Dpp signaling within the germarium.
(A) Control, (B) Wnt4C1/Df(2L)BSC291, (C) c587-gal4/+; UAS-dshRNAi/+ and (D) c587-gal4/+; UAS-sgg/+ ovaries carrying a Dad-LacZ enhancer trap stained for Hts (green), LacZ (red) and DNA (blue). RNA in situ hybridization of dpp and Hts immunostaning were performed on (E) control, (F) Wnt4C1/Df(2L)BSC291, (G) c587-gal4/+; UAS-dshRNAi/+ and (H) c587-gal4/+; UAS-sgg/+ ovaries. Arrows point to cells expressing dpp mRNA. (E) In control germaria, dpp transcripts were mostly detected in the cap cells. (F-H) Disruption of the canonical Wnt pathway led to an upregulation of dpp transcripts in the escort cells. RNAi knockdown of dpp partially rescued the tumorous phenotype of (I) Wnt4C1/Df(2L)BSC291 and (J) c587-gal4/+; UAS-dshRNAi/+ germaria. (K) Quantification of the suppression of Wnt pathway mutants phenotypes by RNAi knockdown of dpp. (Scale bars, 10 μm).
Because the expression of Dad-LacZ may persist beyond the time when a cell directly experiences BMP signaling, we also examined the expression of phosphorylated Mad (pMAD), a second readout for BMP signal transduction. Like Dad-LacZ, pMAD is normally restricted to GSCs immediately adjacent to the cap cells of wild-type germaria (Figure S4). By contrast, we observed that within 67% of germaria (n=30) from c587-gal4>UAS-dshRNAi females a small subset of single germ cells with round fusomes outside of the normal niche expressed low levels of pMAD upon escort cell knock-down of dsh (Fig. S4). Other single cells with round fusomes within the same tumors did not express detectable levels of pMAD. While these results indicate that some germ cells experience ectopic BMP signaling transduction outside the normal niche upon disruption of Wnt signaling, and are thus similar to GSCs, others may be slightly more differentiated and exist within a pre-cystoblast or cystoblast-like state. To test this possibility, we examined the expression of a highly sensitive bam transcriptional reporter (bamP-GFP) (Chen and McKearin, 2003b) in both a wild-type and Wnt pathway loss-of-function background. In control germaria, we first detect the GFP expression of the reporter in cystoblasts. This expression continued to increase as cells entered into cyst differentiation. By contrast, ectopic single germ cells present upon escort cell knock-down of Wnt pathway components expressed either no or low levels of the bam transcriptional reporter (Fig. S4). Together, these results are largely consistent with recently published findings (Wang et al., 2015) and suggest that loss of Wnt signaling in escort cells results in a varying degree of ectopic BMP signal transduction in germ cells positioned away from the cap cells. This, in turn, results in an expansion of undifferentiated cells that exist within a GSC-like, pre-cystoblast and cystoblast-like state.
To determine whether the expanded domain of Dad-LacZ and pMAD expression reflected ectopic dpp expression, we employed a new, highly sensitive, in situ hybridization protocol that utilizes a series of end-labeled oligo probes. This method revealed an enrichment of dpp mRNA in the cap cells of wild-type ovaries (Fig. 5E). Wnt4 mutants exhibited modest levels of ectopic dpp mRNA expression in escort cells (Fig. 5F). Likewise, RNAi knock-down of dsh and over-expression of sgg also resulted in an expansion of dpp mRNA within escort cells (Fig. 5G,H). To test the functional significance of the observed ectopic dpp expression, we tested whether loss of dpp in the escort cells modified the GSC expansion phenotype of the Wnt pathway mutants. Expressing UAS-dppRNAi in escort cells suppressed the Wnt4 mutant and c587-gal4>UAS-dshRNAi mutant phenotypes, so fewer germaria contained greater than 5 single cells with round fusomes (Fig. 5I-K). These results together suggest that the Wnt pathway promotes germ cell differentiation, at least in part, by repressing dpp expression within the escort cells of adult ovaries.
Wnt signaling along the anterior-posterior axis of the germarium
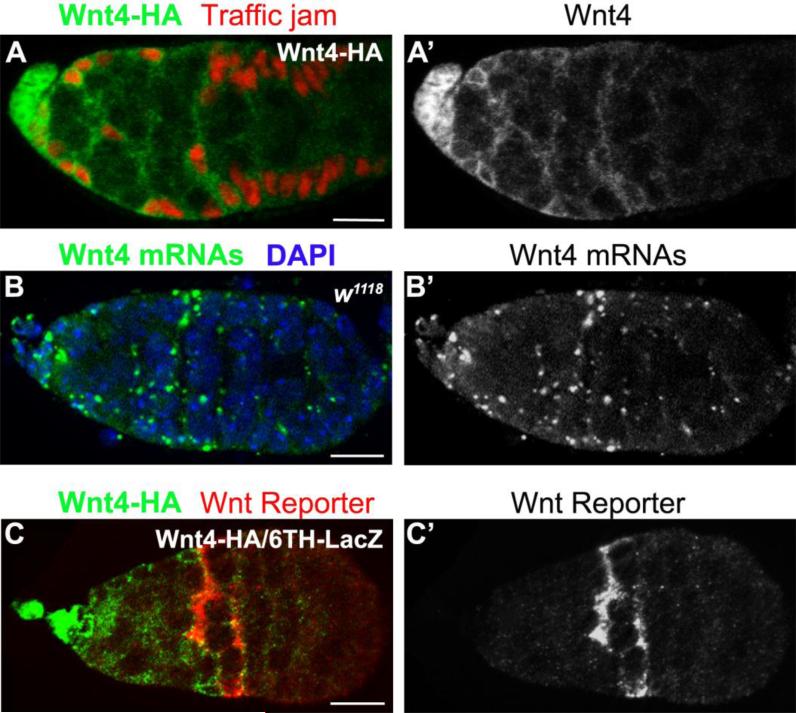
To gain a better understanding of which cells produce Wnt4 and which cells respond to this ligand, we examined the expression of the HA-tagged Wnt4 in our rescuing genomic construct (Fig. S1). Staining the resulting lines for the HA epitope and Traffic Jam (Tj), a marker for somatic cells in the germarium, revealed that cap cells and anterior escort cells expressed high levels of the Wnt4 tagged genomic transgene (Fig. 6A). Expression of Wnt4 was lower in more posteriorly positioned escort cells. The expression pattern of this tagged transgene was largely consistent with the expression of Wnt4 mRNA, as visualized by in situ hybridization (Fig. 6B).
Fig. 6. Expression of Wnt4 and a Wnt pathway reporter in the germarium.
(A) Germaria of transgenic flies harboring a genomic Wnt4 HA-tagged construct were co-stained for HA (green) and Traffic jam (red). Wnt4-HA was detected at high levels in the terminal filament, the cap cells as well as in the anterior escort cells and at lower levels in the more posterior escort cells. (B) The localization of Wnt4 mRNAs (green) correlated with the Wnt4-HA staining. (C) Germaria expressing the HA-tagged Wnt4 genomic transgene and a Wnt reporter 6TH-LacZ were co-stained for HA (green) and β-galactosidase (red). The 6TH-LacZ Wnt reporter labeled mostly escort cells and follicle stem cells located posterior to the cells expressing the Wnt4-HA transgene. However, we did observe cases where cells displayed high levels of both Wnt4-HA and of the Wnt reporter. (Scale bars, 10 μm).
Next we examined the expression pattern of a previously described Wnt pathway reporter (6TH) within wild-type germaria (Chang et al., 2008). This reporter places the β-galactosidase gene under control of six TCF binding sites flanked by helper sites (Chang et al., 2008). We observed expression of this reporter in a small number of cells within each germarium. These Wnt reporter expressing cells tended to be positioned along the escort cell and follicle stem cell boundary (Fig. 6C), but occasionally more anteriorly positioned Wnt reporter positive escort cells were also noted (Fig. S5A). Loss of Wnt4 or dsh extinguished expression of the reporter in all cells while over-expression of armS10 increased the number of 6TH expressing cells (Fig. S5B-D). Again these positive cells tended to lie along the escort cells and follicle stem cell border, despite the expression of the armS10 transgene throughout the escort cell population. These results suggest that unidentified mechanisms restrict the number of escort cells that respond to Wnt4, or that 6TH does not fully report all Wnt signaling within the germarium. Double labeling experiments using the HA-tagged Wnt4 genomic transgene and the Wnt reporter tended to show tight juxtaposition between cells expressing moderate levels of Wnt4 and those cells that expressed the reporter (Fig 6C). Occasionally we observed individual cells that expressed Wnt4 and relatively high levels of the reporter. These data suggest that Wnt4 may tend to act in a paracrine manner to induce a response in more posteriorly positioned somatic cells. However, given that Wnt4 and the reporter are expressed together in individual escort cells, we cannot rule out the possibility that Wnt4 also acts in an autocrine fashion in certain circumstances.
The BMP pathway antagonizes the Wnt pathway within germaria
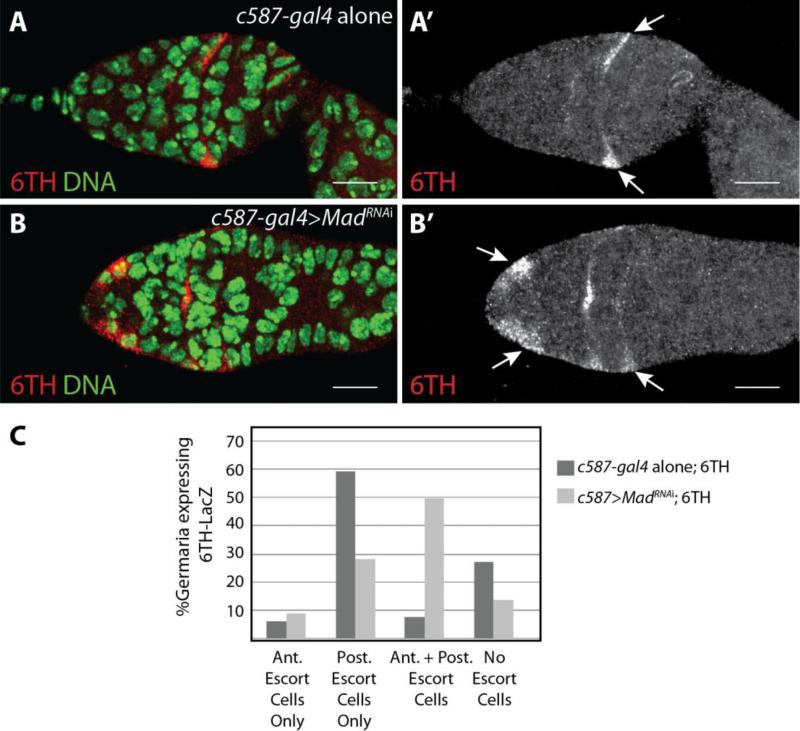
Previous studies have shown that the BMP and Wnt signaling pathways antagonize each other in specific contexts, resulting in mutually exclusive domains of pathway activation (Jiang and Struhl, 1996). Given our observations that armS10 over-expression resulted in greater expression of the 6TH Wnt pathway reporter in posterior escort cells but not in anterior escort cells (Fig. S5), we considered the possibility that Dpp expressed by the cap cells activates the BMP signaling pathway in neighboring anterior escort cells of the germarium, which in turn represses Wnt signaling within these cells. To test this possibility, we disrupted BMP signaling within escort cells by knocking down the expression of Mothers against dpp (Mad) using the c587-gal4 driver in combination with a UAS-MadRNAi transgene. Reduced levels of Mad resulted in an expansion of 6TH Wnt reporter expression, suggesting that loss of BMP signaling results in ectopic Wnt pathway activation within anterior somatic cells of the germarium (Fig. 7).
Fig. 7. Loss of Mad results in expanded Wnt signaling in the germarium.
(A) c587-gal4 alone control and (B) c587-gal4>UAS-MadRNAi knockdown germaria carrying the 6TH-LacZ Wnt reporter were stained for β-galactosidase (red). Knockdown of Mad led to an increase of the number of anterior escort cells expressing the Wnt reporter. Arrows point to cells expressing the 6TH reporter. (C) Graph showing quantification of the position of cells that express the 6TH Wnt reporter (c587-gal4 control n=148; c587-gal4>MadRNAi n=135). (Scale bars, 10 μm).
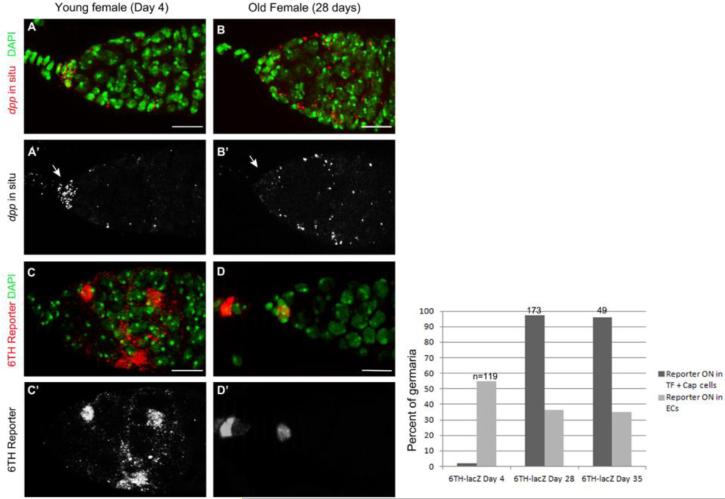
Decreased BMP signal production in aged flies contributes to a decline in GSC niche function (Pan et al., 2007). We speculated that changes in Wnt signaling response might contribute to age-related changes in niche function to some degree. To test this idea, we first compared dpp expression in young and aged germaria using in situ hybridization (Fig. 8). Germaria from young females clearly exhibited high levels of dpp expression in cap cells and far lower and sporadic expression in the escort cells. In contrast, cap cells from aged females display reduced levels of dpp expression while escort cells exhibit increased expression (Fig. 8). These results are consistent with previous results (Rojas-Rios et al., 2012; Song et al., 2004). The changes in dpp mRNA expression within specific somatic cells within the germarium correlate with changes in the expression of the Wnt reporter. As noted above, in young flies expression of the 6TH Wnt reporter is observed in posterior escort cells and occasionally in anterior escort cells, but very rarely in cap cells or terminal filament cells (Fig. 6 and 7). Aged germaria exhibited a dramatic change in reporter expression so that fewer escort cells expressed the reporter while an increased number of cap cells and terminal filament cells exhibited very high levels of expression (Fig. 8). These results suggest that changes in both BMP and Wnt signaling may contribute to a decline in niche activity during the course of aging.
Fig. 8. Expression of dpp and a Wnt reporter change during aging.
RNA in situ hybridization of dpp was performed on germaria from females (A) aged for 4 days or (B) for 28 days. The level of dpp transcripts decreased in the cap cells and increased in the escort cells upon aging. Wnt reporter expression exhibits the opposite pattern with an increase of Wnt activity in the cap cells and a reduction in the escort cells in the germaria of old females (D) compared to young females (C). The number of germaria exhibiting Wnt activity either in the terminal filament (TF) and the cap cells or in the escort cells was scored in 4 day, 28 day and 35 day-old females (E). (Scale bars, 10 μm).
Discussion
The dynamic regulation of adult stem cell numbers and activity often depends on the size and signaling output of the specialized niches within which they reside. A growing body of evidence suggests that stem cell niches may exhibit a certain degree of plasticity, expanding and contracting in response to local homeostatic needs and systemic cues (Rojas-Rios and Gonzalez-Reyes, 2013). Characterizing the diverse mechanisms that affect niche size is critical for understanding the control of stem cell self-renewal, proliferation and differentiation in vivo. Here we provide evidence that the Wnt signaling pathway, driven by the Wnt4 ligand, and perhaps other Wnts as well (Wang et al., 2015), represses the normally niche cell specific expression of dpp in the escort cells of the Drosophila ovary. Interestingly, wild-type cap cell express high levels of both dpp mRNA and Wnt4, yet Wnt pathway activation is most readily seen in posterior escort cells and early follicle cells. These results indicate that the Wnt pathway plays an unexpected role in regulating BMP signal production in somatic cells of germaria and that cells neighboring the stem cell microenvironment influence whether one another can produce niche specific factors. Lastly, we show that the cells responding to Wnt signaling change with age, strongly correlated with a decrease in niche function in older females.
Wnt4 represses dpp expression in escort cells
Drosophila Wnt4 is most closely related to vertebrate Wnts 9, 14 and 15 (Prud'homme et al., 2002) and has been shown to be involved in the development of dorsal epidermis, retina, heart and ovaries as well as in the left-right asymmetric morphogenesis of the midgut (Cohen et al., 2002; Hamada-Kawaguchi et al., 2014; Inaki et al., 2007; Kuroda et al., 2012; Wu et al., 2013). Wnt4 was shown to bind to both the Fz1 and Fz2 receptors (Wu and Nusse, 2002) and to signal through both the canonical and non-canonical Wnt pathways, depending on cellular contexts (Cohen et al., 2002; Hamada-Kawaguchi et al., 2014; Wu et al., 2013). Importantly, Wnt4 has been previously shown to regulate morphological events during Drosophila gonad development (Cohen et al., 2002). Consistent with a recent report (Hamada-Kawaguchi et al., 2014), our data support a model in which canonical Wnt signaling functions in the escort cells. However, unlike the aforementioned study, our findings suggest that Wnt signaling serves to limit dpp expression within somatic cells. We observe that loss of several different components of the Wnt pathway result in an expansion of single cells with round fusomes, more in line with a second recently published study (Wang et al., 2015). These undifferentiated cells express Dad-LacZ and do not express Bam protein, although variable expression of pMAD and a bam transcriptional reporter suggests that the germ cell tumors that arise upon loss of escort cell Wnt signaling contain a mixture of GSCs, pre-cystoblasts and cystoblast-like cells. We provide evidence that dpp expression expands in escort cells in the absence of Wnt signaling and that loss of dpp suppresses Wnt related phenotypes in the ovary. However, the complexity of the observed phenotypes may indicate that loss of Wnt signaling disrupts multiple Dpp-dependent and potentially Dpp-independent processes. For example, we observe an expansion of undifferentiated germ cells in germaria from young c587-gal4>UAS-dshRNAi females that still have escort cell extensions, suggesting that bulk loss of escort cell protrusions does not initiate the described phenotypes. However, over time the phenotype worsens, as escort cell extensions are lost, indicating that the ability of escort cells to maintain close contact with multicellular cysts plays an important role in the continued development of germ cells (Kirilly et al., 2011). Ectopic expression of dpp may disrupt the normal morphology of escort cells, giving rise to a complex phenotype involving both low levels of aberrant BMP signal transduction in germ cells and disruption of other processes that depend on contact between escort cells and germ cells (Kirilly et al., 2011; Wang et al., 2015). Future work will be needed to further clarify how germ cells and escort cells communicate and influence the activity and morphology of one another.
Somatic Cells Communicate with Each Other to Limit Niche-Specific Signaling
We observe that somatic cells of the germarium exhibit graded expression of Wnt4, from high levels in the terminal filament and cap cells to lower levels in the posterior escort cells. Despite high levels of Wnt4 expression in the anterior of the germarium, the 6TH Wnt reporter is most readily detected in posterior escort cells, follicle stem cells and early follicle stem cell daughters. Signal transduction reporters cannot always be expected to reflect the entirety of signaling in any given tissue because of the molecular and context dependent complexities that exist in vivo (Barolo, 2006). However, despite this caveat, the expression of the 6TH reporter in germaria appears at least responsive to levels of Wnt signal transduction at some level since it goes away in Wnt pathway mutants and increases in posterior escort cells upon armS10 over-expression. Interestingly, the reporter did not come on in young anterior escort cells upon armS10 over-expression or upon armS10 over-expression coupled with TCF overexpression (data not shown), despite the expression of the c587-gal4 driver throughout the escort cell and early follicle cell population. These observations hint at mechanisms whereby Wnt pathway activation is restricted in some way. The finding that loss of Mad results in expanded 6TH expression suggests that BMP signals emanating from the niche attenuate Wnt pathway activation in surrounding escort cells, and thereby also help to regulate escort cell function.
Wnt Signaling changes with Age
Previous results indicate that niche function within the Drosophila ovary declines with age (Pan et al., 2007). The influence of non-cell-autonomous factors on stem cell aging is not limited to invertebrates. For example, parabiotic studies show that extrinsic factors from young mice can enhance stem cell activity in older mice (Conboy et al., 2005; Conboy and Rando, 2005). Moreover, the ability of mice to regenerate muscle, which depends on the activity of satellite cells, decreases with age. This decrease in the capacity of satellite cells to respond to injury appears to be partially caused by a reduction in Notch gene expresion and an increase in TGF-beta, which in the muscle, represses cell proliferation (Conboy et al., 2003). All of these data highlight the important influence that extrinsic factors have over stem cell maintenance and activity during the course of aging across species.
Results presented here extend our understanding of how Drosophila ovary niche function changes with age. High resolution in situ hybridization reveals that cap cells in young germaria specifically express high levels of dpp. As females age, dpp expression declines in cap cells, accompanied by a concomitant increase in Wnt signaling. Interestingly, we observe low levels of dpp expression in aged escort cells, suggesting that the balanced mutual antagonism between the BMP and Wnt pathways that exists in young escort cells becomes perturbed as these cells age. In regards aged cap cells, we speculate that increased Wnt signaling reinforces the observed loss of dpp expression. These findings suggest that the age-dependent decline in ovarian niche function is not merely a passive process, marked by a general reduction in basic cellular activity, but rather results from changes in signaling dynamics within niche cells and their close neighbors.
Materials and Methods
Fly stocks
Fly stocks were maintained at room temperature (RT) on standard cornmeal-agar medium unless otherwise indicated by experimental design. For RNAi, larvae were allowed to develop at RT for 5 days before being moved to a higher temperature (29°C) in order to increase the efficiency of knock-down. Adult flies were then kept at 29°C for 5 to 7 days before dissection of the ovaries. As the expression of UAS-sgg driven by c587-gal4 during development is lethal, flies were raised at RT until eclosion, adults were then shifted to 29°C for a week before dissection of the ovaries.
The following fly strains were used in this study: w1118 was used as a control; the Wnt Reporter 6TH-LacZ (MK B-31) was provided by K. Cadigan (University of Michigan); the y w hs-FLP122 ; Sp/Cyo ; fzp21Dfz2C2 ri FRT2A/TM2 and y w hs-FLP122 ; wgRF, FRT40A/Cyo ; TM2/TM6B were a gift from G. Struhl (Columbia University); c587-gal4 and Dad-LacZ lines were provided by A. Spradling (Carnegie Institute for Science, Baltimore, MD) ; hs-FLP, Ubi-GFP, FRT19A was a gift from Hugo Bellen (Baylor College of Medicine, Houston) ; Wnt4C1 (#6651), Wnt4EMS23 (#6650) alleles, UAS-Wnt4RNAi (#29442), UAS-fzRNAi (#31311), UAS-fz2RNAi (#31390 and #27562), UAS-Arrow/LRP5/6RNAi (#31473), UAS-dshRNAi (#31306 and #31307), UAS-armRNAi (#31304 and #31305), UAS-Pan/dTCFRNAi (#26743), UAS-sgg (#5361), UAS-ArmS10(#4782), Df(2R)BSC291/Cyo (#23676), y w dsh3 neoFRT19A/FM7 (#6331), UAS-MadRNAi (#31316) were obtained from the Bloomington Stock Center (stock # given in parentheses). The Wnt4::HisHA genomic construct was made according to Carreira-Rosario A. et al, 2013. Briefly, an in vitro recombineering step was performed between the P[acman] Bac CH321-78J12 containing the Wnt4 locus and a cassette containing the His-HA tag followed by a RFP+ marker and flanked by Wnt4 homology arms. Transgenic flies were then generated using the phiC31 integrase transgenesis system with 96E as the landing site. The RFP+ cassette was then excised using the Cre enzyme using the w ;; MKRS, hs-FLP86E/TM6B, Cre stock (Bloomington stock #1501).
Plasmids and Rescue
To construct the pUASt-Wnt4-Flag plasmid, full-length Wnt4-coding sequence was amplified by PCR from the RE26454 clone (Berkeley Drosophila Genome Project), cloned into pENTR/DTOPO (Invitrogen, Carlsbad, CA), and then recombined into pTWF (Terence Murphy, The Drosophila Gateway Vector Collection, Carnegie Institution of Washington, Baltimore, MD). The plasmid was injected into w1118 embryos by Rainbow Transgenics (Camarillo, CA) and flies were selected for the presence of the w+ transgene. Rescue of Wnt4 mutants was performed by driving Wnt4-Flag in ovaries with c587-gal4.
Generation of escort cell clones using MARCM
Escort cell mutant clones for fzp21 fz2C2 and wgRF (a small deletion that removes Wg, Wnt4, Wnt6 and Wnt10) were generated by FLP/FRT-mediated mitotic recombination using the MARCM system to positively mark the clones. For the double fzp21 fz2C2 mutant clones, 6 days after setting up the crosses larvae of the genotype hs-FLP/tubgal4:UAS-GFP;;tubgal80, FRT2A/ fzp21 fz2C2, FRT2A were heat-shocked at 37°C for 1 hr twice a day for 3 consecutive days. For the wgRF mutant clones, larvae of the genotype hs-FLP/tubgal4:UAS-GFP; tubgal80, FRT40A/ wgRF, FRT40A were heat-shocked as mentioned above. hs-FLP/tubgal4:UAS-GFP;;tubgal80, FRT2A/FRT2A and hs-FLP/tubgal4:UAS-GFP; tubgal80, FRT40A/ FRT40A were used as controls. The ovaries of the adults were dissected 7 days after eclosion.
Generation of follicle clones
Escort and follicle mutant clones for dsh3 were generated by FLP/FRT-mediated mitotic recombination. 6 days after setting up the crosses larvae of the genotype hs-FLP, UbiGFP, FRT19A/ dsh3, FRT19A were heat-shocked at 37°C for 1 hr twice a day for 3 consecutive days, and adults were dissected 7 days after eclosion. hs-FLP, UbiGFP, FRT19A/FRT19A were used as controls. To generate follicle mutant clones for dsh3 adult females of 1 to 5 days old of the genotype hs-FLP, UbiGFP, FRT19A/ dsh3, FRT19A were heat-shocked at 37°C for 1 hr twice a day for 3 consecutive days, and adults were dissected 14 days after the last day of heat-shock. Adults of the genotype hs-FLP, UbiGFP, FRT19A/FRT19A were used as controls. To generate follicle mutant clones for wgRF adult females of the genotype hs-FLP; UbiGFP, FRT40A/ wgRF, FRT40A were heat-shocked as mention above for the dsh3 clones and the adults hs-FLP; UbiGFP, FRT40A/FRT40A were used as controls.
Immunostaining
Adult ovaries were dissected, fixed, and stained according to Eliazer et al. (2011), except that ovaries stained for pMAD were fixed for 30 minutes. The images were taken with Zeiss LSM 510 confocal microscope. The following primary antibodies were used: mouse anti-Hts (1B1 from Developmental Studies Hybridoma Bank, dilution at 1:20), rat anti-VASA (Developmental Studies Hybridoma Bank, DSHB, 1:20), mouse anti-Lamin C (LC28.26, DSHB, 1:20), anti-Engrailed (4D9, DSHB, 1:2), mouse anti-Sxl (M114 DSHB, 1:10), rabbit anti-pMAD (Abcam, 1:200) mouse anti-BamC A7 (gift from D. McKearin, 1:20), rabbit anti-Nanos (gift from A. Nakamura, RIKEN, Kobe, Japan), rabbit anti-Spectrin (gift from R. Dubreuil, University of Illinois at Chicago, 1:2,500), mouse anti-β-galactosidase (Promega, 1:1,000), rabbit anti-GFP (Invitrogen, 1:1,000), guinea pig anti-Traffic Jam (gift from D. Godt, University of Toronto, Toronto, ON, Canada, 1:5,000), rat anti-HA (Roche, 3F10). Cy3, Cy5, FITC (Jackson Laboratories) or Alexa 488 (Molecular Probes) fluorescence-conjugated secondary antibodies were used at a 1:200 dilution.
In situ hybridization
In situ hybridization on adult ovaries was performed mostly as described in Raj A. and Tyagi S., Methods in Enzymology, 2010 for Drosophila imaginal discs. This method involves probing target mRNAs using a larger number (>30) of shorter oligonucleotides (20 bases), each of which hybridize to a different portion of the target mRNA. Each of these oligonucleotides are labeled with a single fluorophore at its 3’end. For designing the oligonucleotides, we used the following web-based program (http://www.singlemoleculefish.com/, (Raj and Tyagi, 2010)). Briefly, ovaries were dissected in RNAse free 1XPBS and then fixed in 3.7% formaldehyde for 45 min. After being washed 2 times for 5 min with 1XPBS, they were left in 70% EtOH overnight at 4°C. The next day, ovaries were washed briefly in the Wash Buffer containing 2XSSC RNAse free and 10% of deionized formamide and then incubated overnight at 37°C in the dark with the dpp probe at 50nM diluted in the Hybridization Buffer containing 2XSCC, 10% Dextran sulfate (Sigma D8906), 1μg/μl of yeast tRNA (Sigma R8759), 2mM Vanadyl ribonucleoside complex (NEB, S142), 0.02% RNAse free BSA (Ambion, AM2618) and 10% of deionized formamide. The last day, ovaries were washed 2 times 30 min each 37°C in the dark in the Wash Buffer and then resuspended in a drop of Vectashield containing DAPI (Vector Lab, H-1200).
Supplementary Material
Highlights.
Wnt pathway activation within escort cells promotes germ cell differentiation.
Disruption of the Wnt pathway results in ectopic Dpp signaling within the germarium.
Loss of BMP signal transduction in anterior escort cells results in enhanced Wnt pathway activity.
In aged females, the Wnt pathway becomes active in niche cells, coincident with a decline in niche BMP production.
Acknowledgments
The authors would like to thank Kenneth Cadigan, Gary Struhl, Allan Spradling, Hugo Bellen, Benjamin Ohlstein, the Bloomington Stock Center and the Iowa Developmental Hybridoma Bank for providing reagents. Members of the Buszczak lab provided comments on the manuscript. This work was funded in part by the NIGMS (GM086647), the NIA (AG047318) and the E.E. and Greer Garson Fogelson Endowment (UTSW Medical Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions:
V. M-P. and V.P. designed and conducted experiments, interpreted the data and helped to write the paper. V. M-P. and V.P. carried out experiments and helped to edit the manuscript. S.E. conducted several of the initial genetic experiments. S.S. developed various transgenes and other reagents used throughout the study and M.B. designed the experiments, interpreted the data and helped to write the manuscript.
References
- Barolo S. Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene. 2006;25:7505–7511. doi: 10.1038/sj.onc.1210057. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development. 1999;126:4175–4186. doi: 10.1242/dev.126.18.4175. [DOI] [PubMed] [Google Scholar]
- Chang MV, Chang JL, Gangopadhyay A, Shearer A, Cadigan KM. Activation of wingless targets requires bipartite recognition of DNA by TCF. Curr Biol. 2008;18:1877–1881. doi: 10.1016/j.cub.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Struhl G. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development. 1999;126:5441–5452. doi: 10.1242/dev.126.23.5441. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003a;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003b;130:1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Mariol MC, Wallace RM, Weyers J, Kamberov YG, Pradel J, Wilder EL. DWnt4 regulates cell movement and focal adhesion kinase during Drosophila ovarian morphogenesis. Dev Cell. 2002;2:437–448. doi: 10.1016/s1534-5807(02)00142-9. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. doi: 10.4161/cc.4.3.1518. [DOI] [PubMed] [Google Scholar]
- Coudreuse D, Korswagen HC. The making of Wnt: new insights into Wnt maturation, sorting and secretion. Development. 2007;134:3–12. doi: 10.1242/dev.02699. [DOI] [PubMed] [Google Scholar]
- de Cuevas M, Spradling AC. Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development. 1998;125:2781–2789. doi: 10.1242/dev.125.15.2781. [DOI] [PubMed] [Google Scholar]
- Decotto E, Spradling AC. The Drosophila ovarian and testis stem cell niches: similar somatic stem cells and signals. Dev Cell. 2005;9:501–510. doi: 10.1016/j.devcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Eliazer S, Shalaby NA, Buszczak M. Loss of lysine-specific demethylase 1 nonautonomously causes stem cell tumors in the Drosophila ovary. Proc Natl Acad Sci U S A. 2011;108:7064–7069. doi: 10.1073/pnas.1015874108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes A, Lehmann R. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development. 1998;125:679–690. doi: 10.1242/dev.125.4.679. [DOI] [PubMed] [Google Scholar]
- Forbes AJ, Lin H, Ingham PW, Spradling AC. hedgehog is required for the proliferation and specification of ovarian somatic cells prior to egg chamber formation in Drosophila. Development. 1996a;122:1125–1135. doi: 10.1242/dev.122.4.1125. [DOI] [PubMed] [Google Scholar]
- Forbes AJ, Spradling AC, Ingham PW, Lin H. The role of segment polarity genes during early oogenesis in Drosophila. Development. 1996b;122:3283–3294. doi: 10.1242/dev.122.10.3283. [DOI] [PubMed] [Google Scholar]
- Hamada-Kawaguchi N, Nore BF, Kuwada Y, Smith CI, Yamamoto D. Btk29A promotes Wnt4 signaling in the niche to terminate germ cell proliferation in Drosophila. Science. 2014;343:294–297. doi: 10.1126/science.1244512. [DOI] [PubMed] [Google Scholar]
- Harris RE, Pargett M, Sutcliffe C, Umulis D, Ashe HL. Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev Cell. 2011;20:72–83. doi: 10.1016/j.devcel.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaki M, Yoshikawa S, Thomas JB, Aburatani H, Nose A. Wnt4 is a local repulsive cue that determines synaptic target specificity. Curr Biol. 2007;17:1574–1579. doi: 10.1016/j.cub.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Jiang J, Struhl G. Complementary and mutually exclusive activities of decapentaplegic and wingless organize axial patterning during Drosophila leg development. Cell. 1996;86:401–409. doi: 10.1016/s0092-8674(00)80113-0. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Wang S, Xie T. Self-maintained escort cells form a germline stem cell differentiation niche. Development. 2011;138:5087–5097. doi: 10.1242/dev.067850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Yamada M, Asaoka M, Kitamura T. Essential role of the posterior morphogen nanos for germline development in Drosophila. Nature. 1996;380:708–711. doi: 10.1038/380708a0. [DOI] [PubMed] [Google Scholar]
- Kuroda J, Nakamura M, Yoshida M, Yamamoto H, Maeda T, Taniguchi K, Nakazawa N, Hatori R, Ishio A, Ozaki A, Shimaoka S, Ito T, Iida H, Okumura T, Maeda R, Matsuno K. Canonical Wnt signaling in the visceral muscle is required for left-right asymmetric development of the Drosophila midgut. Mech Dev. 2012;128:625–639. doi: 10.1016/j.mod.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Liu M, Lim TM, Cai Y. The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci Signal. 2010;3:ra57. doi: 10.1126/scisignal.2000740. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annual review of cell and developmental biology. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lopez-Onieva L, Fernandez-Minan A, Gonzalez-Reyes A. Jak/Stat signalling in niche support cells regulates dpp transcription to control germline stem cell maintenance in the Drosophila ovary. Development. 2008;135:533–540. doi: 10.1242/dev.016121. [DOI] [PubMed] [Google Scholar]
- Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKearin DM, Spradling AC. bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- Morris LX, Spradling AC. Long-term live imaging provides new insight into stem cell regulation and germline-soma coordination in the Drosophila ovary. Development. 2011;138:2207–2215. doi: 10.1242/dev.065508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai LM, Orsulic S, Bejsovec A, Peifer M. Negative regulation of Armadillo, a Wingless effector in Drosophila. Development. 1997;124:2255–2266. doi: 10.1242/dev.124.11.2255. [DOI] [PubMed] [Google Scholar]
- Pan L, Chen S, Weng C, Call G, Zhu D, Tang H, Zhang N, Xie T. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1:458–469. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Prud'homme B, Lartillot N, Balavoine G, Adoutte A, Vervoort M. Phylogenetic analysis of the Wnt gene family. Insights from lophotrochozoan members. Curr Biol. 2002;12:1395. doi: 10.1016/s0960-9822(02)01068-0. [DOI] [PubMed] [Google Scholar]
- Raj A, Tyagi S. Detection of individual endogenous RNA transcripts in situ using multiple singly labeled probes. Methods in enzymology. 2010;472:365–386. doi: 10.1016/S0076-6879(10)72004-8. [DOI] [PubMed] [Google Scholar]
- Rojas-Rios P, Gonzalez-Reyes A. The plasticity of stem cell niches: A general property behind tissue homeostasis and repair. Stem Cells. 2013 doi: 10.1002/stem.1621. [DOI] [PubMed] [Google Scholar]
- Rojas-Rios P, Guerrero I, Gonzalez-Reyes A. Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 2012;10:e1001298. doi: 10.1371/journal.pbio.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai-Hernandez P, Nystul TG. A dynamic population of stromal cells contributes to the follicle stem cell niche in the Drosophila ovary. Development. 2013;140:4490–4498. doi: 10.1242/dev.098558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Wood CG, Jones DL, Tazuke SI, Fuller MT. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development. 2002;129:4523–4534. doi: 10.1242/dev.129.19.4523. [DOI] [PubMed] [Google Scholar]
- Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, Xie T. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- Song X, Xie T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 2003;130:3259–3268. doi: 10.1242/dev.00524. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Z, Cai Y. The JAK/STAT pathway positively regulates DPP signaling in the Drosophila germline stem cell niche. J Cell Biol. 2008;180:721–728. doi: 10.1083/jcb.200711022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Gao Y, Song X, Ma X, Zhu X, Mao Y, Yang Z, Ni J, Li H, Malanowski KE, Anoja P, Park J, Haug J, Xie T. Wnt signaling-mediated redox regulation maintains the germ line stem cell differentiation niche. Elife. 2015;4:e08174. doi: 10.7554/eLife.08174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Lin H. Nanos maintains germline stem cell self-renewal by preventing differentiation. Science. 2004;303:2016–2019. doi: 10.1126/science.1093983. [DOI] [PubMed] [Google Scholar]
- Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J Biol Chem. 2002;277:41762–41769. doi: 10.1074/jbc.M207850200. [DOI] [PubMed] [Google Scholar]
- Wu J, Roman AC, Carvajal-Gonzalez JM, Mlodzik M. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat Cell Biol. 2013;15:1045–1055. doi: 10.1038/ncb2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Jia S, Huang S, Wang H, Zhu Y, Mu Y, Kan L, Zheng W, Wu D, Li X, Sun Q, Meng A, Chen D. The Fused/Smurf complex controls the fate of Drosophila germline stem cells by generating a gradient BMP response. Cell. 2010;143:978–990. doi: 10.1016/j.cell.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.