Abstract
In humans, insulin sensitivity varies according to time of day, with decreased values in the evening and at night. Mechanisms responsible for the diurnal variation in insulin sensitivity are unclear. We investigated whether human adipose tissue (AT) expresses intrinsic circadian rhythms in insulin sensitivity that could contribute to this phenomenon. Subcutaneous and visceral AT biopsies were obtained from extremely obese participants (body mass index, 41.8 ± 6.3 kg/m2; 46 ± 11 y) during gastric-bypass surgery. To assess the rhythm in insulin signaling, AKT phosphorylation was determined every 4 h over 24 h in vitro in response to different insulin concentrations (0, 1, 10, and 100 nM). Data revealed that subcutaneous AT exhibited robust circadian rhythms in insulin signaling (P < 0.00001). Insulin sensitivity reached its maximum (acrophase) around noon, being 54% higher than during midnight (P = 0.009). The amplitude of the rhythm was positively correlated with in vivo sleep duration (r = 0.53; P = 0.023) and negatively correlated with in vivo bedtime (r = −0.54; P = 0.020). No circadian rhythms were detected in visceral AT (P = 0.643). Here, we demonstrate the relevance of the time of the day for how sensitive AT is to the effects of insulin. Subcutaneous AT shows an endogenous circadian rhythm in insulin sensitivity that could provide an underlying mechanism for the daily rhythm in systemic insulin sensitivity.—Carrasco-Benso, M. P., Rivero-Gutierrez, B., Lopez-Minguez, J., Anzola, A., Diez-Noguera, A., Madrid, J. A., Lujan, J. A., Martínez-Augustin, O., Scheer, F. A. J. L., Garaulet, M. Human adipose tissue expresses intrinsic circadian rhythm in insulin sensitivity.
Keywords: obesity, glucose tolerance, diabetes
In humans, glucose tolerance varies with time of day (1). Plasma glucose excursions in response to meals, oral glucose, and intravenous glucose are markedly higher in the evening and night than in the morning (2). Diminished insulin sensitivity and decreased insulin secretion both contribute to reduced glucose tolerance later in the day (3); however, mechanisms responsible for this diurnal variation in glucose homeostasis are not clear. Moreover, to determine the existence of an endogenous circadian pattern, care must be taken to minimize confounding effects of variability in meal size and composition, degree of physical activity, sleep patterns, and other major factors that influence glucose tolerance (4).
Another important question that arises from these studies is to what extent these daily fluctuations in glucose tolerance/insulin sensitivity are caused by the endogenous circadian system vs. caused by the behavioral cycle. Most studies performed in humans are not designed to test for the existence of an endogenous circadian pattern in insulin secretion or action, independent of daily rhythms in behaviors and in the environment. Endogenous circadian rhythmicity in humans is generated by the suprachiasmatic nucleus (SCN; the central clock) and peripheral oscillators in virtually all organs and cells in our body. Although these peripheral tissues have intrinsic clocks, there is only limited information about their physiologic functions (5, 6). Studies performed in experimental animals have shown that an intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis (7). In mice, the liver clock also contributes to glucose homeostasis by driving a daily rhythm of hepatic glucose production that counterbalances the daily cycle of nonfeeding and feeding (5). In humans, however, very little is known about the contribution of the peripheral clock in adipose tissue (AT) to insulin sensitivity. In humans, AT is one of the largest organs in the body and accounts for 5 to >50% of bodyweight in lean and extremely obese individuals, respectively. AT, besides its function as mechanical and thermal insulation of vital organs and as an important long-term energy store, is a metabolically active tissue and a key player in regulation of glucose metabolism (8). We previously demonstrated in ex vivo human AT explants the existence of a peripheral circadian clock that can function independently of SCN control (9); however, whether this has significance for AT function, specifically insulin sensitivity, is unknown.
Thus, the aim of the current study was to determine whether human AT exhibits intrinsic circadian rhythms in insulin sensitivity. For this purpose, we stimulated primary explants of both subcutaneous and visceral AT that were obtained from 18 extremely obese participants with several insulin concentrations (0, 1, 10, 100 nM) at different times during the day and night (every 4 h, totaling ∼1000 explants). The experimental design enabled the determination of the effects of the peripheral clocks in AT in the absence of influence from SCN and from other confounding factors, such as circulating levels of nutrients (e.g., glucose, fatty acids, and triglycerides), the autonomic nervous system, and hormones (e.g., insulin and glucocorticoids).
MATERIALS AND METHODS
Study protocol
Protocols were approved by the Institutional Review Board of Virgen de la Arrixaca University Hospital (Murcia, Spain), and participants provided written informed consent before biopsies were obtained.
Participants
Subcutaneous and visceral abdominal AT biopsies were obtained from extremely obese individuals (9 men and 9 women), age 46 ± 11 yr (mean ± sd) and with a body mass index (BMI) of 41.8 ± 6.3 kg/m2, who underwent laparoscopic gastric bypass surgery at the General Surgery Service of Virgen de la Arrixaca Hospital. The study population included diabetic (n = 9) and nondiabetic participants (n = 9). Diabetic participants were chronically treated with one or more antidiabetic drugs, including on the day of surgery: 3 were treated with injected glp-1 (liraglutide) and 8 with oral antidiabetics (metformin, repaglinide, linagliptin, or glicazide) to maintain near-normal glycemia. Other medications for both diabetic and nondiabetic participants included statins (2 participants), antihypertensive drugs (9 participants), antianxiety medications (3 participants), and anti-inflammatory drugs (1 participant). Characteristics of the population are represented in Table 1. The day before surgery, all participants had lunch at 14:30 and dinner at 21:00 h (consistent with average meal times in Spain) to standardize habitual synchronization of peripheral clocks, after which participants fasted until surgery the next day, which finished between approximately 12:00 and 13:00 h for all participants. AT biopsies were obtained from the 2 AT depots (subcutaneous and visceral) at the end of the surgical procedure. Anthropometry, meal timing, metabolic syndrome (MetS) measures, and sleep characteristics were assessed.
TABLE 1.
General participant characteristics
| Characteristic | Value | |
|---|---|---|
| Age (yr) | 46 ± 11 | |
| BMI (kg/m2) | 42 ± 6 | |
| Anthropometry | ||
| Bicipital skinfold (cm) | 31.73 ± 8.24 | |
| Tricipital skinfold (cm) | 40.5 ± 5.81 | |
| Subscapular skinfold (cm) | 38.19 ± 6.8 | |
| Suprailliac skinfold (cm) | 36.94 ± 6.08 | |
| Total body fat, skinfolds (%) | 33.27 ± 2 | |
| Total body fat, impedance (%) | 41.06 ± 8.87 | |
| Hip circumference (cm) | 130.53 ± 13.81 | |
| Thigh circumference (cm) | 74.94 ± 10.52 | |
| WHR | 0.98 ± 0.11 | |
| WTR | 1.73 ± 0.28 | |
| Abdominal sagittal diameter (cm) | 42.29 ± 6.28 | |
| Abdominal coronal diameter (cm) | 46.06 ± 4.94 | |
| MetS | ||
| Waist circumference (cm) | 126.57 ± 15.32 | |
| Triglycerides (mM) | 1.51 ± 0.82 | |
| Glucose (mM) | 6.43 ± 1.87 | |
| HDL-c (mM) | 1.26 ± 0.46 | |
| Diastolic blood pressure (mmHg) | 82.78 ± 6.18 | |
| Systolic blood pressure (mmHg) | 138.22 ± 15.16 | |
| MetS score | 3.18 ± 1.38 | |
| Other | ||
| Total cholesterol (mM) | 4.23 ± 1.24 | |
| AST (IU/L) | 19 ± 8.65 | |
| ALT (IU/L) | 29 ± 16.91 | |
| Sleep | ||
| Sleep onset (hh:mm) | 0:29 ± 1:05 | |
| Sleep duration (h) | 7.15 ± 1.44 | |
| Meal timing | ||
| Breakfast onset (hh:mm) | 8:51 ± 1:03 | |
| Lunch onset (hh:mm) | 14:40 ± 0:36 | |
| Dinner onset (hh:mm) | 21:43 ± 0:49 |
Data are represented as means ± sd of total population (n = 18). ALT, alanine transaminase (also know as glutamic-pyruvate transaminase); AST, aspartate transaminase (also known as glutamic-oxaloacetic transaminase); WHR, waist-to-hip ratio; WTR, waist-to-thigh ratio.
Anthropometry and MetS characteristics
Weight was determined in participants, who wore light clothes and were without shoes, using a digital electronic weighing scale. Height was determined using a Harpenden digital stadiometer (range, 0.70–2.05 m; Holtain Ltd., Crymych, United Kingdom) with the participant standing and the head in the Frankfurt plane. From these data, BMI was calculated according to the formula, weight/height2 (kg/m2). Total body fat (percent) was measured by bioimpedance with a Tanita Model TBF-300 (Tanita Corporation of America, Arlington Heights, IL, USA). The following skinfolds were measured: biceps, triceps, suprailiac, and subscapular. A Harpenden caliper (Holtain) with a constant pressure of 10 g/mm2 was used. Body fat distribution was evaluated by waist circumference at the umbilicus level and hip circumference at the level of the widest circumference over the great trochanters (10). Oblique thigh circumference was also measured. Waist-to-hip ratio and waist-to-thigh ratio were calculated from these measurements. All measurements were obtained 3 times by the same anthropometrist on the right side, with the participant upright and relaxed. Sagittal diameter and coronal diameter were measured at the level of the iliac crest (L4–5) by using a Holtain Kahn abdominal caliper. Fasting plasma concentrations of triacylglycerides, total cholesterol, HDL cholesterol, LDL cholesterol, aspartate transaminase, and alanine transaminase were determined with commercial kits (Roche Diagnostics, Mannheim, Germany). Arterial pressure was measured with a mercury sphygmomanometer while seated for at least 10 min. For each participant, MetS score was calculated as the number of components of MetS on the basis of thresholds for waist circumference, fasting glucose, triacylglycerides, HDL-c, and systolic or diastolic blood pressure, with a maximum value of 5 points (11).
Sleep characteristics and meal timing
The same interviewer asked all participants for their usual sleep habits and food schedule. Questions included habitual time to bed, number of awakenings during the night, time of sleep offset, sleep duration, and habitual time at which each of the 3 main meals of the day (breakfast, lunch, and dinner) were started.
Insulin-signaling assay
Insulin signaling in human AT was assessed in vitro over 24 h and in response to several insulin concentrations (0, 1, 10, and 100 nM). In each participant, 1 large adipose sample for each depot (subcutaneous and visceral) was equally divided into 6 parts, one part for each of the 6 time points at the following circadian times (CTs): CT0 (wake time), CT4, CT8, CT12, CT16, CT20. Assessment of insulin signaling in human subcutaneous AT from a representative individual over 24 h and in response to different insulin concentrations in vitro is represented in Supplemental Fig. 1. pAKT and tAKT is shown in response to 4 insulin concentrations (0, 1, 10, and 100 nM) during the 24-h day. Densitometric quantification of the dose–response relationship of the pAKT-to-tAKT ratio in response to insulin stimulation at 1 representative time point (08:00 h) is also shown.
A seventh time point was also studied (CT24) for a subsample of 15 participants to determine whether insulin sensitivity was affected by in vitro incubation duration, independent of circadian phase (comparing CT0 with CT24). We found a similar circadian pattern (Supplemental Fig. 2) and no significant differences between CT0 and CT24 (P = 0.49), which indicated that insulin sensitivity was not significantly affected by in vitro incubation duration.
Each of these biopsy parts was then further divided into 4 subparts, one subpart for each of the 4 insulin concentrations per time point (6 time points), which resulted in 24 explants per depot (subcutaneous and visceral), 48 in total per participant (∼864 explants across all participants). Different explants were cultured for different durations, increased by 4-h intervals, before each insulin-dosing experiment (10 min of treatment with insulin for each concentration). This resulted in insulin sensitivity estimates with 4 insulin concentrations at each of the 6 times of the day at the following CTs: CT0 (08:00 h), CT4 (12:00 h), CT8 (16:00 h), CT12 (20:00 h), CT16 (00:00 h), and CT20 h (04:00 h). CT0 was defined as 08:00 h, which was the approximate average habitual wake time of the participants. Phosphorylation of AKT was determined by dividing the density of the pAKT value by the tAKT value (loading control) as determined by Western blot. Insulin sensitivity was defined as the relative change in AKT phosphorylation in the presence of insulin compared with the absence of insulin stimulation. An increase in the pAKT-to-tAKT ratio in the presence of insulin compared with the absence of insulin indicates a greater cellular response for a given dose of insulin.
To compare results between diabetic and nondiabetic participants, we ran 1 sample from the same CT (CT4, selected because this was the acrophase), both unstimulated and after stimulation with insulin (10 nM) from each participant in the same gel.
Culture conditions
Immediately after surgery, biopsies were placed in medium and within 0.5 h were placed in culture dishes at 37°C for 24 h in a humidified atmosphere that contained 7% CO2. Explants of ∼1500 mg were cut into pieces of 1–2 mm3 to enhance the contact of AT with medium and were placed in 2.5 ml DMEM that was supplemented with 10% fetal bovine serum (Thermo Fisher Scientific Life Sciences, Waltham, MA, USA), glucose (4.5 g/L), and a mixture of penicillin-streptomycin-glutamine (Thermo Fisher Scientific Life Sciences).
On the next day at 08:00 h, before the start of the experiment, medium was changed with DMEM that was supplemented with 1 g/L glucose and a mixture of penicillin-streptomycin-glutamine (Thermo Fisher Scientific Life Sciences), but without fetal bovine serum. Every 4 h, different explants were treated with the 4 different concentrations of insulin (Supplemental Fig. 1). After 10 min of treatment with insulin, AT explants were collected in cryotubes and frozen at −80°C for later analysis of the pAKT-to-tAKT ratio by Western blot.
Western blot
Explants were homogenized in RIPA buffer (0.1% SDS, 0.1% sodium deoxycholate, and 1% Triton X-100 in PBS) with protease and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO, USA; and Santa Cruz Biotechnology, Dallas, TX, USA, respectively). Homogenates were sonicated and centrifuged (20 min, 4°C, 7000 g). Protein concentration was measured in supernatants by using bicinchoninic acid assay; Laemmli sample buffer (Bio-Rad, Hercules, CA, USA) was then added, and samples were heated at 95°C for 5 min. Samples were separated by SDS-PAGE and transferred to nitrocellulose membranes that were then blocked in TBST (Tris-buffered saline that contained 0.1% Tween and 5% nonfat dried milk) and immunoblotted by using a 1:2000 dilution of Ser473 rabbit monoclonal anti-pAKT antibody (Cell Signaling Technology, Danvers, MA, USA). After washing the membranes, they were incubated with horseradish peroxide–conjugated goat anti-rabbit antibody (Sigma-Aldrich) in TBST. Antibody binding was visualized by using the Pierce chemiluminescent Western blotting detection system (PerkinElmer, Waltham, MA, USA). Membranes were blocked and washed with TBST. Finally, they were reprobed with 1:1000 dilution of rabbit polyclonal anti-tAKT antibody (Cell Signaling Technology).
Supplemental Fig. 3 shows assessment of protein quantity by using increasing amounts of human AT lysates. Western blot signals are shown to be linear until ∼50 µg, after which tAKT reaches a plateau, wheras pAKT increases without sign of saturation. Thus, to remain within the linear section of the quantity–response curve for both pAKT and tAKT, all experiments were run with 30 µg total protein.
Densitometry
Densitometry was performed for immunoblots by using ImageJ, version 1.44 (NIH, Bethesda, MD, USA) to quantify densities of pAKT and tAKT bands. Total AKT levels were used as a protein-loading control to normalize for any variation in protein content between samples. Because there were too many samples for each tissue and each individual to be run in the same gel, and to normalize values of samples run in different blots, a control sample of the same tissue and individual was run in each gel.
Statistics
Statistical differences in general characteristics between diabetic and nondiabetic participants were analyzed by Student’s t test (data not shown). Because there were no significant differences between diabetic and nondiabetic participants for any subject characteristic, nor for AT insulin sensitivity (P = 0.786; Supplemental Fig. 4), all participants were analyzed together.
To investigate the presence of a circadian rhythm in AT insulin sensitivity, a least squares periodic regression (12) was used to fit a sinusoidal function to the data (T = 24 h). Differences in insulin sensitivity between noon and midnight were analyzed with 2-tailed paired Student’s t test.
Moreover, to test whether there was a real circadian modulation or a linear trend in insulin sensitivity during the 24-h period, we performed a linear regression model on the normalized pAKT-to-tAKT ratio using all 7 data points, including 3 fixed factors: 2 fixed factors to fit the 24-h rhythmicity, cosine and sine, which together determine the phase and amplitude of the fit, and 1 fixed factor to fit any linear change (JMP Pro 12, SAS software; SAS Institute, Cary NC, USA).
Additional Pearson’s correlation analyses were performed to assess the potential associations between rhythm characteristics and age, degree of obesity (BMI), abdominal obesity, MetS components, timing of sleep and food intake, and sleep duration. All statistical analyses were carried out by using IBM SPSS Statistics for Windows (version 20.0; Armonk, NY, USA). Level of significance for all statistical tests and hypotheses was set at P < 0.050.
RESULTS
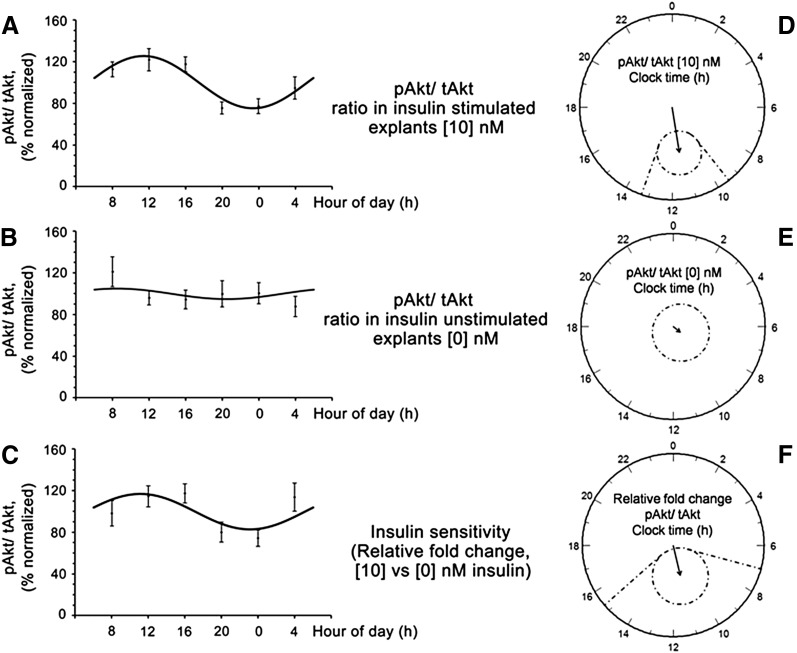
AT explants from the subcutaneous depot exhibited robust circadian rhythms in insulin signaling both at 10 nM (P < 0.001) and at 100 nM (P < 0.010; Fig. 1). Further statistical analyses, simultaneously fitting a 24-h cosine curve and a linear component in a single model using the 7 time points, demonstrated that a 24-h periodic fit significantly accounted for data variability (P = 0.029), whereas no significant linear change over 24 h was found (P = 0.40) demonstrating a true circadian modulation.
Figure 1.
Changes in insulin signaling (pAKT/tAKT) across the day. Subcutaneous AT in stimulated (10 nM) (A) and unstimulated (0 nM) explants (B), and in the insulin sensitivity pattern (relative percentage of change in AKT phosphorylation in insulin-stimulated explants with respect to the unstimulated explants) (C), and their respective polar (clock-like) representations (n = 18) (D–F). Raw data are represented by black dots; solid lines represent fitted 24-h sinusoidal curve of the population. Polar representation of the estimates of the parameters of the rhythm for 0 and 10 nM and insulin sensitivity are also shown. In polar plots, circles represent 24 h and the radius corresponds to 50% units. Vector length is amplitude of the rhythm and it points to its acrophase. Dotted ellipses show the 95% confidence limits for vectors (if the confidence limits include the center, the rhythm is not statistically significant), and straight dotted lines are the corresponding confidence intervals for the acrophases. Least squares periodic regression (12) was used to fit a sinusoidal function to the data; 10 nM (amplitude, 25%; acrophase, 11:23; P < 0.001); 0 nM (amplitude, 5%; P = 0.69); insulin sensitivity (amplitude, 17%; acrophase, 11:06; P = 0.022).
A 10-nM insulin concentration was selected for representation on the basis of the largest circadian modulation of pAKT-to-tAKT ratio at 10 nM compared with 1 and 100 nM. Of interest, insulin sensitivity in subcutaneous AT reached its maximum value (acrophase) around noon being 54% higher than that at midnight (P = 0.009; Fig. 1F); however, no circadian rhythms were detected in visceral AT (P = 0.64; Supplemental Fig. 5).
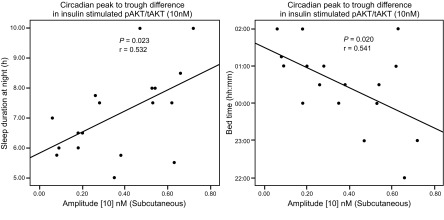
Another remarkable outcome of the current work is that the amplitude of the circadian rhythm in subcutaneous AKT phosphorylation was significantly and positively correlated with sleep duration (r = 0.53; P = 0.023), with larger amplitude relating to a longer duration (Fig. 2, left) and negatively correlated with the bedtime of the participants ( r = −0.54; P = 0.020), with larger amplitude relating to an earlier bedtime (Fig. 2, right), whereas no significant correlations were found between amplitude of the circadian rhythm and other characteristics of the participants, such as age, BMI, and abdominal obesity for both subcutaneous and visceral AT (P > 0.050).
Figure 2.
Bedtime (right), sleep duration (left), and amplitude of the rhythm. Correlations between sleep duration and bedtime of the participants and amplitude of the rhythm in insulin-stimulated pAKT/tAKT (r = 0.532, P = 0.023; and r = −0.541, P = 0.020, respectively; n = 18). (For bedtime, only 17 points are visualized because data from 2 participants overlapped.)
DISCUSSION
This is the first study, to our knowledge, to demonstrate the presence in human AT of a robust circadian rhythm that contributes to the effectiveness of a hormone on tissue function. Here, we demonstrate that insulin sensitivity in human subcutaneaous AT displays a circadian rhythm and that it reaches its maximum (acrophase) around noon with sensitivity 54% higher than during midnight.
Of interest, the amplitude of the rhythm was positively correlated with in vivo sleep duration and negatively with in vivo bedtime.
An important consideration is that this rhythm persists ex vivo for approximately 2 d after surgery and is present even when removed from the influence of the SCN, other neurohumoral factors, or variations in circulating levels of nutrients (13).
Current results in subcutaneous AT confirm previous hypotheses that the circadian clock within the adipocyte plays a significant role by altering sensitivity of the adipocyte to specific stimuli (i.e., insulin) throughout the day (13). These data may help explain in vivo results in humans in which plasma glucose excursions in response to meals, oral glucose, or intravenous glucose are markedly higher in the evening than in the morning (2–4, 14, 15), and also that insulin sensitivity is higher in the morning hours, whereas insulin action is impaired late in the evening (1, 14).
Similar to our results in AT that show minimum values of insulin sensitivity at midnight, previous experimental protocols that involved intravenous glucose infusion at a constant rate for 24–30 h have shown that circulating glucose concentrations increase as the evening progresses and reach a maximum near the middle of the night at an average clock time of ∼02:00 h (range, 00:45–03:50 h) (16).
Moreover, studies performed in conditions similar to the current experiment (AT explants in vitro and extremely obese participants) show that adiponectin, a cytokine that is exclusively produced in AT and seems to have a protective role against insulin resistance (17), reaches its maximum expression level in subcutaneous AT in the morning hours (∼10:00 h) (18, 19), which is in concordance with current results that show that insulin sensitivity reaches its maximum (acrophase) around noon. It has been shown that adiponectin is regulated by the circadian clock (20) and that its rhythm is not driven by the feeding/fasting cycle (21).
An important outcome from the current experiment is that the daily rhythm in insulin signaling seems to be intrinsic to AT and is not dependent on daily rhythms in circulating levels of nutrients. Indeed, our findings are consistent with daily rhythms in glucose tolerance that have been shown to be primarily the result of the circadian system as opposed to the behavioral cycle (15). Moreover, current data, in part, may explain the observations that eating in misalignment with the biologic clock, such as eating late at night and during shift work, is associated with increased risk for diabetes (22–24).
This intrinsic circadian rhythm in subcutaneous AT could provide an underlying mechanism to help explain the daily rhythm in systemic insulin sensitivity. This contribution may be particularly relevant for extremely obese participants with AT that accounts for >40% of bodyweight, as is the case for participants selected for the current study. Apart from AT, skeletal muscle (∼45% of total body mass), together with gastrointestinal absorption, hepatic glucose output, or non-insulin-dependent glucose metabolic pathways, among others, may also contribute the systemic insulin sensitivity (14, 15). However, the function of these molecular clocks in humans has only now started to be uncovered (6, 25).
Another remarkable outcome of the current work is that amplitude of the circadian rhythm in subcutaneous AT was positively correlated with in vivo sleep duration and negatively with in vivo bedtime. In recent years, evidence has accumulated to support a role for sleep disturbances, including insufficient sleep, poor sleep quality, and sleep apnea, as independent risk factors for the development and exacerbation of insulin resistance (26, 27). Findings from several independent experimental studies consistently show that sleep disturbances result in decreased insulin sensitivity. Of particular relevance for in vitro subcutaneous human adipocytes, sleep restriction results in an insulin-resistant state: insulin sensitivity, as assessed by phosphorylation of AKT in response to insulin, was 30% lower during sleep restriction than during normal sleep (28).
Previous results in similar experiments performed in other human peripheral oscillators—skin fibroblasts, pancreatic islets, skeletal myotubes, etc.—also show correlation between oscillator properties assessed in vitro and participants’ phenotype observed in vivo—chronotype, obesity, metabolic alterations, etc. (29, 30)—and suggest that circadian molecular oscillators are plastic: they can change their properties according to their environment (31). An alternative hypothesis is that the clock properties are actually influencing these phenotypes.
One limitation of the current experiment is that it was performed in a sample of extremely obese participants, and we cannot extrapolate it to normal-weight participants. The high number of experiments performed (a total of ∼1000 explants), however, requires a large amount of AT, and biopsies of visceral AT requires access to the peritoneal cavity, both of which are difficult to achieve in normal weight participants.
The current study underscores the potential relevance of circadian studies in primary human cells for metabolic diseases. Experiments performed in vitro are useful because, although they do not capture the multifactorial aspect of the in vivo environment, they allow analysis of oscillator function in the absence of influences from SCN and other confounding factors, and, therefore, they reflect inherent characteristics of molecular clocks (6).
In summary, we have demonstrated for the first time, to our knowledge, that insulin sensitivity in human subcutaneous AT shows an endogenous circadian rhythm, with maximum sensitivity at noon. Results may help to better understand the intricate relationships between the circadian clock, glucose metabolism, and obesity in humans.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Spanish Government of Economy and Competitiveness (Grant SAF2014-52480-R) and from European Regional Development Fund (ERDF) (to M.G.), Grant SAF2011-22812 (to O.M.-A.), the Seneca Foundation from the Government of Murcia (15123/PI/10; to M.G.), and by U.S. National Institutes of Health Grants R01-DK099512 (National Institute of Diabetes and Digestive and Kidney Diseases) and R01-HL118601 (National Heart, Lung, and Blood Institute) (to F.A.J.L.S.). Author contributions: M. P. Carrasco-Benso conducted the experiments, performed the cultures, analyzed data, and wrote the paper; B. Rivero-Gutierrez performed Western blot experiments; J. Lopez-Minguez performed the cultures; A. Anzola performed Western blot experiments; A. Diez-Noguera analyzed circadian data; J. A. Madrid gave advice for circadian aspects; J. A. Lujan recruited the study participants and performed the biopsies; O. Martínez-Augustin directed the Western blot experiments; and F. A. J. L. Scheer and M. Garaulet designed research, wrote the paper, and have primary responsibility for final content.
Glossary
- AT
adipose tissue
- BMI
body mass index
- CT
circadian time
- MetS
metabolic syndrome
- SCN
suprachiasmatic nucleus
- TBST
Tris-buffered saline that contained 0.1% Tween and 5% nonfat dried milk
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Van Cauter E., Blackman J. D., Roland D., Spire J. P., Refetoff S., Polonsky K. S. (1991) Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J. Clin. Invest. 88, 934–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll K. F., Nestel P. J. (1973) Diurnal variation in glucose tolerance and in insulin secretion in man. Diabetes 22, 333–348 [DOI] [PubMed] [Google Scholar]
- 3.Service F. J., Hall L. D., Westland R. E., O’Brien P. C., Go V. L., Haymond M. W., Rizza R. A. (1983) Effects of size, time of day and sequence of meal ingestion on carbohydrate tolerance in normal subjects. Diabetologia 25, 316–321 [DOI] [PubMed] [Google Scholar]
- 4.Saad A., Dalla Man C., Nandy D. K., Levine J. A., Bharucha A. E., Rizza R. A., Basu R., Carter R. E., Cobelli C., Kudva Y. C., Basu A. (2012) Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 61, 2691–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamia K. A., Storch K. F., Weitz C. J. (2008) Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. USA 105, 15172–15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saini C., Brown S. A., Dibner C. (2015) Human peripheral clocks: applications for studying circadian phenotypes in physiology and pathophysiology. Front. Neurol. 6, 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadacca L. A., Lamia K. A., deLemos A. S., Blum B., Weitz C. J. (2011) An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia 54, 120–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Spek R., Kreier F., Fliers E., Kalsbeek A. (2012) Circadian rhythms in white adipose tissue. Prog. Brain Res. 199, 183–201 [DOI] [PubMed] [Google Scholar]
- 9.Gómez-Santos C., Gómez-Abellán P., Madrid J. A., Hernández-Morante J. J., Lujan J. A., Ordovas J. M., Garaulet M. (2009) Circadian rhythm of clock genes in human adipose explants. Obesity (Silver Spring) 17, 1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garaulet M., Hernández-Morante J. J., Tébar F. J., Zamora S. (2006) Anthropometric indexes for visceral fat estimation in overweight/obese women attending to age and menopausal status. J. Physiol. Biochem. 62, 245–252 [DOI] [PubMed] [Google Scholar]
- 11.Corbalán-Tutau D., Madrid J. A., Nicolás F., Garaulet M. (2014) Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiol. Behav. 123, 231–235 [DOI] [PubMed] [Google Scholar]
- 12.Batschelet E., Batschelet E. (1981) Circular Statistics in Biology, Vol. 111, Academic Press, London [Google Scholar]
- 13.Bray M. S., Young M. E. (2007) Circadian rhythms in the development of obesity: potential role for the circadian clock within the adipocyte. Obes. Rev. 8, 169–181 [DOI] [PubMed] [Google Scholar]
- 14.Yoshino J., Almeda-Valdes P., Patterson B. W., Okunade A. L., Imai S., Mittendorfer B., Klein S. (2014) Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole-body and cellular fatty acid metabolism in metabolically normal women. J. Clin. Endocrinol. Metab. 99, E1666–E1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris C. J., Yang J. N., Garcia J. I., Myers S., Bozzi I., Wang W., Buxton O. M., Shea S. A., Scheer F. A. (2015) Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc. Natl. Acad. Sci. USA 112, E2225–E2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro E. T., Tillil H., Polonsky K. S., Fang V. S., Rubenstein A. H., Van Cauter E. (1988) Oscillations in insulin secretion during constant glucose infusion in normal man: relationship to changes in plasma glucose. J. Clin. Endocrinol. Metab. 67, 307–314 [DOI] [PubMed] [Google Scholar]
- 17.Garaulet M., Hernández-Morante J. J., de Heredia F. P., Tébar F. J. (2007) Adiponectin, the controversial hormone. Public Health Nutr. 10(10A), 1145–1150 [DOI] [PubMed] [Google Scholar]
- 18.Garaulet M., Ordovás J. M., Gómez-Abellán P., Martínez J. A., Madrid J. A. (2011) An approximation to the temporal order in endogenous circadian rhythms of genes implicated in human adipose tissue metabolism. J. Cell. Physiol. 226, 2075–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gómez-Abellán P., Gómez-Santos C., Madrid J. A., Milagro F. I., Campion J., Martínez J. A., Ordovás J. M., Garaulet M. (2010) Circadian expression of adiponectin and its receptors in human adipose tissue. Endocrinology 151, 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnea M., Chapnik N., Genzer Y., Froy O. (2015) The circadian clock machinery controls adiponectin expression. Mol. Cell. Endocrinol. 399, 284–287 [DOI] [PubMed] [Google Scholar]
- 21.Scheer F. A., Chan J. L., Fargnoli J., Chamberland J., Arampatzi K., Shea S. A., Blackburn G. L., Mantzoros C. S. (2010) Day/night variations of high-molecular-weight adiponectin and lipocalin-2 in healthy men studied under fed and fasted conditions. Diabetologia 53, 2401–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakubowicz D., Barnea M., Wainstein J., Froy O. (2013) High Caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 21, 2504–2512 [DOI] [PubMed] [Google Scholar]
- 23.Nakajima K., Suwa K. (2015) Association of hyperglycemia in a general Japanese population with late-night-dinner eating alone, but not breakfast skipping alone. J. Diabetes Metab. Disord. 14, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheer F. A., Hilton M. F., Mantzoros C. S., Shea S. A. (2009) Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 106, 4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harfmann B. D., Schroder E. A., Esser K. A. (2015) Circadian rhythms, the molecular clock, and skeletal muscle. J. Biol. Rhythms 30, 84–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Cauter E. (2011) Sleep disturbances and insulin resistance. Diabet. Med. 28, 1455–1462 [DOI] [PubMed] [Google Scholar]
- 27.Anothaisintawee T., Reutrakul S., Van Cauter E., Thakkinstian A. (2015) Sleep disturbances compared to traditional risk factors for diabetes development: systematic review and meta-analysis. Sleep Med. Rev. 30, 11–24 [DOI] [PubMed] [Google Scholar]
- 28.Broussard J. L., Ehrmann D. A., Van Cauter E., Tasali E., Brady M. J. (2012) Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann. Intern. Med. 157, 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown S. A., Kunz D., Dumas A., Westermark P. O., Vanselow K., Tilmann-Wahnschaffe A., Herzel H., Kramer A. (2008) Molecular insights into human daily behavior. Proc. Natl. Acad. Sci. USA 105, 1602–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagani L., Semenova E. A., Moriggi E., Revell V. L., Hack L. M., Lockley S. W., Arendt J., Skene D. J., Meier F., Izakovic J., Wirz-Justice A., Cajochen C., Sergeeva O. J., Cheresiz S. V., Danilenko K. V., Eckert A., Brown S. A. (2010) The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PLoS One 5, e13376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheer F. A., Wright K. P. Jr., Kronauer R. E., Czeisler C. A. (2007) Plasticity of the intrinsic period of the human circadian timing system. PLoS One 2, e721 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.