Abstract
HIV-infected individuals have activated monocytes with an IFNα phenotype and elevated levels of circulating LPS. These individuals also have a risk of premature cardiovascular disease. The effect of activated monocyte exosomes (Exos) on endothelial cells is unknown. To determine whether Exos from immune-activated monocytes could alter endothelial cell expression and contribute to monocyte/macrophage transmigration and adhesion, we isolated Exos from monocytes stimulated with IFNα, LPS, or both (I/L). We show that monocyte Exos contain different inflammatory microRNA cargo depending on stimulation. When LPS Exos or I/L Exos were added to HUVECs, we found a significant increase in adhesion molecule ICAM-1, chemokine ligand (CCL)-2, and cytokine IL-6 mRNAs and proteins compared with cells treated with IFNα Exos or Exos derived from unstimulated monocytes. Inhibition of transcription factor NF-κB, a common inflammatory cytokine pathway, prevented induction of CCL2, IL6, and ICAM1. Inhibition of TLR4 resulted in differential blockage of the targets. Our results demonstrate for the first time that primary human monocyte Exos enter endothelial cells and cause dysfunction via the TLR4 and NF-κB pathways, which may contribute to heart disease in HIV infection and other diseases involving chronic immune activation.—Tang, N., Sun, B., Gupta, A., Rempel, H., Pulliam, L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-κB in endothelial cells.
Keywords: CCL2, ICAM-1, atherosclerosis, HIV, miRNA
In HIV-infected persons who are receiving antiretroviral therapy (ART), there is an increased long-term risk of cardiovascular disease (CVD), with atherosclerosis being a prominent comorbidity (1, 2). HIV-infected individuals have a higher rate of CVD at a younger age (3). HIV-infected individuals also exhibit immune activation, having monocytes with type I IFNα expression profiles and circulating levels of LPS that are higher than control noninfected individuals (4–6).
Atherosclerosis is a chronic inflammatory disease characterized by monocyte adherence to endothelium, migration into the arterial wall, and lipid accumulation. Under normal conditions, endothelial cells are quiescent and do not interact with monocytes. However, with increased age and in an inflammatory environment, activated endothelial cells express adhesion molecules, such as intercellular cell adhesion molecule (ICAM)-1, which bind monocytes (7). In addition, monocytes, smooth muscle cells, and HUVECs up-regulate expression of monocyte chemoattractant protein (MCP)-1 [also known as chemokine ligand (CCL)-2] (8, 9). The combined up-regulation of ICAM-1 and CCL2 help initiate the formation of atherosclerotic plaques by inducing the migration and adhesion of monocytes to the subendothelial space. There in the intima, the monocytes scavenge oxidized lipid particles, transform into foam cells, and secrete inflammatory cytokines and proteases, such as matrix metalloprotease, contributing to the activation of endothelial cells and the progression of atherosclerosis (10). Once activated, endothelial cells can also secrete proinflammatory cytokines, such as IL-1β and -6, further fueling the atherosclerotic process (11, 12).
Exosomes (Exos) are bilipid membranous vesicles that are secreted from cells upon fusion of multivesicular endosomes/bodies with the plasma membrane (13). They are ∼40–100 nm in size and contain RNA, proteins, and lipids. Most cells, including those of the vascular system, such as red blood cells, platelets, endothelial cells, monocytes, lymphocytes, dendritic cells, and mast cells, secrete Exos under normal conditions. However, under pathologic or stress conditions, Exo secretion may increase, exosomal content may be altered, or both (14). Exos are characterized and identified by integrins and tetraspanins (CD9, CD63, CD81, and CD82), proteins involved in membrane transport and fusion (annexins, Rab proteins, and flotillin), proteins associated with multivesicular body biogenesis (Alix, TSG101), and heat shock protein (HSP)-70 and -90 (15). Although they were originally considered cellular debris, it is becoming clear that Exos are not a result of casual sampling. Rather, cargo is selectively packaged by dedicated packing mechanisms and delivered to target cells via multiple trafficking modalities (16).
Because emerging evidence suggests that extracellular vesicles, including Exos, participate in communication between blood cells and vascular tissues during atherosclerosis (17), we sought to recapitulate in vitro the immune activation observed in HIV persons on ART and determine whether the Exos derived from immune-activated monocytes play a role in CVD.
MATERIALS AND METHODS
Monocyte purification and characterization
Healthy human blood samples were obtained from Blood Centers of the Pacific (San Francisco, CA, USA), and cells were isolated within 4 h from leukocyte reduction filters. The cells were diluted with PBS containing 2 mM EDTA and incubated with RosetteSep Monocyte Enrichment Cocktail (StemCell, Vancouver, BC, Canada) for 30 min at room temperature. The blood was further diluted and layered over Ficoll (GE Healthcare, Pittsburgh, PA, USA). The layered blood was centrifuged at 1200 g for 20 min at room temperature. Monocytes were collected at the PBS/Ficoll interface and washed 2 times in PBS+2 mM EDTA at 300 g. The monocytes were analyzed for purity by flow cytometry on the FACSCalibur instrument (BD Biosciences, Franklin Lakes, NJ, USA) with a minimum of 10,000 events.
Monocyte activation
Purified monocytes were cultured overnight in ultra–low-attachment 6-well plates with rotation at 97 rpm in RPMI Medium 1640 (Thermo Fisher Scientific Life Sciences, Rockford, IL, USA) supplemented with 10% fetal bovine serum (FBS) and 1× antibiotics. For stimulation, IFNα (PBL Assay Science, Piscataway, NJ, USA) was added at 100 U/ml for 4 h, and LPS (Sigma-Aldrich, St. Louis, MO, USA) was added at 1 ng/ml for 3 h. For the combination treatment, 100 U/ml IFNα was added for 1 h, and 1 ng/ml LPS was added for 3 h.
Exo isolation and characterization
After activation, the monocytes were collected, centrifuged, washed with PBS, and resuspended in Exo-depleted medium consisting of RMPI 1640 supplemented with 10% Exo-depleted FBS (Thermo Fisher Scientific Life Sciences), 100 U/ml penicillin G, 100 μg/ml streptomycin, and 500 pg/ml macrophage colony-stimulating factor. The monocytes were then cultured for 24 h at 37°C, 5% CO2. Supernatants from cultured monocytes were collected and centrifuged at 3000 g for 15 min to remove debris, after which Exoquick-TC (System Biosciences, Mountain View, CA, USA) was added, and the Exo pellet was collected per the manufacturer’s instructions.
Exo particle size and count were determined with the NanoSight LM10 instrument (Malvern Instruments, Malvern, United Kingdom) with a 405 nm laser-equipped sample chamber and were analyzed by Nanoparticle Tracking Analysis (NTA; Malvern Instruments). The sample chamber was completely filled with Exoquick-TC-precipitated Exo pellets resuspended in PBS. Shutter and gain were manually adjusted for optimal detection and were kept at the same settings for all samples. Each sample was analyzed 3 times with NTA, 3.0 software to determine size distribution and vesicle concentration. Each analysis consisted of three 40-s .avi (audio video interleaved) file recordings.
Transmission electron microscopy was performed on the purified Exos (18). In brief, the Exo pellet was resuspended in 2% paraformaldehyde, deposited onto Formvar carbon-coated electron microscopy grids, and left to dry at room temperature for 20 min. Samples were then washed several times with water, stained with saturated uranyl-oxalate solution for 5 min, and embedded in methylcellulose uranyl acetate solution on ice for 10 min. Excess fluid was blotted and the grids were dried at room temperature for 10 min. Visualization of extracellular vesicles was performed using a Technai 10 electron microscope [Field Electron and Ion Co. (FEI), Hillsboro, OR, USA].
For Western blot analysis, monocytes or Exos were lysed with RIPA buffer containing protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). Protein lysate (20–30 μg) was loaded onto a 4–20% Tris-glycine gradient gel, and SDS-PAGE was performed. Separated proteins were transferred to PVDF (EMD Millipore, Darmstadt, Germany) and blocked with 5% milk in Tris-buffered saline (TBS)-Tween 20. Membrane was incubated overnight at 4°C with anti-CD63 antibody (System Biosciences Inc.) and anti-β actin antibody (Thermo Fisher Scientific Life Sciences). The next day, the membrane was washed 4 times with TBS-Tween 20, incubated with HRP-conjugated secondary antibodies in 5% milk in TBS-Tween 20 (System Biosciences Inc. and Bio-Rad Laboratories, Hercules, CA,USA) and washed again 4 times with TBS-Tween 20. Proteins were visualized by using ECL Plus Western blot substrate (Thermo Fisher Scientific Life Sciences).
To determine microRNA (miR) levels, Exos derived from stimulated or nonstimulated (NS) monocytes (as described above) were lysed with Qiazol (Qiagen, Valencia, CA, USA) and the RNA purified with the miRNeasy Mini Kit (Qiagen) to retain short-nucleotide miRs. Reverse transcription was performed with a TaqMan miRNA Reverse Transcription Kit (Thermo Fisher Scientific Life Sciences). PCR was performed on the cDNA with TaqMan assays for miR-155, miR-146a, and -223 (Thermo Fisher Scientific Life Sciences). Quantitative PCR (qPCR) reactions were performed in triplicate with a ViiA 7 instrument (Thermo Fisher Scientific Life Sciences). The cycles were set up using the standard ΔΔCt TaqMan Fast cycles provided with the instrument. PCR threshold values were normalized to the housekeeping gene RNU6-1.
HUVEC culture
HUVECs were purchased and grown in Endothelial Cell Basal Medium-2 with supplement (Lonza, Allendale, NJ, USA) per the manufacturer’s instructions. For Exo treatment experiments, HUVECs were seeded into 12-well cell culture plates or 8-chamber tissue culture treated glass slides and incubated for 24 h, after which the medium was exchanged for Exo-depleted medium, and monocyte Exos were added.
NF-κB/p65 immunofluorescence
HUVECs were seeded into 8-well chamber slides (Thermo Fisher Scientific Life Sciences) for 24 h, after which the medium was exchanged for Exo-depleted medium, and monocyte Exos were added. After 4 h, the cells were washed, fixed with 4% paraformaldehyde in PBS, permeabilized with 0.1% Triton X-100 in PBS, blocked with 5% donkey serum in PBS, and incubated overnight at 4°C with NF-κB/p65 primary antibody (D14E12 XP rabbit monoclonal antibody; Cell Signaling Technology, Danvers, MA, USA). The next day, the cells were washed with PBS containing 0.5% Tween 20 and incubated with Alexa Fluor 568-conjugated donkey anti-rabbit IgG (Thermo Fisher Scientific Life Sciences). Cell nuclei were counterstained with 2 μg/ml of Hoechst 33258 (Thermo Fisher Scientific Life Sciences). Fluorescence images were captured on an Axio Observer Z1 inverted microscope (Zeiss, Thornwood, NY, USA) and analyzed with MetaMorph software (Molecular Devices, Sunnyvale, CA, USA).
qPCR
HUVECs grown in 12-well plates were treated with Exos for 24 h, after which the cells were lysed with Qiazol (Qiagen, Valencia, CA, USA) and stored at −80°C until use for PCR. Total RNA was isolated from the cell lysates with the miRNeasy Mini Kit per the manufacturer’s protocol (Qiagen). Using random hexamers with the total RNA, we performed reverse transcription to generate cDNAs (First-Strand Synthesis Kit; Thermo Fisher Scientific Life Sciences). Subsequently, TaqMan assays for CCL2, ICAM1, IL6, IL1β, TNFα, and GAPDH (Thermo Fisher Scientific Life Sciences) were used in the mRNA expression assays. qPCR reactions were performed in triplicate with a ViiA 7 instrument (Thermo Fisher Scientific Life Sciences). The cycles were set up using the standard ΔΔCt TaqMan Fast cycles provided with the instrument.
Western blot analysis
HUVECs were lysed in RIPA buffer containing protease inhibitor cocktail (Sigma-Aldrich). Proteins were separated by gel electrophoresis (4–20% gradient), transferred to PVDF (EMD-Millipore), and blocked with Odyssey Blocking Buffer (Li-Cor, Lincoln, NE, USA). The membranes were incubated with antibodies to ICAM-1 (Abcam, Cambridge, MA, USA) and anti-β actin (Thermo Fisher Scientific Life Sciences), followed by incubation with IRDye 800CW-conjugated goat anti-mouse and IRDye 680 RD-conjugated goat anti-rabbit antibodies (Li-Cor). Visualization and quantification were performed with the Odyssey CLx Imager and Image Studio software (ver. 4.0; Li-Cor).
ELISA
Monocyte Exos (∼1 × 1011) were added to HUVECs, cultured in Exo-free medium, at 1.5 × 105 cells/ml in 12-well plates for 24 h. Conditioned medium was collected and centrifuged (3000 g) to clear the supernatants, which were subsequently used for cytokine detection by ELISA. Supernatants were analyzed for CCL2 (R&D Systems, Minneapolis, MN, USA), IL-1β, and TNFα (Thermo Fisher Scientific Life Sciences) and IL-6 (R&D and Thermo Fisher Scientific Life Sciences), according to the manufacturers’ instructions. Cytokine concentrations were determined by absorbance in duplicate with a Spectra Max M5 plate reader (Molecular Devices) running Softmax Pro 5 software.
NF-κB and TLR4 inhibition
HUVECs were seeded into a 12- or 24-well plate, incubated for 48 h, and exchanged for Exo-free medium. The HUVECs were then pretreated with the NF-κB inhibitor parthenolide (PTN) or the TLR4-specific inhibitor TAK-242 (CLI-095; both from InvivoGen, San Diego, CA, USA) for 90 min, and monocyte Exos were added for 24 h.
Statistics
In the scatterplots, each dot represents the mean of the replicates. Each horizontal bar represents the mean of the treatment group. Shading in the scatterplots illustrates the range, not the confidence interval. Paired Student’s t tests were used for all analyses. The value P < 0.05 was considered statistically significant. All statistical analyses were performed with Excel software (Microsoft, Inc., Redmond WA, USA).
RESULTS
Characterization of Exos derived from primary human monocytes
Monocytes (∼3 × 108 per chamber) were isolated from normal healthy people, with CD14+/CD16− monocytes being the predominant population (Fig. 1A). Exos released into the conditioned medium were subsequently harvested and analyzed for size by using NTA (Fig. 1B) and electron microscopy (Fig. 1C). By electron microscopy, particles having cup-shaped morphology with sizes in the range of 50–100 nm were observed, consistent with published reports. Upon further characterization by Western blot, we found that the tetraspanin CD63, was highly enriched in the monocyte Exos but was barely detectable in the monocyte whole-cell lysates (Fig. 1D).
Figure 1.
Characterization of Exos. A) Flow cytometry of enriched monocytes stained with anti-CD14PE and anti-CD16PerCP-Cy5.5 antibodies. B) Representative Exo size by NTA (n = 3). Gray error bars: ±1 sem. C) Transmission electron micrograph of Exos. Arrows: cup-shaped morphology characteristic of Exos in fixed samples. D) Western blot of CD63 and β-actin protein expression in monocyte Exos and monocyte cell lysates from 2 donors. Representative experiments are shown.
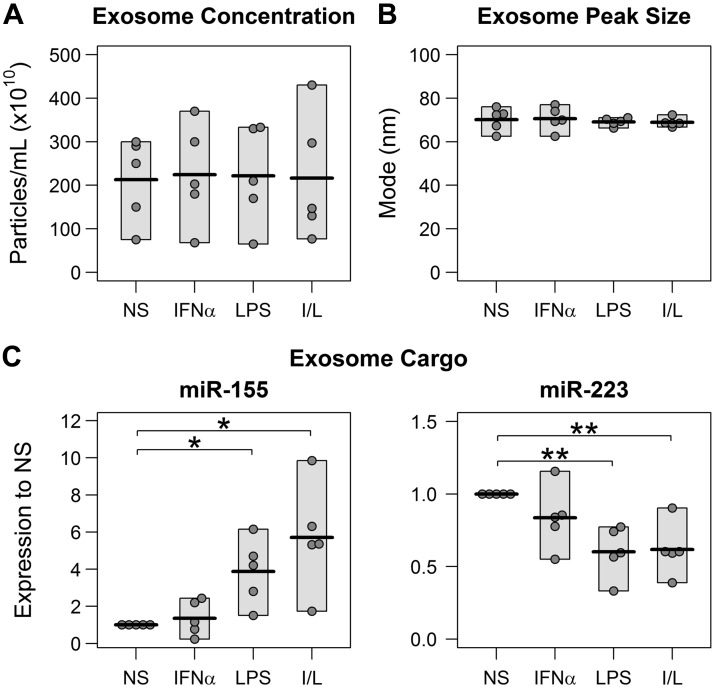
Monocyte stimulation alters Exo cargo but not size or quantity
Because different blood cell types modulate extracellular vesicle secretion in response to environmental changes (19) and because individuals with vascular diseases present with a dramatically increased number of circulating microparticles (20), we queried whether monocytes, activated under different stimuli, showed altered Exo secretion. Using NTA, we determined that unstimulated monocytes secreted ∼2 × 104 Exos per monocyte, which is similar to secretion of Exos from monocytes stimulated with IFNα (INF Exos), LPS (LPS Exos), or both (I/L Exos) (Fig. 2A). The sizes of the extracellular vesicles were also similar between the treatments (Fig. 2B), with the peak population showing a size of 69.7 ± 3.8 nm. The average size of all of the extracellular vesicles was 105.8 ± 5.1 nm. Despite the similarity in size and amount, the exosomal cargo was different between the monocyte treatments. MicroRNA-155 (Fig. 2C) was significantly elevated in the Exos derived from LPS- and/or I/L-stimulated monocytes. In contrast, miR-223 (Fig. 2D) was significantly suppressed after stimulation with LPS or I/L. The difference in miR-146a expression between the treatments (data not shown) was not statistically significant. Differences in Exo miRs-155, -223, and -146a have been associated with chronic monocyte inflammation [reviewed in Marques-Rocha et al. (21)] and are involved in HIV-1 infectivity and replication [reviewed in Swaminathan et al. (22)].
Figure 2.
Stimulation alters Exo cargo but not size or quantity. Monocytes were not stimulated (NS) or were stimulated as follows: 4 h with 100 U/ml IFNα, 3 h with 1 ng/ml LPS, or 1 h with 100 U/ml IFNα, followed by 3 h with 1 ng/ml LPS (I/L). Immediately after stimulation, the monocytes were washed to remove the inducers, and the cells were resuspended in Exo-depleted culture medium. Exos were precipitated from the 24-h clarified conditioned medium and resuspended in PBS. A) NTA for Exo concentration from the NS and stimulated monocytes. B) NTA of Exos for peak particle size. C) qPCR of specific miRs in the NS and stimulated monocyte Exos. (For all panels, n = 5 donors.) All assays were performed in triplicate. Dots represent the means of the replicates. Horizontal bars show the means of the groups. Shading of the scatterplots illustrates the range; 2-sided, paired Student’s t test. *P < 0.05, **P < 0.01.
Activated monocyte-derived Exos induce ICAM-1, CCL2, and IL-6 in endothelial cells through NF-κB mechanism
The transcription factor NF-κB is one of the key regulators of genes involved in the immune and inflammatory response. Therefore, we looked at its activation in endothelial cells after Exo treatment. After incubation with monocyte Exos, HUVECs clearly internalized the nanovesicles (Fig. 3A). When fluorescently stained for the p65 subunit of NF-κB, untreated endothelial cells or those treated with Exos derived from unstimulated monocytes showed minimal cytoplasmic and no nuclear NF-κB/p65 staining (Fig. 3B). In contrast, endothelial cells treated with Exos derived from LPS- or I/L-stimulated monocytes showed significant nuclear staining for NF-κB/p65, indicating activation and translocation of NF-κB. HUVECs incubated with IFN Exos did not show nuclear NF-κB staining, despite increased cytoplasmic levels.
Figure 3.
Monocyte Exos enter HUVECs and activate NF-κB. A) HUVECs were incubated with Dil-labeled monocyte Exos for 4 h (red), and the nuclei were counterstained with DAPI (blue). Magnification, ×1000. B) HUVECs were incubated for 4 h with Exos derived from nonstimulated and stimulated monocytes, as described in Fig. 2, immunostained for NF-κB/p65 (red), and counterstained with Hoechst (blue). Arrows: NF-κB/p65 nuclear staining. Blood samples were from 6 donors. A representative experiment is shown. Magnification, ×200.
We next looked at downstream targets of NF-κB activation. Using qPCR, we found significantly induced expression of ICAM1, CCL2, and IL6 mRNAs in the endothelial cells treated with Exos derived from monocytes stimulated with LPS or I/L, but not with IFN (Fig. 4A). This up-regulation was also present at the protein level when assessed by Western blot and ELISA (Fig. 4B). We did not detect either IL-1β or TNFα proteins by ELISA. By qPCR, TNFα was not detectable, and IL1β was minimally amplified (data not shown). LPS-treated monocytes secrete large quantities of TNFα, an effective inflammatory inducer of endothelial cells. To demonstrate that activation in the Exo-treated HUVECs was not the result of TNFα carryover from the activated monocyte supernatant, we tested the purified exosomal preparations by ELISA. We found no detectable TNFα in the exosomal preparations added to the HUVECs (data not shown). We also titrated the amount of monocyte Exos added and found a dose-dependent induction of the target genes in the HUVECs (Fig. 4C). To demonstrate that the increases seen in the endothelial cells arose from de novo synthesis and not simply a reflection of cargo brought in by the monocyte Exos, we compared the gene expression profiles of the monocyte Exos with the Exo-treated endothelial cells. There was minimal CCL2 mRNA in the untreated HUVECs and barely detectable amounts in the Exos themselves. However, in the LPS or I/L Exo-treated endothelial cells, CCL2 was highly elevated (Fig. 4D). ICAM1 and IL6 mRNA levels in the Exos were too low to be reliably detected and therefore could not be compared with the Exo-treated HUVECs.
Figure 4.
Immune-activated monocyte-derived Exos induce ICAM-1, CCL2, and IL-6 in HUVECs. A) Exo-treated HUVEC lysates were collected, and qPCR was performed, to determine mRNA expression of ICAM1, CCL2, IL6. (IFN, n = 6 donors; HUVECs, NS, LPS, I/L, n = 11 donors). B) Protein expression by Western blot (ICAM-1, n = 3 donors, representative blot shown) and ELISA (CCL2 and IL-6: n = 4 donors for IFN group; HUVECs, NS, LPS, and I/L groups: n = 8 donors). C) Exos derived from LPS- and I/L-stimulated monocytes were serially diluted in PBS, and equal volumes were added to HUVECs and then analyzed by qPCR to demonstrate dose response (n =1 donor). D) CCL2 gene expression by qPCR in untreated HUVECs, monocyte Exos (LPS-Exos, I/L-Exos), and Exo-treated HUVECs (LPS-Exo-HUVECs, I/L-Exo-HUVECs, n = 3 donors). HUVEC lysates were collected 24 h after incubation with Exos, except for those used for Western blot analysis, which were collected 48 h after addition of Exos. All qPCRs were performed in triplicate and ELISAs in duplicate. Dots: means of the replicates; horizontal bars: means of the groups; shading: range; 2-sided, paired Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
Regulation by TLR4 and NF-κB
To gain insight into the mechanism behind the induction of ICAM1, CCL2, and IL6 by the activated monocyte Exos, we first examined the role of NF-κB activation in this process. NF-κB is one of the main signaling pathways involved in inflammation. Using the NF-κB inhibitor PTN, which specifically inhibits NF-κB activation by preventing the degradation of IκB-α and IκB-β (23), we found that induction of ICAM1, CCL2, and IL6 by the activated monocyte Exos was reduced in a dose-dependent manner (Fig. 5). At subcytotoxic levels of ≤10 μM PTN, we saw maximum reduction of ICAM1 expression in the range of 42–87%, depending on the blood donor. For CCL2, the maximum reduction was 38–78%. For IL-6, it was 17–69%. The slightly larger decrease in ICAM1 vs. CCL2 may indicate that NF-κB plays a larger role in ICAM1 induction than in CCL2 induction.
Figure 5.
Inhibition of NF-κB reduces Exo-mediated adhesion molecule and cytokine induction in a dose-dependent manner. HUVECs were preincubated for 90 min with various concentrations of NF-κB inhibitor PTN (0, 3, and 10 μM), and then Exos derived from nonstimulated (NS) or stimulated monocytes (LPS, I/L) were added for 24 h. HUVEC lysates were then collected and analyzed by qPCR for ICAM1, CCL2, and IL6 gene expression. qPCR was performed in triplicate for each of 3 different blood donors; shading, range; 1-sided, paired Student’s t test. *P < 0.05.
Because the induction levels were qualitatively similar between LPS Exo and I/L Exo stimulation, we asked whether the activated monocyte Exos activate a TLR pathway, leading to the subsequent activation of NF-κB. HUVECs express predominantly TLR4, with little or undetectable TLR2 (24). We next examined the effect of preincubation of the Exo-treated HUVECs with the TLR4-specific small molecule inhibitor TAK-242. TAK-242 is a cell-permeable cyclohexane carboxylate that disrupts TLR4 interaction with the adaptor molecules toll/interleukin 1 receptor (TIR) domain–containing adaptor protein and TIR-domain-containing adaptor protein inducing interferon-β–related adaptor molecule, by selectively binding to TLR4, but not to TLR-1 to -3 or TLR 5 to 10 (25). TAK-242 significantly inhibited ICAM1 and CCL2 induction but not IL6 induction (Fig. 6). Maximum inhibition of ICAM1 and CCL2 by TAK-242 was 26–38% and 11–32%, respectively, depending on the donor. The amount of inhibition observed for TAK-242 was less than that observed for PTN, suggesting that monocyte Exo-mediated activation of NF-κB and the subsequent up-regulation of ICAM1, CCL2, and IL6 occur via multiple pathways, one of which involves TLR4.
Figure 6.
Inhibition of TLR4 partially blocks monocyte Exo-mediated induction. HUVECs were preincubated for 90 min with various concentrations of TLR4-specific inhibitor TAK-242 (0, 3, and 10 μM), followed by the addition of monocyte Exos for 24 h. HUVEC lysates were then collected, and qPCR was performed in triplicate for each of 3 blood donors. Shading: range; 1-sided, paired Student’s t test.. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
Evidence is emerging that extracellular vesicles can act as conveyors of immune responses (19). At present, less than a handful of studies have been published on the effect of monocyte Exos on target cells. In most of these studies, extracellular vesicles, including microparticles, from THP1 acute monocytic leukemia cells were used (26–29). Microparticles are distinct from Exos, in that they are larger and originate from a different cellular compartment. Moreover, THP1 cells respond differently to stimulation than do normal, primary monocytes (30). One study was an investigation of the effect of primary, human monocyte Exos on osteo differentiation of mesenchymal stem cells (31). The strengths of the present study are the use of primary monocytes, treatment factors associated with HIV infection known to activate monocytes, and isolation and characterization of Exos and their impact on target, primary endothelial cells. To our knowledge, this is the first study to show that primary, human monocyte Exos enter endothelial cells and cause dysfunction.
The results in this study confirm studies of other cell types showing primarily constitutive release of Exos, whose size and quantity do not change significantly with stimulation (19, 32–35), although there are reports of inducible Exo release (19, 36). Exo release is very different from microvesicle secretion, which blebs from the plasma membrane and is increased during cellular stimulation (37–39). Although we found no differences in size and quantity of Exos released with the various stimulations, we did find differences in monocyte Exo cargo with different resultant effects on endothelial cells. In terms of its effect on NF-κB, IFN Exo treatment increased expression but did not activate NF-κB/p65. This increase in NF-κB/p65 expression without activation has also been observed during the differentiation of human peripheral blood monocytes to macrophages (40), and may represent a cytoplasmic reservoir of NF-κB that is available for rapid and robust response upon inducer-mediated stimulation akin to cellular priming. This observation may explain why miR-155 expression is enhanced in Exos derived from monocytes that were first primed with IFNα followed by subsequent stimulation with LPS (Fig. 2C).
We also found that unlike IFN Exos, both LPS Exos and I/L Exos induced expression of ICAM-1, CCL2, and IL-6 in endothelial cells, principally via NF-κB activation. Blockage of NF-κB led to ∼90% inhibition of ICAM1, CCL2, and IL6 expression. For ICAM1 and CCL2 (but not IL6), the inductions were in part caused by TLR4 signaling, in that TLR4 blockage led to partial inhibition (≤38%) of the inductions. TLR4 did not appear to play a role in the Exo-mediated induction of IL6, as TAK-242 had no effect, indicating that another pathway activatesNF-κB and induces IL6. Because Exos are complex vessels containing a variety of biologically active molecules, it is reasonable to postulate that delivery of activated monocyte Exos to endothelial cells will affect more than 1 signaling pathway.
It is tempting to speculate about other pathways that may have contributed to our observed results, particularly miRs, given that they are highly enriched in Exos. miR-223 is not detectable in normal HUVECs but it is the predominant miR in Exos derived from monocytes and macrophages (41, 42). It has been shown to down-regulate TLR-mediated IL-6 production via signal transducer and activator of transcription-3 in macrophages (43). This miR also down-regulates ICAM-1 in endothelial cells (44). Other studies have shown an emerging role for miR-223 in cardiovascular pathophysiology functioning as cardioprotective and anti-inflammatory (45). Our finding that ICAM-1, CCL2, and IL-6 inductions are associated with decreased miR-223 in the LPS- and I/L-stimulated monocyte Exos is consistent with the roles suggested for miR-223. Moreover, if miR-223 affects other TLR pathways and activating STATs, it may help explain why a specific inhibition of TLR4 had no effect on Exo-mediated IL6 induction.
microRNA-155 participates in inflammation and TLR signaling. In monocytes and macrophages exposed to LPS and a variety of cytokines including IFN, miR-155 is rapidly and substantially induced (46, 47). Although there are reports that miR-155 represses NF-κB, recent studies show it to activate NF-κB (48, 49) and promote atherosclerosis. In mouse studies, miR-155 was found to be specifically expressed in atherosclerotic plaques and proinflammatory macrophages, where it was induced by treatment with mildly oxidized (mox)LDL and IFNγ (50). Combined stimulation of miR-155−/− mouse macrophages with moxLDL and IFNγ also led to reduced CCL2 expression. MicroRNA 155 may promote foam cell formation. Tian et al. (51) found that ApoE−/− mice fed a high-fat diet exhibited higher plasma miR-155; macrophages from these mice showed much higher miR-155 expression. In vitro overexpression of miR-155 in macrophages led to both enhanced lipid uptake and reactive oxygen species (ROS) production, whereas inhibition of miR-155 presented the opposite effect. These studies, together with our data on endothelial cell induction of ICAM-1, CCL2, and IL-6 associated with increased exosomal miR-155, suggest that miR-155, delivered by monocyte Exos, participates in atherogenesis and CVD.
There is other cargo in the monocyte Exos that may participate in the inductions that we observed in the endothelial cells including mRNAs, proteins, and lipids. A recent report showed that LPS-stimulated macrophages secrete Exos with proinflammatory cytokines (52). Given the complexity of the Exo cargo, which can vary, depending on cellular stimulation and environment, future work in this area would benefit from a thorough nucleic acid, proteomic, and lipidomic analysis of the stimulated and unstimulated monocyte Exos. In addition, in vivo studies using monocyte Exos derived from individuals, with and without CVD and HIV infection, are needed to substantiate our in vitro findings.
Not only do HIV-infected individuals have increased circulating LPS, but also their monocytes have an IFNα phenotype with elevated scavenger receptor (SR)-A expression and demonstrate enhanced lipid uptake. Monocyte exposure to IFNα in vitro increases SR-A expression, acetylated LDL uptake, and increases ROS (53). This result, coupled with the findings from the current study, leads us to posit that activated monocytes release Exos, which up-regulate ICAM-1 and CCL2, thereby facilitating migration of activated monocytes through the endothelium. Once in the intima, the activated monocyte/macrophages become foam cells through the enhanced ability to internalize LDL. IL-6 stimulation by monocyte-derived Exos potentiates inflammation, further fueling atherogenesis.
Persistent immune activation is integral to the pathologic course of CVD. In HIV infection, activated monocytes secrete inflammatory cytokines and other soluble plasma biomarkers. This report describes yet another mechanism stemming from an activated monocyte that can both alter chemotaxis and activate endothelial cells through Exo delivery. Exos released from immune-activated monocytes can travel to distant endothelial barriers, enter, and stimulate chemotaxis, all without the parent cell. These findings demonstrate both the pathogenic potential of Exos and the possibilities for therapeutic use.
ACKNOWLEDGMENTS
The authors thank Ivy Hsieh (Veterans Administration Medical Center) for performing the electron microscopy, Dr. Kang Li [University of California, San Francisco (UCSF)] for technical assistance with the NF-κB microscopy studies, Dr. Robert Raffai (UCSF) for helpful discussions, and Dr. Craig Meyer (UCSF) for assistance with statistical analyses. These studies were supported in part by the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grant R21HL129853 and NIH National Institute of Mental Health Grant R01MH096673 (both to L.P.). Author contributions: N. Tang, B. Sun, and A. Gupta performed the research; N. Tang, H. Rempel, and L. Pulliam analyzed the data; N. Tang and L. Pulliam wrote the manuscript; and B. Sun performed the statistical analyses. The authors declare no conflicts of interest.
Glossary
- ART
antiretroviral therapy
- CCL
chemokine (C-C motif) ligand
- CVD
cardiovascular disease
- Exo
exosome
- I/L Exo
exosome from monocyte stimulated with both IFNα and LPS
- FBS
fetal bovine serum
- HSP
heat shock protein
- ICAM
intercellular adhesion molecule
- IFN Exo
exosome from monocyte stimulated with IFNα
- LPS Exo
exosome from monocyte stimulated with LPS
- miR
microRNA
- moxLDL
mildly oxidized LDL
- NS Exo
exosome from nonstimulated monocyte
- NTA
nanoparticle tracking analysis
- PTN
parthenolide
- qPCR
quantitative PCR
- TBS
Tris-buffered saline
REFERENCES
- 1.Post W. S., Budoff M., Kingsley L., Palella F. J. Jr., Witt M. D., Li X., George R. T., Brown T. T., Jacobson L. P. (2014) Associations between HIV infection and subclinical coronary atherosclerosis. Ann. Intern. Med. 160, 458–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ssinabulya I., Kayima J., Longenecker C., Luwedde M., Semitala F., Kambugu A., Ameda F., Bugeza S., McComsey G., Freers J., Nakanjako D. (2014) Subclinical atherosclerosis among HIV-infected adults attending HIV/AIDS care at two large ambulatory HIV clinics in Uganda. PLoS One 9, e89537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boccara F., Lang S., Meuleman C., Ederhy S., Mary-Krause M., Costagliola D., Capeau J., Cohen A. (2013) HIV and coronary heart disease: time for a better understanding. J. Am. Coll. Cardiol. 61, 511–523 [DOI] [PubMed] [Google Scholar]
- 4.Rempel H., Sun B., Calosing C., Pillai S. K., Pulliam L. (2010) Interferon-alpha drives monocyte gene expression in chronic unsuppressed HIV-1 infection. AIDS 24, 1415–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilton J. C., Johnson A. J., Luskin M. R., Manion M. M., Yang J., Adelsberger J. W., Lempicki R. A., Hallahan C. W., McLaughlin M., Mican J. M., Metcalf J. A., Iyasere C., Connors M. (2006) Diminished production of monocyte proinflammatory cytokines during human immunodeficiency virus viremia is mediated by type I interferons. J. Virol. 80, 11486–11497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenchley J. M., Price D. A., Schacker T. W., Asher T. E., Silvestri G., Rao S., Kazzaz Z., Bornstein E., Lambotte O., Altmann D., Blazar B. R., Rodriguez B., Teixeira-Johnson L., Landay A., Martin J. N., Hecht F. M., Picker L. J., Lederman M. M., Deeks S. G., Douek D. C. (2006) Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371 [DOI] [PubMed] [Google Scholar]
- 7.Golias C., Tsoutsi E., Matziridis A., Makridis P., Batistatou A., Charalabopoulos K. (2007) Leukocyte and endothelial cell adhesion molecules in inflammation focusing on inflammatory heart disease [review]. In Vivo 21, 757–769 [PubMed] [Google Scholar]
- 8.Rollins B. J., Yoshimura T., Leonard E. J., Pober J. S. (1990) Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am. J. Pathol. 136, 1229–1233 [PMC free article] [PubMed] [Google Scholar]
- 9.Sica A., Wang J. M., Colotta F., Dejana E., Mantovani A., Oppenheim J. J., Larsen C. G., Zachariae C. O., Matsushima K. (1990) Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J. Immunol. 144, 3034–3038 [PubMed] [Google Scholar]
- 10.Ilhan F., Kalkanli S. T. (2015) Atherosclerosis and the role of immune cells. World J. Clin. Cases 3, 345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tedgui A., Mallat Z. (2006) Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev. 86, 515–581 [DOI] [PubMed] [Google Scholar]
- 12.Huber S. A., Sakkinen P., Conze D., Hardin N., Tracy R. (1999) Interleukin-6 exacerbates early atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 19, 2364–2367 [DOI] [PubMed] [Google Scholar]
- 13.Théry C., Zitvogel L., Amigorena S. (2002) Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 [DOI] [PubMed] [Google Scholar]
- 14.De Jong O. G., Verhaar M. C., Chen Y., Vader P., Gremmels H., Posthuma G., Schiffelers R. M., Gucek M., van Balkom B. W. (2012) Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J. Extracell. Vesicles 1, 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A., Pulliam L. (2014) Exosomes as mediators of neuroinflammation. J. Neuroinflammation 11, 68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Villarroya-Beltri C., Baixauli F., Gutiérrez-Vázquez C., Sánchez-Madrid F., Mittelbrunn M. (2014) Sorting it out: regulation of exosome loading. Semin. Cancer Biol. 28, 3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber H. J., Holvoet P. (2015) Exosomes: emerging roles in communication between blood cells and vascular tissues during atherosclerosis. Curr. Opin. Lipidol. 26, 412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Théry, C., Amigorena, S., Raposo, G., and Clayton, A. (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Chapter 3, Unit 3, 22 [DOI] [PubMed] [Google Scholar]
- 19.Théry C., Ostrowski M., Segura E. (2009) Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 9, 581–593 [DOI] [PubMed] [Google Scholar]
- 20.Mallat Z., Benamer H., Hugel B., Benessiano J., Steg P. G., Freyssinet J. M., Tedgui A. (2000) Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 101, 841–843 [DOI] [PubMed] [Google Scholar]
- 21.Marques-Rocha J. L., Samblas M., Milagro F. I., Bressan J., Martínez J. A., Marti A. (2015) Noncoding RNAs, cytokines, and inflammation-related diseases. FASEB J. 29, 3595–3611 [DOI] [PubMed] [Google Scholar]
- 22.Swaminathan G., Navas-Martín S., Martín-García J. (2014) MicroRNAs and HIV-1 infection: antiviral activities and beyond. J. Mol. Biol. 426, 1178–1197 [DOI] [PubMed] [Google Scholar]
- 23.Hehner S. P., Heinrich M., Bork P. M., Vogt M., Ratter F., Lehmann V., Schulze-Osthoff K., Dröge W., Schmitz M. L. (1998) Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J. Biol. Chem. 273, 1288–1297 [DOI] [PubMed] [Google Scholar]
- 24.Faure E., Equils O., Sieling P. A., Thomas L., Zhang F. X., Kirschning C. J., Polentarutti N., Muzio M., Arditi M. (2000) Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells: differential expression of TLR-4 and TLR-2 in endothelial cells. J. Biol. Chem. 275, 11058–11063 [DOI] [PubMed] [Google Scholar]
- 25.Matsunaga N., Tsuchimori N., Matsumoto T., Ii M. (2011) TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol. Pharmacol. 79, 34–41 [DOI] [PubMed] [Google Scholar]
- 26.Aharon A., Tamari T., Brenner B. (2008) Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb. Haemost. 100, 878–885 [DOI] [PubMed] [Google Scholar]
- 27.Wang J. G., Williams J. C., Davis B. K., Jacobson K., Doerschuk C. M., Ting J. P., Mackman N. (2011) Monocytic microparticles activate endothelial cells in an IL-1β-dependent manner. Blood 118, 2366–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y., Liu D., Chen X., Li J., Li L., Bian Z., Sun F., Lu J., Yin Y., Cai X., Sun Q., Wang K., Ba Y., Wang Q., Wang D., Yang J., Liu P., Xu T., Yan Q., Zhang J., Zen K., Zhang C. Y. (2010) Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol. Cell 39, 133–144 [DOI] [PubMed] [Google Scholar]
- 29.Ramakrishnan D. P., Hajj-Ali R. A., Chen Y., Silverstein R. L. (2016) Extracellular vesicles activate a CD36-dependent signaling pathway to inhibit microvascular endothelial cell migration and tube formation. Arterioscler. Thromb. Vasc. Biol. 3, 534–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schildberger A., Rossmanith E., Eichhorn T., Strassl K., Weber V. (2013) Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediators Inflamm. 2013, 697972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekström K., Omar O., Granéli C., Wang X., Vazirisani F., Thomsen P. (2013) Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS One 8, e75227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goetzl E. J., Goetzl L., Karliner J. S., Tang N., Pulliam L. (2016) Human plasma platelet-derived exosomes: effects of aspirin. FASEB J. 30, 2058–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatnagar S., Shinagawa K., Castellino F. J., Schorey J. S. (2007) Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 110, 3234–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4, 594–600 [DOI] [PubMed] [Google Scholar]
- 35.Raposo G., Nijman H. W., Stoorvogel W., Liejendekker R., Harding C. V., Melief C. J., Geuze H. J. (1996) B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Record M., Subra C., Silvente-Poirot S., Poirot M. (2011) Exosomes as intercellular signalosomes and pharmacological effectors. Biochem. Pharmacol. 81, 1171–1182 [DOI] [PubMed] [Google Scholar]
- 37.Distler J. H., Pisetsky D. S., Huber L. C., Kalden J. R., Gay S., Distler O. (2005) Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum. 52, 3337–3348 [DOI] [PubMed] [Google Scholar]
- 38.Mause S. F., Weber C. (2010) Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ. Res. 107, 1047–1057 [DOI] [PubMed] [Google Scholar]
- 39.Montoro-García S., Shantsila E., Marín F., Blann A., Lip G. Y. (2011) Circulating microparticles: new insights into the biochemical basis of microparticle release and activity. Basic Res. Cardiol. 106, 911–923 [DOI] [PubMed] [Google Scholar]
- 40.Conti L., Hiscott J., Papacchini M., Roulston A., Wainberg M. A., Belardelli F., Gessani S. (1997) Induction of relA(p65) and I kappa B alpha subunit expression during differentiation of human peripheral blood monocytes to macrophages. Cell Growth Diff. 8, 435–442 [PubMed] [Google Scholar]
- 41.Pulliam L., Gupta A. (2015) Modulation of cellular function through immune-activated exosomes. DNA Cell Biol. 34, 459–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ismail N., Wang Y., Dakhlallah D., Moldovan L., Agarwal K., Batte K., Shah P., Wisler J., Eubank T. D., Tridandapani S., Paulaitis M. E., Piper M. G., Marsh C. B. (2013) Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood 121, 984–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Q., Wang H., Liu Y., Song Y., Lai L., Han Q., Cao X., Wang Q. (2012) Inducible microRNA-223 down-regulation promotes TLR-triggered IL-6 and IL-1β production in macrophages by targeting STAT3. PLoS One 7, e42971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabet F., Vickers K. C., Cuesta Torres L. F., Wiese C. B., Shoucri B. M., Lambert G., Catherinet C., Prado-Lourenco L., Levin M. G., Thacker S., Sethupathy P., Barter P. J., Remaley A. T., Rye K. A. (2014) HDL-transferred microRNA-223 regulates ICAM-1 expression in endothelial cells. Nat. Commun. 5, 3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangrez A. Y., Kumari M., Frey N. (2013) An emerging role of microRNA miR-223 in cardiovascular pathophysiology. microRNAs Cardiovasc. Res. 1, 23-33 [Google Scholar]
- 46.Taganov K. D., Boldin M. P., Chang K. J., Baltimore D. (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 103, 12481–12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Connell R. M., Taganov K. D., Boldin M. P., Cheng G., Baltimore D. (2007) MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 104, 1604–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma X., Becker Buscaglia L. E., Barker J. R., Li Y. (2011) MicroRNAs in NF-kappaB signaling. J. Mol. Cell Biol. 3, 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bala S., Marcos M., Kodys K., Csak T., Catalano D., Mandrekar P., Szabo G. (2011) Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor alpha (TNFalpha) production via increased mRNA half-life in alcoholic liver disease. J. Biol. Chem. 286, 1436–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nazari-Jahantigh M., Wei Y., Noels H., Akhtar S., Zhou Z., Koenen R. R., Heyll K., Gremse F., Kiessling F., Grommes J., Weber C., Schober A. (2012) MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J. Clin. Invest. 122, 4190–4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian F. J., An L. N., Wang G. K., Zhu J. Q., Li Q., Zhang Y. Y., Zeng A., Zou J., Zhu R. F., Han X. S., Shen N., Yang H. T., Zhao X. X., Huang S., Qin Y. W., Jing Q. (2014) Elevated microRNA-155 promotes foam cell formation by targeting HBP1 in atherogenesis. Cardiovasc. Res. 103, 100–110 [DOI] [PubMed] [Google Scholar]
- 52.McDonald M. K., Tian Y., Qureshi R. A., Gormley M., Ertel A., Gao R., Aradillas Lopez E., Alexander G. M., Sacan A., Fortina P., Ajit S. K. (2014) Functional significance of macrophage-derived exosomes in inflammation and pain. Pain 155, 1527–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pulliam L., Calosing C., Sun B., Grunfeld C., Rempel H. (2014) Monocyte activation from interferon-alpha in HIV infection increases acetylated LDL uptake and ROS production. J. Interferon Cytokine Res. 34, 822–828 [DOI] [PMC free article] [PubMed] [Google Scholar]