Abstract
Dopamine signaling is involved in a variety of neurobiological processes that contribute to learning and memory. D1-like dopamine receptors (including D1 and D5 receptors) are thought to be involved in memory and reward processes, but pharmacological approaches have been limited in their ability to distinguish between D1 and D5 receptors. Here, we examine the effects of a specific knockout of D1 receptors in associative learning tasks involving aversive (shock) or appetitive (cocaine) unconditioned stimuli. We find that D1 knockout mice show similar levels of cued and contextual fear conditioning to WT controls following conditioning protocols involving one, two, or four shocks. D1 knockout mice show increased generalization of fear conditioning and extinction across contexts, revealed as increased freezing to a novel context following conditioning and decreased freezing to an extinguished cue during a contextual renewal test. Further, D1 knockout mice show mild enhancements in extinction following an injection of SKF81297, a D1/D5 receptor agonist, suggesting a role for D5 receptors in extinction enhancements induced by nonspecific pharmacological agonists. Finally, although D1 knockout mice show decreased locomotion induced by cocaine, they are able to form a cocaine-induced conditioned place preference. We discuss these findings in terms of the role of dopamine D1 receptors in general learning and memory processes.
Keywords: Dopamine, D1 receptor, D5 receptor, fear, cocaine, learning, extinction
Dopamine neurons generate a wide range of firing responses that have been hypothesized to encode features that guide motivation and learning, such as reward value (Tobler et al., 2005), effort (Hamid et al., 2016), and prediction error (Schultz & Dickinson, 2000; Eshel et al., 2016). Phasic dopamine signals, which occur in response to acute stimulation via pharmacological, opto/chemogenetic, or environmental input, appear to be particularly important for learning (Steinberg et al., 2013; Tsai, et al. 2009; Waelti et al., 2001). These signals cause a release of dopamine that then binds to different classes of G protein-coupled receptors in dopamine neuron terminal regions. These receptors fall into two broad classes -- D1-like dopamine receptors (including D1 and D5) that initiate Gαs or Gαolf signaling and D2-like dopamine receptors (including D2, D3, and D4) that initiate Gαi or Gαo signaling (Beaulieu & Gainetdinov, 2011). Although a great deal is known about how dopamine acts on these receptors, identifying D1-specific effects in learning processes has been difficult because most pharmacological approaches cannot specifically distinguish between D1 and D5 receptors. D1 and D5 receptors are differentially expressed across the brain; thus, specifying the action of one particular receptor subtype would allow for greater precision in therapeutic development.
Pharmacological approaches have found that antagonists and agonists of D1/5 receptors can modulate cocaine-induced conditioned place preference (CPP; Cervo & Samanin, 1995), fear conditioning (Inoue et al., 2000), and fear extinction (Hikind & Maroun, 2008; Abraham, et al. 2016). These studies suggest that alterations in phasic signaling through D1-like receptors could have effects on fear and reward processing, but there are currently no pharmacological agents available to distinguish the contributions of D1 and D5 receptors to these behaviors. Thus, genetic approaches are required to examine the specific contribution of D1 receptors to learning (Holmes et al., 2004; Wall et al., 2011).
Previous studies have not shown a consistent effect of D1 receptor knockout (D1 KO) on fear conditioning. For example, El-Ghundi et al. (2001) demonstrated that expression of contextual fear is unimpaired on the first test day in D1 KO, but that fear remains during repeated extinction tests; that is, D1 KO prevented extinction of contextual fear. In contrast, Ortiz et al. (2010) found impaired cued fear conditioning and Fadok et al. (2009), found impaired fear-potentiated startle in D1 KO mice. There are procedural variations within these studies that could lead to the different observed outcomes, such as the use of different types of fear conditioning procedures (contextual fear, cued fear, or fear-potentiated startle) that require distinct behavioral responses and circuits. Additionally, increased novelty-induced locomotion in D1 KO mice compared to wildtype (Karlsson et al., 2008) could interfere with freezing or potentiated startle measurements. One procedural difference between Ortiz et al. (2010) and El-Ghundi et al. (2001) that could decrease expression of fear was the use of one- or two-shock conditioning protocols. When given a one-shock protocol, as in Ortiz et al. (2010), D1 KO mice showed decreased freezing, but with two footshocks, El-Ghundi et al. (2001) did not observe differences between wildtype or D1 KO mice. Thus, further work is needed to evaluate the effects of D1 KO on learning and memory processes.
In the following experiments, we characterized the behavior of D1 KO mice in fear conditioning and fear extinction to determine whether associative learning can be acquired and maintained without D1 receptors. Based on the studies described above, we tested the effect of both a one-shock and a two-shock cued fear conditioning protocol to determine whether the strength of conditioning impacts the expression of fear in D1 KO mice. The use of cued fear conditioning also allows for an examination of fear responses during CS-off and CS-on periods, which could show whether there are differences in generalized freezing that would impact the expression of fear in D1 KO mice. Additionally, we assess further contextual modulation of extinction by examining renewal of fear after extinction in D1 KO mice. To determine generality of contextual learning effects, we measured cocaine conditioned place preference (CPP) in D1 KO mice (Miner et al., 1995). Finally, we used the D1 KO mice to try to isolate whether pharmacological enhancements of extinction induced by a D1/5 agonist (SKF81297; Fiorenza et al., 2012; Rey et al., 2014; Abraham et al., 2016) occur in the absence of the D1 receptor. If they do, it would suggest that D5 receptors are sufficient to mediate these pharmacological effects. We find that contextual and cued fear learning, shock reactivity, and contextual reward learning are retained in D1 KO mice. However, D1 KO mice show decreased contextual fear renewal. We also find that there may be some contribution of D5 receptors to D1/5 agonist-mediated enhancements of fear extinction, but the long-term enhancement of extinction is likely to be modulated by D1 receptor activation.
Materials and Methods
Subjects
Wildtype (male = 19, female = 25), heterozygote (m = 23, f = 22) and D1 receptor knockout mice (m = 11, f = 21) were used in these experiments ranging from three to eight months of age. D1 knockout mice (D1 KO; Drd1aCre/Cre) were generated by insertion of Cre recombinase at the initiation codon of the Drd1a gene locus, resulting in the deletion of the Drd1a gene (Heusner et al., 2008). Mice were generated on a 129/Sv background, but backcrossed to C57BL/6 for >10 generations prior to experimental manipulations. Animals were maintained in the laboratory as heterozygote mice (D1 HET; Drd1aCre/+) and heterozygote pairs were bred to produce wildtype littermates (WT) and homozygous D1 KO mice. Genotypes were determined through polymerase chain reaction (PCR) amplification of DNA isolated from ear punches using primers targeting Drd1a.
One to three months following weaning, mice were transferred to the Oregon Health & Science University vivarium and given at least 3 weeks in the vivarium prior to experimental use. As reported previously by Fadok et al. (2009), D1 KO mice used in this study showed lower body weight compared to wildtype or heterozygotes (KO: 17–21 g; HET: 24–30 g; WT: 25–30 g), which may be related to deficits in food pellet consumption in early life. These differences in weight led to the use of a special diet for cages housing D1 KO mice (DietGel Recovery; Clear H20) until postnatal day 80 in all experiments. All D1 KO mice were placed on a regular laboratory diet (irradiated Pico Lab Rodent diet 5053) following postnatal day 80 and experiments occurred at least 2 weeks following removal of the special diet. WT and D1 HET mice (25–30 g) were fed regular laboratory diet throughout the entirety of experiments, although some wildtype and heterozygote mice had access to the diet gel in addition to standard lab diet during early life because genotypes were intermixed within home cages. D1 HET controls were run in addition to WT littermate controls to determine whether there may be some effect of reduction of D1 receptor signaling on behavior, as previously shown by El-Ghundi et al. (2001). Naïve mice were used for all fear conditioning/extinction experiments. Mice with previous fear conditioning experience were used for testing shock reactivity and cocaine conditioned place preference. Animals were housed two to five per cage and genotypes were mixed within cages. Polycarbonate cages with 1/8″ corn cob bedding on the floor and 2″ square nestlets were held in a ventilated rack (Thoren Caging Systems), and animals were given access to food and water ad libitum. Vivarium and experiment room temperatures were maintained at 22°C ± 1°C, and subjects were maintained on a 12-h light–dark cycle (lights on 0600 h–1800 h). Animals were moved from the vivarium to the experiment room 60 min before the start of an experiment, and experiments were conducted between 900 and 1700 h. All experimental procedures were approved by the OHSU Institutional Animal Care and Use Committee and were conducted in accordance with National Institutes of Health (NIH) “Principles of Laboratory Animal Care” (NIH Publication No. 86-23, revised 1985).
Drugs
Cocaine (20 mg/kg) was dissolved in saline. Drugs were administered intraperitoneally (i.p.) in a volume of 10 mL/kg. SKF 81297 (Tocris Bioscience, Bristol, UK) was dissolved in saline (0.9% NaCl) at 10 mg/kg with gentle heating.
Apparatus
Fear conditioning
Four Coulbourn Instruments (Whitehall, PA) mouse-conditioning chambers (H10-11M-TC) were used with a Plexiglass cylinder (21.5 cm in diameter and 23 cm in height) placed on the chamber floor. Scrambled shock (2 sec, 0.35 mA) was delivered to the grid floor by a computer controlled shock generator (Coulbourn H13-15). For cued conditioning, an 85 dB white noise CS was administered through a sound generator (Coulbourn A12-33). Above the Plexiglas cylinder, an automated infrared activity monitor (Coulbourn H24-61) recorded activity in Graphic State 3.01 software. Contextual fear conditioning studies were conducted in these chambers, and fear conditioning and context testing occurred in these chambers for cued fear studies (Context A). Testing for cued conditioning was conducted in a separate room in rectangular conditioning chambers (Context B; Med-Associates, St. Albans, VT). For cued fear experiments, all data (in both Context A and Context B) were hand-scored due to the lack of an automated measurement system in Context B. For contextual fear experiments, an automated activity monitor was used to assess freezing behavior in Context A. Contexts were cleaned with 95% ethanol following each session. Fear renewal was tested in Context A.
Conditioned Place Preference
Eight sound- and light-attenuating cabinets contained clear acrylic cages (30 cm X 15 cm X 15 cm) with two distinct removable floor types (grid or hole) as interchangeable halves. Position in the box (left/right side) and general activity were assessed by EthoVision XT 5 software (Noldus, Leesburg, VA) that records and analyzes the position of the center point of the mouse within the apparatus via a ceiling mounted camera.
Behavioral Procedures
One-shock cued fear conditioning
This experiment used a single trial conditioning protocol (i.e., one CS-US pairing) to determine whether fear expression in D1 KO mice would be impaired after weak fear conditioning. On Day 1, naïve subjects (n = 2 male D1 KO, 8 male D1 HET, 7 male WT, 4 female D1 KO, 4 female D1 HET, 3 female WT) received a 6.5-min exposure to Context A with a 30 second noise CS (85 dB) at 2 min co-terminating with shock (2 s, 0.35 mA; Acq). Due to the variation of animal numbers within sex and genotype, male and female subjects were pooled within genotype for this analysis. In general, low viability of the D1 KO mice disrupted balanced distribution of animal numbers throughout the experiments. On Day 2, mice received a 6-min nonreinforced exposure to Context A (Ctx Ext). On Days 3 and 4, subjects were placed in Context B for 15-min with 3-min CS presentations at 3 and 9 min (Test 1 and 2).
Two-shock cued fear conditioning, extinction, and renewal
This experiment compared D1 KO, D1 HET and WT mice on acquisition, extinction, and renewal of fear. On Day 1, naïve subjects (n = 7 male D1 KO, 8 male D1 HET, 8 male WT, 7 female D1 KO, 8 female D1 HET, 8 female WT) received a 6.5-min exposure to Context A with a 30-second noise CS (85 dB) at 2 and 4 min co-terminating with shock (2 s, 0.35 mA; Acq). On Day 2, mice received a 6-min nonreinforced exposure to Context A (Ctx Ext). This test functioned as a measure of contextual fear conditioning, and also served to reduce context-evoked freezing. On Days 3–8, mice were placed in Context B for 15-min with 3-min CS presentations at 3 and 9 min (Test 1–6). On Day 9, to measure fear renewal, mice were returned to Context A for a 15-min session with 3-min CS presentations at 3 and 9 min (Ren.).
Shock reactivity
To determine whether there were gross differences in unconditioned responses to the shock itself, this experiment examined responses at increasing intensities of shock. Animals (n= 3 male D1 KO, 5 male D1 HET, 5 male WT, 3 female D1 KO, 4 female D1 HET, 5 female WT) were given footshocks (2 s) increasing in strength (0.05 mA increments) from 0.05 mA to 0.25 mA with three deliveries of each shock. Shock levels were confirmed using a Med Associates multimeter (ENV-420). Animals received 5 s between each shock delivery. Following the third shock, animals received a 10 s interval prior to the delivery of a shock of higher strength. Mice that jumped in response to at least one of three shocks delivered (within each shock strength) were recorded as a “yes” response and classified as showing shock reactivity. The experimenter was blinded to the genotype of the animals, although knockout mice were visually identifiable based on consistently smaller body weights. The experiment was terminated at 0.25 mA (0.10 mA below conditioning strength) to prevent unnecessary stress to the animals; all animals responded consistently (with jumping) to shock starting at 0.15 mA. Shock reactivity testing occurred >4 weeks after mice had been used in fear conditioning and cocaine conditioned place preference studies.
Effect of D1/5 agonism on contextual fear extinction in D1 knockout mice
To determine whether the previously observed enhancements of fear extinction induced by a D1/D5 agonist (Abraham et al., 2016) could be attributed to pharmacological activation of D5 receptors, we tested the effect of SKF 81297 on fear extinction in D1 KO. On Day 1, naïve subjects received a 12-min exposure to the context with four unsignaled shocks (2s, 0.35 mA), delivered at 2.5, 5, 9, and 11.5 min. Groups were matched within genotypes following acquisition to ensure equal terminal freezing levels across SKF 81297 dose assignments (Acq). For female SKF-treated animals, n = 6 D1 KO, 5 D1 HET, and 6 WT. For male SKF-treated animals, n = 1 D1 KO, 4 D1 HET, and 2 WT. For female saline treated animals, n = 4 D1 KO, 5 D1 HET, 8 WT. For male saline treated animals, n = 1 D1 KO, 3 D1 HET, and 2 WT. Males and females were pooled for statistical analysis. On Day 2, mice received a 12-min nonreinforced exposure to the context (Ext). Immediately after the extinction session, mice received an injection of SKF81297 (10 mg/kg) or vehicle and were returned to the homecage. Groups were matched within genotypes as closely as possible following extinction to ensure equal levels of terminal freezing before SKF 81297 administration. On Test Day, mice received a 12-min nonreinforced exposure to the context.
Cocaine conditioned place preference in D1 knockout mice
This experiment tested whether D1 KO mice can acquire a cocaine conditioned place preference. An unbiased CPP apparatus and procedure was used (Hitchcock, Cunningham, & Lattal, 2014). Mice were first habituated (5-min pretest) to the conditioning chamber with both floors present. Animals (n = 5 male D1 KO, 7 male D1 HET, 5 male WT, 9 female D1 KO, 10 female D1 HET, 10 female WT) were assigned to counterbalanced groups that received cocaine (20 mg/kg) or saline immediately before exposure to a grid or hole floor. All subjects had received fear conditioning >4 weeks prior to conditioned place preference experiment and no bias was observed to hole or grid floors during the preconditioning test. During conditioning, animals were confined to one half of the CPP apparatus with grid or hole floor for 15-min. Mice received two pairings of pre-session cocaine (20 mg/kg i.p.) and two pairings of pre-session saline, counterbalanced for order and floor type, on alternating days. To minimize the possibility of a side preference, floor placement was alternated such that animals received a pairing of cocaine or saline on both right and left sides of the chamber. Twenty-four hr following the last conditioning session, mice were given a 15-min exposure to the CPP apparatus with both floors to assess preference for the drug-paired side (Preference Test).
Data analysis
Fear expression was determined by freezing response within the context. Freezing was defined as an episode of at least 3 sec of inactivity. Freezing for cued fear is reported as percentage of time freezing during the CS-on period. Locomotor data for cued fear conditioning was measured by automated activity counts in Context A prior to CS presentation. For contextual fear, total freezing time was divided by 12 min to calculate percentage of time freezing in each day. Locomotor activity in the CPP apparatus was measured by total distance moved in cm, and reported as distance moved (cm) per min. Conditioned place preference was measured in Ethovision XT 5 (Noldus) by tracking the center point of the mouse for time (s) spent on drug-paired floor (CS+) or vehicle-paired floor (CS−) per minute of test session. Average time on CS+ floor was used to calculate percentage of time spent on CS+ floor over the whole session. Grid/hole (G+/G−) analysis examined time (s) spent on grid floor per min during the Preference Test in animals that received cocaine-conditioning sessions on grid floor (G+) compared to animals that received cocaine-conditioning sessions on hole floor (G−; Cunningham et al., 2006). Data analyses were performed with Prism 6 and SPSS Statistics 22 (IBM). Data in Figure 1 were analyzed with a one-way analysis of variance (ANOVA) on each day. Data in Figures 2 and 4 were analyzed using a two-way ANOVA on each day with genotype and sex as between-subjects factors. Data from Figure 3 were analyzed using a two-way ANOVA on each day with genotype and drug treatment as between subject factors. G+/G− data in Figure 4c were analyzed using a three-way ANOVA, with genotype, sex, and floor assignment as between-subjects factors. Sex was not included as a statistical factor when there were fewer than n = 3 animals per sex within a genotype. Post hoc comparisons follwoing ANOVAs were performed using a Dunnett’s test to compare D1 HET and WT to D1 KO behavior (Figures 2 and 4) or Sidak’s test to assess SKF 81297 treatment compared to saline treatment at each time point (Figure 3).
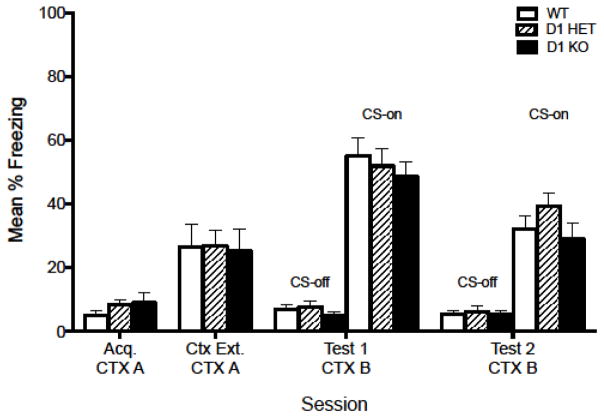
Figure 1. One shock cued fear conditioning is retained in D1 knockout mice.
Mice did not show differences in freezing between genotypes in Acquisition (Acq.), Contextual Extinction (Ctx. Ext.), Test 1 or Test 2 during CS-on or CS-off periods. Male and female wildtype (WT), heterozygote (D1 HET), knockout (D1 KO) mice were pooled within genotypes for statistical analysis.
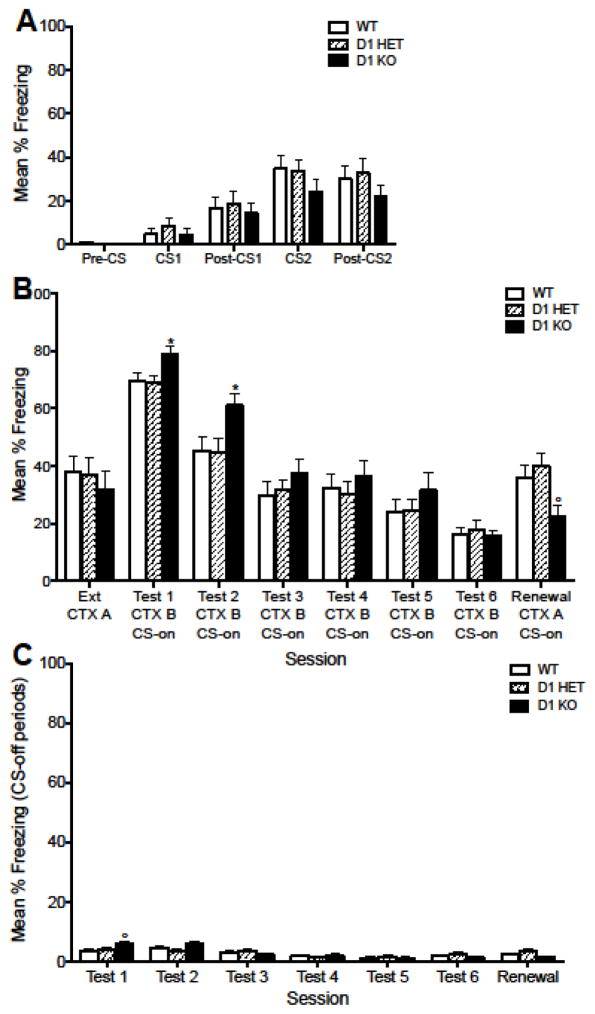
Figure 2. Cued fear conditioning is retained in D1 knockout mice.
(A) Freezing during fear conditioning before (pre-CS), during (CS1 and CS2) and after (post-CS) the two conditioning trials in wildtype (WT), heterozygote (D1 HET), and knockout (D1 KO) mice. (B) Average freezing to the context during acquisition (Acq CTX A) and during an extinction test 24 hr later (Ext CTX A), and freezing to the CS during daily cued extinction sessions in Context B (Tests 1–6 CTX B), and during a test in the acquisition context (Renewal CTX A). (C) Freezing during CS-off periods during the cued extinction tests. D1 KO mice showed increased freezing in response to the CS on Test 1 (compared to WT) and on Test 2 (compared to WT). D1 KO mice showed a trend towards increased freezing during CS-off periods on Test 1. D1 KO mice showed a non-significant decrease in freezing during CS presentations compared to WT during Renewal. Error bars indicate SEM. (*) p < 0.05 significant difference compared to WT. (°) p = non-significant trend for KO compared to WT.
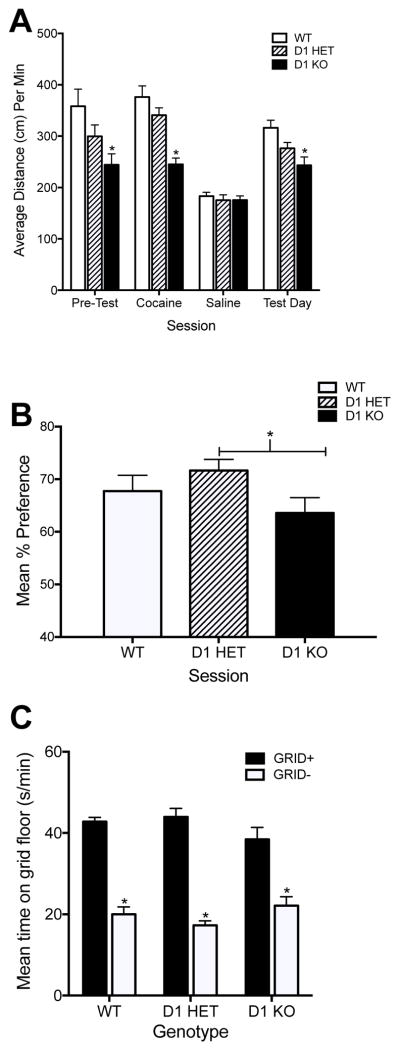
Figure 4. Cocaine conditioned place preference in D1 knockout mice.
Panel A shows locomotor activity of WT, D1 HET and D1 KO during pre-test, training days, and preference test day. Panel B shows percent preference for the cocaine-paired floor during the test day in male and female wildtype (WT), heterozygotes (D1 HET) and knockouts (D1 KO). Panel C shows grid+/grid− analysis of WT, D1 HET and D1 KO during the preference test day. During pre-test, cocaine conditioning sessions, and Test Day, knockout mice showed decreased locomotor activity compared to wildtype mice. Knockout mice showed significantly less preference for cocaine-paired floor compared to heterozygote mice in Panel B. Grid+/grid− analysis did not indicate a main effect of genotype, but showed that all genotypes acquired a conditioned place preference when comparing animals that received cocaine on grid floor (grid+) against animals that received saline on grid floor (grid−) during training. Error bars indicate SEM. (*) p < 0.05 significant difference compared to D1 HET or WT.
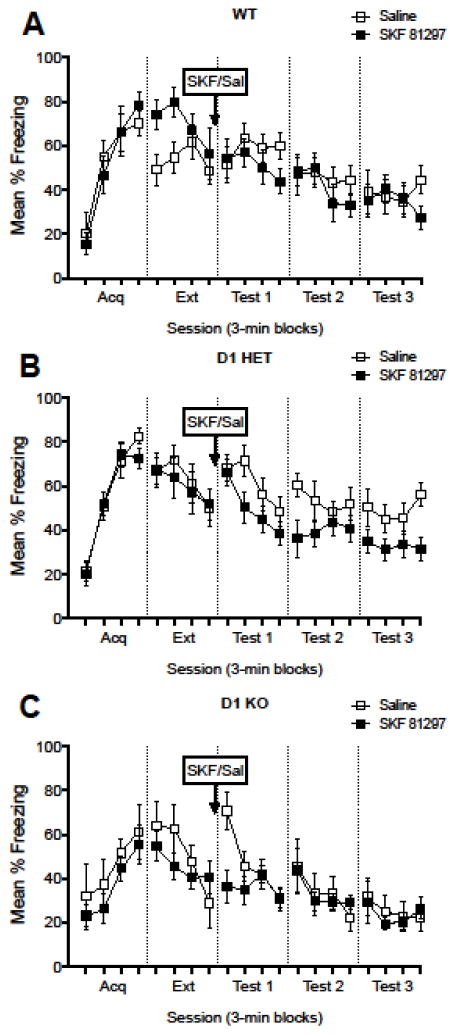
Figure 3. D1 agonist (SKF 81297) effects on contextual fear extinction in D1 knockout mice.
Mice were treated with SKF 81297 or saline following extinction. WT and D1 HET mice treated with SKF 81297 generally showed non-significant decreases in freezing from Test 1–3. D1 KO mice showed a decrease in freezing during the first block of Test 1, but no differences were apparent on Test 2 or Test 3.
Results
One trial cued fear conditioning
This experiment examined whether fear learning following a single CS-US pairing is impaired in D1 KO mice compared to WT or D1 HET mice (Ortiz et al., 2010). Due to the small number of knockout mice available for this study, animals were collapsed across sex for statistical power. Figure 1 shows there were no significant effects of genotype on Acquisition (Acq.), Contextual Extinction (Ctx Ext), Test 1, or Test 2 during CS-on or CS-off periods. This experiment demonstrates that fear learning is retained in D1 KO mice following a brief conditioning session.
D1 knockout does not alter shock reactivity
This experiment addressed whether there may be differences in shock reactivity between genotypes that could affect fear conditioning. Table 1 shows the percentage of subjects jumping in response to shock. At 0.05 mA, there was no effect of genotype or sex, and no interaction between genotype and sex. At 0.10 mA there was no effect of sex, but there was a significant effect of genotype (F (2,19) = 4.071, p = 0.0337) and a significant interaction between sex and genotype (F(2,19) = 4.071, p = 0.0337). Post-hoc analysis of genotype confirmed that D1 HET mice showed lower shock reactivity than did WT mice (p = 0.0179) to 0.10 mA shock. There was no significant difference (p = 0.107) between KO and WT mice at 0.10 mA shock. When separated by sex for post-hoc analysis, there was no significant effect of genotype on shock reactivity. At 0.15 mA and above, there was no effect of genotype or sex and no interaction between sex and genotype. There were no gross genotype-specific differences in shock reactivity; D1 KO, heterozygote, and wildtype mice consistently responded to footshock starting at 0.15 mA, which was below the 0.35 mA footshock used for conditioning in these studies.
Table 1. Shock reactivity in D1 knockout mice.
Table shows percent of subjects showing jumping responses at each shock strength. Male wildtype, D1 heterozygote and D1 knockout (MWT, MHET, MKO) showed consistent jumping responses starting at 0.10 mA. Female wildtype, D1 heterozygote, and D1 knockout (FWT, FHET, FKO) showed consistent jumping responses starting at 0.15 mA. * indicates a significant difference between MWT and MHET for shock reactivity at 0.10 mA
| Genotype | Shock Strength | ||||
|---|---|---|---|---|---|
| 0.05 mA | 0.10 mA | 0.15 mA | 0.20 mA | 0.25 mA | |
| MWT | 20% | 100% | 100% | 100% | 100% |
| MHET | 0% | 40% | 100% | 100% | 100% |
| MKO | 33% | 100% | 100% | 100% | 100% |
| FWT | 20% | 100% | 100% | 100% | 100% |
| FHET | 50% | 100% | 100% | 100% | 100% |
| FKO | 33% | 100% | 100% | 100% | 100% |
Cued fear conditioning, extinction, and renewal
To further characterize the effect of D1 knockout on fear conditioning, this experiment examined conditioning with two CS-US trials. This served to increase freezing levels, which then allowed us to investigate effects of D1 knockout on extinction and renewal. Figure 2a shows freezing during the acquisition session prior to (Pre-CS), during (CS1; CS2), and following (Post-CS1; Post-CS2) CS-US presentations. There was no significant effect of genotype on acquisition of fear. To determine whether there were locomotor activity differences between genotypes during initial exposure to the context, mean activity counts were examined prior to the first CS-US presentation. There was no significant effect of genotype, but there was a main effect of sex (F (1,40) = 7.337, p = 0.0099) during the 2-min pre-CS presentation period (females = 450 ± 13.2; males = 500 ± 12.3) and no significant interaction between sex and genotype.
Figure 2b shows freezing during CS-on periods and Figure 2c shows freezing during CS-off periods during test sessions. During Contextual Extinction (Ctx Ext in Fig. 2b), there was a main effect of sex (F (1,40) = 6.167, p = 0.0173), with female mice generally showing higher freezing (44%) to the conditioning context than male mice (28%). There was no significant effect of genotype on freezing, and no significant interaction between genotype and sex during Contextual Extinction. The sex-specific increases in freezing in female mice compared to male mice did not persist to the following Test 1 in Context B (Test 1 in Fig 2b). However, there was a main effect of genotype during Test 1 (F (2,40) = 3.628, p = 0.0357), and post-hoc analysis revealed a trend indicating that the genotype effect was driven by increased freezing during the CS-on period in D1 KO mice compared to WT mice (p = 0.053). There was no significant main effect of sex or interaction between genotype and sex during Test 1. Analysis of the CS-off period during Test 1 (Fig. 2c) indicated no significant differences between genotype or sex, or interaction between genotype and sex, on freezing in the absence of the CS in Context B. However, there was a trend towards a genotype effect on freezing during CS-off periods (F (2,40) = 2.860, p = 0.069), and post-hoc analysis indicated that D1 KO mice showed significantly higher freezing during baseline periods compared to WT mice (p = 0.046). This suggests that the observed increases in freezing during CS-on periods in Test 1 may be driven at least in part by differences in generalized freezing to the context, rather than an enhancement of fear acquisition in D1 KO mice.
During CS-on periods in Test 2, there was no significant effect of sex and no interaction between sex and genotype, but there was a main effect of genotype on freezing (F (2,40) = 3.934, p = 0.0276). A post-hoc analysis revealed that D1 KO mice showed significantly higher freezing compared to WT mice (p = 0.038). There were no significant effects of genotype or sex and no interaction between sex and genotype during the CS-off period in Test 2, indicating that fear expression differences on Test 2 during the CS-on period cannot be solely attributed to different baseline levels of freezing between genotypes. There were no significant differences in freezing between males and females or genotypes during CS-on or CS-off periods on any day from Test 3 to Test 6.
When returned to the conditioning context to test for renewal of fear, there was a main effect of genotype during CS-on periods (F (2,40) = 4.202, p = .0221), with a non-significant trend for differences between wildtype and knockout mice (p = 0.066). There was no main effect of sex and no interaction during Renewal CS-on periods (Ren. in Fig. 2a). There were no significant main effects of sex or genotype on freezing during CS-off periods and no interaction between sex and genotype (Ren. in Fig. 2b). Together, these data indicate that fear acquisition and expression is retained in D1 KO mice, and fear expression may be slightly enhanced in D1 KO mice during initial test days. There is a trend for a genotype effect on fear renewal, suggesting that knockout of the D1 receptor leads to that enhanced acquisition of fear decreases fear renewal or that D1 KO mice may have a general deficit in contextual discrimination.
D1/5 receptor agonist mediated extinction enhancements may require D5 receptors
The first two experiments found no differences in context-evoked freezing in D1 KO, D1 HET, and WT mice. The next experiment served two purposes. First, we examined whether increasing the amount of freezing in WT mice would reveal a deficit in freezing in D1 KO mice at higher ends of the performance scale. Second, we evaluated whether we could replicate our previous demonstration of extinction enhancements with a D1/D5 receptor agonist in D1 KO mice. An effect in these mice would mean that the pharmacological effects may be mediated by the D5 receptor.
One challenge for assessing the effect of activation of D1-like receptors is that there are currently no pharmacological tools to distinguish D1 from D5 receptors (Holmes et al., 2004). Because D1 KO mice do not express the D1 receptor, evaluating the effects of a D1/5 receptor agonist (SKF 81297) in D1 KO mice may reveal a D1- or D5-receptor specific mechanism for extinction enhancements induced by this agonist. Due to low numbers of males available for this study, males and females were pooled for analysis to increase statistical power. During acquisition, there was a non-significant trend for a main effect of genotype (F (2,41) = 2.736, p = 0.0767) and a trend toward decreased freezing behavior was observed in D1 KO mice compared to D1 HET mice (p = 0.0607; Figure 3, Acq). There was no significant main effect of genotype or drug treatment, and no interaction between genotype and drug treatment during Extinction (Figure 3, Ext). During Test 1, there was a significant effect of drug treatment (F (1,41) = 5.329, p = 0.0261) and genotype (F (2,41) = 3.936, p = 0.0273), but no interaction between genotype and drug treatment (F (2,41) = 0.1125, p = 0.8939). Post-hoc analysis of Test 1 showed significant differences in SKF 81297-treated D1 KO mice compared to both WT (p = 0.0390) and D1 HET (p = 0.0226) mice treated with saline. There were no significant effects of drug treatment or genotype during Test 2 and no significant interaction between drug treatment and genotype. During Test 3, there was a significant effect of genotype (F (2,41) = 3.645, p = 0.0349), and no significant effect of drug treatment and no interaction between drug treatment and genotype. Post-hoc analysis showed that D1 KO animals were significantly (p = 0.0364) different from heterozygote mice during Test 3.
To further explore whether D1 agonism may have enhanced extinction in any group, we examined behavior within all genotypes during Test Days 1–3 in 3-min blocks (Figure 3, Tests 1–3). A two-way ANOVA with block as a repeated measure and drug treatment showed that there was a significant main effect of block in all genotypes, but an interaction (F (11,110) = 2.077, p = 0.0278) between block and drug treatment was detected only in knockout mice. Post-hoc analyses revealed that saline and SKF 81297-treated knockout mice were significantly different (p = 0.0064) during the first 3-min block of Test 1. This suggests that there may be a contribution of D5 receptors to the observed effects of D1/5 agonists. The effect was relatively transient, but may be limited by the high floor that mice reach during extinction of fear (Lattal & Maughan, 2012).
D1 knockout effect on cocaine conditioned place preference
The fear conditioning experiments did not reveal deficits in acquisition or retention of cued or contextual fear in D1 KO mice. This suggests that the D1 receptor may not be needed to associate discrete stimuli or contexts with aversive unconditioned stimuli. There was some evidence in those experiments for increased generalization between the conditioning and extinction contexts at the outset of extinction (increased pre-CS freezing) and between the extinction and the testing context at the end of extinction (decreased CS freezing). The next experiment assessed whether D1 KO mice could learn an appetitive context discrimination between a context associated with cocaine and a different context associated with saline in a conditioned place preference procedure.
All mice were matched following a pre-test to ensure no bias to grid or hole floors and given two conditioning sessions with cocaine and saline over four days. Examination of locomotor behavior during pre-test (Fig. 4A) indicated that there was a main effect of sex (F (1,40) = 10.96, p = 0.002), with females showing less locomotor activity than males, and a main effect of genotype (F (2,40) = 4.153, p = 0.0230), with no interaction effect. Post hoc analysis confirmed that D1 KO mice showed significantly less locomotor activity than WT mice (p = 0.004) during pre-test. Assessment of locomotor behavior during cocaine conditioning sessions showed that there was a significant effect of genotype (F (2,40) = 12.64, p < 0.0001) and no effect of sex and no interaction between sex and genotype. Post-hoc analysis confirmed that D1 KO mice showed significantly less locomotor response to cocaine compared to WT mice (p < 0.0001). There was no effect of genotype or sex on locomotor behavior during both saline conditioning days and no interaction between genotype and sex. During the preference test, there was no effect of sex and no interaction between sex and genotype, but there was a main effect of genotype (F (2,40) = 4.806, p = 0.0135). Post-hoc analysis confirmed that D1 KO mice showed significantly less locomotor activity compared to wildtype mice (p = 0.001).
When tested for preference (Preference Test in Figure 4B), there was a main effect of genotype on percent preference (F (2,40) = 3.618, p = 0.036). There was no effect of sex and no interaction between sex and genotype during the preference test. Post-hoc analysis showed that the genotype effect was significant in D1 HET mice compared to D1 KO mice (p = 0.028), with heterozygotes showing greater preference than knockout mice.
To further assess preference within each genotype, we examined time spent on the grid floor during the test day between animals that received cocaine on the grid floor (G+) compared to animals that received cocaine on the hole floor (G−) during conditioning (Cunningham et al., 2006). A significant difference between grid-paired (G+) compared to hole-paired (G−) mice indicates whether animals acquired a conditioned place preference. There was a significant effect of floor (F (1,34) = 153.22, p < 0.0001), but no effect of sex or genotype and no significant interactions between floor, sex or genotype. Figure 4C shows that all genotypes showed a preference for the cocaine-paired grid floor (G+) compared to saline-paired grid floor (G−).
Discussion
There are several key findings in these experiments that help to define the role that D1 receptors may have in learning processes. First, D1 KO mice were unimpaired in cued fear conditioning with either one or two trials or contextual conditioning with one, two, or four trials, suggesting that associative learning may occur in the absence of D1 receptors. Second, this effect was replicated in an appetitive contextual conditioning task, in which D1 KO mice formed a preference for contexts paired with cocaine over contexts paired with saline. Although there was some evidence that this preference was not as robust in KO mice as in D1 HET mice, the finding of a reliable preference in the knockout mice suggests that they are able to form associations between contexts and unconditioned stimuli of positive or negative valence. Third, although D1 KO mice showed rates of extinction that were comparable to D1 HET and WT mice, the knockout mice showed less contextual renewal when returned back to the conditioning context. One possible account for this effect is that the absence of the D1 receptor causes increased generalization between contexts, which is also consistent with the increased freezing that we observed during CS-off periods in a novel context after acquisition. The findings that cued and contextual fear conditioning and cocaine CPP are retained in D1 KO mice suggest that associative learning can be maintained in animals lacking the D1 receptor.
D1 receptors are important in a number of motivated behaviors, and loss of this signaling system appears to have deleterious effects on body weight and locomotor behavior. As reported by Fadok et al. (2009) and Karlsson et al. (2008), D1 KO mice exhibit decreased food consumption in the absence of a palatable food replacement, and decreased body weight compared to heterozygote and wildtype mice. These effects on body weight were observed within the D1 KO mice used in our experiments. Similar to Fadok et al. (2009), there were no differences in shock reactivity between genotypes at shock intensities used for fear conditioning. Although startle response can be used to broadly measure shock reactivity, subtler differences may be revealed between genotypes based on distance traveled during shock (Wiltgen et al., 2006). However, D1 KO mice in these studies had decreased basal locomotor activity, preventing the use of a velocity measurement to assess shock reactivity. D1 KO mice showed decreased locomotor activity during an initial 5-min pre-test and during the post-conditioning preference test in the CPP assay. In contrast, Karlsson et al. (2008) observed consistently increased locomotor activity over a two-hour open-field test. Both that study and ours used mice that were backcrossed for several generations to C57BL/6 mice, but it is possible that other differences, such as size of the locomotor chamber (Poon et al., 1997), contributed to the different effects. Our CPP chamber (30 cm X 15 cm X 15 cm) is smaller than the open-field chamber (40 cm X 35 cm X 35 cm) used in Karlsson et al. (2008), but the exact cause for the differing locomotor effects of D1 knockout is unclear.
One key difference between the animals used in this study and other D1 KO mice is that the D1 KO mice in this study express Cre-recombinase in place of the D1 receptor. The effect of linking Cre-recombinase expression to the D1 receptor promoter is unknown, but Forni et al. (2006) demonstrated that increased Cre-expression in neuronal progenitors has toxic effects on neuron development. This effect only emerges when Cre-recombinase is present at high levels within the nucleus, and has little effect when Cre-recombinase is stored in the cytoplasm. As Cre-recombinase may not translocate to the cytoplasm similar to D1 receptors, there may be effects of Cre-recombinase expression in the nucleus during development leading to behavioral changes. However, the D1 promoter driven expression of Cre-recombinase is unlikely to explain the differences in behavior between these studies compared to previous studies of D1 KO mice, as extensive use of Cre-recombinase lines with other promoters (Tye & Deisseroth, 2012) indicates that Cre-recombinase expression alone has little impact on behavior.
The primary finding of the studies presented here is that associative learning is retained in D1 KO mice. We first tested the effect of a single CS-US pairing on fear conditioning (Ortiz, et al. 2010) and observed no differences in acquisition or retention of cued fear across genotypes. Contextual fear also did not appear to be strongly affected by D1 KO, in concordance with El Ghundi et al. (2001). However, there was no clear impairment of extinction in D1 KO animals as reported by El-Ghundi et al. (2001) and there were non-significant decreases in freezing in knockout mice during contextual fear acquisition compared to other genotypes; a directional effect consistent with previous findings in D1 KO mice (Fadok et al., 2009; Ortiz et al., 2010).
When trained with two CS-US pairings, D1 KO mice trended towards increased freezing compared to wildtype during Test 1 (Figure 2a) and showed significantly higher freezing than wildtype during Test 2. This could be considered an enhancement of fear acquisition, but the general decreases in locomotor behavior and increased freezing during CS-off periods in Test 1 prevent the clear determination of whether the increases in freezing are conclusively showing enhanced acquisition of fear. One possibility for the observed acquisition of fear is that the general decrease in locomotion (as observed during the pre-test in conditioned place preference) could artificially inflate fear responses measured as freezing. However, there was no observed decrease in locomotor behavior between genotypes during the pre-CS period in acquisition. Although this suggests that general decreases in locomotor behavior alone would not be a likely explanation for effects on acquisition of fear, we need to be cautious in overinterpreting a null effect in pre-CS freezing during later tests, which was near the floor.
One finding in the two-shock cued fear study suggesting that the increased expression of fear in D1 KO mice is related to learning, rather than differences in behavioral expression, is the decreased renewal of fear in D1 KO mice, consistent with effects of D1/5 receptor antagonism on renewal of reward-seeking behaviors (Hamlin et al., 2007). If D1 KO mice showed consistently high levels of freezing across all tests, including renewal, it would be difficult to assess whether fear expression or learning is impacted. However, D1 KO mice only show increased freezing during Test 1 and Test 2, then show relatively equal or slightly higher levels of fear responding compared to other genotypes. When tested for fear renewal, the D1 KO animals trend towards less freezing, suggesting that acquisition and extinction of fear may generalize across contexts in these mice. One possibility for the weakened renewal effect is that the high levels of fear at the outset of extinction resulted in an increased prediction error between the expected (shock) and obtained (no shock) outcome, which may have promoted extinction and weakened contextual renewal. This type of deepened and persistent extinction effect with high levels of fear at the outset of extinction has been reported in other studies (e.g., Leung and Westbrook, 2008).
In summary, it does not appear that the D1 receptor is required for acquisition or extinction of cued fear, but it may be involved in context discrimination and contextual modulation of fear. An advantage of a genetic approach to the D1 receptor is that knockouts are specific to that receptor, whereas pharmacological approaches are not specific, targeting D1 and D5 receptors. We have previously reported that pharmacological activation of the D1/5 receptor enhances fear extinction in male C57BL/6 mice (Abraham et al., 2016). To test whether this effect required D1 receptors, we examined the effects of the D1/5 agonist SKF81297 in the D1 knockout mice. We did not find a robust effect of D1/5 receptor activation following contextual fear extinction within any genotype. It is likely that the low freezing in saline-treated mice prior to drug administration during the first extinction session prevented the detection of a D1/5 agonist mediated extinction enhancement in WT mice. Although male C57BL/6 mice have previously shown consistent extinction enhancement effects with D1/5 receptor agonism, there were only a limited number of male mice available in this study to overcome baseline differences in extinction. In female WT mice, we did not observe a strong effect of D1/5 receptor agonism on fear extinction. Thus, the experiment with SKF81297, although suggestive of a role for D5 receptors in extinction enhancements, is still somewhat inconclusive.
To directly examine reward learning, we tested whether D1 KO mice could acquire cocaine conditioned place preference. Miner et al. (1995), using a biased apparatus, demonstrated that cocaine conditioned place preference is equivalent between wildtype, heterozygote, and D1 KO mice. We show that D1 KO mice can acquire cocaine conditioned place preference in an unbiased apparatus. Although there was no difference in preference between heterozygotes and wildtypes, preference for the cocaine-paired floor was lower in D1 KO mice compared to heterozygote mice. This difference between heterozygote and D1 KO mice was not observed in Miner et al. (1995). There may be some contribution of D1 receptors to cocaine conditioned place preference, but the absence of D1 receptors does not entirely prevent reward learning, as D1 KO mice showed reliable preference for the cocaine-associated floor. Cocaine conditioned place preference can be retained within dopamine deficient mice through serotonergic mechanisms (Hnasko et al., 2007), so there may be similar compensatory mechanisms that maintain reward learning in D1 KO mice. There may also be changes within the dopamine system following D1 knockout, such as increased expression of D5 receptors to maintain normal behaviors. The examination of these compensatory changes following D1 receptor deletion is beyond the scope of these studies, but one possible reason for the discrepancies between the studies presented here and previous literature could be different mechanisms of compensating for the loss of D1 receptors between different D1 KO lines.
The overarching aim of these experiments was to determine whether the loss of dopamine signaling through D1 receptors could affect fear or reward learning. Our studies show that global deletion of D1 receptors in mice generally has little effect on fear conditioning, with some evidence for increased expression of freezing in the presence and absence of a CS when tested in a novel context. Although an effect occurred on cocaine-induced locomotor activity, there was little effect on cocaine-induced CPP. These findings add to a literature of somewhat discrepant results with D1 KO mice. These differences may be due to procedural differences, differences in behavioral expression, alternative mechanisms of compensation used by different genetic lines, or slight differences in the genetic background used in different mouse lines (Holmes et al., 2004). To fully capture the role of D1 receptors in behavior, alternative strategies may be needed, such as the use of RNA silencing (Ortiz et al., 2010), optogenetic (Land et al., 2014) or chemogenetic (Ferguson et al., 2011) control of D1 receptor expressing neurons in particular brain regions, and further development of agonists and antagonists selective for D1 or D5 receptors.
Highlights.
D1 knockout mice show similar levels of cued and contextual fear conditioning to WT controls following conditioning protocols involving one, two, or four shocks.
D1 knockout mice show increased generalization of fear conditioning and extinction across contexts, revealed as increased freezing to a novel context following conditioning and decreased freezing to an extinguished cue during a contextual renewal test.
D1 knockout mice show mild enhancements in extinction following an injection of SKF81297, a D1/D5 receptor agonist, suggesting a role for D5 receptors in extinction enhancements induced by nonspecific pharmacological agonists.
Although D1 knockout mice show decreased locomotion induced by cocaine, they are able to form a cocaine-induced conditioned place preference.
Acknowledgments
This work was supported by an APA Dissertation Award (ADA), NIH grants DA007262 (ADA), DA018165 (KML), DA025922 (KML), US Department of the Army/DOD-TATRC W81XWH-12-2-0048 (KML), the Merit Review Award BX000810 from the US Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development (K.A.N.). ADA’s present address is Department of Pharmacology, University of Washington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AD, Neve KA, Lattal KM. Activation of D1/5 Dopamine Receptors: A Common Mechanism for Enhancing Extinction of Fear and Reward-Seeking Behaviors. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673(2):242–50. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1(4):1662–70. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- El-Ghundi M, O’Dowd BF, George SR. Prolonged fear responses in mice lacking dopamine D1 receptor. Brain Res. 2001;892(1):86–93. doi: 10.1016/s0006-8993(00)03234-0. [DOI] [PubMed] [Google Scholar]
- Eshel N, Tian J, Bukwich M, Uchida N. Dopamine neurons share common response function for reward prediction error. Nat Neurosci. 2016;19(3):479–486. doi: 10.1038/nn.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Dickerson TM, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J Neurosci. 2009;29(36):11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2010;14(1):22–4. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenza NG, Rosa J, Izquierdo I, Myskiw JC. Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res. 2012;232(1):210–216. doi: 10.1016/j.bbr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Forni PE, Scuoppo C, Imayoshi I, Taulli R, Dastrù W, Sala V, … Ponzetto C. High levels of Cre expression in neuronal progenitors cause defects in brain development leading to microencephaly and hydrocephaly. J Neurosci. 2006;26(37):9593–602. doi: 10.1523/JNEUROSCI.2815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD. Mesolimbic dopamine signals the value of work. Nat Neurosci. 2016;19(1):117–126. doi: 10.1038/nn.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146(2):525–36. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Heusner CL, Beutler LR, Houser CR, Palmiter RD. Deletion of GAD67 in dopamine receptor-1 expressing cells causes specific motor deficits. Genesis. 2008;46(7):357–67. doi: 10.1002/dvg.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikind N, Maroun M. Microinfusion of the D1 receptor antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobiol Learn Mem. 2008;90(1):217–222. doi: 10.1016/j.nlm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Hitchcock LN, Cunningham CL, Lattal KM. Cue configuration effects in acquisition and extinction of a cocaine-induced place preference. Behavioral Neuroscience. 2014;128(2):217–227. doi: 10.1037/a0036287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;27(46):12484–8. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Lachowicz JE, Sibley DR. Phenotypic analysis of dopamine receptor knockout mice; recent insights into the functional specificity of dopamine receptor subtypes. Neuropharmacology. 2004;47(8):1117–34. doi: 10.1016/j.neuropharm.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Inoue T, Izumi T, Maki Y, Muraki I, Koyama T. Effect of the dopamine D(1/5) antagonist SCH 23390 on the acquisition of conditioned fear. Pharmacol Biochem Behav. 2000;66(3):573–578. doi: 10.1016/s0091-3057(00)00254-9. [DOI] [PubMed] [Google Scholar]
- Karlsson RM, Hefner KR, Sibley DR, Holmes A. Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl) 2008;200(1):117–27. doi: 10.1007/s00213-008-1165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, DiLeone RJ. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 2014;17(2):248–53. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Maughan DK. A parametric analysis of factors affecting acquisition and extinction of contextual fear in C57BL/6 and DBA/2 mice. Behav Processes. 2012;90(1):49–57. doi: 10.1016/j.beproc.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HT, Westbrook RF. Spontaneous recovery of extinguished fear responses deepens their extinction: a role for error-correction mechanisms. J Exp Psychol Anim Behav Process. 2008;34(4):461–74. doi: 10.1037/0097-7403.34.4.461. [DOI] [PubMed] [Google Scholar]
- Miner LL, Drago J, Chamberlain PM, Donovan D, Uhl GR. Retained cocaine conditioned place preference in D1 receptor deficient mice. Neuroreport. 1995;6(17):2314–6. doi: 10.1097/00001756-199511270-00011. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. In Guide for the care and use of laboratory animals. Public Health Service, National Institutes of Health; 1985. NIH Publication 86-23 Revised. [Google Scholar]
- Ortiz O, Delgado-Garcia JM, Espadas I, Bahi A, Trullas R, Dreyer JL, Gruart A, Moratalla R. Associative learning and CA3-CA1 synaptic plasticity are impaired in D1R null, Drd1a-/- mice and in hippocampal siRNA silenced Drd1a mice. J Neurosci. 2010;30(37):12288–12300. doi: 10.1523/JNEUROSCI.2655-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon AM, Wu BM, Poon PW, Cheung EP, Chan FH, Lam FK. Effect of cage size on ultradian locomotor rhythms of laboratory mice. Physiol Behav. 1997;62(6):1253–8. doi: 10.1016/s0031-9384(97)00305-3. [DOI] [PubMed] [Google Scholar]
- Rey CD, Lipps J, Shansky RM. Dopamine d1 receptor activation rescues extinction impairments in low-estrogen female rats and induces cortical layer-specific activation changes in prefrontal-amygdala circuits. Neuropsychopharmacology. 2014;39(5):1282–9. doi: 10.1038/npp.2013.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16(7):966–973. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324(5930):1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13(4):251–66. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;41(6842):43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Wall VZ, Parker JG, Fadok JP, Darvas M, Zweifel L, Palmiter RD. A behavioral genetics approach to understanding D1 receptor involvement in phasic dopamine signaling. Mol Cell Neurosci. 2011;46(1):21–31. doi: 10.1016/j.mcn.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. J Neurosci. 2006;26(20):5484–91. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]