Abstract
Post transplant lymphoproliferative disorder (PTLD) causes significant morbidity and mortality in pediatric recipients of liver transplantation (LT).
Objective
Describe the incidence of PTLD and symptomatic Epstein Barr virus disease (SEBV) in a large multicenter cohort of children who have undergone LT focused on the risk factors and changes in incidence over time.
Methods
Prospective determination of SEBV and PTLD in 2283 subjects who had undergone a first LT with at least 1 year of follow up in the Studies of Pediatric Liver Transplantation database. SEBV was defined by specific criteria and PTLD required tissue confirmation. Incidences of SEBV and PTLD were determined by Kaplan Meier. Univariate and multivariate modeling of risk factors were performed using standard methods.
Results
SEBV occurred in 199, of whom 174 (87.4%) were EBV negative at LT. 75 patients developed PTLD of whom 64 (85.3%) were EBV negative at LT. Of 2048 patients with at least 2 years of follow-up, 8.3% developed SEBV and 2.8% PTLD by the second year post-LT. There was a lower incidence of SEBV (11.3% vs 5.9, p<0.0001) and PTLD (4.2 vs. 1.7, p=0.0011) in 2002-07 compared to 1995-2001. Tacrolimus and cyclosporine trough blood levels in the first year post transplant were significantly lower and fewer children were receiving steroids in 02-07. Era of LT, EBV negative recipient status, younger age, biliary atresia and frequent rejection episodes were associated with an increased risk for SEBV and PTLD in univariate analysis. Age, biliary atresia and EBV recipient status were correlated. In multivariate analysis era of LT, EBV recipient status and frequent rejection episodes was associated with SEBV and PTLD.
Conclusions
The incidence of SEBV and PTLD is decreasing in pediatric LT recipients coincident with a reduction in immunosuppression. Younger recipients and those with multiple rejections remain at higher risk for SEBV and PTLD.
Post transplant lymphoproliferative disorder (PTLD) is a significant source of morbidity and mortality in pediatric recipients of liver transplantation with a reported incidence of 6-20% and a mortality of 12-60% (1-6). First recognized in the late 1960's, most cases of PTLD are associated with infection with Epstein Barr Virus (EBV) and represent a spectrum of disease from polyclonal PTLD to lymphoma (7). PTLD related to EBV infection is most frequent in the first one to two years following liver transplantation with non EBV related PTLD more frequent later (8). Risk factors for PTLD have been reported to include: EBV seronegativity of the recipient (9), age of the recipient (1, 2), older donors, high levels of immunosuppression (1), immunosuppression regimen (2, 10) and use of anti-lymphocyte therapies (11). These risk factors have been reported in single center experiences and may reflect center effects.
There have been significant changes in the management of pediatric liver transplant recipients since the early reports on PTLD. Some of these changes may increase the risk of PTLD, such as an increase in the use of newer and/or potentially more potent immunosuppressive agents (10, 13) as well as an increase in the use of partial grafts (from either split livers or live donors) with a resultant older donor age leading to a higher risk of an EBV positive donor for the younger pediatric recipients who are more likely to be EBV naïve. In contrast, some changes might be expected to reduce the risk for PTLD, such as the development of molecular monitoring for EBV infection (14) and, subsequent responses to this information such as reduction of immune suppression and the use of as yet unproven preventative and preemptive interventions like antiviral therapy (15-17).
We report the incidence of symptomatic EBV infection (SEBV) and histologically confirmed PTLD utilizing a standardized definition in 2283 pediatric liver transplant recipients from 1995 to 2008 prospectively enrolled and followed for at least one year as part of the Studies of Pediatric Liver Transplantation (SPLIT). The goal of this study is to report the incidence and describe the factors that may be related to longitudinal changes in PTLD and SEBV in a large diverse pediatric population of liver transplant recipients.
Methods
The SPLIT data registry is a multi-center data registry for children receiving liver transplantation in the United States and Canada. As of May 31, 2008, the SPLIT registry database contained data on 2283 children who had undergone a first liver-only transplantation at 1 of 45 SPLIT centers with follow up for at least one year. As described previously, all of the participating SPLIT centers had institutional review board and/or research ethics board approval for data collection and analysis (18). Individual informed consent was obtained from parents and/or guardians. For analysis of changes in prevalence over time, we a priori assigned time periods to 1995-2001 (prior to PELD) and 2001-2007 (after PELD).
Data Collection
Detailed data was collected at listing, transplant and during follow up. After liver transplantation, follow-up data was submitted by each participating center to the SPLIT data coordinating center every 6 months for 2 years and annually thereafter until the subject's 18th birthday. One-year follow up was defined as a follow-up visit at 12 ± 3 months following transplant. These regular follow-up forms requested data elements on demographics, blood chemistry, hospitalizations, school status and performance, infections, post transplant complications, immunosuppression, and other medication regimens. Specific events including death, retransplantation, allograft rejection, symptomatic EBV infection and histologically confirmed PTLD were reported on separate forms. Missing data indicates nothing entered in the data forms and unknown data indicates that the testing was not done or the result was not known. For EBV status, missing data was treated as unknown for analyses.
SEBV was determined locally and was defined as seroconversion (development of a new positive VCA IgM in a previously documented negative patient) or development of a positive viral load, with either histologic evidence of EBV infection (as determined by the local pathologist) or symptoms associated with EBV infection (fever, leukopenia, atypical lymphocytosis, exudative tonsillitis and/or adenopathy, or hepatitis). PTLD was defined locally based on the minimum requirement of a tissue biopsy considered diagnostic of PTLD by the local pathologist based on the published classification scheme (19). EBV PCR was determined by the method in place at the local site.
For patients with SEBV or PTLD after liver transplantation, the following information was requested using a specific EBV/PTLD form: EBV/PTLD symptoms, diagnostic criteria, immunosuppression prior to diagnosis, initial treatment of the EBV disease or PTLD, and use of monoclonal/polyclonal antibody therapy for induction or rejection that were reviewed for inclusion in the database. Each new diagnosis of SEBV or PTLD required a new enrollment. Following SEBV or PTLD diagnosis, data were captured every three months until resolution, up to a maximum of 12 months post diagnosis, exit or death. Date of follow-up or resolution, immunosuppression, and other treatments were queried in the follow-up assessments. In the data analysis of this study, for patients with multiple enrollments, only the first diagnosis with associated follow-up visits was considered. Since maternal EBV antibodies can cross the placenta and remain with the infant until about 12 months of age, for the purposes of this analysis, the EBV status of the recipient and donor were classified by serology for those >12 months of age and as negative for those ≤12 months of age.
For the purposes of this study, the SEBV group included those patients with or without PTLD and PTLD is therefore a subset of the SEBV group.
Statistical Methods
Kaplan-Meier estimates was used to calculate and plot time to the first SEBV or PTLD. Univariate statistical analyses included Chi-square or Fisher exact test to make comparisons of baseline categorical variables between SEBV/PTLD patients and non-SEBV/PTLD patients, Wilcoxon rank sum test for comparison of means of continuous factors. Hazard ratios and p values for risk factors for time to the first symptomatic EBV or PTLD were estimated by using a univariate and multivariate Cox proportional hazards model. Demographic factors, such as era of transplant, age, gender, race, primary diagnosis, donor age; baseline characteristics, including EBV status for recipient and donor, PELD score, organ type, patient hospitalization status at the time of liver transplantation, CMV status for recipient, medical treatments, and rejection were assessed in the univariate model. To develop a multivariate model, factors significant at 0.10 level in the univariate analyses were included in the initial multivariate model. We investigated the correlation between univariate factors including age at transplant, primary diagnosis and recipient EBV status. The final multivariate model was derived using the stepwise backward elimination procedure. Factors remaining significant at p ≤ 0.05 were kept in the final model. Multivariate analysis was performed using separate models excluding 2 of the 3 individual correlated factors (age, recipient EBV status and primary diagnosis). N, mean, median, and standard deviation were calculated and p value was estimated using Wilcoxon rank sum test to compare the trough level of calcineurin inhibitors between era of transplant. All statistical analyses were performed using the SAS System (Windows, v 9.2, SAS Institute, Cary, North Carolina). Note that the number of rejections was analyzed as a time-varying covariate. In such analyses, a patient's rejection status varies over time and rejections occurring after the outcome of interest are not included.
Results
A total 2283 children undergoing liver transplantation since 1995 with at least one year of follow up, were included in this study. The baseline data for all subjects and those with SEBV or PTLD are shown in Table 1. The donor EBV status was unknown for 37.9% of all subjects and 43.2% of those with SEBV. This was in part due to sites not performing EBV screening. There was a difference between era 1 and era 2 with 45.4% unknown in era 1 vs. 32.7% in era 2 for all patients and 46.3% (era 1) vs. 38.5% (era 2) for patients with SEBV. In contrast, the EBV status of the recipient was unknown in only 3.9% of all subjects and 3.0% of those with SEBV. SEBV occurred in 199 subjects post transplant, of whom, 174 (87.4%) were EBV negative at the time of transplant. One hundred twenty four had SEBV without PTLD of whom 88 (71%) developed SEBV within 12 months of transplant. Of the 199 subjects with SEBV, 75 (37.7%) developed PTLD of whom 46 (61.3%) developed PTLD within 12 months of transplant. Of those who developed PTLD, 64 (85.3%) were EBV negative at the time of transplant, which is higher compared to 1524 of the 2208 (69%) who did not develop PTLD (p=0.0003). Similarly of those who developed SEBV, 174 (87.4%) were EBV negative at the time of transplant, which is higher compared to 1414 of the 2084 (67.8%) who did not develop SEBV (p<0.0001). Fifty-two percent of the EBV negative patients developing PTLD received an organ from a donor with unknown EBV status. Patients with SEBV and PTLD were younger than the overall cohort.
Table 1.
Baseline Characteristics for Patients with Symptomatic EBV and PTLD Disease
| All Patients | Symptomatic EBV | PTLD | ||||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | P Value# | N | % | P Value# | |
| Total | 2283 | 100.0 | 199 | 100.0 | 75 | 100.0 | ||
| Gender | 1 | 0.0 | 0 | 0.0 | 0.23 | 0 | 0.0 | 0.41 |
| Missing | ||||||||
| Male | 1057 | 46.3 | 84 | 42.2 | 31 | 41.3 | ||
| Female | 1225 | 53.7 | 115 | 57.8 | 44 | 58.7 | ||
| EBV Status of Recipient and Donor | 54 | 2.4 | 2 | 1.0 | <.0001 | 1 | 1.3 | 0.0003 |
| R and D EBV unknown* | ||||||||
| R EBV +/ D EBV + | 209 | 9.2 | 8 | 4.0 | 5 | 6.7 | ||
| R EBV +/ D EBV − | 106 | 4.6 | 1 | 0.5 | 0 | 0.0 | ||
| R EBV +/ D EBV unknown* | 290 | 12.7 | 10 | 5.0 | 3 | 4.0 | ||
| R EBV −/ D EBV + | 484 | 21.2 | 42 | 21.1 | 13 | 17.3 | ||
| R EBV −/ D EBV − | 583 | 25.5 | 58 | 29.1 | 18 | 24.0 | ||
| R EBV −/ D EBV unknown* | 521 | 22.8 | 74 | 37.2 | 33 | 44.0 | ||
| R EBV unknown/ D EBV +* | 13 | 0.6 | 0 | 0.0 | 0 | 0.0 | ||
| R EBV unknown/ D EBV −* | 23 | 1.0 | 4 | 2.0 | 2 | 2.7 | ||
| Immunosuppression at Transplant | 94 | 4.1 | 6 | 3.0 | 0.85 | 2 | 2.7 | 0.65 |
| Missing | ||||||||
| CsA Base | 432 | 18.9 | 39 | 19.6 | 17 | 22.7 | ||
| Tac Base | 1596 | 69.9 | 138 | 69.3 | 52 | 69.3 | ||
| Other | 161 | 7.1 | 16 | 8.0 | 4 | 5.3 | ||
| Age at Transplant (mean ± SD) (year) | 2282 | 4.9 ± 5.3 | 199 | 2.7 ± 3.6 | <.0001 | 75 | 2.4 ± 3.2 | 0.0002 |
| Mo from transplant to SEBV/PTLD | -- | 199 | 11.2 ± 10.6 | -- | 75 | 12.7 ± 12.9 | -- | |
EBV unknown: Recipient / donor did not have EBV testing or data was missing.
Comparisons between SEBV and non SEBV or PTLD and non PTLD subjects
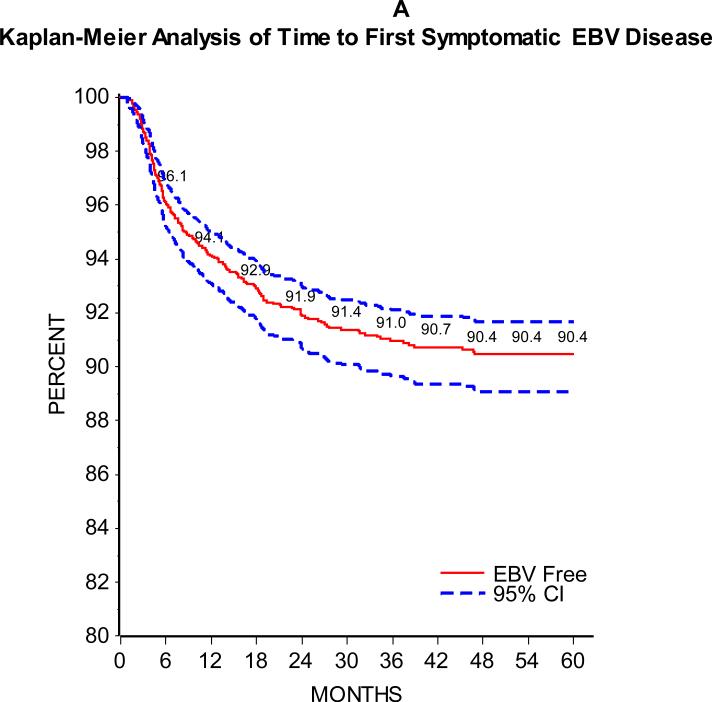
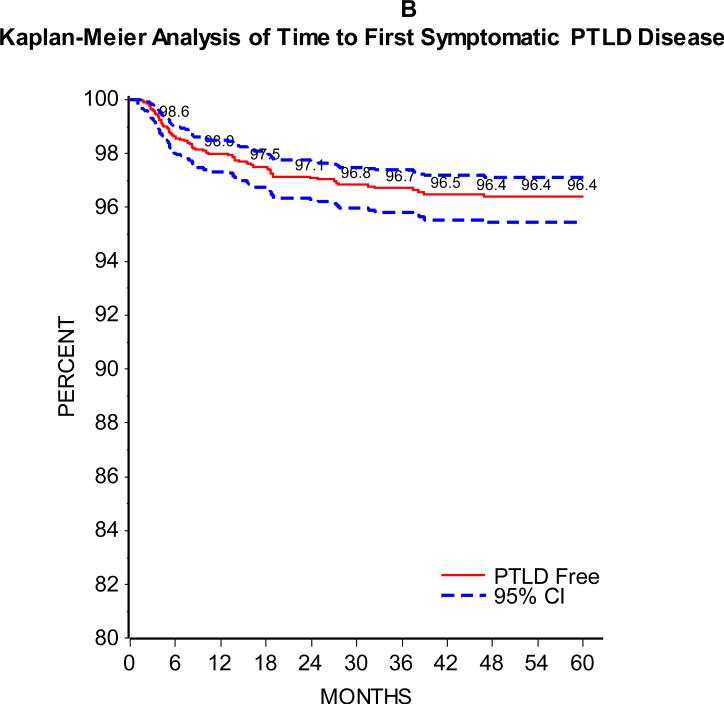
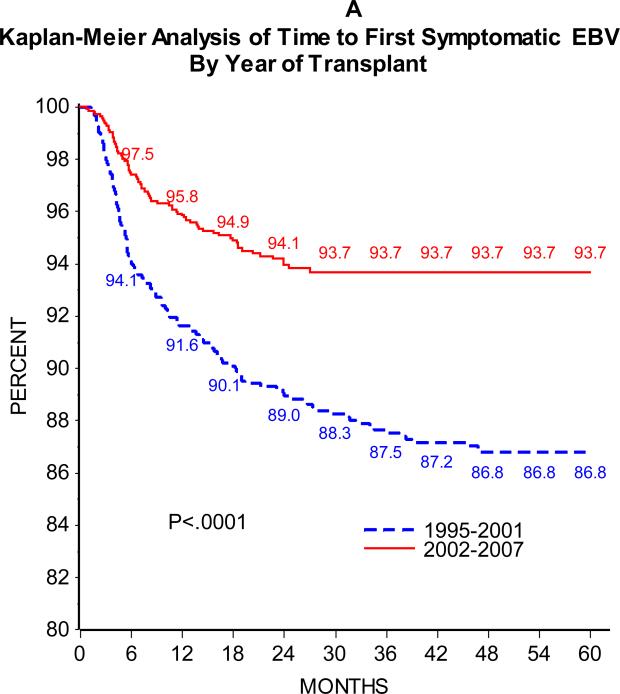
As can be seen in Figure 1, the majority of patients developed SEBV disease (Figure 1A) or PTLD (Figure 1B) in the first 24 months following transplant. Of 2283 patients with at least one year of follow-up and 2048 with at least two years of follow-up, 5.9% and 8.3% developed SEBV and 2.0% and 2.8% developed PTLD by the first one and two years post-transplant, respectively. There was an effect of era on the incidence of SEBV and PTLD, with a significantly higher one and two year prevalence of both SEBV and PTLD in patients transplanted from 1995 to 2001 compared to those transplanted from 2002 to 2007 (Table 2 and Figures 2A and B).
Figure 1A.
shows the Kaplan-Meier estimates of time from transplant to development of first symptomatic EBV disease with 95% confidence intervals.
Figure 1B.
displays a Kaplan-Meier analysis for time to first incidence of PTLD with 95% confidence intervals.
Table 2.
Incidence of Symptomatic EBV and PTLD
| Patients with at least one year of follow-up | Patients with at Least Two Years of Follow-up | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | EBV in the First Year Post Transplant | PTLD in the First Year Post Transplant | Total | EBV in the First Two Years Post Transplant | PTLD in the First Two Years Post Transplant | |||||||||
| N | N | Incidence (%) | P value | N | Incidence (%) | P value | N | N | Incidence (%) | P value | N | Incidence (%) | P value | |
| Total | 2283 | 134 | 5.9 | 46 | 2.0 | 2048 | 170 | 8.3 | 58 | 2.8 | ||||
| Era of Transplant | 934 | 78 | 8.4 | <.0001 | 29 | 3.1 | 0.0035 | 900 | 102 | 11.3 | <.0001 | 38 | 4.2 | 0.0011 |
| 1995-2001 | ||||||||||||||
| 2002-2007 | 1349 | 56 | 4.2 | 17 | 1.3 | 1148 | 68 | 5.9 | 20 | 1.7 | ||||
Figure 2A.
shows the Kaplan-Meier estimates of time from transplant to development of first symptomatic EBV disease by era of transplant (1995-2001 solid red line and 2002-2008 dashed blue line)
Figure 2B.
displays a Kaplan-Meier analysis of time from transplant to development of first PTLD by era of transplant (1995-2001 solid red line and 2002-2008 dashed blue line)
We next performed univariate analysis of time to SEBV and PTLD, which is presented in table 3. Factors found to be significant for both SEBV and PTLD include: era of transplant, recipient EBV status, age at transplant, primary diagnosis, and number of of rejection episodes. Since the number of patients with 4 or more rejection episodes is very few, we combined 3 or more rejection episodes into one group. None of the following oft cited factors were associated with a risk for PTLD: donor EBV status, recipient CMV status, antiviral treatments or IVIG, early rejection or any rejection episode. Importantly, the donor EBV status was unknown in 43.2% of the SEBV and 49.3% of the PTLD cases, which likely affected the outcome of the role of this factor in the univariate and multivariate analyses. There was a significant relationship between primary diagnosis, age at transplant and recipient EBV status, thus the multivariate model was performed with only one of the three factors age at transplant, primary diagnosis or recipient EBV status. There were no differences in the other resultant factors and thus the multivariate analysis results using recipient EBV status are shown in Table 4. The factors associated with an increased risk of SEBV and PTLD were limited to era of transplantation, having more than one rejection episode and recipient with negative EBV status at transplant.
Table 3.
Univariate Survival Analysis of Time to EBV and PTLD (N=2283)
| Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|
| EBV (N=199) | PTLD (N=75) | |||||||
| Factor | Comparison Group | Reference Group | Hazard Ratio | p-value | Overall p value | Hazard Ratio | p-value | Overall p value |
| Era of Transplant | 1995-2001 | 2002-2007 | 2.06 | <.0001 | <.0001 | 2.24 | 0.0009 | 0.0009 |
| Gender | Female | Male | 1.17 | 0.26 | 0.26 | 1.22 | 0.40 | 0.40 |
| Organ Type | Live | Cadaver | 1.22 | 0.27 | 0.27 | 1.24 | 0.46 | 0.46 |
| Monoclonal AB Use at Transplant |
No | Yes | 1.62 | 0.020 | 0.020 | 1.82 | 0.092 | 0.092 |
| Antiviral Use at Transplant | Yes | No | 0.95 | 0.84 | 0.84 | 1.07 | 0.87 | 0.87 |
| IVIg Use at Transplant | Yes | No | 0.97 | 0.86 | 0.86 | 0.89 | 0.72 | 0.72 |
| CMV at Transplant | Yes | No | 1.37 | 0.35 | 0.35 | 2.04 | 0.12 | 0.12 |
| EBV Status of Recipient | Recipient EBV negative |
Recipient EBV positive |
3.58 | <.0001 | <.0001 | 3.02 | 0.0032 | 0.010 |
| Recipient EBV unknown |
3.87 | 0.014 | 4.45 | 0.059 | ||||
| EBV Status of Donor | Donor EBV positive | Donor EBV negative | 0.83 | 0.31 | 0.14 | 0.94 | 0.84 | 0.11 |
| Donor EBV unknown |
1.17 | 0.34 | 1.58 | 0.10 | ||||
| Age At Transplant | (0,12) mos | 13+ Yrs | 5.47 | <.0001 | <.0001 | 12.57 | 0.013 | 0.0037 |
| [1,5) Yrs | 4.15 | 0.0003 | 12.88 | 0.012 | ||||
| [5,13) Yrs | 2.06 | 0.091 | 4.52 | 0.15 | ||||
| Race | Black | White | 1.33 | 0.13 | 0.50 | 1.69 | 0.081 | 0.30 |
| Hispanic | 1.12 | 0.58 | 1.40 | 0.30 | ||||
| Other | 1.00 | 0.99 | 1.47 | 0.30 | ||||
| Primary Diagnosis | Biliary Atresia | Other chronic liver disease |
1.99 | <.0001 | <.0001 | 2.38 | 0.0007 | 0.0002 |
| Fulminant L. Failure | 0.76 | 0.3601 | 0.49 | 0.2397 | ||||
| ICU Status | Cont. Hospital. | Cont. Med. care | 0.90 | 0.59 | 0.32 | 0.68 | 0.26 | 0.14 |
| ICU | 0.76 | 0.14 | 0.56 | 0.073 | ||||
| Immunosuppression at Transplant |
CsA Base | Tac Base | 0.97 | 0.88 | 0.92 | 1.11 | 0.72 | 0.74 |
| Other | 1.10 | 0.71 | 0.72 | 0.53 | ||||
| Donor Age | 0-11 mos | >=18 yrs | 0.84 | 0.57 | 0.21 | 1.08 | 0.87 | 0.43 |
| 1-17 yrs | 1.24 | 0.18 | 1.40 | 0.21 | ||||
| Calculated PELD Score | < 0 | >= 20 | 0.98 | 0.93 | 0.99 | 1.28 | 0.49 | 0.91 |
| [0, 10) | 1.03 | 0.89 | 1.12 | 0.74 | ||||
| [10, 20) | 1.01 | 0.95 | 1.20 | 0.60 | ||||
| Rejection Status | Yes | No | 1.17 | 0.27 | 0.27 | 1.43 | 0.12 | 0.12 |
|
Number of Rejection Episodes |
1 | 0 | 0.90 | 0.54 | 0.0002 | 1.04 | 0.89 | 0.0036 |
| 2 | 1.96 | 0.0028 | 2.39 | 0.013 | ||||
| ≥3 | 2.62 | 0.0014 | 3.58 | 0.0030 | ||||
Table 4.
Multivariate Analysis of Time to Symptomatic EBV and PTLD
| Factor | Comparison Group | Reference Group | EBV (N=2228, Case=197) | PTLD (N=2228, Case=74) | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | P value | Overall p value | Hazard Ratio | P value | Overall p value | |||
| Era of Transplant | 1995-2001 | 2002-2007 | 2.00 | -- | <.0001 | 2.11 | -- | 0.0028 |
| Number of Rejection Episodes | 1 | 0 | 0.83 | 0.27 | 0.0014 | 0.92 | 0.75 | 0.016 |
| 2 | 1.74 | 0.015 | 2.07 | 0.039 | ||||
| ≥3 | 2.19 | 0.0098 | 2.86 | 0.015 | ||||
| EBV Status of Recipient | Negative | Positive | 3.51 | < 0.0001 | <0.0001 | 2.94 | 0.0040 | 0.12 |
| Unknown | 4.04 | 0.011 | 4.34 | 0.64 | ||||
Factors included in the initial model: Era of Transplant, Age at Transplant, Monoclonal AB Use at Transplant, EBV Status of Recipient, and Number of Rejection Episodes. Primary Diagnosis and age at transplant were not included due to correlation with EBV Status of Recipient.
We sought to explore factors that might account for the reduced incidence of SEBV and PTLD in the two eras. Overall the immunosuppression regimens did change between the two eras. The percentage of subjects with cyclosporine based immunosuppression decreased from 34.0% in era 1 to 8.5% in era 2 and tacrolimus use increased from 54.6% to 80.5%. Induction therapy was used in 11.7% in era 1 and 27.4% in era 2. Steroid use in the regimens did not change. There was no difference in the percentage of recipients who were EBV negative at the time of transplant between the two eras (71.2% v 68.4% p=0.09). In the earlier era, donors were slightly older and there were more live donors. In the later era, there were more cadaveric split liver donors. To assess the relative intensity of immunosuppression, we compared the mean and median trough levels of tacrolimus or cyclosporine at 7 days and 1, 6 and 12 months post transplant. Among all evaluable subjects in the database, there were significantly lower trough levels of tacrolimus at 7 days and 1, 6 and 12 months (p value<0.01) and of cyclosporine at 7 days and 1 month (p value <0.01) in 2002-2007 compared to the earlier era (Table 5). There was a significantly lower percentage of patients receiving steroids at 1, 6 and 12 months post transplant (p=0.03 at 1 month, <.0001 at 6 and 12 months) in 2002-2007 compared to the earlier era. There was no difference in the mean or median trough levels of tacrolimus or cyclosporine before 12 months post transplant between the patients who were EBV negative at the time or transplant and those who were EBV positive or whose status was unknown in either era. There were significantly more rejection episodes in the earlier era compared to the more recent era and more rejection episodes in the recipients with EBV positive/unknown in that earlier era (Table 6).
Table 5.
Trough levels of Calcineurin inhibitors by Era of Transplant
| Time post transplant | Medication | 1995-2001 | 2002-2007 | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Median | N | Mean | SD | Median | |||
| Day 7 | Cyclosporine | 304 | 328.3 | 157.3 | 308.0 | 103 | 275.6 | 197.2 | 257.0 | 0.0006 |
| Tacrolimus | 537 | 12.9 | 6.9 | 11.8 | 1107 | 11.6 | 5.9 | 10.6 | 0.0005 | |
| Month 1 | Cyclosporine | 234 | 315.6 | 128.5 | 302.0 | 83 | 273.3 | 103.8 | 270.0 | 0.0078 |
| Tacrolimus | 574 | 11.9 | 6.6 | 10.7 | 1068 | 11.1 | 4.6 | 10.2 | 0.0093 | |
| Month 6 | Cyclosporine | 214 | 230.7 | 141.3 | 208.5 | 58 | 209.9 | 82.9 | 206.5 | 0.61 |
| Tacrolimus | 613 | 9.2 | 3.8 | 8.7 | 1114 | 8.5 | 3.8 | 8.0 | 0.0003 | |
| Month 12 | Cyclosporine | 190 | 185.3 | 93.8 | 167.5 | 55 | 216.7 | 145.8 | 172.0 | 0.26 |
| Tacrolimus | 611 | 8.1 | 3.9 | 7.2 | 998 | 7.4 | 3.4 | 7.0 | 0.0005 | |
Table 6.
Number of Rejection Episodes by Era of Transplant and Recipient EBV Status
| 1995-2001 | 2002-2007 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recipient EBV positive/unknown | Recipient EBV negative | P value | Recipient EBV positive/unknown | Recipient EBV negative | P Value | |||||
| N | % | N | % | N | % | N | % | |||
| Total | 269 | 100.0 | 665 | 100.0 | 426 | 100.0 | 923 | 100.0 | ||
| Number of Rejection Episodes | 87 | 32.3 | 256 | 38.5 | 0.073 | 217 | 50.9 | 506 | 54.8 | 0.43 |
| 0 | ||||||||||
| 1 | 79 | 29.4 | 212 | 31.9 | 140 | 32.9 | 274 | 29.7 | ||
| 2 | 46 | 17.1 | 92 | 13.8 | 42 | 9.9 | 93 | 10.1 | ||
| 3+ | 57 | 21.2 | 105 | 15.8 | 27 | 6.3 | 50 | 5.4 | ||
EBV monitoring and treatment paradigms based on the levels of circulating EBV viral load have evolved in the last 10 years. We did not begin to systematically collect this data until 2006. We have examined the use of EBV monitoring for the diagnosis of SEBV and this was not significantly different between the two eras.
Outcome
The outcomes: clearance of disease, death, and graft loss for patients with SEBV and PTLD are shown in Table 7. One hundred and forty-two of 199 SEBV cases (71.4%) and 60 of 75 PTLD cases (80.0%) resolved with a mean time to resolution of 5.83 and 5.68 months, respectively, Twelve (6.0%) patients with SEBV and 5 (6.0 %) patients with PTLD died with unresolved SEBV and PTLD, respectively. Twenty-one (10.6%) patients with SEBV and 7 (9.3%) with PTLD experienced graft loss (death or rejection post SEBV/PTLD diagnosis). Similar rates of resolution, death and graft loss were seen in the subset of patients with SEBV who did not have PTLD compared to the larger SEBV group and those with PTLD. There was no significant era effect on outcome.
Table 7.
Morbidity, Graft Loss, Recurrence of Disease, and Recovery after a Diagnosis of symptomatic EBV or PTLD By Era of Transplant
| All Symptomatic EBV | PTLD | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 1995-2001 | 2002-2007 | P value | Total | 1995-2001 | 2002-2007 | P value | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |||
| Total | 199 | 100.0 | 121 | 100.0 | 78 | 100.0 | 75 | 100.0 | 48 | 100.0 | 27 | 100.0 | ||
| Recovery* | 142 | 71.4 | 93 | 76.9 | 49 | 62.8 | Log rank 0.53 | 60 | 80.0 | 38 | 79.2 | 22 | 81.5 | Log rank 0.0354 |
| Resolved | ||||||||||||||
| Unresolved, < 12 Mo F/U | 19 | 10.1 | 9 | 7.4 | 10 | 12.8 | 8 | 10.7 | 5 | 10.4 | 3 | 11.1 | ||
| Unresolved, ≥12 Mo F/U | 35 | 18.1 | 17 | 14.1 | 18 | 23.1 | 7 | 9.3 | 5 | 10.4 | 2 | 7.4 | ||
| Death Prior to Resolution | 3 | 0.5 | 2 | 1.6 | 1 | 1.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Death | 187 | 94.0 | 113 | 93.4 | 74 | 94.9 | Log rank 0.82 | 70 | 93.3 | 45 | 93.8 | 25 | 92.6 | Log rank 0.14 |
| No | ||||||||||||||
| Yes | 12 | 6.0 | 8 | 6.6 | 4 | 5.1 | 5 | 6.7 | 3 | 6.3 | 2 | 7.4 | ||
| Graft Loss Status (Death or re-Transplant after diagnosis of EBV/PTLD) | 178 | 89.4 | 106 | 87.6 | 72 | 92.3 | Log rank 0.78 | 68 | 90.7 | 43 | 89.6 | 25 | 92.6 | Log rank 0.56 |
| No | ||||||||||||||
| Yes | 21 | 10.6 | 15 | 12.4 | 6 | 7.7 | 7 | 9.3 | 5 | 10.4 | 2 | 7.4 | ||
| Recurrence of Disease | 188 | 94.5 | 112 | 92.6 | 76 | 97.4 | Chi-square 0.21 | 72 | 96.0 | 45 | 93.8 | 27 | 100.0 | Chi-square 0.55 |
| No | ||||||||||||||
| Yes | 11 | 5.5 | 9 | 7.4 | 2 | 2.6 | 3 | 4.0 | 3 | 6.3 | 0 | 0.0 | ||
| Follow-up Time (yrs) (mean±SD) | 199 | 4.4±2.8 | 121 | 5.7±2.7 | 78 | 2.4±1.4 | Kruskal - Wallis Test* <.0001 | 75 | 4.2±2.9 | 48 | 5.7±2.5 | 27 | 1.7±1.2 | Kruskal - Wallis Test* <.0001 |
non-parametric
Discussion
PTLD is a multifactorial process in children undergoing liver transplantation that is related to the interplay between immunosuppression and an EBV infection. As previously described and as expected, this study demonstrated that children who are EBV naïve at the time of transplant and those who have frequent episodes of rejection are at the highest risk for the development of SEBV and PTLD.
Several important observations come from this large multicenter study. There has been a significant reduction in the risk of the development of SEBV and PTLD during the first 2 years following liver transplantation in the more recent era (2002-2007) compared to the earlier era (1995-2001) with equal number of patients and risks. This suggests some factor or factors in management has changed the natural history of the development of PTLD.
This study provides strong evidence that the primary reason for the reduction in incidence of SEBV and PTLD is that there has been a general reduction in the overall “level of immunosuppression” in pediatric patients. There were significantly lower early levels of tacrolimus and cyclosporine and less steroid use in the patients in the more recent era, consistent with an overall reduction in immunosuppression during the recent era. Despite these lower levels, the frequency of rejection in all patients was lower in the more recent era with an apparent reduction in the level of immunosuppression.
Recent data from the SPLIT registry has suggested that the major mortality associated with pediatric liver transplantation is related to infections implicating potentially excessive immunosuppression (20). This is reinforced by our finding that the risk of both SEBV disease and PTLD was greater in patients with more than one rejection episode in the first year post transplant. These data indicate the relationship between the effect of increased levels of immunosuppression and the risk for SEBV and PTLD. With the knowledge from previous studies of the importance of the EBV status of the recipient, specifically the increased risk in an EBV naïve recipient, selective changes in the immunosuppressive management of these patients likely contributed to the decreasing incidence of both SEBV disease and PTLD.
There was a slight reduction in the use of older donors in the more recent era that was also reflected in less live donors. This is another factor that may contribute to the reduced incidence of PTLD, but given that most of the recipients were EBV naïve, immunosuppression is still likely the major factor for the decline in PTLD.
Another potential factor is the use of techniques to monitor EBV burden, specifically EBV viral load measurements in blood to identify transplant recipients who may be at increased risk for development of disease. Unfortunately, most of our data comes from before we prospectively determined EBV monitoring and management resultant from that monitoring. This is an area that needs further study. However, we speculate that EBV molecular monitoring leads to changes in immunosuppression prior to the development of symptomatic disease that may in part account for the decreasing incidence of both SEBV disease and PTLD in the recent era. Many centers have developed strategies for either prophylactic (antiviral treatments or alterations in immunosuppression regimen in higher risk patients) or preemptive (antiviral treatments or alterations in immunosuppression regimen in patients with asymptomatic elevated EBV viral load) strategies (15, 21). The use of these strategies has increased with the increased availability of EBV monitoring. However, antiviral use at the time of transplant (a surrogate for prophylactic treatment) was not found to be a significant factor in the univariate analysis. This may be complicated by the overlap between CMV prophylaxis and EBV prophylaxis, but does suggest that antiviral prophylaxis in this group did not alter the risk of either SEBV disease or PTLD independent of the level of immunosuppression.
Unfortunately, while the incidence of PTLD has decreased in the recent era, the outcome for those who developed PTLD in this study has not significantly improved over time. Possible implications of this finding are that PTLD represents a threshold beyond which outcome is already determined. It may also indicate that our treatment modalities have potentially improved resolution of PTLD or SEBV, but lead to an imbalance in graft survival. An alternative explanation is that the presence of SEBV or PTLD indicates a level of immunosuppression imbalance that leads to complications of infections as we have previously reported (20).
The primary risk factors for SEBV and PTLD in multivariate analysis were recipient EBV negative status, era of transplant and number of rejection episodes. The era of transplant and number of rejection episodes are strong indicators of the level of immunosuppression. EBV status of the recipient is tightly correlated with a younger age at transplant and a diagnosis of biliary atresia, which may explain the variation in these risk factors in other studies.
This large study of pediatric liver transplant recipients demonstrates that the incidence of SEBV and PTLD is clearly decreasing in the more recent era. The reasons behind this decline are likely multifactorial and complex. However, the primary factor is more attention to the level of immunosuppression and a global decrease in the level of immunosuppression in the more recent era. Future research should focus on the management of patients with subclinical EBV targeting younger patients, EBV naïve recipients and those who have more rejection episodes who seem to be at the highest risk for SEBV and PTLD.
Acknowledgements
SPLIT is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant U01-DK061693-01A1 and by Astellas Pharma US, Inc, and Roche Laboratories (unrestricted grant). The SPLIT Research Group is composed of the following centers, investigators, and coordinators: Alfred I Dupont Hospital for Children, Wilmington, DE (S. Dunn, J. Menendez, V. Gopalareddy); Boston Children's Hospital, Boston, MA (M. Jonas, L. Krawczuk); Cardinal Glennon Children's Hospital, St Louis University, St Louis, MO (R. Kane, H. Solomon, E. Phillips); Children's Healthcare of Atlanta, Atlanta, GA (T. Heffron, J. DePaolo, T. Pillen, G. Smallwood, L. Davis, D. Welch); Children's Hospital Cincinnati, Cincinnati, OH (J. Bucuvalas, W. Balistreri, F. Ryckman, A. Howkins, S. Krug, G. Arya); Children's Hospital, University of Colorado School of Medicine, Aurora, CO (M. Narkewicz, R. Sokol, F. Karrer, K. Orban-Eller); Children's Hospital of Philadelphia, Philadelphia, PA (E. Rand, C. Goodsell); Children's Hospital of Pittsburgh, Pittsburgh, PA (G. Mazariegos, L. Chien, A. Smith); Children's Hospital Western Ontario, London, Ontario, Canada (P. Atkinson); Children's Hospital of Wisconsin, Wauwatosa, WI (G. Telega, S. Lerret, J. Nebel); Children's Medical Center of Dallas, Dallas, TX (J. Roden, N. Mittal, B. Friedman, L. Arnott, D. Cross, L. Cutright, E. Faye); Children's Memorial Medical Center, Chicago, IL (E. Alonso, R. Superina, P. Whitington, J. Lokar, K. Neighbors, S. Kelly); Children's Mercy Hospital, Kansas City, MO (W. Andrews, J. Daniel, V. Fioravanti, A. Tendick); Duke University School of Medicine, Durham, NC (D. Desai, S. Jarvis); Hospital for Sick Children Toronto, Toronto, Ontario, Canada (E. DeLuca, A. Fecteau, V. Ng); Indiana University Medical Center, Indianapolis, IN (J. Tector, J. Lim, J. Molleston, J. Pearson); Johns Hopkins Hospital, Baltimore, MD (P. DeRusso, P. Colombani, M. Alford, C. Bhave, L. Wilson, R. Jurao); Le Bonheur Children's Medical Center, Memphis, TN (H. Shokough-Amiri, H. Grewal, S. Powell); Mayo Medical School, Rochester, MN (D. Freese, J. Weckwerth, J. Greseth, L. Pearson, L. Young); Medical College of Virginia, Richmond, VA (R. Fisher, M. Akyeampong, M. Benka, A. Lassiter); Mount Sinai Medical Center, New York, NY (S. Emre, B. Shneider, S. Cuellar, R. Gagliardi); New York Presbyterian Hospital, New York, NY (S. Lobritto, L. Smith, P. Harren, K. Ventura); Primary Children's Medical Center, Salt Lake City, UT (L. Book, M. O'Gorman, C. Kawai, L. Bruschke, J. Kraus, R. Thorson); Sainte-Justine Hospital, Montreal, Quebec, Canada (F. Alvarez, S. Martin, C. Viau); St Christopher's, Philadelphia, PA (H. Soriano, K. Falkenstein); St Louis Children's Hospital, St Louis, MO (R. Shepherd, J. Lowell, M. Nadler); Stanford University Medical Center, Palo Alto, CA (K. Cox, W. Berquist, M. Kreisl, S. Alvarez, R. Berquist); Texas Children's Hospital, Houston, TX (S. Karpen, J. Mayo, J. Goss, B. Carter, D. Ybarra); University of California Los Angeles Medical Center, Los Angeles, CA (S. McDiarmid, S. Fiest); University of Alberta, Edmonton, Alberta, Canada (S. Gilmour, L. Greet, N. Kneteman); University of California, San Diego, CA (J. Lavine, A. Khanna, R. Clawson); University of California, San Francisco, CA (P. Rosenthal, D. Filipowski, J. Roberts); University of Chicago, Chicago, IL (J. Millis, P. Boone); University of Florida-Shands, Gainesville, FL (R. Gonzalez-Peralta, M. Hodik, M. Langham); University of Miami, Jackson Memorial Hospital, Miami, FL (A. Tzakis, T. Kato, D. Weppler, L. Cooper, M. Gonzalez, A. Santiago); University of Michigan, Ann Arbor, MI (J. Lopez, J. Magee, V. Shieck); University of Minnesota, Minneapolis, MN (A. Humar, B. Durand); University of Nebraska, Omaha, NE (A. Langnas, D. Antonson, J. Botha, W. Grant, D. Sundan, D. Andersen, B. Fleckten, K. Seipel, B. Shaw, C. Torres); University of North Carolina, Chapel Hill, NC (J. Fair, J. Prinzhorn, P. McIver, J. Young, A. Raizada); University of Rochester, Strong Memorial Hospital, Rochester, NY (T. Shisler, M. Orloff, T. Rossi, C. Hill- Sober); University of Texas, HSC San Antonia, San Antonio, TX (G. Halff, J. Silva); University of Washington, Seattle, WA (S. Horslen, K. Larocque); and University of Wisconsin, Madison, WI (M. Kalayoglu, A. D'Alessandro, N. Erickson, R. Judd, S. Knechtle, E. Spaith).
References
- 1.Cox KL, Lawrence-Miyasaki LS, Garcia-Kennedy R, Lennette ET, Martinez OM, Krams SM, et al. An increased incidence of Epstein-Barr virus infection and lymphoproliferative disorder in young children on FK506 after liver transplantation. Transplantation. 1995;59:524–9. [PubMed] [Google Scholar]
- 2.Guthery SL, Heubi JE, Bucuvalas JC, Gross TG, Ryckman FC, Alonso MH, et al. Determination of risk factors for Epstein-Barr virus-associated posttransplant lymphoproliferative disorder in pediatric liver transplant recipients using objective case ascertainment. Transplantation. 2003;75:987–93. doi: 10.1097/01.TP.0000057244.03192.BD. [DOI] [PubMed] [Google Scholar]
- 3.Sokal EM, Antunes H, Beguin C, Bodeus M, Wallemacq P, de Ville de Goyet J, et al. Early signs and risk factors for the increased incidence of Epstein-Barr virus-related posttransplant lymphoproliferative diseases in pediatric liver transplant recipients treated with tacrolimus. Transplantation. 1997;64:1438–42. doi: 10.1097/00007890-199711270-00011. [DOI] [PubMed] [Google Scholar]
- 4.Newell KA, Alonso EM, Whitington PF, Bruce DS, Millis JM, Piper JB, et al. Posttransplant lymphoproliferative disease in pediatric liver transplantation. Interplay between primary Epstein-Barr virus infection and immunosuppression. Transplantation. 1996;62:370–5. doi: 10.1097/00007890-199608150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Cacciarelli TV, Reyes J, Jaffe R, Mazariegos GV, Jain A, Fung JJ, et al. Primary tacrolimus (FK506) therapy and the long-term risk of post-transplant lymphoproliferative disease in pediatric liver transplant recipients. Pediatr Transplant. 2001;5:359–64. doi: 10.1034/j.1399-3046.2001.00021.x. [DOI] [PubMed] [Google Scholar]
- 6.Younes BS, McDiarmid SV, Martin MG, Vargas JH, Goss JA, Busuttil RW, et al. The effect of immunosuppression on posttransplant lymphoproliferative disease in pediatric liver transplant patients. Transplantation. 2000;70:94–9. [PubMed] [Google Scholar]
- 7.Tsao L, Hsi ED. The clinicopathologic spectrum of posttransplantation lymphoproliferative disorders. Arch Pathol Lab Med. 2007;131:1209–18. doi: 10.5858/2007-131-1209-TCSOPL. [DOI] [PubMed] [Google Scholar]
- 8.Kremers WK, Devarbhavi HC, Wiesner RH, Krom RA, Macon WR, Habermann TM. Post-transplant lymphoproliferative disorders following liver transplantation: incidence, risk factors and survival. Am J Transplant. 2006;6:1017–24. doi: 10.1111/j.1600-6143.2006.01294.x. [DOI] [PubMed] [Google Scholar]
- 9.Ho M, Jaffe R, Miller G, Breinig MK, Dummer JS, Makowka L, et al. The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestations in children. Transplantation. 1988;45:719–27. doi: 10.1097/00007890-198804000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao S, Cox KL, Berquist W, Hayashi M, Concepcion W, Hammes GB, et al. Long-term outcomes in pediatric liver recipients: comparison between cyclosporin A and tacrolimus. Pediatr Transplant. 1999;3:22–6. doi: 10.1034/j.1399-3046.1999.00002.x. [DOI] [PubMed] [Google Scholar]
- 11.Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O'Sullivan EJ, Johnson MR, Heroux AL, et al. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N Engl J Med. 1990;323:1723–8. doi: 10.1056/NEJM199012203232502. [DOI] [PubMed] [Google Scholar]
- 12.Holmes RD, Sokol RJ. Epstein-Barr virus and post-transplant lymphoproliferative disease. Pediatr Transplant. 2002;6:456–64. doi: 10.1034/j.1399-3046.2002.02043.x. [DOI] [PubMed] [Google Scholar]
- 13.Otte JB. History of pediatric liver transplantation. Where are we coming from? Where do we stand? Pediatr Transplant. 2002;6:378–87. doi: 10.1034/j.1399-3046.2002.01082.x. [DOI] [PubMed] [Google Scholar]
- 14.Gridelli B, Spada M, Riva S, Colledan M, Segalin A, Lucianetti A, et al. Circulating Epstein-Barr virus DNA to monitor lymphoproliferative disease following pediatric liver transplantation. Transpl Int. 2000;13(Suppl 1):S399–401. doi: 10.1007/s001470050370. [DOI] [PubMed] [Google Scholar]
- 15.McDiarmid SV, Jordan S, Kim GS, Toyoda M, Goss JA, Vargas JH, et al. Prevention and preemptive therapy of postransplant lymphoproliferative disease in pediatric liver recipients. Transplantation. 1998;66:1604–11. doi: 10.1097/00007890-199812270-00006. [DOI] [PubMed] [Google Scholar]
- 16.Green M, Michaels MG, Katz BZ, Burroughs M, Gerber D, Shneider BL, et al. CMV-IVIG for prevention of Epstein Barr virus disease and posttransplant lymphoproliferative disease in pediatric liver transplant recipients. Am J Transplant. 2006;6:1906–12. doi: 10.1111/j.1600-6143.2006.01394.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee TC, Savoldo B, Rooney CM, Heslop HE, Gee AP, Caldwell Y, et al. Quantitative EBV viral loads and immunosuppression alterations can decrease PTLD incidence in pediatric liver transplant recipients. Am J Transplant. 2005;5:2222–8. doi: 10.1111/j.1600-6143.2005.01002.x. [DOI] [PubMed] [Google Scholar]
- 18.Studies of Pediatric Liver Transplantation (SPLIT): year 2000 outcomes. Transplantation. 2001;72:463–76. doi: 10.1097/00007890-200108150-00018. [DOI] [PubMed] [Google Scholar]
- 19.Harris NL, Swerdlow SH, Frizzera G, M KD. Post-transplant lymphoproliferative disorders. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. World Health Organization Classification of Tumors. IARC Press; Lyon, France: 2001. pp. 264–9. [Google Scholar]
- 20.Shepherd RW, Turmelle Y, Nadler M, Lowell JA, Narkewicz MR, McDiarmid SV, et al. Risk factors for rejection and infection in pediatric liver transplantation. Am J Transplant. 2008;8:396–403. doi: 10.1111/j.1600-6143.2007.02068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes RD, Orban-Eller K, Karrer FR, Rowe DT, Narkewicz MR, Sokol RJ. Response of elevated Epstein-Barr virus DNA levels to therapeutic changes in pediatric liver transplant patients: 56-month follow up and outcome. Transplantation. 2002;74:367–72. doi: 10.1097/00007890-200208150-00013. [DOI] [PubMed] [Google Scholar]