Abstract
TLRs are critical for innate immunity, but excessive activation can lead to tissue damage and disease. Specialized proresolving mediators (SPMs), including resolvin D2 (RvD2), promote the active resolution of inflammation. How SPMs regulate early LPS signaling, including activation of TLR4, is unknown. We treated human THP-1 monocytic cells and primary human blood monocytes with RvD2 and LPS to evaluate modulation of TLRs. miRNA-146a overexpression and inhibition were used to dissect the mechanism of RvD2-mediated actions. We validated our studies using ELISAs for cytokines, PCR, Western blot analysis, and flow cytometry. Cells treated with 0.1% ethanol (control for RvD2) and/or PBS (control for LPS), and control microRNA mimics and inhibitors were used as controls. RvD2 reduced LPS-induced cytokines and TLR4 expression in human monocytes by up to 75%. In THP-1 cells, RvD2 reduced expression of TLR4, lymphocyte antigen 96 (MD-2), and downstream signals (MyD88, TRIF, and TAK1). These effects were partially mediated through RvD2 induction of microRNA-146a, and RvD2’s actions were blocked by microRNA-146a inhibition. These new findings reveal the ability of RvD2 to reduce TLR4 expression and attenuate LPS-induced inflammation, providing a new area of SPM activity to investigate in this major area of therapeutic research.—Croasdell, A., Sime, P. J., Phipps, R. P. Resolvin D2 decreases TLR4 expression to mediate resolution in human monocytes.
Keywords: inflammation, LPS, MD-2, microRNA-146a, SPM
TLRs are one of the essential components of the immune response; they are particularly critical in the innate immune response to invading pathogens. More than a dozen TLRs have been identified that respond to a variety of pathogens, including bacteria, viruses, and fungi. These TLRs act through conserved pathways, signaling through myeloid differentiation primary response gene 88 (MyD88) or TIR-domain-containing adapter-inducing IFN-β (TRIF) to induce an inflammatory response (1). Many of these TLRs are expressed by human monocytes. Monocytes are one of the first responders and a primary mediator of pathogen-induced inflammation (2). In particular, monocytes highly express TLR4, the primary TLR responsible for responding to LPS, the major cell wall component of gram-negative bacteria.
LPS induces a strong proinflammatory response through activation of the TLR4 signaling cascade. CD14 and LPS-binding protein (LBP) are responsible for presenting LPS to TLR4, which is complexed with lymphocyte antigen 96 (MD-2, myeloid differentiation 2) (1, 3, 4). A TLR4/MD-2 homodimer forms and through both MyD88 and TRIF/TNF receptor–associated factor (TRAF) signaling, TLR4 activates NF-κB, AP-1, and IFN responses (1, 3, 4). Although this responsiveness to invading pathogens is an important protective response, prolonged activation of TLR4 by LPS can lead to numerous health complications (5–7). Short term, excessive TLR4 activation contributes to enhanced cell death, cytokine storm, systemic inflammatory responses, and septic shock (5, 8, 9). Long term, TLR4 activation has also been implicated in a variety of diseases, including pneumonia, chronic obstructive pulmonary disease, and asthma (9–11). It is noteworthy that a phenomenon termed “tolerance” occurs in vitro in response to LPS where cells that undergo repeated LPS stimulations become tolerant to this activation and fail to produce proinflammatory cytokines or mount an immune response (12, 13). Although the clinical existence and importance of tolerance is highly debated, it demonstrates the complexity of TLR4 regulation, and the importance of appropriate activation of TLR4 to avoid insufficient or excessive responsiveness.
Recently, endogenous lipid derived mediators termed specialized proresolving mediators (SPMs) have been identified as key players in the resolution of inflammation. SPMs are derivatives of polyunsaturated fatty acids and are divided into subclasses based on their metabolic pathway and structures, including lipoxins, resolvins, protectins, and maresins (14). These molecules have both anti-inflammatory and proresolving actions, and it is clear that SPMs are not immunosuppressive. SPMs can induce a paradigm shift in monocyte function, enhancing apoptotic neutrophil clearance, promoting production of proresolving cytokines, and enhancing phagocytosis (14–17). SPMs have been shown to play roles in mediating multiple inflammatory diseases, including several lung related diseases such as those linked to cigarette smoke exposure, asthma, and fibrosis (15, 16, 18).
Along with these proinflammatory environmental insults, there is a growing interest in the efficacy of SPMs against viral and bacterial infections. SPMs, primarily lipoxins and D-series resolvins, can dampen LPS-induced inflammation in mouse models of acute lung injury. Resolvin D1 (RvD1) and other SPMs dampen proinflammatory cytokines and cellular influx to reduce LPS-induced tissue destruction and mortality in mice (19, 20). SPMs have even been effective against live bacteria, promoting resolution in several models of sepsis and nontypeable Haemophilus influenzae lung infection, restoring lung physiology and reducing the need for antibiotics in infected mice (21–23). Despite these results showing that SPMs are effective at attenuating bacterial induced, and specifically LPS-induced inflammation, very little is known about the effects of SPMs on TLR expression and signaling, especially in human cells. Based on the nonimmunosuppressive nature of resolvins and their efficacy in acting on macrophages in other inflammatory models, we hypothesized that resolvin D2 (RvD2) dampens TLR4 expression to attenuate LPS-induced inflammation. Given the role of LPS in promoting pathogen-induced inflammation and the many diseases for which LPS plays an important role, understanding how SPMs may affect TLR4 signaling is an important area of research.
MATERIALS AND METHODS
Materials
RvD2 (10007279) was purchased from Cayman Chemicals (Ann Arbor, MI, USA). Polyinosinic/polycytidylic acid [poly(I:C); tlrl-pic] was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Pam3CSK (NBP2-25297) was purchased from Novus Biologicals (Littleton, CO, USA). LPS 0111:B4 (L4391), anti-TLR4 antibody (PRS3141), LPS 055:B5 (6529), and hyaluronan (S0326) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-MD-2 antibody (ab24182) and DyLight488 secondary antibody (ab96883) were purchased from Abcam (Cambridge, MA, USA). AF488 isotype control antibody (sc-45068) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti–G-protein coupled receptor 18 (GPR18) antibody (NBP2-24918) was purchased from Imgenex (San Diego, CA, USA). Anti-CD14 antibody (555398) and ELISA components for IL-6 (554543 and 554546) and TNF-α (555212) were purchased from BD Biosciences (San Jose, CA, USA). ELISA antibodies for IL-8 (M-801, M-802-B) were purchased from Endogen (Farmingdale, NY, USA). Anti-actin antibody (CP-01) was purchased from Calbiochem (San Diego, CA, USA). Secondary Western blot antibodies (115-035-146, 111-035-144) were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). PBS (14200-075) and RPMI 1640 medium (11875-119) were purchased from Thermo Fisher Scientific.
RvD2 treatment and LPS exposure
For human monocytes, peripheral blood mononuclear cells were isolated as described previously (24). Human peripheral blood monocytes were further isolated by adherence to tissue culture dishes for 2 h. Human monocytes were first treated with 1 to 100 nM of RvD2 or vehicle (0.1% EtOH in PBS) for 1 h. After RvD2 treatment, cells were exposed to 20 ng/ml LPS (0111:B4), 20 ng/ml poly(I:C), or 20 ng/ml Pam3CSK. For assessment of TLRs, MD-2, and MyD88 mRNA and TLR4 protein, 1 h after activation cells were washed twice with 1× PBS and lysed with CW lysis buffer (50 mM Tris-HCl, 2% SDS). Supernatants were collected 24 h after exposure and cytokines were assessed by ELISA according to manufacturer’s protocols (IL-6, IL-8, and TNF-α).
In separate experiments, THP-1 cells were plated in 12-well plates in serum-free medium for 24 h. After serum starvation, monocytes were treated with 1, 10, or 100 nM RvD2 or vehicle (0.1% EtOH in PBS) for 1 h before exposure to 20 ng/ml LPS (011:B4) or vehicle (1× PBS). To assess TLR4 activation with multiple stimuli, cells were additionally treated with LPS 055:B5 or hyaluronan. After activation, THP-1 cells were washed twice with 1× PBS and lysed with CW Buffer (50 mM Tris-HCl, 2% SDS).
Flow cytometry for GPR18 receptor expression
THP-1 cells were treated as described above. Four hours after LPS exposure, cells were collected, washed, and stained with 1:50 dilution of GPR18 antibody. Monocytes were then washed and stained with a secondary DyLight 488 antibody at a 1:200 dilution. GPR18 receptor expression was determined by flow cytometry using a 2-laser, 4-color BD Accuri C6 (BD Biosciences) and FlowJo analysis software (FlowJo, LLC, Ashland, OR, USA). Unstained and isotype controls were included to verify expression changes.
Assessment of TLR4 and associated genes
RNA was isolated using the Qiagen miRNeasy kit (217004; Germantown, MD, USA) according to manufacturer’s protocols. RNA was quantified by NanoDrop Instrument and cDNA synthesized using a iScript kit according to manufacturer’s protocols (170-8891; Bio-Rad, Hercules, CA, USA). mRNA levels of GPR18, TLR4, MyD88, TRIF, sterile α and TIR motif containing 1 (SARM1), transforming growth factor β-activated kinase 1 (TAK1), LBP, MD-2, gp96, and PRAT4A, IL-1β, NLRP3, and caspase-1 were determined by semiquantitative PCR using the CFX Connect Real Time Detection System from Bio-Rad. Bio-Rad CFX Manager software was used to calculate Ct values according to standard curves. All mRNAs were normalized to 18S mRNA as a control. CD14 was assessed by flow cytometry as previously described (15). Western blots analyses were performed as described previously to detect TLR4 and MD-2 protein expression 4 h after LPS exposure (15).
miRNA evaluation and transfections
All microRNA (miR) reagents were purchased from Applied Biosystems Life Technologies (Grand Island, NY, USA). Primers for hsa-miR-146a, -146b, -155, -27a, and -223 (4427975, 4427975, 4427975, 4427975, 4427975) were used for cDNA and RNA synthesis. miRNA-specific cDNA was synthesized according to manufacturer’s protocol using the TaqMan MicroRNA Reverse Transcriptase Kit (4366595). miRNA levels were assessed by PCR using the TaqMan Universal PCR Master Mix (4304437). Bio-Rad CFX Manager software was used to calculate Ct values according to standard curves. miRNA values were normalized to U6 as a control gene.
For miR-146a mimic and inhibitor experiments, THP-1 cells were plated in 12-well plates in RPMI 1640 medium with 1% fetal bovine serum. Cells were transfected with 30 μM of a miR-146a mimic (4464066) or a control mimic (4464058), or a miR-146a inhibitor (4464084) or control inhibitor (4464076) for 24 h using lipofectamine 2000 (11668-019; Thermo Fisher Scientific). In brief, mimic/ inhibitor or lipofectamine were added to separate tubes of 100 μl/sample of Opti-Mem medium and incubated at room temperature for 5 min. Mimic/inhibitor and lipofectamine were then mixed in a 1:1 ratio and incubated at room temperature for an additional 20 min before addition of 200 μl lipofectamine mix to each sample. After transfection, cells were washed with 1× PBS and then treated as described above with RvD2 and LPS. The effects of miR-146a mimic or inhibitor were assessed on IL-8, TNF-α, and TLR4 expression.
Statistical analysis
All experiments were repeated 3 to 5 times with THP-1 cells or with 3 to 6 human donors in independent experiments. Results are expressed as means ± sem. Statistical analyses on normally distributed data were performed by Student's t test or 1- or 2-way ANOVA with Bonferroni’s posttest correction for multiple comparisons by Prism software (GraphPad Software, La Jolla, CA, USA).
RESULTS
RvD2 decreases LPS-induced cytokine production and TLR4 in human monocytes
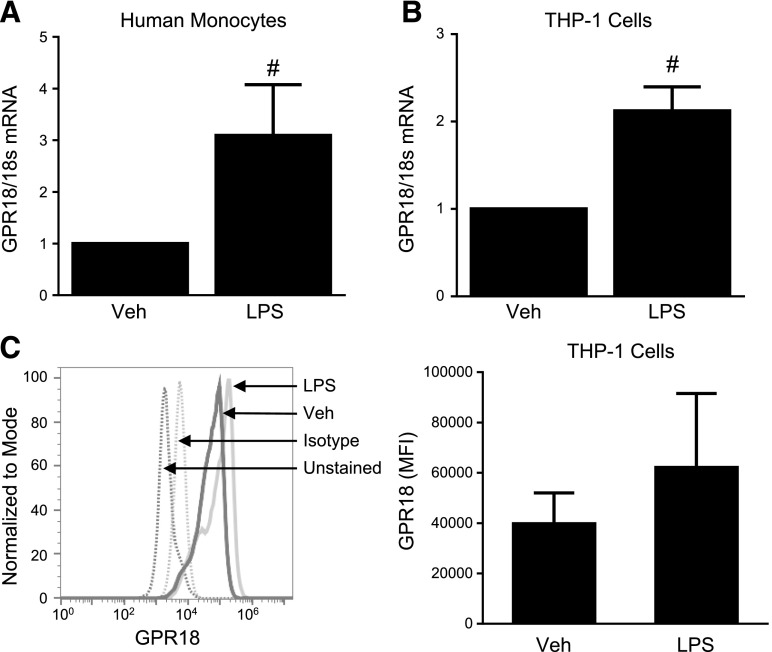
Human monocytes and macrophages contribute to the synthesis of multiple SPMs, including D-series resolvins (25). In particular, RvD2 has shown efficacy at attenuating inflammation and acting through monocytes/macrophages to mediate its effects (15, 17, 26–28). Along with producing RvD2, these cells have recently been shown to express the RvD2 receptor, GPR18 (29). LPS treatment of both THP-1 cells and human monocytes increased expression of GPR18 mRNA and protein, likely initiating a feedback loop (Fig. 1). These results, along with the known production of RvD2 by human monocytes/macrophages, led us to next investigate whether RvD2 could alter inflammatory responses induced by LPS and other TLR ligands.
Figure 1.
RvD2 receptor GPR18 is increased with LPS treatment. Human blood monocytes (A) and THP-1 cells (B, C) were treated with 20 ng/ml LPS. At 4 h after activation, levels of GPR18 mRNA were assessed by RT-PCR (A, B), or protein expression was determined by flow cytometry (C). Statistical significance was determined by Student's t test, n = 3–4 independent experiments or individual donors. #P < 0.05 compared to vehicle.
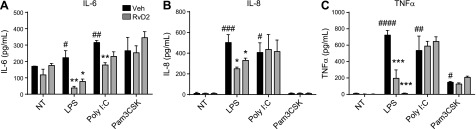
To evaluate the potential efficacy of RvD2 in attenuating TLR-ligand induced inflammation, human monocytes were treated with a range of TLR ligands and cytokine production was assessed. Ligands for TLR2–4 were chosen based on the high expression level of these TLRs in human monocytes (1, 2). RvD2 failed to have a significant impact on IL-6, IL-8, or TNF-α production induced by TLR2 (Pam3CSK) and TLR3 [poly(I:C)] ligands. However, TLR4 (LPS)-induced cytokine production was significantly reduced (Fig. 2).
Figure 2.
RvD2 dampens LPS-induced cytokine production by human monocytes. Human blood monocytes were treated with 10 or 100 nM RvD2 followed by 20 ng/ml LPS, poly(I:C), or Pam3CSK. At 24 h, activation levels of IL-6 (A), IL-8 (B), and TNF-α (C) were assessed by ELISA. Statistical significance was determined by 2-way ANOVA, n = 3–4 individual donors. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 compared with vehicle/vehicle; *P < 0.05, **P < 0.01, ***P < 0.001 compared with activator/vehicle.
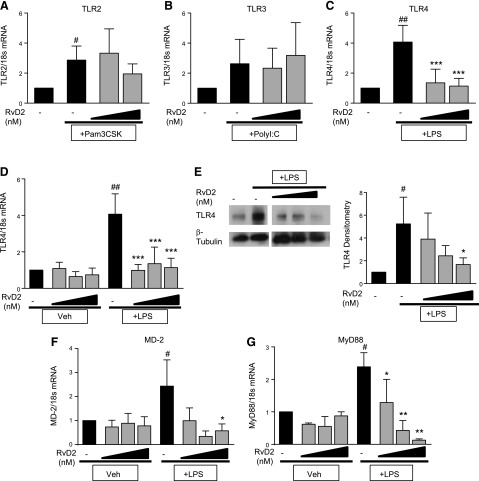
Based on these data, we then evaluated the effect of RvD2 on TLR expression. Similar to our observed cytokine effects, RvD2 did not dampen TLR2 or TLR3 mRNA expression (Fig. 3A, B). RvD2, however, did decrease TLR4 mRNA expression in human monocytes stimulated with LPS (Fig. 3C). We further explored whether RvD2 would alter baseline expression of TLR4 or whether these effects were only seen with LPS activation. RvD2 did not alter basal TLR4 expression in these cells, and only attenuated LPS-induced TLR4 levels (Fig. 3D). Along with mRNA, we evaluated TLR4 protein expression by Western blot analysis 1 h after LPS exposure. RvD2 additionally decreased TLR4 protein expression (Fig. 3E). RvD2 further decreased mRNA expression of MD-2, an important binding partner necessary for TLR4-mediated responses to LPS, and MyD88, a downstream signaling molecule (Fig. 3F, G), indicating that these observed decreases in TLR4 also affect subsequent signals.
Figure 3.
RvD2 dampens LPS-induced TLR4 expression in human monocytes. Human blood monocytes were treated with 10 or 100 nM RvD2 followed by 20 ng/ml LPS, poly(I:C), or Pam3CSK. A–C) TLR2 (A), TLR3 (B), and TLR4 (C) mRNA expression were determined 1 h after activator stimulation by RT-PCR. D–G) Effects of 1–100 nM RvD2 alone and with LPS treatment on TLR4 (D), MD-2 (F), and MyD88 (G) mRNA were also assessed; TLR4 protein expression was determined by Western blot analysis 1 h after LPS exposure (E) (representative image shown, densitometry for n = 4). Statistical significance was determined by 2-way ANOVA, n = 4–5 individual donors. #P < 0.05, ##P < 0.01 compared with vehicle/vehicle; *P < 0.05, **P < 0.01, ***P < 0.001 compared with activator/vehicle.
RvD2 decreases TLR4 and downstream signaling genes in THP-1 monocytes
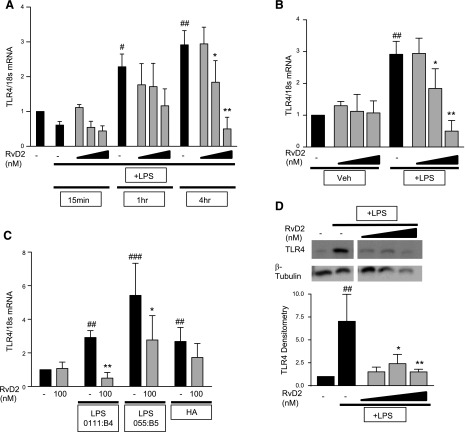
To allow for more in depth studies, we used THP-1 cells, a human monocytic cell line, to investigate the role of RvD2 in modulating TLR4 expression. LPS induced TLR4 mRNA expression in a time-dependent manner, with the strongest induction at 4 h after LPS exposure (Fig. 4A). RvD2 did not decrease TLR4 mRNA at 15 min or 1 h, but significantly reduced mRNA at 4 h postexposure (Fig. 4A). Similar to human monocytes, RvD2 alone did not decrease basal TLR4 mRNA, but only decreased LPS-stimulated TLR4 4 h after LPS exposure (Fig. 4B). These reductions were not specific to a particular form of LPS, as 100 nM RvD2 reduced TLR4 mRNA induced by LPS O55:B5 and hyaluronic acid (Fig. 4C). Along with mRNA, RvD2 reduced TLR4 protein expression 4 h after LPS exposure (Fig. 4D).
Figure 4.
RvD2 decreases TLR4 expression in time-dependent manner in THP-1 monocytic cells. A) THP-1 cells were treated with 1–100 nM RvD2 followed by 20 ng/ml LPS. TLR4 mRNA was assessed 15 min, 1 h, and 4 h after LPS stimulation. B) Effects of 1–100 nM RvD2 alone and with LPS treatment on TLR4 mRNA were also assessed 4 h after LPS exposure (same data shown as that for vehicle/vehicle, vehicle/LPS, and RvD2/LPS groups). C) THP-1 cells were also treated with 100 nM RvD2 followed by 20 ng/ml LPS (either 0111:B4 or 055:B5) or hyaluronan, and TLR4 mRNA was assessed after 4 h. D) TLR4 protein expression was determined by Western blot analysis 4 h after LPS exposure (representative image shown, densitometry for n = 4). Statistical significance was determined by 2-way ANOVA, n = 3–5 independent experiments. #P < 0.05, ##P < 0.01, ###P < 0.001 compared with vehicle/vehicle; *P < 0.05, **P < 0.01 compared with activator/vehicle.
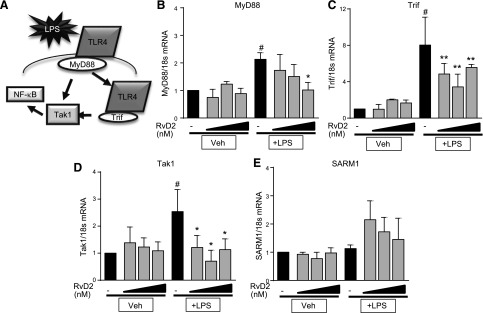
We next investigated whether these changes in TLR4 expression affected downstream signaling pathways (Fig. 5A). RvD2 can decrease NF-κB expression, the main endpoint of TLR4 signaling (15, 30). TLR4 is the only TLR to signal through both the MyD88 and TRIF signaling pathways, and indeed, we observed that LPS increased mRNA expression of both of these genes (Fig. 5B, C). RvD2 dose-dependently decreased expression of both MyD88 and TRIF after LPS exposure (Fig. 5B, C). Additionally, RvD2 dampened LPS-induced increases in TAK1, an intermediary gene that is downstream of both MyD88 and TRIF, which promotes NF-κB signaling (Fig. 5D). We also evaluated expression of SARM1, a negative regulator of TRIF expression and found that RvD2-treated cells had trending increases in SARM1 expression, though these changes were not statistically significant (Fig. 5E).
Figure 5.
RvD2 decreases mRNA expression of TLR4 downstream signaling molecules in THP-1 monocytic cells. A) Schematic of TLR4 downstream signaling. B–D) THP-1 cells were treated with 1–100 nM RvD2 followed by 20 ng/ml LPS for 4 h. Expression of downstream signaling genes MyD88 (B), TRIF (C), TAK1 (D), and SARM1 (E) was determined by RT-PCR. Statistical significance was determined by 2-way ANOVA, n = 3–5 independent experiments. #P < 0.05 compared with vehicle/vehicle; *P < 0.05, **P < 0.01 compared with LPS/vehicle.
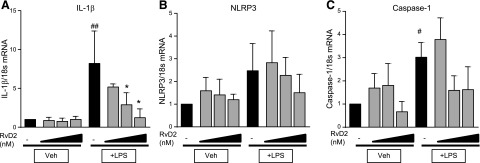
In addition to downstream signals, TLR4 ligand binding triggers activation of the inflammasome. We assessed expression of 3 players in inflammasome activation- IL-1β, NLRP3, and caspase-1. LPS strongly induced IL-1β expression, which was dose-dependently reduced by RvD2 treatment (Fig. 6A). In contrast, LPS only moderately increased expression of NLRP3 and caspase-1 mRNA (Fig. 6B, C). RvD2 did not alter expression of either of these proteins (Fig. 6B, C). These data indicate that RvD2 may primarily be acting on pathways outside of inflammasome activation.
Figure 6.
RvD2 decreases expression of IL-1β in THP-1 monocytic cells. THP-1 cells were treated with 1–100 nM RvD2 followed by 20 ng/ml LPS for 4 h. Expression of IL-1β (A), NLRP3 (B), and caspase-1 (C) was determined by RT-PCR. Statistical significance was determined by 2-way ANOVA, n = 4 independent experiments. #P < 0.05, ##P < 0.01 compared with vehicle/vehicle; *P < 0.05 compared with LPS/vehicle.
RvD2 decreases MD-2 expression in THP-1 cells
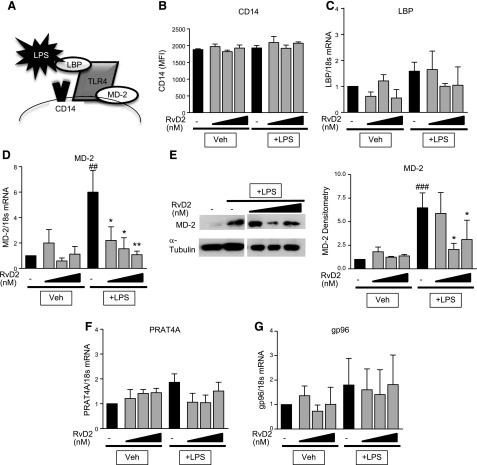
There are many additional signaling molecules necessary for mediating TLR4 responses to LPS (Fig. 7A). Two of these molecules, CD14 and LBP, are not necessary for cellular responsiveness to LPS, but they greatly increase the binding efficiency of LPS and TLR4 to increase sensitivity (4). Neither LPS nor RvD2 altered CD14 expression or LBP (Fig. 7B, C). In contrast, LPS strongly induced both mRNA and protein expression of MD-2 (Fig. 7D, E). MD-2 is a critical cofactor for LPS involved in trafficking, formation of TLR4 homodimers, and LPS signaling and is necessary for TLR4 responsiveness to LPS (3, 4, 31–33). RvD2 treatment dose-dependently reduced LPS-induced expression of MD-2 mRNA and protein (Fig. 7D, E).
Figure 7.
RvD2 decreases expression of MD-2 in THP-1 monocytic cells. A) Schematic of TLR4 binding complex. THP-1 cells were treated with 1–100 nM RvD2 followed by 20 ng/ml LPS for 4 h. B) CD14 surface expression was determined by flow cytometry. C–G) Expression of LBP (C), MD-2 (D), PRAT4A (F), and gp96 (G) was determined by RT-PCR; MD-2 protein expression was determined by Western blot analysis (E) (representative image shown, densitometry for n = 3). Statistical significance was determined by 2-way ANOVA, n = 3–5 independent experiments. ##P < 0.01, ###P < 0.001 compared with vehicle/vehicle; *P < 0.05, **P < 0.01 compared with LPS/vehicle.
Along with other proteins that aid in LPS signaling, multiple genes are involved in promoting proper trafficking of TLR4 to the cell surface. Two main genes, gp96 and PRAT4A, are responsible for proper folding of TLR4 to allow for appropriate transport (34–36). Neither LPS nor RvD2, though, altered expression of these genes in THP-1 cells (Fig. 7F, G), indicating that the effects of RvD2 are not on the trafficking component of the TLR4 pathway. Overall, these data demonstrate that RvD2 specifically targets the TLR4/MD-2 complex, rather than altering LPS presentation or TLR4 trafficking to promote resolution and lower proinflammatory responses to LPS.
RvD2 increases miRNA146a to promote TLR4 degradation
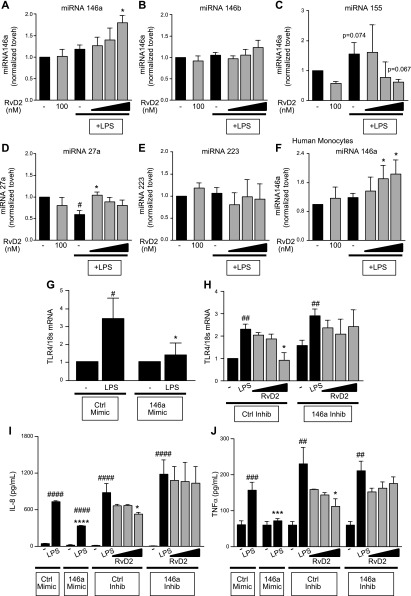
TLR4 expression is regulated by a variety of genes and signaling pathways, providing a large number of possible targets which RvD2 could be acting upon. In particular, there is a growing body of literature regarding the regulation of TLR4 by microRNAs. Several miRNAs can regulate TLR4 expression, inflammasome signaling, and inflammatory cytokines (37). We evaluated several candidate miRNAs to observe the effects of LPS and RvD2. RvD2 treatment of THP-1 cells followed by LPS activation increased expression of miR-146a, but not miR146b, indicating one possible mechanism by which RvD2 decreases TLR4 expression as these miRNAs both directly target TLR4 (Fig. 8A, B). LPS alone led to trending increases in miR155 and decreases in miR27a; both of these effects were abrogated by RvD2 treatment (Fig. 8C, D). Neither LPS nor RvD2 altered miR223, a regulator of inflammasome activation (Fig. 8E) (38). Given these observed changes and known targets of the selected miRNAs, miR-146a appeared to be the most likely candidate for negatively regulating TLR4 in this model. These findings were confirmed in human monocytes, where RvD2 dose-dependently increased miR-146a (Fig. 8F).
Figure 8.
RvD2 increases miR-146a, which decreases TLR4 expression. THP-1 cells or human monocytes were treated with 1–100 nM RvD2 followed by 20 ng/ml LPS for 4 h. A–E) miRNA-146a (A), -146b (B), -155 (C), -27a (D), and -223 (E) expression levels were determined by RT-PCR for THP-1 cells. F) miR-146a expression levels in human monocytes were also evaluated. THP-1 cells were transfected further with control or miR-146a mimics or inhibitors before RvD2 and LPS treatment. G) THP-1 cells transfected with miR-146a mimic had reduced TLR4 expression compared with control transfection. H) In contrast, inhibition of miR-146a partially blocked effects of RvD2 in decreasing TLR4 expression. I, J) The 146a mimic reduced IL-8 (I) and TNF-α (J) cytokine production, whereas inhibition of miR-146a prevented RvD2 reductions in these cytokines. Statistical significance was determined by 2-way ANOVA, n = 3–5 independent experiments. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.001 compared with corresponding vehicle group; *P < 0.05, ***P < 0.001, ****P < 0.0001 compared with control/LPS.
Overexpression of miR-146a by transfection demonstrated similar outcomes as RvD2 treatment, wherein the miR-146a mimic decreased TLR4 expression (Fig. 8G). Additionally, we blocked miR-146a expression by transfecting THP-1 cells with a miR-146a inhibitor. Inhibition of miR-146a resulted in strong LPS-induced increases in TLR4 (Fig. 8H). MD-2 expression was not altered by transfection with either a miR-146a mimic or a miR-146a inhibitor (data not shown). Furthermore, inhibition of miR-146a partially blocked RvD2 decreases in TLR4 (Fig. 8H). Similar results were observed with LPS-induction of IL-8 and TNF-α; miR-146a mimic reduced LPS-induction of these cytokines, and inhibition of miR-146a prevented RvD2-reductions of IL-8 and TNF-α (Fig. 8I, J). Taken together, these data demonstrate that RvD2 is acting in part by increasing miR-146a expression to decrease TLR4 and mediating resolution.
DISCUSSION
SPMs represent a novel class of nonimmunosuppressive small lipid molecules with high clinical potential for treatment of inflammatory diseases. Here, we have shown that RvD2 dampens proinflammatory cytokine production induced by LPS in human monocytes. These actions are stimuli-dependent, as RvD2 only acted to decrease LPS-induced cytokines and TLR4 expression, and not TLRs or cytokines induced by poly(I:C) or Pam3CSK. In addition to human peripheral blood monocytes, THP-1 cells were similarly responsive to SPMs and RvD2 decreased TLR4 expression in a time-dependent manner. RvD2 also decreased components of the downstream TLR4 signaling pathways and selectively dampened MD-2 expression. These changes were mediated in part by RvD2 induction of miR-146a, and inhibition of this microRNA blocked the efficacy of RvD2. Taken together, these data demonstrate that RvD2 dampens TLR4 expression to promote the resolution of LPS-induced inflammation.
LPS increased expression of GPR18, the primary receptor for RvD2. Other studies have shown that SPM receptors (specifically GPR32 and ALX/FPR2) are dysregulated in chronic disease (39, 40). These data point to increased expression of SPMs receptors as a compensatory mechanism or feedback loop in response to inflammation. It is currently unknown whether LPS specifically stimulates monocyte production of SPMs, though these cells are known to produce multiple mediators. In mice infected with Escherichia coli, elevated SPMs and intermediates were detected at time points that correlated with increased macrophage infiltration (21). Similar results were observed with other stimuli (including thioglycolate and zymosan), pointing to a common mechanism of SPM induction (41). SPMs are also elevated in peripheral blood in sepsis patients (42). This collection of literature shows that SPMs are produced in the presence of E. coli and other TLR ligands, and SPM production and macrophage cellular levels correlate in these microbial models, which suggest that LPS specifically is likely to induce SPM production by monocytes in conjunction with other cell types necessary for transcellular synthesis.
In our studies, RvD2 acted specifically on LPS-induced inflammation to attenuate cytokine production and LPS expression. Notably, this decrease did not happen until at least several hours after LPS stimulation. This suggests that RvD2 did not fully block TLR4 responsiveness, but rather acted in a feedback mechanism to prevent excessive TLR4 activation. In many circumstances, SPM addition alone (without stimuli) has no effect compared to vehicle treatment. In vivo, an inflammatory stimuli is often required to initiate the inflammatory response, but also to trigger resolution signals. Eicosanoids, including both SPMs and proinflammatory mediators, produced in the early stages of inflammation can form feedback loops to promote the shift to anti-inflammatory lipids (43, 44). Furthermore, SPMs are proresolving, meaning they promote an active paradigm shift to proresolution cytokines, enhanced phagocytosis, and wound healing. This is distinct from classic anti-inflammatory approaches, which are immunosuppressive and blunt initial inflammatory signaling; these therapies can hinder resolution signaling because they block inflammatory signals that may promote a feedback loop to increase resolution signals. Therefore, on the basis of our knowledge of resolution and these data, we propose the following model. First, LPS increases TLR4 and initiates TLR4 signaling, a protective response to a bacterial mimetic. This increases GPR18 expression (and may promote SPM production in vivo), which allows for enhanced binding of exogenous RvD2 to initiate resolution. RvD2 increases, likely among other mechanisms, miRNAs (including miR-146a), which target TLR4 expression. These resolution signals result in reductions of TLR4 and downstream mediators, eventually culminating in reduced inflammatory cytokine expression and a prevention of excessive inflammation. Excessive bacterial infiltration, of which LPS/TLR4 activation is a contributor, leads to septic shock, and prolonged LPS activation may contribute to tolerance phenomena and chronic inflammatory diseases, so shutting down these pathways to promote quicker resolution would be an advantageous therapeutic pathway. In sum, RvD2 does not block initial TLR4 responses, but acts to prevent prolonged TLR4 activation and thereby excessive inflammation.
The specificity of RvD2 regarding attenuation of only LPS-induced inflammation is interesting. There are multiple SPMs, which each act through unique receptors, and what promotes production of one SPM over another is not fully understood. SPMs are derived from ω-3 and ω-6 fatty acids, precursors that can undergo “class switching” to produce both pro- and anti-inflammatory molecules in a temporal manner and under different stimuli (43). It is possible that other SPMs at different time points may promote resolution of inflammation initiated by other TLR ligands. There is some evidence for the link between SPMs and other TLRs in the literature. Activation of TLR7 with R-828 stimulates production of SPMs in mouse and human macrophages, which then act in a feedback loop to promote resolution of allergic airway inflammation (45). It is noteworthy that these studies were also stimulus dependent as LPS did not induce production of SPMs and lipoxygenase activity in this model. Additionally, activation of the lipoxin receptor ALX using non-lipoxin A4 (LxA4) agonists decreases TLR2 activity in a model of allergic airway inflammation (46). These studies underscore that SPMs and TLRs act to regulate expression of each other, and suggest that there may be some specificity regarding which SPMs may be efficacious in different diseases induced by TLR ligands.
Along with decreasing TLR4 expression, downstream signaling components are also decreased with RvD2 treatment. The observed decreases in MyD88, TRIF, and TAK1 confirm the functional effect of decreased TLR4 expression by highlighting that the downstream mediators are also decreased. Although we did observe trending increases in SARM1 with RvD2 treatment, these changes were not significant, indicating that this may not be a primary mechanism by which RvD2 reduces TRIF expression. Previously, we showed in epithelial cells that RvD1 can dampen TAK1, highlighting another potential target for mediating resolution (47). Even further downstream from these signaling components, TLR4 eventually leads to activation of the NF-κB pathway. SPMs have also been shown to act on the NF-κB pathway to mediate their effects (15, 21, 30, 48). RvD1 reduced NF-κB expression in mice with LPS-induced acute lung injury (19). Specifically in human macrophages, RvD2 dampens cigarette smoke–induced NF-κB expression and promotes alternative activation of RelB (15). SPMs may be directly acting to dampen NF-κB expression, which could then act as a feedback mechanism to decrease TLR4, and/or may decrease TLR4 through other mechanisms which then result in reduced NF-κB expression. This may also explain why RvD2 dampened IL-1b expression, but did not alter other inflammasome components. IL-1β has multiple inflammatory roles and can be induced by several different pathways, including NF-κB signaling. SPM dampening of NF-kB signaling, could lead to reduced IL-1β gene expression, showing that RvD2’s actions on IL-1β may be more reflective of SPMs general anti-inflammatory capabilities and effects on NF-κB signaling than inflammasome signaling. It is also possible that the doses and time points observed were not optimal for inflammasome activation, as other studies have shown that SPMs are effective in models where inflammasome activation is key. In all cases, it is clear that SPMs modulate multiple stages of the TLR4 signaling pathway to mediate their anti-inflammatory effects.
RvD2 also decreased expression of MD-2, the first evidence for SPM regulation of this molecule. This finding was particularly interesting, as RvD2 did not affect expression of other genes involved in in LPS binding to TLR4 (CD14 and LBP). Several possible explanations exist for why MD-2 regulation may be different. First, CD14 and LBP greatly enhance TLR4 responsiveness to LPS and efficiency of signaling, but are not necessary for this response, as MD-2-knockout mice display the same phenotype as TLR4-knockout mice, and CD14-knockout mice can still respond to LPS (3, 33, 49). Furthermore, in acute-phase responses, high concentrations of LBP have been shown to inhibit LPS recognition to help prevent septic shock, so there may be beneficial roles for this protein in inhibiting inflammation (50). In contrast, MD-2 is required for proper trafficking of LPS to the cell surface and for formation of TLR4 multimers necessary for signaling (3, 31–33, 51); therefore, the difference in regulation may be due to necessity of MD-2. Second, SPM/LPS treatments were conducted in serum-free conditions to minimize binding up of SPMs by serum components. Although we still detected low levels of CD14 and LBP, expression of these components would be greatly increased in the presence of serum and differences in expression may have been observed with greater expression. However, given that we observed LPS-induced TLR4 and cytokine production, we are confident that these results in serum-free media are still reflective of normal biologic responses. Third, given the physical association of MD-2 to TLR4, it is possible that MD-2 is simply decreased as a result of less available TLR4 to bind to MD-2.
Furthermore, MD-2 is critical for appropriate glycosylation of TLR4. Reduced MD-2 could thereby lead to reductions in mature TLR4, contributing to the lower TLR4 levels seen here. We did not evaluate specific forms of TLR4 in these experiments (immature compared to mature TLR4 and/or glycosylation status), but given that we observed reductions in both TLR4 mRNA and TLR4 protein, we expect SPMs reductions in TLR4 to be, at least in part, mediated by mechanisms independent of glycosylation. Although MD-2 is the primary protein indicated in regulating TLR4 glycosylation, there is some evidence that PRAT4A also contributes to this process, which we found to be unmodified in our studies. This provides further evidence suggesting that SPMs act independently of TLR4 modulation, although further investigation of TLR4 glycosylation, folding, and general modification would be needed to fully support this hypothesis.
Little is known about the mechanisms responsible for MD-2 regulation and degradation, but this protein is inducible and therefore must undergo some degree of regulation (52, 53). Further complicating this association is the fact that MD-2 has multiple TLR4 independent actions and is able to bind to molecules independent of TLR4 which then act as antagonists to canonical TLR4 signaling (54). Finally, while miR-146a has not been shown to target MD-2, RvD2 may be up-regulating other miRs to specifically decrease expression of MD-2 or target a molecule that regulates MD-2, independent of RvD2’s actions on TLR4. For instance, miR30a has been shown to target STAT1 (which induces MD-2), thereby decreasing MD-2 expression (53). Clearly, the regulation of MD-2 in regards to TLR4 signaling is still not fully understood, and the possible link between SPMs and MD-2 expression bears further investigation.
RvD2 acts on multiple miRNAs to dampen TLR4 expression and promote resolution. microRNAs in general and in regards to TLRs are an expanding area of research. Multiple miRs have been identified which target TLR4 or components of the TLR4 signaling pathway (37). These include miR-146a, miR146b, miR155, miR132, miR27b, and more (37). Some of these miRs can also be induced by LPS or activation of TLR4, demonstrating the cyclic regulation of TLR4 signaling. We identified several targets (miR-146a, miR155, and miR27a) which RvD2 altered. RvD2 treated cells had trending reductions in miR155, which is induced by TLR4 and other TLR ligands and impairs negative regulators of inflammatory signaling; thus, reductions in miR155 may contribute to overall reduced inflammatory responses. LPS also slightly reduced miR27, a reduction which RvD2 prevented (55–57). miR27 is primarily linked to adipocyte differentiation, with increased miR27a decreasing adipogenesis, but has additionally been shown to be down-regulated by TLR4 induction, resulting in reduced IL-10, an anti-inflammatory cytokine important in M2 macrophage activation (58). miR-146a is both regulated by and negatively regulates TLR4, MyD88, NF-κB, other TLRs, and a range of proinflammatory proteins, as well positively regulating RelB (37, 59–64). The effects of regulation have been specifically identified in macrophages; THP-1 cells that are exogenously transfected with a miR-146a mimic had decreased production of TNF-α, IL-6, and IL-1β (62). Inhibiting miR-146a similarly prevented miR-146a induced shifts to resolution, and led to higher expression of cytokines and TRAF6 (62). miR-146a also promotes M2 macrophage polarization, which SPMs have additionally been shown to do (65). SPMs have been shown to induce several miRs in other inflammatory models, indicating that miR regulation may be a key mechanism of SPM action (66, 67). Our data, however, are the first to show RvD2 induction of miR-146a, uncovering a previously unknown possible mechanism for RvD2’s actions.
Overall, our studies provide the first evidence of SPM regulation of TLR4 signaling. The temporal regulation of TLR4 is particularly interesting, and highlights the nonimmunosuppressive nature of RvD2. Furthermore, this is the first evidence for regulation of MD-2 and identification of miR-146a as a new microRNA through which RvD2 may mediate its actions. This investigation brings to light new potential therapeutic targets to address septic shock and chronic diseases associated with LPS. RvD2 and SPMs have high therapeutic potential for addressing pathogen-mediated diseases, and these studies demonstrate the first evidence that they may act in novel ways to regulate TLR signaling, an important area of resolution research.
ACKNOWLEDGMENTS
The authors thank the University of Rochester Medical Center Blood Bank and A. Casey for phlebotomy assistance in collecting blood donations, C. Woeller for his guidance on the microRNA experiments, and the University of Rochester Medical Center Flow Cytometry Core for its flow cytometry assistance. This work was supported by the U.S. National Institutes of Health (NIH) National Institute of Environmental Health Sciences Grants T32ES007026 and P30ES01247; NIH National Heart, Lung, and Blood Institute Grants R01HL120908 and T32HL066988; NIH Clinical and Translational Science Institute (CTSI) Incubator Grant UL1RR024160, NIH CTSI Trainee Pilot Grant 8UL1TR000042; and the PhRMA Foundation. Author contributions: A. Croasdell designed and performed experiments, analyzed and interpreted data, and wrote the manuscript. P. Sime and R. Phipps designed experiments, interpreted data, and wrote the manuscript. All authors are members of the Lung Biology and Disease Program (University of Rochester School of Medicine and Dentistry).
Glossary
- GPR18
G-protein coupled receptor 18
- LBP
LPS-binding protein
- MD-2
lymphocyte antigen 96
- miR
microRNA
- MyD88
myeloid differentiation primary response gene 88
- poly(I:C)
polyinosinic/polycytidylic acid
- RvD1
resolvin D1
- RvD2
resolvin D2
- SARM1
sterile α and TIR motif containing 1
- SPM
specialized proresolving mediator
- TAK1
transforming growth factor β-activated kinase 1
- TRIF
TIR-domain-containing adapter-inducing IFN-β
REFERENCES
- 1.Ostuni R., Zanoni I., Granucci F. (2010) Deciphering the complexity of Toll-like receptor signaling. Cell. Mol. Life Sci. 67, 4109–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maris N. A., Dessing M. C., de Vos A. F., Bresser P., van der Zee J. S., Jansen H. M., Spek C. A., van der Poll T. (2006) Toll-like receptor mRNA levels in alveolar macrophages after inhalation of endotoxin. Eur. Respir. J. 28, 622–626 [DOI] [PubMed] [Google Scholar]
- 3.Nagai Y., Akashi S., Nagafuku M., Ogata M., Iwakura Y., Akira S., Kitamura T., Kosugi A., Kimoto M., Miyake K. (2002) Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat. Immunol. 3, 667–672 [DOI] [PubMed] [Google Scholar]
- 4.Szatmary Z. (2012) Molecular biology of Toll-like receptors. Gen. Physiol. Biophys. 31, 357–366 [DOI] [PubMed] [Google Scholar]
- 5.Hall M. J., Williams S. N., DeFrances C. J., Golosinskiy A. (2011) Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief 1–8 [PubMed] [Google Scholar]
- 6.Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 7.Kovach M. A., Standiford T. J. (2011) Toll like receptors in diseases of the lung. Int. Immunopharmacol. 11, 1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y., Xiang M., Yuan Y., Xiao G., Zhang J., Jiang Y., Vodovotz Y., Billiar T. R., Wilson M. A., Fan J. (2009) Hemorrhagic shock augments lung endothelial cell activation: role of temporal alterations of TLR4 and TLR2. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1670–R1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lafferty E. I., Qureshi S. T., Schnare M. (2010) The role of Toll-like receptors in acute and chronic lung inflammation. J. Inflamm. (Lond.) 7, 57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pace E., Giarratano A., Ferraro M., Bruno A., Siena L., Mangione S., Johnson M., Gjomarkaj M. (2011) TLR4 upregulation underpins airway neutrophilia in smokers with chronic obstructive pulmonary disease and acute respiratory failure. Hum. Immunol. 72, 54–62 [DOI] [PubMed] [Google Scholar]
- 11.Tesse R., Pandey R. C., Kabesch M. (2011) Genetic variations in Toll-like receptor pathway genes influence asthma and atopy. Allergy 66, 307–316 [DOI] [PubMed] [Google Scholar]
- 12.Biswas S. K., Lopez-Collazo E. (2009) Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 [DOI] [PubMed] [Google Scholar]
- 13.Nahid M. A., Satoh M., Chan E. K. (2011) MicroRNA in TLR signaling and endotoxin tolerance. Cell. Mol. Immunol. 8, 388–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley C. D., Gilroy D. W., Serhan C. N. (2014) Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Croasdell, A., Thatcher, T. H., Kottmann, R. M., Colas, R. A., Dalli, J., Serhan, C. N., Sime, P. J., and Phipps, R. P. (2015) Resolvins attenuate inflammation and promote resolution in cigarette smoke–exposed human macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 309, L888–L901. [DOI] [PMC free article] [PubMed]
- 16.Hsiao H. M., Sapinoro R. E., Thatcher T. H., Croasdell A., Levy E. P., Fulton R. A., Olsen K. C., Pollock S. J., Serhan C. N., Phipps R. P., Sime P. J. (2013) A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One 8, e58258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalli J., Serhan C. N. (2012) Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 120, e60–e72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogerio A. P., Haworth O., Croze R., Oh S. F., Uddin M., Carlo T., Pfeffer M. A., Priluck R., Serhan C. N., Levy B. D. (2012) Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. J. Immunol. 189, 1983–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B., Gong X., Wan J. Y., Zhang L., Zhang Z., Li H. Z., Min S. (2011) Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm. Pharmacol. Ther. 24, 434–441 [DOI] [PubMed] [Google Scholar]
- 20.Palmer C. D., Mancuso C. J., Weiss J. P., Serhan C. N., Guinan E. C., Levy O. (2011) 17(R)-Resolvin D1 differentially regulates TLR4-mediated responses of primary human macrophages to purified LPS and live E. coli. J. Leukoc. Biol. 90, 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiang N., Fredman G., Bäckhed F., Oh S. F., Vickery T., Schmidt B. A., Serhan C. N. (2012) Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 484, 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdulnour R. E., Sham H. P., Douda D. N., Colas R. A., Dalli J., Bai Y., Ai X., Serhan C. N., Levy B. D. (2015) Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. [E-pub ahead of print] Mucosal Immunol. doi: 10.1038/mi.2015.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Croasdell, A., Lacy, S. H., and Thatcher, T. H. (2016) Resolvin D1 dampens pulmonary inflammation and promotes clearance of nontypeable Haemophilus influenzae.J. Immunol. 196, 2742–2752. [DOI] [PMC free article] [PubMed]
- 24.Ramon S., Gao F., Serhan C. N., Phipps R. P. (2012) Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 189, 1036–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serhan C. N. (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gu, Z., Lamont, G. J., Lamont, R. J., Uriarte, S. M., Wang, H., and Scott, D. A. (2016) Resolvin D1, resolvin D2 and maresin 1 activate the GSK3beta anti-inflammatory axis in TLR4-engaged human monocytes. Innate Immun. 22, 186–195. [DOI] [PMC free article] [PubMed]
- 27.Clària J., Dalli J., Yacoubian S., Gao F., Serhan C. N. (2012) Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J. Immunol. 189, 2597–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spite M., Norling L. V., Summers L., Yang R., Cooper D., Petasis N. A., Flower R. J., Perretti M., Serhan C. N. (2009) Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature 461, 1287–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang N., Dalli J., Colas R. A., Serhan C. N. (2015) Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 212, 1203–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen, F., Fan, X. H., Wu, Y. P., Zhu, J. L., Wang, F., Bo, L. L., Li, J. B., Bao, R., and Deng, X. M. (2014) Resolvin D1 improves survival in experimental sepsis through reducing bacterial load and preventing excessive activation of inflammatory response. Eur. J. Clin. Microbiol. Infect. Dis. 33, 457–464. [DOI] [PubMed]
- 31.Park B. S., Song D. H., Kim H. M., Choi B. S., Lee H., Lee J. O. (2009) The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 458, 1191–1195 [DOI] [PubMed] [Google Scholar]
- 32.da Silva Correia J., Ulevitch R. J. (2002) MD-2 and TLR4 N-linked glycosylations are important for a functional lipopolysaccharide receptor. J. Biol. Chem. 277, 1845–1854 [DOI] [PubMed] [Google Scholar]
- 33.Shimazu R., Akashi S., Ogata H., Nagai Y., Fukudome K., Miyake K., Kimoto M. (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med. 189, 1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGettrick A. F., O’Neill L. A. (2010) Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr. Opin. Immunol. 22, 20–27 [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K., Shibata T., Akashi-Takamura S., Kiyokawa T., Wakabayashi Y., Tanimura N., Kobayashi T., Matsumoto F., Fukui R., Kouro T., Nagai Y., Takatsu K., Saitoh S., Miyake K. (2007) A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J. Exp. Med. 204, 2963–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y., Liu B., Dai J., Srivastava P. K., Zammit D. J., Lefrançois L., Li Z. (2007) Heat shock protein gp96 is a master chaperone for Toll-like receptors and is important in the innate function of macrophages. Immunity 26, 215–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He X., Jing Z., Cheng G. (2014) MicroRNAs: new regulators of Toll-like receptor signalling pathways. BioMed Res. Int. 2014, 945169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauernfeind F., Rieger A., Schildberg F. A., Knolle P. A., Schmid-Burgk J. L., Hornung V. (2012) NLRP3 inflammasome activity is negatively controlled by miR-223. J. Immunol. 189, 4175–4181 [DOI] [PubMed] [Google Scholar]
- 39.Hsiao H. M., Thatcher T. H., Colas R. A., Serhan C. N., Phipps R. P., Sime P. J. (2015) Resolvin D1 reduces emphysema and chronic inflammation. Am. J. Pathol. 185, 3189–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto A., Hayashi I., Murakami Y., Sato Y., Kitasato H., Matsushita R., Iizuka N., Urabe K., Itoman M., Hirohata S., Endo H. (2007) Antiinflammatory mediator lipoxin A4 and its receptor in synovitis of patients with rheumatoid arthritis. J. Rheumatol. 34, 2144–2153 [PubMed] [Google Scholar]
- 41.Lastrucci C., Baillif V., Behar A., Al Saati T., Dubourdeau M., Maridonneau-Parini I., Cougoule C. (2015) Molecular and cellular profiles of the resolution phase in a damage-associated molecular pattern (DAMP)-mediated peritonitis model and revelation of leukocyte persistence in peritoneal tissues. FASEB J. 29, 1914–1929 [DOI] [PubMed] [Google Scholar]
- 42.Dalli J., Chiang N., Serhan C. N. (2015) Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 21, 1071–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levy B. D., Clish C. B., Schmidt B., Gronert K., Serhan C. N. (2001) Lipid mediator class switching during acute inflammation: signals in resolution. Nat. Immunol. 2, 612–619 [DOI] [PubMed] [Google Scholar]
- 44.Wu D., Zheng S., Li W., Yang L., Liu Y., Zheng X., Yang Y., Yang L., Wang Q., Smith F. G., Jin S. (2013) Novel biphasic role of resolvin D1 on expression of cyclooxygenase-2 in lipopolysaccharide-stimulated lung fibroblasts is partly through PI3K/AKT and ERK2 pathways. Mediators Inflamm. 2013, 964012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koltsida O., Karamnov S., Pyrillou K., Vickery T., Chairakaki A. D., Tamvakopoulos C., Sideras P., Serhan C. N., Andreakos E. (2013) Toll-like receptor 7 stimulates production of specialized pro-resolving lipid mediators and promotes resolution of airway inflammation. EMBO Mol. Med. 5, 762–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong X., Wu S. H., Zhang L., Chen X. Q. (2015) Roles of lipoxin A4 receptor activation and anti-interleukin-1β antibody on the Toll-like receptor 2/mycloid differentiation factor 88/nuclear factor-κB pathway in airway inflammation induced by ovalbumin. Mol. Med. Rep. 12, 895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsiao H. M., Thatcher T. H., Levy E. P., Fulton R. A., Owens K. M., Phipps R. P., Sime P. J. (2014) Resolvin D1 attenuates polyinosinic–polycytidylic acid–induced inflammatory signaling in human airway epithelial cells via TAK1. J. Immunol. 193, 4980–4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao Z., Dong J., Wu W., Yang T., Wang T., Guo L., Chen L., Xu D., Wen F. (2012) Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir. Res. 13, 110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perera P. Y., Vogel S. N., Detore G. R., Haziot A., Goyert S. M. (1997) CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J. Immunol. 158, 4422–4429 [PubMed] [Google Scholar]
- 50.Lamping N., Dettmer R., Schröder N. W., Pfeil D., Hallatschek W., Burger R., Schumann R. R. (1998) LPS-binding protein protects mice from septic shock caused by LPS or gram-negative bacteria. J. Clin. Invest. 101, 2065–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tumurkhuu G., Dagvadorj J., Jones H. D., Chen S., Shimada K., Crother T. R., Arditi M. (2015) Alternatively spliced myeloid differentiation protein-2 inhibits TLR4-mediated lung inflammation. J. Immunol. 194, 1686–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jia H. P., Kline J. N., Penisten A., Apicella M. A., Gioannini T. L., Weiss J., McCray P. B. Jr (2004) Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am. J. Physiol. Lung Cell. Mol. Physiol. 287, L428–L437 [DOI] [PubMed] [Google Scholar]
- 53.Wang Y., Li T., Wu B., Liu H., Luo J., Feng D., Shi Y. (2014) STAT1 regulates MD-2 expression in monocytes of sepsis via miR-30a. Inflammation 37, 1903–1911 [DOI] [PubMed] [Google Scholar]
- 54.Miyake K. (2006) Roles for accessory molecules in microbial recognition by Toll-like receptors. J. Endotoxin Res. 12, 195–204 [DOI] [PubMed] [Google Scholar]
- 55.Tili E., Michaille J. J., Cimino A., Costinean S., Dumitru C. D., Adair B., Fabbri M., Alder H., Liu C. G., Calin G. A., Croce C. M. (2007) Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J. Immunol. 179, 5082–5089 [DOI] [PubMed] [Google Scholar]
- 56.O’Connell R. M., Taganov K. D., Boldin M. P., Cheng G., Baltimore D. (2007) MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 104, 1604–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ceppi M., Pereira P. M., Dunand-Sauthier I., Barras E., Reith W., Santos M. A., Pierre P. (2009) MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl. Acad. Sci. USA 106, 2735–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie N., Cui H., Banerjee S., Tan Z., Salomao R., Fu M., Abraham E., Thannickal V. J., Liu G. (2014) miR-27a regulates inflammatory response of macrophages by targeting IL-10. J. Immunol. 193, 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zago M., Rico de Souza A., Hecht E., Rousseau S., Hamid Q., Eidelman D. H., Baglole C. J. (2014) The NF-κB family member RelB regulates microRNA miR-146a to suppress cigarette smoke-induced COX-2 protein expression in lung fibroblasts. Toxicol. Lett. 226, 107–116 [DOI] [PubMed] [Google Scholar]
- 60.El Gazzar M., Church A., Liu T., McCall C. E. (2011) MicroRNA-146a regulates both transcription silencing and translation disruption of TNF-α during TLR4-induced gene reprogramming. J. Leukoc. Biol. 90, 509–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boldin M. P., Taganov K. D., Rao D. S., Yang L., Zhao J. L., Kalwani M., Garcia-Flores Y., Luong M., Devrekanli A., Xu J., Sun G., Tay J., Linsley P. S., Baltimore D. (2011) miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J. Exp. Med. 208, 1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nahid M. A., Pauley K. M., Satoh M., Chan E. K. (2009) miR-146a is critical for endotoxin-induced tolerance: implication in innate immunity. J. Biol. Chem. 284, 34590–34599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taganov K. D., Boldin M. P., Chang K. J., Baltimore D. (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 103, 12481–12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McMillan D. H., Woeller C. F., Thatcher T. H., Spinelli S. L., Maggirwar S. B., Sime P. J., Phipps R. P. (2013) Attenuation of inflammatory mediator production by the NF-κB member RelB is mediated by microRNA-146a in lung fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 304, L774–L781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang, C., Liu, X. J., QunZhou, Xie, J., Ma, T. T., Meng, X. M., and Li, J. (2016) MiR-146a modulates macrophage polarization by inhibiting Notch1 pathway in RAW264.7 macrophages. Int. Immunopharmacol. 32, 46–54 [DOI] [PubMed] [Google Scholar]
- 66.Krishnamoorthy S., Recchiuti A., Chiang N., Fredman G., Serhan C. N. (2012) Resolvin D1 receptor stereoselectivity and regulation of inflammation and proresolving microRNAs. Am. J. Pathol. 180, 2018–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Recchiuti A., Krishnamoorthy S., Fredman G., Chiang N., Serhan C. N. (2011) MicroRNAs in resolution of acute inflammation: identification of novel resolvin D1-miRNA circuits. FASEB J. 25, 544–560 [DOI] [PMC free article] [PubMed] [Google Scholar]