Abstract
Cachexia is a devastating syndrome that causes morbidity and mortality in a large number of patients with cancer. However, the mechanisms of cancer cachexia remain poorly understood. Accumulation of misfolded proteins in the endoplasmic reticulum (ER) causes stress. The ER responds to this stress through activating certain pathways commonly known as the unfolding protein response (UPR). The main function of UPR is to restore homeostasis, but excessive or prolonged activation of UPR can lead to pathologic conditions. In this study, we examined the role of ER stress and UPR in regulation of skeletal muscle mass in naïve conditions and during cancer cachexia. Our results demonstrate that multiple markers of ER stress are highly activated in skeletal muscle of Lewis lung carcinoma (LLC) and ApcMin/+ mouse models of cancer cachexia. Treatment of mice with 4-phenylbutyrate (4-PBA), a chemical chaperon and a potent inhibitor of ER stress, significantly reduced skeletal muscle strength and mass in both control and LLC-bearing mice. Blocking the UPR also increased the proportion of fast-type fibers in soleus muscle of both control and LLC-bearing mice. Inhibition of UPR reduced the activity of Akt/mTOR pathway and increased the expression of the components of the ubiquitin–proteasome system and autophagy in LLC-bearing mice. Moreover, we found that the inhibition of UPR causes severe atrophy in cultured myotubes. Our study provides initial evidence that ER stress and UPR pathways are essential for maintaining skeletal muscle mass and strength and for protection against cancer cachexia.—Bohnert, K. R., Gallot, Y. S., Sato, S., Xiong, G., Hindi, S. M., Kumar, A. Inhibition of ER stress and unfolding protein response pathways causes skeletal muscle wasting during cancer cachexia.
Keywords: Lewis lung carcinoma, PERK, XBP-1, mTOR, autophagy
Cachexia is a multifactorial syndrome that affects most patients with advanced cancer, especially colon, lung, and pancreatic cancers (1). The syndrome is characterized by progressive weight loss due to significant loss of skeletal muscle mass, with or without adipose tissue wasting (2). The occurrence of cachexia correlates with poor prognosis and negatively impacts patient management, tolerance to antineoplastic therapies, and quality of life. Cancer cachexia cannot be reversed by nutritional support alone (1). Therefore, development of a therapy for cancer cachexia is critical in enhancing the quality of life and survival of patients with cancer (1–3).
Skeletal muscle wasting during cancer cachexia occurs through multiple mechanisms including an imbalance in the rate of protein synthesis and degradation (2). The ubiquitin–proteasome system (UPS) is the primary mechanism that causes the degradation of thin and thick filament proteins in many wasting conditions, including cancer cachexia (4–6). Inhibition of UPS or its specific components has been found to attenuate muscle loss in many experimental animal models of chronic diseases, including cancer (5, 7, 8). The autophagy–lysosomal system (ALS) is another important mechanism by which many misfolded proteins and defunct organelles are cleared within the cells. Although a basal level of autophagy is critical for skeletal muscle homeostasis, spurious activation of autophagy can lead to muscle wasting in many conditions (9). A recent study has shown that the activation of ALS contributes to loss of skeletal muscle mass in models of cancer cachexia (10). The activity of these proteolytic systems is regulated by the activation of NF-κB, AMPK, and p38 MAPK pathways (4–6, 11–13). In contrast, PI3K/Akt/mTOR is the major signaling pathway that increases the rate of protein synthesis in skeletal muscle in response to growth factors and functional overload (14). Activation of this pathway also inhibits protein degradation through inhibiting the activation of UPS and ALS (14). In recent years, several inflammatory cytokines and other host- or tumor-derived factors have been identified that mediate the activation of various proteolytic systems and inhibit protein synthesis in skeletal muscle in models of cancer cachexia (15–18). However, the molecular mechanisms that lead to the loss of muscle mass during cancer cachexia remain less understood.
ER is a membrane-bound organelle that is responsible for proper folding, processing, and trafficking of proteins within the cell. Any misfolded protein in the ER may induce ER stress. To deal with this stress, cells initiate the unfolding protein response (UPR), which is mediated by 3 types of ER transmembrane proteins: inositol-requiring protein (IRE)-1, RNA-dependent protein kinase-like ER eukaryotic translation initiation factor 2 α kinase (PERK), and activating transcription factor (ATF)-6 (19–21). When the cell is not under stress, these proteins are maintained in a relatively inactive state by binding to immunoglobulin heavy chain binding protein/glucose-regulating protein 78 (BiP/GRP78), an important ER chaperone. In response to ER stress, BiP disassociates from these proteins and preferentially binds to the misfolded proteins in the ER lumen. Upon release from BiP, PERK is autophosphorylated, leading to a cascade of signals including phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) and translation of ATF4, which induces transcription of C/EBP homologous protein (CHOP) (21–23). PERK/eiF2α axis is also involved in the termination of the UPR after the stress has been relieved by activation of growth arrest and DNA damage-inducible protein (GADD34) (24). Upon ER stress, IRE1 becomes activated by autophosphorylation, which promotes splicing of a 26-base intron from X-box binding protein (XBP)-1 mRNA (25). Spliced (s)XBP-1 increases ER chaperones and other components to assist in the folding capacity of the ER (26). ATF6 moves from ER to the Golgi apparatus to be cleaved by site-1 (S1P) and -2 protease (S2P) (27). The cleaved N-terminal fragment of ATF6 is then transported to the nucleus, where it acts in combination with ATF4 and sXBP-1 to increase the levels of proteins that function to alleviate the ER protein folding capacity (25). Activation of all 3 pathways executes ER stress by regulating gene expression. Although the primary role of UPR is to restore ER function, the failed recovery from ER stress leads to UPR-mediated cell death (19–21, 25). Indeed, the contribution of UPR-activated cell death has now been reported in various disease states, including ischemic stroke, multiple sclerosis, and Alzheimer’s disease (19–21).
ER-induced UPR appears to play an important role in myogenesis, evidenced by the findings that the ATF6 arm of UPR is activated during myogenic differentiation. ATF6 mediates selective apoptosis of a subpopulation of myoblasts that may be vulnerable to cellular stresses. Inhibition of ER stress signaling blocks apoptosis and myoblast differentiation (28), whereas inducers of ER stress selectively eliminate vulnerable myoblasts in cultures, and the surviving cells more efficiently differentiate into contracting myotubes (29). In adult skeletal muscle, increased expression of ER stress markers such as GRP78, GRP94, GADD34, ATF4, CHOP, and sXBP-1 has been observed after a single bout of exhaustive treadmill running (30). The role of ER stress in exercise-induced muscle adaptation is supported by the findings that recovery from acute exercise is compromised in ATF6α-null mice (30). Conversely, activation of ER stress is also linked with inflammation and insulin resistance in skeletal muscle (31). Attenuation of ER stress enhances insulin sensitivity, increases glucose uptake, and reduces glucose concentration in diabetic mice (32, 33). Recently, ER stress has been observed in myopathies such as myotonic dystrophy type 1 (34), sporadic inclusion body myositis (35, 36), and limb–girdle muscular dystrophy 1C (37). ER stress has also been found to be induced in skeletal muscle of aged mice (38). We have reported that the markers of ER stress and UPR are highly activated in skeletal muscle in response to starvation (39). However, the role of ER stress in regulation of skeletal muscle mass in catabolic conditions including cancer cachexia remains largely unknown.
The primary goal of this study was to investigate whether ER stress is activated and what role it plays in skeletal muscle wasting during cancer cachexia. To evaluate the role of ER stress, we used 4-phenylbutyrate (4-PBA), a chemical chaperon that is known to attenuate ER stress both in vivo and in vitro (32, 40). Our results demonstrate that ER stress is induced in skeletal muscle of Lewis lung carcinoma (LLC) and ApcMin/+ models of cancer cachexia. Our results also demonstrate that treatment with 4-PBA hastens the loss of skeletal muscle mass in the LLC model of cancer cachexia. More important, we have found that the inhibition of ER stress using 4-PBA leads to muscle wasting in naïve mice and in cultured primary myotubes.
MATERIALS AND METHODS
Animals
C57BL/6 and ApcMin/+ mice were originally purchased from Jackson Laboratories (Bar Harbor, ME, USA), and breeding was maintained at the University of Louisville animal resource facility. ApcMin/+ mice were killed at the age of 26 wk to study the activation of markers of ER stress. For the cancer cachexia model, LLC cells (2 × 106 cells in 100 µl saline; American Type Culture Collection, Manassas, VA, USA) were injected subcutaneously into the flanks of 3-mo-old C57BL/6 mice (41). For the study of muscle atrophy, the mice were weighed daily and euthanized 18 d after implantation of LLC. In one experiment, the mice were given an injection of 4-PBA (100 mg/kg body weight, i.p.) daily until the end of the experiment.
Histology and morphometric analysis
Individual TA and soleus muscles were isolated from mice, snap frozen in liquid nitrogen, and sectioned with a microtome cryostat. For the assessment of muscle morphology and to quantify fiber cross-sectional area (CSA), 10-μm-thick transverse sections of TA and soleus muscle were stained with hematoxylin and eosin (H&E). The sections were examined under an Eclipse TE 2000-U microscope (Nikon, Tokyo, Japan). Fiber CSA was analyzed in H&E-stained muscle sections using ImageJ software [National Institutes of Health (NIH), Bethesda, MD, USA]. For each muscle, the distribution of fiber CSA was calculated by analyzing ∼220 myofibers.
Muscle fiber-type immunostaining
To determine the composition of different types of fibers in the soleus muscle of mice, transverse cross sections were made and blocked in 5% goat serum and 2% bovine serum albumin (BSA) for 30 min, followed by incubation for 1 h with monoclonal antibodies against type I, IIa, and IIb MyHC isoforms using clone BA-D5, SC-7, and BF-F3, respectively (Developmental Studies Hybridoma Bank, Iowa City, IA, USA). Secondary antibody used was goat anti-mouse IgG2b conjugated with Alexa-350, goat anti-mouse IgG1 conjugate with Alexa-568 and goat anti-mouse IgM conjugated with Alexa-488. Finally, the fluorescence was captured with an Eclipse TE 2000-U microscope (Nikon), the images were merged, and the percentage of each type of fibers in whole muscle section was recorded.
Preparation of LLC cell–conditioned medium
LLC cells (American Type Culture Collection) were seeded in 100 mm cell culture plates in growth medium (DMEM containing 10% fetal bovine serum) at a density of 5000 cells/cm2. Supplementary growth medium was added to each plate after 2 d of plating. LLC cell cultures contain a heterogeneous mix of adherent and floating cells. After 4 d, we removed growth medium, and floating cells were harvested by centrifugation at 800 g, 5 min. Pelleted cells and 10 ml differentiation medium were added back to the plate containing the adherent cells. After 24 h, conditioned medium (CM) was harvested and cleared of cells and debris by centrifugation (800 g, 5 min). The CM was passed through 0.45 μm filters, and aliquots were frozen in liquid nitrogen for later use. For myotube treatments, CM was diluted 1:4 with fresh differentiation medium.
Indirect immunofluorescence and analysis of myotube size
Mouse primary myotubes were prepared according to a published protocol (42). After treatment with LLC-CM and/or 4-PBA, the myotubes were fixed with paraformaldehyde and blocked in 1% BSA in PBS for 1 h and incubated with anti-MF20 (1:250; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) in blocking solution at 4°C overnight. A brief PBS wash was applied before incubation with Alexa Fluor 568-conjugated secondary antibody (1:3000; Thermo Fisher Scientific Life Sciences, Carlsbad, CA, USA) for 1 h at room temperature. The cultures were washed 3 times for 15 min with PBS followed by incubation with DAPI (1:5000) for 3 min and subsequent PBS washes. The myotubes were then visualized at room temperature on an Eclipse TE 2000-U microscope equipped with a Digital Sight DS-Fi1 camera (Nikon). Images were captured, and the diameter of the myotubes was measured with ImageJ software (NIH). The myotube diameter was quantified as follows: 10 fields were chosen randomly, and 10 myotubes were measured per field. The average diameter per myotube was calculated as the mean of the 3 measurements taken along the length of the myotube.
Surface sensing of translation assay
Protein synthesis was measured by nonisotope-labeled surface sensing of translation (Sunset), a validated method (43). Myotubes were treated with LLC-CM and/or 4-PBA for 12 h, followed by addition of 0.1 μM puromycin for 30 min. The cells were collected, protein extracts were made, and newly synthesized protein was detected by Western blot with anti-puromycin (1:1000; EMB Millipore Darmstadt, Germany) as the primary antibody.
AMPK assay
Enzymatic activity of AMPK in skeletal muscle tissue extracts was measured with a commercially available kit, according to the protocol suggested by the manufacturer (MBL International, Woburn, MA, USA).
Total RNA extraction and qRT-PCR assay
RNA isolation and qRT-PCR were performed by using a published method (41, 44). In brief, total RNA was extracted from gastrocnemius (GA) and TA muscles of mice or cultured primary myotubes isolated with TRIzol reagent (Thermo Fisher Scientific Life Sciences) and an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturers’ protocols. First-strand cDNA for PCR analyses was made with a commercially available kit (Thermo Fisher Scientific Life Sciences). The quantification of mRNA expression was performed using the SYBR Green dye (Thermo Fisher Scientific Life Sciences) method on a sequence-detection system (model 7300; Thermo Fisher Scientific Life Sciences). Primers were designed with Vector NTI software (Thermo Fisher Scientific Life Sciences) and are available from the authors on request. Data normalization was accomplished with the endogenous control (β-actin), and the normalized values were subjected to a 2−ΔΔCt formula to calculate the fold change between control and experimental groups.
Analysis of sXBP-1
To measure the unspliced (u)XBP-1 and spliced (s)XBP-1, we prepared cDNA from skeletal muscle of control and LLC-bearing mice and subjected it to a semi-quantitative RT-PCR (qRT-PCR) assay (39). The sequences of the primers were 5′-TTA CGG GAG AAA ACT CAC GGC-3′ (forward) and 5′-GGG TCC AAC TTG TCC AGA ATG C-3′ (reverse). The primer’s annealing temperature was 56°C, and reaction mixtures containing 100 ng of cDNA proceeded for 35 cycles. The PCR products were run on a 2% agarose gel to identify the presence of uXBP-1 and sXBP-1 cDNA.
Western blot analysis
Estimation of the presence of various proteins was quantitated by performing Western blot analysis. TA or GA muscle of mice or primary myotubes were washed with sterile PBS and homogenized in lysis buffer: 50 mM Tris-Cl (pH 8.0), 200 mM NaCl, 50 mM NaF, 1 mM dithiothreitol, 1 mM sodium orthovanadate, 0.3% IGEPAL, and protease inhibitors. Approximately 100 μg protein was resolved in each lane on 10% SDS-polyacrylamide gels, electrotransferred onto nitrocellulose membranes, and probed with the following antibodies: anti-phospho-eIF2α (1:1000; Cell Signaling Technology, Danvers, MA, USA), anti- eIF2α (Cell Signaling Technology 1:1000), anti- CHOP (1:500; Cell Signaling Technology), anti-GADD34 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-ATF6 (1:500; Santa Cruz Biotechnology), anti-MyHC (1:1000; DSHB), anti-troponin T (1:500; Sigma-Aldrich, St. Louis, MO, USA), anti-tropomyosin (1:500; Sigma-Aldrich), anti-sarcomeric α-actin (1:500; Sigma-Aldrich), anti-IRE1α (1:1000; Cell Signaling Technology), anti-sXBP-1 (1:1000; Cell Signaling Technology), anti-ubiquitin (1:500; Santa Cruz Biotechnology), anti-LC3B1/2 (1:500; Cell Signaling Technology), anti-Beclin1 (1:500; Cell Signaling Technology), anti-p62 (1:500; MBL International), anti-phospho-Akt (Ser473) (1:500; Cell Signaling Technology), anti-Akt(1:500; Cell Signaling Technology), anti-phospho-mTOR (1:500; Cell Signaling Technology), anti-mTOR (1:500; Cell Signaling Technology), anti-phospho-p70S6K (1:500; Cell Signaling Technology), anti-p70S6K (1:500; Cell Signaling Technology), anti-phospho-ribosomal protein (rp)S6 (1:500; Cell Signaling Technology), anti-rpS6 (1:000; Cell Signaling Technology), anti-α-tubulin (1:1000; Cell Signaling Technology), and anti-GAPDH (1:2000; Cell Signaling Technology). Antibodies were detected by chemiluminescence. Quantitative estimation of the bands’ intensity was performed with ImageJ software (NIH).
Grip-strength test
We used a digital grip-strength meter (Columbus Instruments, Columbus, OH, USA) to measure forelimb or total 4-limb grip strength of mice by following a known protocol (45). Mice were acclimatized for 5 min before the grip-strength test began. The mouse was allowed to grab the metal pull bar with the forepaws and in a separate experiment with all 4 paws. The mouse tail was then gently pulled backward in the horizontal plane until it could no long grasp the bar. The force at the time of release was recorded as the peak tension. Each mouse was tested 5 times with a 20–40 s break between tests. The average peak tension from 3 best attempts normalized against total body weight was defined as forelimb grip strength.
Statistical analysis
Results are expressed as means ± sd. For statistical analysis, we used paired Student’s t test or analysis of variance to compare quantitative data populations with normal distribution and equal variance. A result reaching P < 0.05 was considered statistically significant unless otherwise specified.
RESULTS
Markers of ER stress are induced in skeletal muscle of mouse models of cancer cachexia
We first investigated how the expression of ER stress markers is altered in skeletal muscles of mice in response to cancer growth. There are several mouse models, but LLC and ApcMin/+ mice are commonly used to study cancer cachexia (4, 6, 11, 46, 47). C57BL/6 mice were inoculated with 2 × 106 LLC cells in the left flank. The mice were euthanized 18 d after implantation of LLC and right hindlimb muscles were analyzed. Markers of ER stress were measured by performing qRT-PCR and Western blot. As shown in Fig. 1A, mRNA levels of IRE1α, XBP-1, ATF6, and DR5 were significantly increased in the GA muscle of LLC-bearing mice. Western blot analysis showed that the phosphorylation of p-eIF2α, a phosphorylation target of PERK, was significantly elevated in skeletal muscle of LLC-bearing mice. Moreover, the protein levels of CHOP were significantly increased in skeletal muscle of LLC-bearing mice (Fig. 1B, C). In response to ER stress, XBP-1 mRNA is spliced by IRE1 to produce a highly active transcription factor that induces UPR (48). We evaluated the splicing of XBP-1 mRNA by performing semi-qRT-PCR using primers that detect both unspliced and spliced mRNAs. As shown in Fig 1D, there was a considerable increase in levels of sXBP-1 in GA muscle of LLC-bearing mice. We next used an ApcMin/+ genetic mouse model of cancer cachexia to determine whether the induction of markers of ER stress was limited to a specific type of cancer. Results showed that mRNA levels of PERK, IRE1α, XBP-1, ATF6, GRP78, GRP94, GADD34, and DR5 were significantly increased in GA muscle of 4-mo-old ApcMin/+ transgenic mice compared to littermate control mice (Fig. 1E). These results suggest that ER stress is elevated in skeletal muscle in models of cancer cachexia.
Figure 1.
Activation of ER stress in mouse models of cancer cachexia. LLC cells were implanted in the left flank of wild-type mice, and tumor growth was monitored. Right hindlimb muscles were isolated and used to measure the levels of various markers of ER stress. A) Relative mRNA levels of PERK, IRE1α, XBP-1, ATF6, GRP78, GRP94, GADD34, and DR5 in GA muscle of control and LLC-bearing mice. B) Western blot analysis of levels of phosphorylated and total eIF2α and total CHOP and of total GADD34 protein in GA muscle of control and LLC-bearing mice. C) Densitometry quantification of phosphorylated vs. total eIF2α ratio and CHOP in GA muscle of control and LLC-bearing mice. D) Spliced (s)XBP-1 and unspliced (u)XBP-1 levels in control and LLC-bearing mice measured by semiquantitative RT-PCR assay using primers that detected both sXBP-1 and uXBP-1. Black vertical lines in gel images indicate that intervening lanes have been spliced out. E) Relative mRNA levels of PERK, IRE1α, XBP-1, ATF6, GRP78, GRP94, GADD34, and DR5 in GA muscle of 4-mo-old control and ApcMin/+ mice measured by qRT-PCR. Error bars represent sd (n = 4/group); unpaired Student’s t test. *P < 0.05, vs. control mice.
Tumor-derived factors increase markers of ER stress in skeletal muscle
Although we found increased activation and expression of markers of ER stress in skeletal muscle of the models of cancer cachexia, it is not clear whether host- or tumor-derived factors mediate such activation. To resolve this question, we investigated whether treatment with LLC-CM can also activate markers of ER stress in cultured myotubes. Fully differentiated mouse primary myotubes were treated with LLC-CM in a 1:4 ratio for 24 h, followed by a study of the markers of ER stress. Treatment of myotubes with LLC-CM significantly increased the mRNA levels of PERK, ATF6, GRP94, GADD34, CHOP, ATF4, and DR5 measured by the qRT-PCR technique (Fig. 2A). We also performed Western blot analysis to determine the levels of phosphorylated eIF2α protein. This analysis showed that the levels of phosphorylated eIF2α were significantly elevated in myotubes upon treatment with LLC-CM (Fig. 2B, C). Moreover, the levels of CHOP and ATF6 proteins were also significantly increased in myotubes treated with LLC-CM. By contrast, the levels of GADD34 protein remained comparable between control and LLC-CM-treated myotubes (Fig. 2B). These results suggest that tumor-derived factors are responsible for the increased ER stress in skeletal muscle in models of cancer cachexia.
Figure 2.
Activation of UPR markers in cultured myotubes treated with LLC-CM. Primary myotubes prepared from WT mice were treated with LLC-CM in a 1:4 ratio for 24 h and processed for a qRT-PCR assay or Western blot analysis. A) Relative mRNA levels of PERK, ATF6, GRP94, GADD34, CHOP, ATF4, and DR5 in control and LLC-CM-treated myotubes. B) Immunoblots demonstrate the levels of phosphorylated and total eIF2α and ATF6, CHOP, and GADD34 protein in control and LLC-CM-treated myotubes. C) Densitometry quantification of phosphorylated vs. total p-eIF2α and total ATF6 and CHOP in control and LLC-CM-treated myotubes (n = 3/group). Error bars represent sd; unpaired Student’s t test. *P < 0.05, vs. control cultures.
Inhibition of ER stress diminishes muscle strength in normal and LLC-bearing mice
Although we observed increased activation of the markers of ER stress in models of cancer cachexia, it is not known whether ER stress works to promote muscle wasting or it antagonizes atrophying signals. We next investigated the effect of inhibition of ER stress in normal and LLC-bearing mice. In several previous studies 4-PBA has been used to block ER stress in animal models of various diseases (36, 37). Mice were given intraperitoneal injections of 4-PBA (100 mg/kg body weight, i.p.) daily until 18 d after inoculation of LLC cells. Control mice received only PBS. The average body weight of LLC-bearing mice was significantly lower than that of control mice at 18 d (Fig. 3A). Treatment with 4-PBA significantly reduced the body weight of normal mice. Furthermore, there was a trend toward further reduction in average body weight of LLC-bearing mice upon treatment with 4-PBA. We next measured the muscle strength of these mice using a grip-strength meter. As expected, LLC-bearing mice displayed a significant decrease in maximum and average forelimb, as well as total (4-paw) grip strength compared to control mice (Fig. 3B–E). Consistent with body weight, treatment of normal mice with 4-PBA resulted in a significant loss in grip strength. Indeed, loss of grip strength was comparable between control mice treated with 4-PBA and untreated LLC-bearing mice. Moreover, treatment with 4-PBA further reduced grip strength in LLC-bearing mice.
Figure 3.
ER stress inhibitor 4-PBA causes loss of muscle strength and mass in naïve conditions and in LLC-bearing mice. C57BL6 12-wk-old mice were inoculated with 2 × 106 LLC cells in the left flank. They were also treated daily with 4-PBA (100 mg/kg body weight, i.p.). After 18 d, muscle strength, body weight, and wet muscle mass were measured. A) Average body weight of mice in each group. B, C) Maximum (B) and average (C) forelimb grip strength of mice in each group. D, E) Maximum (D) and average (E) total 4-paw grip strength of mice in each group. F–H) Average wet weight of isolated soleus (F), TA (G), and GA (H) muscle in each group. I) Wet weight of tumor after 18 d of inoculation with LLC in vehicle alone or 4-PBA-treated mice (n = 5/group). Error bars represent sd; paired Student’s t test. *P < 0.05, vs. control mice; #P < 0.05, vs. LLC-inoculated mice.
We next isolated individual hindlimb muscles and measured their wet weight. Wet weight of tibialis anterior (TA) and soleus muscle was found to be significantly reduced in 4-PBA-treated normal mice; LLC-bearing mice; or 4-PBA-treated, LLC-bearing mice compared to control mice treated with vehicle alone (Fig. 3F, G). Furthermore, wet weight of the GA muscle was significantly reduced in 4-PBA-treated, LLC-bearing mice compared to mice in the other 3 groups (Fig. 3H). We also investigated whether increased muscle loss in 4-PBA-treated, LLC-bearing mice was due to increase in tumor growth. We found unexpectedly that treatment with 4-PBA reduced the size of tumor in mice (Fig. 3I). These results suggest that inhibition of ER stress in naïve and LLC-bearing mice exaggerates the loss of skeletal muscle mass and strength.
Inhibition of ER stress causes skeletal muscle wasting in normal and LLC-bearing mice
Skeletal muscle wasting is characterized by loss of fiber CSA without having any effect on number of fibers (14). We next investigated how inhibition of ER stress using 4-PBA affects fiber CSA in normal and LLC-bearing mice. For this analysis, we prepared TA and soleus muscle transverse sections and performed H&E staining followed by quantification of average fiber CSA. This analysis showed that average fiber CSA of TA (Fig. 4A, B) and soleus muscle (Fig. 4C, D) decreased significantly in LLC-bearing mice compared to control mice confirming a cachectic phenotype in these mice. Consistent with wet muscle weight, we found that treatment with 4-PBA alone significantly reduced fiber CSA in both TA and soleus muscle of normal mice. Moreover, treatment with 4-PBA further reduced average fiber CSA in both these muscles of LLC-bearing mice (Fig. 4A–D).
Figure 4.
Inhibition of ER stress by 4-PBA exacerbates skeletal muscle atrophy in control and LLC-bearing mice. A) Representative photomicrographs of H&E-stained sections of mouse TA muscle. Scale bar, 10 µm. B) Quantification of average fiber CSA of TA muscle in control and LLC-bearing mice, with or without 4-PBA treatment. C) Representative photomicrographs of H&E-stained sections of soleus muscle. Scale bar, 10 µm. D) Quantification of average fiber CSA of soleus muscle in control and LLC-bearing mice, with or without 4-PBA treatment. E) GA muscle of vehicle, 4-PBA, LLC, and LLC+4-PBA mice were processed for Western blot analysis to detect levels of specific muscle proteins. Representative immunoblots demonstrate the levels of MyHC, troponin, tropomyosin, sarcomeric α-actin, and unrelated protein α-tubulin. F) Densitometry quantification of MyHC. G) Representative immunoblots demonstrate that the levels of phosphorylated and total eIF2α, total IRE1, total sXBP-1, and ATF6 were reduced by treatment with 4-PBA, confirming suppression of ER stress. H) Transcript levels of ATF4 in GA muscle of vehicle, 4-PBA alone, LLC alone, and LLC+4-PBA groups measured by performing qRT-PCR assay. Black vertical lines in immunoblots indicate that intervening lanes have been spliced out (n = 5/group). Error bars represent sd; paired Student’s t test. *P < 0.05, vs. mice treated with vehicle alone; #P < 0.05 vs. LLC-inoculated mice.
Studies have shown that skeletal muscle wasting in response to multiple stimuli including cancer cachexia involves degradation of specific muscle proteins, such as MyHC fast type (44, 49). Protein extracts were prepared from GA muscle of mice, and levels of various thin and thick filament protein were measured by Western blot analysis. Levels of MyHC were significantly reduced in skeletal muscle of LLC-bearing mice and 4-PBA-treated normal or LLC-bearing mice compared with that in normal mice treated with vehicle alone (Fig. 4E, F). By contrast, the levels of troponin, tropomyosin, and sarcomeric α-actin were comparable between all the groups (Fig. 4E, quantification not shown). Our Western blot analysis also confirmed that the major markers of ER stress (i.e., p-eIF2, IRE1, sXBP-1, and ATF6) were considerably reduced in skeletal muscle of 4-PBA-treated mice (Fig. 4G). ATF4 is regulated by the PERK arm of ER stress (20). One study has shown that ATF4 is involved in starvation-induced muscle atrophy (50). By performing qRT-PCR, we measured mRNA levels of ATF4 and found that the levels of ATF4 were drastically increased in skeletal muscle of LLC-bearing mice. Treatment with 4-PBA blunted the LLC-induced expression of ATF4 in skeletal muscle of mice. Whether ATF4 has any role in regulation of muscle mass during cancer growth remains unknown, but inhibition of expression of ATF4 confirms that 4-PBA effectively inhibits the markers of UPR in skeletal muscle of mice. The collective results suggest that inhibition of ER stress/UPR pathways causes muscle wasting in normal mice and produces a more severe cachetic phenotype in LLC-bearing mice.
ER stress is necessary for maintaining the oxidative phenotype of skeletal muscle
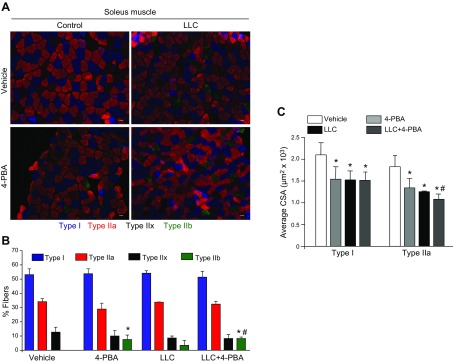
Skeletal muscle wasting in many conditions is associated with a slow-to-fast fiber type transition (51). There are also several reports suggesting that fast-type (glycolytic) fibers undergo atrophy at a faster rate than do slow-type (oxidative) fibers (52). Induction of cancer cachexia through C-26 tumor cells in mice has been shown to cause a transition from slow-to-fast type fibers in soleus muscle (53). To investigate whether inhibition of ER stress affects the composition of slow- and fast-type fibers in skeletal muscle, we prepared soleus muscle cross sections and stained them with anti-myosin heavy chain (MyHC) types I, IIa, and IIb. Representative images of all 4 groups are presented in (Fig. 5A). Consistent with published reports, soleus muscle of normal mice contained mainly type I and IIa (both oxidative) fibers with almost no type IIb (glycolytic) fibers. Treatment of mice with 4-PBA alone or inoculation with LLC cells significantly increased the number of type IIb fibers in soleus muscle of mice. Moreover, treatment of LLC-bearing mice with 4-PBA further increased the proportion of type IIb fibers in soleus muscle (Fig 5B). We also investigated whether the inhibition of ER stress reduces the CSA of type I or IIa fibers or of both. Quantitative estimation of stained soleus muscle section showed that 4-PBA or inoculation of LLC alone significantly reduced average CSA of type I and IIa fibers in mice. Combination of 4-PBA and LLC had no additional effects on the reduction of average CSA of type I fibers. By contrast, the average CSA of type IIa fibers was significantly reduced compared to 4-PBA alone or LLC alone (Fig. 5C). These results suggest that ER stress/UPR maintains the proportion and size of slow-type fibers in skeletal muscle.
Figure 5.
Blocking ER stress increases the proportion of fast-type fibers in normal and LLC-bearing mice. Soleus muscle sections prepared from vehicle, 4-PBA, LLC, and LLC+4-PBA mice were subjected to triple immunostaining against MyHC I, IIA, and IIB protein. A) Representative photomicrographs of triple-stained sections of soleus muscle. Scale bar, 50 µm. B) Quantification of the percentage of each fiber type in different groups. C) Average CSA of type I and IIa fibers in soleus muscle of mice in each group (n = 5 in each group). Error bars represent sd; paired Student’s t test. *P < 0.05, vs. control mice; #P < 0.05, vs. LLC-inoculated mice.
Inhibition of ER stress activates proteolytic pathways in skeletal muscle
The UPS and autophagy are 2 major proteolytic systems that cause degradation of muscle protein during cancer growth and in many other catabolic conditions (4, 10). We next investigated whether inhibition of ER stress affects the activation of these pathways in skeletal muscle of mice. To understand the overall levels of ubiquitinylation, we prepared muscle extracts and performed immunoblot analysis with an antibody against ubiquitin. There was a significant increase in the conjugation of muscle proteins to ubiquitin in LLC-bearing mice. Furthermore, the levels of ubiquitinated proteins were considerably increased in skeletal muscle of 4-PBA-treated mice compared with those treated with vehicle alone. Treatment with 4-PBA reduced the levels of ubiquitinated protein in LLC-bearing mice (Fig. 6A). We also measured mRNA levels of the E3 ubiquitin ligases MAFBx, MuRF1, MUSA1, and TRAF6, which are induced in skeletal muscle and have been shown to mediate degradation of muscle proteins in multiple catabolic conditions (4, 14, 39, 41, 54, 55). Consistent with published reports, there was a significant increase in the mRNA levels of MAFBx, MuRF1, and TRAF6 in skeletal muscle of LLC-bearing mice compared to control mice. In contrast, the mRNA levels of MUSA1 were reduced in LLC-bearing mice (Fig. 6B). There was also a trend toward increased expression of MAFBx in skeletal muscle of mice treated with 4-PBA, although it was not statistically significant. Transcript levels of MuRF1 remained unchanged, whereas the levels of TRAF6 and MUSA1 were found to be significantly reduced in skeletal muscle of mice treated with 4-PBA alone compared to vehicle alone. Treatment with 4-PBA significantly reduced the mRNA levels of MAFBx, MuRF1, and TRAF6 in skeletal muscle of LLC-bearing mice.
Figure 6.
The effect of 4-PBA treatment on the activation of ubiquitin proteasome system and autophagy. GA muscle of vehicle, 4-PBA, LLC, and LLC+4-PBA mice were processed for Western blot and qRT-PCR analyses. A) Representative immunoblots demonstrate the levels of ubiquitinated proteins in GA muscle of mice in each group. B) Relative mRNA levels of MAFBx, MuRF1, MUSA1, and TRAF6 in GA muscle measured by performing qRT-PCR assay. C) Representative immunoblots demonstrates the levels of LC3B-I and -II, Beclin1, p62, and the unrelated protein GAPDH in GA muscle of mice. D) Densitometry quantification of the ratio of LC3B-II vs. LC3B-I and total levels of Beclin1, and p62. Black vertical lines in immunoblots indicate that intervening lanes have been spliced out (n = 4/group). Error bars represent sd; paired Student’s t test. *P < 0.05, vs. control mice; #P < 0.05, vs. LLC inoculated mice.
We next measured the markers of autophagy in skeletal muscle of mice in each group. During autophagy, microtubule-associated protein 1A/1B LC3B-I is converted to LC3B-II through lipidation, allowing LC3B to become associated with autophagic vesicles. Furthermore, p62 is a bona fide substrate of autophagy, and its levels are reduced upon activation of autophagy (9). Our analysis showed that there was no significant difference in the ratio of LC3B-II/LC3B-I protein in skeletal muscle of control and 4-PBA-treated mice. However, the ratio of LC3B-II to -I was significantly higher in skeletal muscle of LLC-bearing mice and was further increased by treatment with 4-PBA. We did not find a significant difference in the protein levels of another autophagy-related protein, Beclin1 (Fig. 6C, D); however, the levels of p62 protein were significantly reduced in skeletal muscle of mice treated with 4-PBA alone, LLC alone, or LLC+4-PBA, suggesting activation of autophagy in all the 3 groups (Fig. 6C, D). Our collective results suggest that inhibition of ER stress leads to the activation of the UPS and autophagy system in skeletal muscle.
Role of ER stress in the activation of the Akt/mTOR pathway and AMPK in skeletal muscle
Akt/mTOR is one of the most important signaling pathways that promote protein synthesis in skeletal muscle. Furthermore, activation of this pathway inhibits the activity of UPS and ALS in skeletal muscle (14). We next sought to determine whether the inhibition of ER stress affects the activity of the Akt/mTOR pathway in skeletal muscle. There was no significant difference in phosphorylated or total levels of Akt in different groups (Fig. 7A, B). Treatment with 4-PBA significantly reduced the levels of phosphorylated mTOR, p70S6K, and rpS6 in skeletal muscle of mice. Levels of phosphorylated p70S6K and rpS6 were also found to be significantly reduced in LLC-bearing mice, and 4-PBA further reduced the levels of phosphorylated rpS6 protein. AMPK is an important signaling protein that induces the activity of proteolytic pathways and inhibits protein synthesis through negatively regulating mTOR (14). Using a commercially available AMPK activity assays kit, we measured the enzymatic activity of AMPK in skeletal muscle of mice in all 4 groups. We found that the activity of AMPK increased significantly in skeletal muscle of mice in the 4-PBA alone, LLC, or LLC+4-PBA groups, compared to the mice treated with vehicle alone (Fig. 7C).
Figure 7.
Effects of 4-PBA on activation of Akt/mTOR and AMPK signaling pathways in skeletal muscle of normal and LLC-bearing mice. GA muscle of vehicle-, 4-PBA-, LLC-, and LLC+4-PBA-treated mice were processed by Western blot analysis. A) Representative immunoblots demonstrate the levels of phosphorylated and total Akt, mTOR, p70S6 kinase (p70S6K), rpS6, and the unrelated protein α-tubulin. Black vertical lines indicate that intervening lanes have been spliced out. B) Quantification of phosphorylated and total Akt, mTOR, p70S6K, and rpS6 levels after normalizing with α-tubulin in GA muscle of vehicle-, 4-PBA-, LLC-, and LLC+4-PBA-mice. C) Fold change in enzymatic activity of AMPK in GA muscle of vehicle-, 4-PBA-, LLC-, and LLC+4-PBA-treated mice measured with a commercially available kit (n = 4/group). Error bars represent sd; paired Student’s t test. *P < 0.05, vs. control mice; #P < 0.05, vs. LLC-inoculated mice.
Inhibition of ER stress causes atrophy in cultured primary myotubes
LLC-CM has been shown to induce atrophy in cultured myotubes (6). To further understand the role of ER stress and tumor-derived factors in induction of atrophy, we treated primary myotubes with 4-PBA (5 mM), with or without LLC-CM (1:4 ratio), for 24 h. Results showed that treatment with 4-PBA alone or LLC-CM caused a significant reduction in myotube diameter. Loss of myotube diameter was further increased by combination of 4-PBA and LLC-CM (Fig. 8A, B). By performing Western blot analysis, we confirmed that the markers of ER stress/UPR, such as phosphorylation of eIF2α and levels of sXBP-1 and total XBP-1 are reduced upon treatment of myotubes with 4-PBA (Fig. 8C). We also measured the mRNA levels of some of the markers of UPS and autophagy at 12 and 24 h after treatment of myotubes with 4-PBA and LLC-CM. The mRNA levels of MuRF1, MAFBx, MUSA1, TRAF6, LC3B, and Beclin1 were significantly increased at 12 h after treatment of myotubes with 4-PBA (Fig. 8D). Levels of MAFBx, MUSA1, TRAF6, and LC3B (but not MuRF1 and Beclin1) remained significantly elevated at 24 h after start of 4-PBA treatment. LLC-CM alone significantly increased the mRNA of only Beclin1 after 12 h of treatment. However, at 24 h, the mRNA levels of MuRF1, MAFBx, and LC3B were significantly higher in the myotubes treated with LLC-CM compared with the control cultures. We found that 4-PBA significantly reduced the mRNA levels of MuRF1 in LLC-CM-treated myotubes without having a significant effect on the mRNA levels of MAFBx, TRAF6, and LC3B at 12 or 24 h. We also measured the rate of protein synthesis in cultured myotubes using the Sunset assay. As shown in Fig. 8E, treatment with 4-PBA for 12 h considerably reduced the rate of protein synthesis in cultured myotubes. Studies have shown that the addition of LLC-CM initially increases protein synthesis, potentially to counter atrophy signals, whereas long-term (>48 h) treatment inhibits protein synthesis in myotubes (56). We found a modest up-regulation in protein synthesis by treatment of myotubes with LLC-CM. However, 4-PBA reduced the LLC-CM-induced protein synthesis in cultured myotubes. We also measured the phosphorylation of Akt, mTOR, and rpS6 proteins. As shown in Fig. 8F, treatment with 4-PBA increased the phosphorylation of Akt but reduced the levels of phosphorylated mTOR and rpS6 proteins in myotubes. Consistent with protein synthesis, LLC-CM increased the activation of mTOR and rpS6 in myotubes within 12 h, which was blunted by treatment with 4-PBA. The collective results suggest that the inhibition of ER stress causes atrophy in cultured myotubes potentially through perturbing the activity of various proteolytic pathways and suppressing protein synthesis.
Figure 8.
Inhibition of ER stress causes atrophy in cultured primary myotubes. Differentiated myotubes were treated with vehicle alone, 4-PBA (5 mM), LLC-CM (1:4 ratio), or LLC-CM+4-PBA for 24 h. A) Representative photomicrographs of myotubes in each group taken at 24 h. Scale bar, 10 µm. B) Average myotube diameter in each group. C) Immunoblots demonstrate the levels of phosphorylated and total eIF2α, sXBP-1, and tXBP-1 in cultured myotubes after treatment with 4-PBA (5 mM) for 24 h. D) Relative mRNA levels of the E3 ubiquitin ligases MuRF1, MAFBx, MUSA1, and TRAF6 and the autophagy marker LC3B and Beclin1 in vehicle, 4-PBA, LLC-CM, or LLC-CM+4-PBA-treated myotubes measured after 12 and 24 h. E) Representative immunoblot (top) from the Sunset assay demonstrating relative amounts of newly synthesized protein in each condition. Equal loading of protein in each lane was confirmed by staining nitrocellulose membrane with Ponceau S dye (bottom). F) Immunoblots demonstrate the levels of phosphorylated and total Akt, mTOR, and rpS6 after 12 h in the indicated groups (n = 3/group). Error bars represent sd; paired Student’s t test *P < 0.05, vs. control cultures; #P < 0.05, vs. LLC-CM-treated myotube cultures at corresponding time points.
DISCUSSION
Recent studies have shown that ER stress and UPR pathways get activated in skeletal muscle in response to both physiologic and pathologic stimuli (57). With the exception of a study in which the role of the ATF6 arm of UPR in an acute exercise-induced adaptive response was investigated (30), there has been no published report investigating the direct role of ER stress in regulation of skeletal muscle mass in adult animals. In this study, many markers of ER stress were significantly increased in skeletal muscle of 2 mouse models of cancer cachexia (Fig. 1). 4-PBA effectively inhibits ER stress and is one of the most commonly used agents to block all 3 arms of UPR in different conditions (32, 40), and we therefore used this chemical chaperon to identify the potential role of ER stress in regulation of skeletal muscle in both naïve conditions and the LLC model of cancer cachexia. We found that inhibition of ER stress through prolonged administration of 4-PBA caused loss of skeletal muscle mass and strength in adult animals (Figs. 3 and 4). Our study also provided initial evidence that the activation of ER stress and UPR may be an important mechanism for preventing additional loss of skeletal muscle mass during cancer cachexia. These findings are consistent with the presumption that the main role of UPR is to restore ER function and hence improve proper protein folding in stress conditions (19, 20). The requirement of individual UPR pathways in homeostasis is evident by the findings that genetic ablation of PERK, IREα or ATF6α/β causes growth retardation, pancreatic dysfunction, and embryonic lethality in mice (26, 58–60). Therefore, inhibition of ER stress-induced UPR may disrupt homeostasis, which eventually results in loss of skeletal muscle mass. Alternatively, it is possible that the components of UPR pathways engage in cross-talk with other signaling pathways that are important for the acquisition and maintenance of skeletal muscle mass. Indeed, our results demonstrate that the inhibition of ER stress using 4-PBA inhibits the phosphorylation of mTOR and its downstream phosphorylation targets in skeletal muscle both in vivo and in vitro (Figs. 7A, B, and 8E).
Although we observed increased expression of the markers of ER stress in skeletal muscle of mouse models of cancer cachexia, it remains unknown whether increased ER stress is a common phenomenon in atrophying skeletal muscle in all catabolic conditions. Another report has suggested that the markers of ER stress are not altered during unloading-induced skeletal muscle atrophy (61). Moreover, we found no difference in the levels of expression of ER stress/UPR markers in undenervated and 7 d denervated skeletal muscle of mice (data not shown), indicating that ER stress may not have any significant role in disuse muscle atrophy. The prototypical markers of ER stress and UPR in skeletal muscle are induced during starvation-induced skeletal muscle atrophy (39). The increased expression of markers of ER stress in atrophying skeletal muscle during cancer (Figs. 1, 2) and starvation (39) suggests that ER stress is associated with the regulation of skeletal muscle mass in certain conditions, especially those that involve chronic inflammation and metabolic perturbations. Indeed, there are reports suggesting increased ER stress in muscle diseases such as myositis, which involves chronic inflammation, swelling, and weakness of skeletal muscle (35, 36). Increased expression of ER stress markers has also been observed in the myopathy associated with myasthenia gravis (57, 62).
Prolonged activation and unresolved ER stress can lead to many pathologic conditions in different tissues, including skeletal muscle. For example, activation of ER stress has been associated with development of insulin resistance in skeletal muscle (63). Indeed, we have reported that treatment with tunicamycin or thapsigargin, the inducers of ER stress, significantly increases (∼2-fold) the transcript levels of the components of UPS (e.g., MAFBx and MuRF1) and autophagy (e.g., LC3B and Beclin1) and represses expression of MyHC in cultured myotubes (39). Compared to the UPS and autophagy markers, tunicamycin or thapsigargin caused a dramatic (>20-fold) increase in the markers of ER stress, suggesting that very high levels of ER stress can initiate the atrophy program leading to loss of skeletal muscle mass (39). However, it is also possible that a small (in physiologic range) elevation of ER stress-associated UPR protects cells from undergoing further damage. Indeed, our results in this study demonstrate that activation of ER stress/UPR in skeletal muscle preserves skeletal muscle mass and strength. Treatment of LLC-bearing mice with 4-PBA led to a further reduction in both muscle strength and weight (Fig. 3). Similar results were obtained when cultured myotubes were treated with a combination of LLC-CM and 4-PBA (Fig. 8A, B). Inhibition of ER stress using 4-PBA resulted in lower tumor weight in mice (Fig. 3I), suggesting that the additional loss of muscle mass with administration of 4-PBA in LLC-implanted mice is not caused by the additional growth of the tumor. These results further emphasize the protective role of ER stress and UPR in tumor-induced muscle wasting.
Skeletal muscle atrophy is also associated with a change in expression of myosin isoforms. Specifically, cancer growth has been shown to cause a shift toward type IIb muscle fibers in the soleus muscle (53). Our results confirm a slow-to-fast fiber type transition in soleus muscle of LLC-bearing mice (Fig. 5). We found that treatment with 4-PBA also causes a slow-to-fast fiber type transition in the naïve conditions and leads to an even higher number of type IIb fibers in soleus muscle of LLC-bearing mice (Fig. 5). Although the underpinning mechanisms remain unknown, these results suggest that ER stress plays an important role in maintenance of oxidative fibers in skeletal muscle in vivo.
Activation of the UPS and ALS has been observed in numerous catabolic states (4, 8, 9). It has been observed that E3 ubiquitin ligases such as MuRF1, MAFBx, MUSA1, and TRAF6 are necessary in catalyzing the conjugation of ubiquitin to target protein (4, 14, 41, 54). Once the protein is tagged with ubiquitin chains, they are subjected to degradation in the proteasome (4). As expected, implantation of LLC in mice resulted in an increase in the levels of ubiquitinated proteins (Fig. 6A) and significantly higher expression of MAFBx, MuRF1, and TRAF6. We found that the levels of MUSA1 were significantly reduced in skeletal muscle of LLC-bearing mice (Fig. 6B). Treatment of wild-type mice with 4-PBA alone also increased the levels of ubiquitinated proteins (Fig. 6A). Although statistical significance could not be achieved with the number of animals used in each group, there was a trend toward increased mRNA levels of MAFBx and MuRF1 in skeletal muscle of mice treated with 4-PBA (Fig. 6B). By contrast, the levels of MUSA1 and TRAF6 were significantly reduced in skeletal muscle of mice treated with 4-PBA alone (Fig. 6B). Treatment with 4-PBA reduced the levels of ubiquitinated proteins (Fig. 6A) and mRNA levels of MAFBx, MuRF1, and TRAF6 (Fig. 6B) in LLC-implanted mice. Myotubes treated with LLC-CM and 4-PBA exhibited similar inhibition in MAFBx and MuRF1 (Fig. 8C). These results indicate that the inhibition of ER stress perturbs homeostasis in skeletal muscle in naïve conditions, leading to enhanced protein degradation, potentially through UPS. It is also possible that some components of ER stress are involved in the activation of UPS in tumor-bearing animals and the inhibition of ER stress/UPR attenuates the activity of UPS in tumor-bearing mice. However, more severe muscle atrophy in LLC-bearing mice upon treatment with 4-PBA indicates that, even though UPS is inhibited, other catabolic mechanisms may get activated, resulting in overall higher loss of skeletal muscle mass. One such mechanism appears to be the activation of autophagy. The markers of autophagy are increased in skeletal muscle of normal mice treated with 4-PBA alone or in LLC-bearing mice. Although 4-PBA reduced the levels of ubiquitinated protein, it did not inhibit the markers of autophagy in LLC-bearing mice (Fig. 6C, D).
Although we observed muscle atrophy and increased levels of polyubiquitinated proteins, we found no significant increase in the mRNA levels of MAFBx, MuRF1, TRAF6, or MUSA1 in skeletal muscle of 4-PBA-treated mice. We cannot rule out the possibility that the inhibition of physiologic ER stress leads to the increased activity of some other E2 and E3 enzymes of UPS leading to elevated levels of polyubiquitinated proteins in skeletal muscle. Moreover, it is possible that continued inhibition of some E3 ubiquitin ligase, such as MUSA1 and TRAF6, as observed in 4-PBA-treated mice (Fig. 6B) upregulates other E3 ligases in skeletal muscle as a part of a compensatory mechanism. In fact, a few recently published reports demonstrate that proteasome activity is significantly increased in the MuRF1-knockout mice compared to that in control mice in 14 d denervated muscle or during aging, even though muscle atrophy is significantly rescued in MuRF1-KO mice (64, 65). There is also a possibility that the expression of some of these E3 ubiquitin ligases is increased at an early time point after treatment of mice with 4-PBA. This hypothesis is partly supported by our results demonstrating that 4-PBA significantly increased the levels of MAFBx, MuRF1, TRAF6, and MUSA1 within 12 h (Fig. 8D). Nevertheless, the results presented in this study suggest that ER stress/UPR pathways differentially regulate polyubiquitination and the expression of various E3 ubiquitin ligases in skeletal muscle in naïve and cachectic conditions.
Another potential mechanism by which inhibition of ER stress causes muscle atrophy is through inhibition of the mTOR signaling pathway. Akt is known to regulate skeletal muscle hypertrophy through phosphorylation and activation of downstream targets, such as mTOR and rpS6, leading to increased protein synthesis (66). Although Akt is an upstream activator in response to growth factors, mTOR can also be activated through Akt-independent mechanisms (13). Our results suggest that although phosphorylation of Akt was not much affected, the phosphorylation of mTOR, p70S6K, and rpS6 kinase was significantly reduced in LLC-bearing mice (Fig. 7A, B). Similar to the muscle atrophy phenotype, we found that 4-PBA further reduced the phosphorylation of mTOR, p70S6K, and rpS6 protein in normal and LLC-bearing mice (Fig. 7A, B). The AMPK, a highly conserved heterotrimeric kinase complex composed of a catalytic (α) subunit and 2 regulatory (β and γ) subunits, is activated under conditions of energy stress when intracellular ATP levels decline and intracellular AMP increases, as occurs during nutrient deprivation, hypoxia, and physical exercise (67). Accumulating evidence suggests that mTOR and AMPK represents 2 antagonistic forces governing muscle adaptation in response to different stimuli. Specifically, activation of AMPK inhibits mTOR in skeletal muscle, leading to reduced protein synthesis and increased activation of proteolytic pathways (68). Our analysis showed that the activity of AMPK is significantly increased in skeletal muscle of mice treated with 4-PBA, LLC, or combination of LLC and 4-PBA (Fig. 7C). Although the exact mechanisms by which inhibition of ER stress increases the activation of AMPK and inhibits mTOR remain unknown, an interplay between AMPK and mTOR through the components of UPR could be an important mechanism of the regulation of muscle mass in the naïve and catabolic states.
We also examined the rate of protein synthesis using the Sunset assay and found that treatment of 4-PBA drastically reduced protein synthesis in cultured myotubes (Fig. 8E). Furthermore, 4-PBA significantly reduced the phosphorylation of mTOR and rpS6 protein in cultured myotubes (Fig. 8F). However, when treated with LLC-CM alone, protein synthesis and phosphorylation of mTOR and rpS6 protein were increased (Fig. 8E, F), perhaps because of the short time (12 h) during which the myotubes were subjected to the LLC-CM. The myotubes could have been attempting to rescue the atrophy after addition of LLC-CM. However, treatment with 4-PBA reduced LLC-CM-induced protein synthesis and phosphorylation of mTOR and rpS6 protein in cultured myotubes. Taken together, these results further support a physiologic role of ER stress in promoting activation of the mTOR pathway and protein synthesis in skeletal muscle.
One caveat of the present study is that we used 4-PBA to block ER stress and UPR pathways in vivo. Although 4-PBA is one of the most commonly used inhibitors of ER stress, prolonged use can influence other metabolic pathways in addition to inhibition of ER stress. A recent study has demonstrated that 4-PBA increases the expression of glucose transporter-4 through inhibiting histone deacetylase 5, leading to increased glucose metabolism in cultured C2C12 myotubes (69). Certainly, further investigation is needed to determine whether 4-PBA causes muscle wasting in mice through perturbation of other pathways in addition to inhibition of ER stress.
In summary, our results suggest that ER stress-induced UPR is essential for maintenance of skeletal muscle mass and strength in both naïve and tumor-bearing mice. Although the exact roles remain enigmatic, it is possible that different arms of the UPR provide different signaling outcomes in skeletal muscle. Moreover, different parts of UPR may play distinct roles in naïve and catabolic states, including cancer cachexia. It is also possible that an alteration in a specific arm of UPR would be beneficial to the muscle, even in catabolic states. Future research using genetic mouse models will tease out whether distinct mechanisms are used by different arms of UPR to regulate skeletal muscle mass in the conditions of muscle hypertrophy, atrophy, and disease. Because of the complexity of ER stress/UPR and its many targets, a better understanding of its regulatory role is of significant clinical importance for developing new therapeutic strategies for treatment of various skeletal muscle disorders.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR059810 and AR068313 and National Institute on Aging Grant AG029623 (to A.K.). Author contributions: K. R. Bohnert, S. M. Hindi, and A. Kumar designed research and analyzed data; K. R. Bohnert, Y. S. Gallot, S. Sato, G. Xiong, and S. M. Hindi performed research; and K. R. Bohnert and A. Kumar wrote the manuscript. The authors report no conflicts of interest.
Glossary
- 4-PBA
4-phenylbutyrate
- ALS
autophagy-lysosome system
- ATF
activating transcription factor
- BSA
bovine serum albumin
- BiP
immunoglobulin heavy-chain binding protein
- CHOP
C/EBP homologous protein
- CM
conditioned medium
- CSA
cross-sectional area
- eIF
eukaryotic translation initiation factor
- ER
endoplasmic reticulum
- GA
gastrocnemius muscle
- GADD34
growth arrest and DNA damage-inducible protein
- GRP78
glucose-regulating protein 78
- H&E
hematoxylin and eosin
- IRE1
inositol-requiring protein 1
- LC3B
light chain 3B
- LLC
Lewis lung carcinoma
- mTOR
mammalian target of rapamycin
- MyHC
myosin heavy chain
- PERK
RNA-dependent protein kinase-like ER eukaryotic translation initiation factor 2 α kinase
- rpS6
ribosomal protein S6
- qRT-PCR
quantitative RT-PCR
- sXBP
spliced X-box binding protein
- Sunset
surface sensing of translation
- TA
tibialis anterior muscle
- UPR
unfolding protein response
- UPS
ubiquitin–proteasome system
- XBP
X-box binding protein
REFERENCES
- 1.Fearon K., Strasser F., Anker S. D., Bosaeus I., Bruera E., Fainsinger R. L., Jatoi A., Loprinzi C., MacDonald N., Mantovani G., Davis M., Muscaritoli M., Ottery F., Radbruch L., Ravasco P., Walsh D., Wilcock A., Kaasa S., Baracos V. E. (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 [DOI] [PubMed] [Google Scholar]
- 2.Fearon K. C., Glass D. J., Guttridge D. C. (2012) Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 16, 153–166 [DOI] [PubMed] [Google Scholar]
- 3.Johns N., Stephens N. A., Fearon K. C. (2013) Muscle wasting in cancer. Int. J. Biochem. Cell Biol. 45, 2215–2229 [DOI] [PubMed] [Google Scholar]
- 4.Sandri M. (2015) Protein breakdown in cancer cachexia. [E-pub ahead of print] Semin. Cell Dev. Biol. 10.1016/j.semcdb.2015.11.002 S1084-9521(15)00248-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhang G., Jin B., Li Y. P. (2011) C/EBPβ mediates tumour-induced ubiquitin ligase atrogin1/MAFbx upregulation and muscle wasting. EMBO J. 30, 4323–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang G., Lin R. K., Kwon Y. T., Li Y. P. (2013) Signaling mechanism of tumor cell-induced up-regulation of E3 ubiquitin ligase UBR2. FASEB J. 27, 2893–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. (2004) In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15, 1101–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz A. L., Ciechanover A. (1999) The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annu. Rev. Med. 50, 57–74 [DOI] [PubMed] [Google Scholar]
- 9.Sandri M. (2010) Autophagy in skeletal muscle. FEBS Lett. 584, 1411–1416 [DOI] [PubMed] [Google Scholar]
- 10.Penna F., Costamagna D., Pin F., Camperi A., Fanzani A., Chiarpotto E. M., Cavallini G., Bonelli G., Baccino F. M., Costelli P. (2013) Autophagic degradation contributes to muscle wasting in cancer cachexia. Am. J. Pathol. 182, 1367–1378 [DOI] [PubMed] [Google Scholar]
- 11.Cai D., Frantz J. D., Tawa N. E. Jr., Melendez P. A., Oh B. C., Lidov H. G., Hasselgren P. O., Frontera W. R., Lee J., Glass D. J., Shoelson S. E. (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119, 285–298 [DOI] [PubMed] [Google Scholar]
- 12.Cornwell E. W., Mirbod A., Wu C. L., Kandarian S. C., Jackman R. W. (2014) C26 cancer-induced muscle wasting is IKKβ-dependent and NF-kappaB-independent. PLoS One 9, e87776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Judge S. M., Wu C. L., Beharry A. W., Roberts B. M., Ferreira L. F., Kandarian S. C., Judge A. R. (2014) Genome-wide identification of FoxO-dependent gene networks in skeletal muscle during C26 cancer cachexia. BMC Cancer 14, 997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egerman M. A., Glass D. J. (2014) Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 49, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian M., Nishijima Y., Asp M. L., Stout M. B., Reiser P. J., Belury M. A. (2010) Cardiac alterations in cancer-induced cachexia in mice. Int. J. Oncol. 37, 347–353 [DOI] [PubMed] [Google Scholar]
- 16.Yu A. P., Pei X. M., Sin T. K., Yip S. P., Yung B. Y., Chan L. W., Wong C. S., Siu P. M. (2014) Acylated and unacylated ghrelin inhibit doxorubicin-induced apoptosis in skeletal muscle. Acta Physiol. (Oxf.) 211, 201–213 [DOI] [PubMed] [Google Scholar]
- 17.Roxburgh C. S., McMillan D. C. (2010) Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol. 6, 149–163 [DOI] [PubMed] [Google Scholar]
- 18.Seto D. N., Kandarian S. C., Jackman R. W. (2015) A key role for leukemia inhibitory factor in C26 cancer cachexia. J. Biol. Chem. 290, 19976–19986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M., Kaufman R. J. (2014) The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 14, 581–597 [DOI] [PubMed] [Google Scholar]
- 20.Wu J., Kaufman R. J. (2006) From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 13, 374–384 [DOI] [PubMed] [Google Scholar]
- 21.Hetz C. (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 [DOI] [PubMed] [Google Scholar]
- 22.Harding H. P., Zhang Y., Ron D. (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 [DOI] [PubMed] [Google Scholar]
- 23.Ma Y., Brewer J. W., Diehl J. A., Hendershot L. M. (2002) Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J. Mol. Biol. 318, 1351–1365 [DOI] [PubMed] [Google Scholar]
- 24.Ron D., Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 25.Flamment M., Hajduch E., Ferré P., Foufelle F. (2012) New insights into ER stress-induced insulin resistance. Trends Endocrinol. Metab. 23, 381–390 [DOI] [PubMed] [Google Scholar]
- 26.Tirasophon W., Welihinda A. A., Kaufman R. J. (1998) A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 12, 1812–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haze K., Yoshida H., Yanagi H., Yura T., Mori K. (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakanishi K., Sudo T., Morishima N. (2005) Endoplasmic reticulum stress signaling transmitted by ATF6 mediates apoptosis during muscle development. J. Cell Biol. 169, 555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakanishi K., Dohmae N., Morishima N. (2007) Endoplasmic reticulum stress increases myofiber formation in vitro. FASEB J. 21, 2994–3003 [DOI] [PubMed] [Google Scholar]
- 30.Wu J., Ruas J. L., Estall J. L., Rasbach K. A., Choi J. H., Ye L., Boström P., Tyra H. M., Crawford R. W., Campbell K. P., Rutkowski D. T., Kaufman R. J., Spiegelman B. M. (2011) The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α/ATF6α complex. Cell Metab. 13, 160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koh H. J., Toyoda T., Didesch M. M., Lee M. Y., Sleeman M. W., Kulkarni R. N., Musi N., Hirshman M. F., Goodyear L. J. (2013) Tribbles 3 mediates endoplasmic reticulum stress-induced insulin resistance in skeletal muscle. Nat. Commun. 4, 1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R. O., Görgün C. Z., Hotamisligil G. S. (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cnop M., Foufelle F., Velloso L. A. (2012) Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 18, 59–68 [DOI] [PubMed] [Google Scholar]
- 34.Ikezoe K., Nakamori M., Furuya H., Arahata H., Kanemoto S., Kimura T., Imaizumi K., Takahashi M. P., Sakoda S., Fujii N., Kira J. (2007) Endoplasmic reticulum stress in myotonic dystrophy type 1 muscle. Acta Neuropathol. 114, 527–535 [DOI] [PubMed] [Google Scholar]
- 35.Nagaraju K., Casciola-Rosen L., Lundberg I., Rawat R., Cutting S., Thapliyal R., Chang J., Dwivedi S., Mitsak M., Chen Y. W., Plotz P., Rosen A., Hoffman E., Raben N. (2005) Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 52, 1824–1835 [DOI] [PubMed] [Google Scholar]
- 36.Vattemi G., Engel W. K., McFerrin J., Askanas V. (2004) Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am. J. Pathol. 164, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuga A., Ohsawa Y., Okada T., Kanda F., Kanagawa M., Toda T., Sunada Y. (2011) Endoplasmic reticulum stress response in P104L mutant caveolin-3 transgenic mice. Hum. Mol. Genet. 20, 2975–2983 [DOI] [PubMed] [Google Scholar]
- 38.Chalil S., Pierre N., Bakker A. D., Manders R. J., Pletsers A., Francaux M., Klein-Nulend J., Jaspers R. T., Deldicque L. (2015) Aging related ER stress is not responsible for anabolic resistance in mouse skeletal muscle. Biochem. Biophys. Res. Commun. 468, 702–707 [DOI] [PubMed] [Google Scholar]
- 39.Paul P. K., Bhatnagar S., Mishra V., Srivastava S., Darnay B. G., Choi Y., Kumar A. (2012) The E3 ubiquitin ligase TRAF6 intercedes in starvation-induced skeletal muscle atrophy through multiple mechanisms. Mol. Cell. Biol. 32, 1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zode G. S., Kuehn M. H., Nishimura D. Y., Searby C. C., Mohan K., Grozdanic S. D., Bugge K., Anderson M. G., Clark A. F., Stone E. M., Sheffield V. C. (2015) Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J. Clin. Invest. 125, 3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paul P. K., Gupta S. K., Bhatnagar S., Panguluri S. K., Darnay B. G., Choi Y., Kumar A. (2010) Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J. Cell Biol. 191, 1395–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hindi S. M., Mishra V., Bhatnagar S., Tajrishi M. M., Ogura Y., Yan Z., Burkly L. C., Zheng T. S., Kumar A. (2014) Regulatory circuitry of TWEAK-Fn14 system and PGC-1α in skeletal muscle atrophy program. FASEB J. 28, 1398–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman C. A., Hornberger T. A. (2013) Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques? Exerc. Sport Sci. Rev. 41, 107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mittal A., Bhatnagar S., Kumar A., Lach-Trifilieff E., Wauters S., Li H., Makonchuk D. Y., Glass D. J., Kumar A. (2010) The TWEAK-Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J. Cell Biol. 188, 833–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato S., Ogura Y., Tajrishi M. M., Kumar A. (2015) Elevated levels of TWEAK in skeletal muscle promote visceral obesity, insulin resistance, and metabolic dysfunction. FASEB J. 29, 988–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baltgalvis K. A., Berger F. G., Pena M. M., Davis J. M., Muga S. J., Carson J. A. (2008) Interleukin-6 and cachexia in ApcMin/+ mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R393–R401 [DOI] [PubMed] [Google Scholar]
- 47.Zhao J., Brault J. J., Schild A., Cao P., Sandri M., Schiaffino S., Lecker S. H., Goldberg A. L. (2007) FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 6, 472–483 [DOI] [PubMed] [Google Scholar]
- 48.Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 [DOI] [PubMed] [Google Scholar]
- 49.Acharyya S., Ladner K. J., Nelsen L. L., Damrauer J., Reiser P. J., Swoap S., Guttridge D. C. (2004) Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Invest. 114, 370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebert S. M., Monteys A. M., Fox D. K., Bongers K. S., Shields B. E., Malmberg S. E., Davidson B. L., Suneja M., Adams C. M. (2010) The transcription factor ATF4 promotes skeletal myofiber atrophy during fasting. Mol. Endocrinol. 24, 790–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Caiozzo V. J., Haddad F., Baker M. J., Herrick R. E., Prietto N., Baldwin K. M. (1996) Microgravity-induced transformations of myosin isoforms and contractile properties of skeletal muscle. J Appl Physiol. 81, 123–132 [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Pessin J. E. (2013) Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 16, 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diffee G. M., Kalfas K., Al-Majid S., McCarthy D. O. (2002) Altered expression of skeletal muscle myosin isoforms in cancer cachexia. Am. J. Physiol. Cell Physiol. 283, C1376–C1382 [DOI] [PubMed] [Google Scholar]
- 54.Bodine S. C., Latres E., Baumhueter S., Lai V. K., Nunez L., Clarke B. A., Poueymirou W. T., Panaro F. J., Na E., Dharmarajan K., Pan Z. Q., Valenzuela D. M., DeChiara T. M., Stitt T. N., Yancopoulos G. D., Glass D. J. (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708 [DOI] [PubMed] [Google Scholar]
- 55.Sartori R., Schirwis E., Blaauw B., Bortolanza S., Zhao J., Enzo E., Stantzou A., Mouisel E., Toniolo L., Ferry A., Stricker S., Goldberg A. L., Dupont S., Piccolo S., Amthor H., Sandri M. (2013) BMP signaling controls muscle mass. Nat. Genet. 45, 1309–1318 [DOI] [PubMed] [Google Scholar]
- 56.Gao S., Carson J. A. (2016) Lewis lung carcinoma regulation of mechanical stretch-induced protein synthesis in cultured myotubes. Am. J. Physiol. Cell Physiol. 310, C66–C79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rayavarapu S., Coley W., Nagaraju K. (2012) Endoplasmic reticulum stress in skeletal muscle homeostasis and disease. Curr. Rheumatol. Rep. 14, 238–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang P., McGrath B., Li S., Frank A., Zambito F., Reinert J., Gannon M., Ma K., McNaughton K., Cavener D. R. (2002) The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 22, 3864–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J., Rutkowski D. T., Dubois M., Swathirajan J., Saunders T., Wang J., Song B., Yau G. D., Kaufman R. J. (2007) ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 13, 351–364 [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto K., Sato T., Matsui T., Sato M., Okada T., Yoshida H., Harada A., Mori K. (2007) Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev. Cell 13, 365–376 [DOI] [PubMed] [Google Scholar]
- 61.Hunter R. B., Mitchell-Felton H., Essig D. A., Kandarian S. C. (2001) Expression of endoplasmic reticulum stress proteins during skeletal muscle disuse atrophy. Am. J. Physiol. Cell Physiol. 281, C1285–C1290 [DOI] [PubMed] [Google Scholar]
- 62.Iwasa K., Nambu Y., Motozaki Y., Furukawa Y., Yoshikawa H., Yamada M. (2014) Increased skeletal muscle expression of the endoplasmic reticulum chaperone GRP78 in patients with myasthenia gravis. J. Neuroimmunol. 273, 72–76 [DOI] [PubMed] [Google Scholar]
- 63.Salvadó L., Coll T., Gómez-Foix A. M., Salmerón E., Barroso E., Palomer X., Vázquez-Carrera M. (2013) Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 56, 1372–1382 [DOI] [PubMed] [Google Scholar]
- 64.Gomes A. V., Waddell D. S., Siu R., Stein M., Dewey S., Furlow J. D., Bodine S. C. (2012) Upregulation of proteasome activity in muscle RING finger 1-null mice following denervation. FASEB J. 26, 2986–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hwee D. T., Baehr L. M., Philp A., Baar K., Bodine S. C. (2014) Maintenance of muscle mass and load-induced growth in Muscle RING Finger 1 null mice with age. Aging Cell 13, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bodine S. C., Stitt T. N., Gonzalez M., Kline W. O., Stover G. L., Bauerlein R., Zlotchenko E., Scrimgeour A., Lawrence J. C., Glass D. J., Yancopoulos G. D. (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3, 1014–1019 [DOI] [PubMed] [Google Scholar]
- 67.Mounier R., Théret M., Lantier L., Foretz M., Viollet B. (2015) Expanding roles for AMPK in skeletal muscle plasticity. Trends Endocrinol. Metab. 26, 275–286 [DOI] [PubMed] [Google Scholar]
- 68.Sandri M. (2008) Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23, 160–170 [DOI] [PubMed] [Google Scholar]
- 69.Hu H., Li L., Wang C., He H., Mao K., Ma X., Shi R., Oh Y., Zhang F., Lu Y., Wu Q., Gu N. (2014) 4-Phenylbutyric acid increases GLUT4 gene expression through suppression of HDAC5 but not endoplasmic reticulum stress. Cell. Physiol. Biochem. 33, 1899–1910 [DOI] [PubMed] [Google Scholar]