Abstract
Early pharmacological studies of Aconitum and Delphinium sp. alkaloids suggested that these neurotoxins act at site 2 of voltage-gated Na+ channel and allosterically modulate its function. Understanding structural requirements for these compounds to exhibit binding activity at voltage-gated Na+ channel has been important in various fields. This paper reports quantum-chemical studies and quantitative structure-activity relationships (QSARs) based on a total of 65 natural alkaloids from two plant species, which includes both blockers and openers of sodium ion channel. A series of 18 antagonist alkaloids (9 blockers and 9 openers) have been studied using AM1 and DFT computational methods in order to reveal their structure-activity (structure-toxicity) relationship at electronic level. An examination of frontier orbitals obtained for ground and protonated forms of the compounds revealed that HOMOs and LUMOs were mainly represented by nitrogen atom and benzyl/benzoylester orbitals with –OH and –OCOCH3 contributions. The results obtained from this research have confirmed the experimental findings suggesting that neurotoxins acting at type 2 receptor site of voltage-dependent sodium channel are activators and blockers with common structural features and differ only in efficacy. The energetic tendency of HOMO-LUMO energy gap can probably distinguish activators and blockers that have been observed. Genetic Algorithm with Multiple Linear Regression Analysis (GA-MLRA) technique was also applied for the generation of two-descriptor QSAR models for the set of 65 blockers. Additionally to the computational studies, the HOMO-LUMO gap descriptor in each obtained QSAR model has confirmed the crucial role of charge transfer in receptor-ligand interactions. A number of other descriptors such as logP, IBEG, nNH2, nHDon, nCO have been selected as complementary ones to LUMO and their role in activity alteration has also been discussed.
Keywords: alkaloids, QSAR, toxicity, antiarrhythmic activity, Na+ channel modulator
1. Introduction
Voltage-gated sodium channels are transmembrane proteins responsible for signal transduction and amplification. They are primary molecular targets for several groups of naturally occurring neurotoxins and a number of drugs (Clare et al., 2000, Catterall, 2001, Baker and Wood, 2001, Taylor and Meldrum, 1995). One of these groups is represented by lipid-soluble neurotoxins targeting type 2 receptor site on voltage-gated Na+ channels. Site 2 toxins are of very diverse chemical structures and a number of studies on mechanisms of their action suggest that they promote Na+ channel opening by indirect allosteric interactions (Cestele and Catterall, 2000, Wang and Wang, 2003). The recent investigations (Wang and Wang, 2003) showed that the receptor site of these neurotoxins appears to be adjacent to or overlap with that for sodium channel blockers (anticonvulsants, antidepressants, local anaesthetics, antiarrhythmics etc).
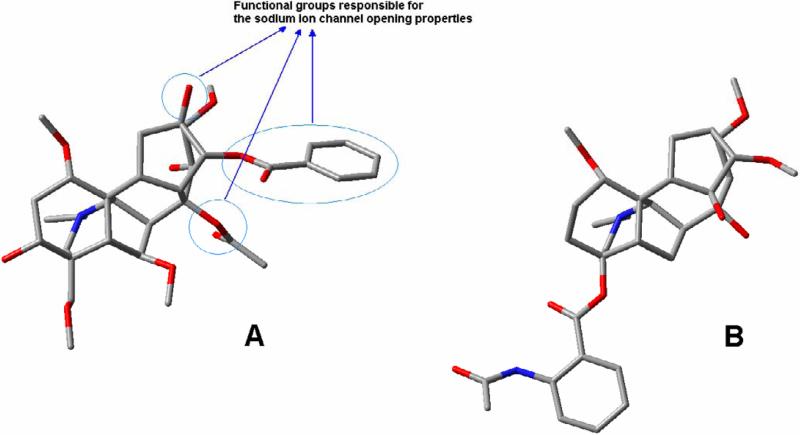
Diterpene alkaloids isolated from Delphinium and Aconitum plant species are compounds of considerable interest as they belong to site 2 neurotoxins (Anger et al., 2001). Paradoxically, despite of similar molecular structures these alkaloids exhibit antagonistic alteration of sodium channel function and therefore different therapeutic action (Benn and Jacyno, 1984, Ameri, 1998, Friese et al., 1997, Wright, 2001, Heubach and Schule, 1998, Dzhakhangirov et al., 1997, Ameri and Simmet, 1999). In particular, aconitine-type compounds are suggested to bind with high affinity to activated sodium channels and shift conformational equilibrium toward the activated state (Catteral, 1980, Mozahayeva et al., 1977). On the contrary, the other group of alkaloids isolated from the same plant species and having very similar (heteratisine and napelline) or even identical (lycoctonine) to aconitine core skeletons are reported to posses strong antinociceptive, antiarrhythmic and antiepiletiform properties due to a blockade of the voltage-dependent sodium channel. Thus, as it was demonstrated earlier (Valeev et al. 1990, Ameri, 1997), arrhythmogenic effect of aconitine can quickly be reversed by antiarrhythmic agent lappaconitine (Fig. 1). The most interesting fact about these two alkaloids is that they both belong to the subgroup comprised of molecules with lycoctonine skeleton (Dzhakhangirov et al.,, 1997). As it was reported elsewhere (Dzhakhangirov et al., 1997), there are four active regions in aconitine molecule: nitrogen atom of lycoctonine skeleton that acquires strong positive charge when protonated in a solution, and three functional residues (hydroxyl group at C13, benzoylester group at C14 and acetyl group at C8) playing a crucial role for exhibiting channel opening properties (Fig.1 A). Interestingly, the absence of any of functional groups mentioned results in blockade of sodium ion channel.
Figure 1.
Aconitine alkaloid molecule (A) with three functional residues responsible for the sodium channel opening activity and its sodium channel antagonist modulator Lappaconitine alkaloid molecule (B)
Large number of various groups of sodium channel binders have been the target of extensive multidisciplinary studies including computational approach. This is mainly due to the proven therapeutic value of voltage-gated sodium channel modulators in local anaesthesia, cardiac arrhythmia, pain, epilepsy, stroke and other disorders (Benn and Jacyno, 1984, Ameri, 1998). A literature survey revealed computational and molecular modelling technique have been used primarily for developing QSARs and pharmacophore model generation (Li and Harte, 2002) as 3D x-ray structure of sodium channel is not available yet. These two techniques are considered valuable tools in design of sodium channel modulators and any other drugs of better efficiency. Moreover, existing parallelism between two or more activities exhibited by Na+ channel binders (for example antiarrhythmic agents posses also local anaesthetic activity (Gupta, 1998)) can impede at development of new active analogues while QSAR methods allows to find factors affecting on secondary activities to develop active compounds without side effects (Unverferth et al., 1998, Chung-Chin Kuo et al., 2000).
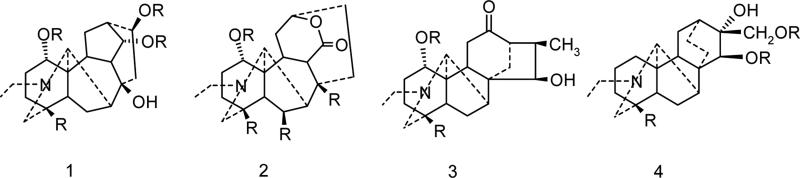
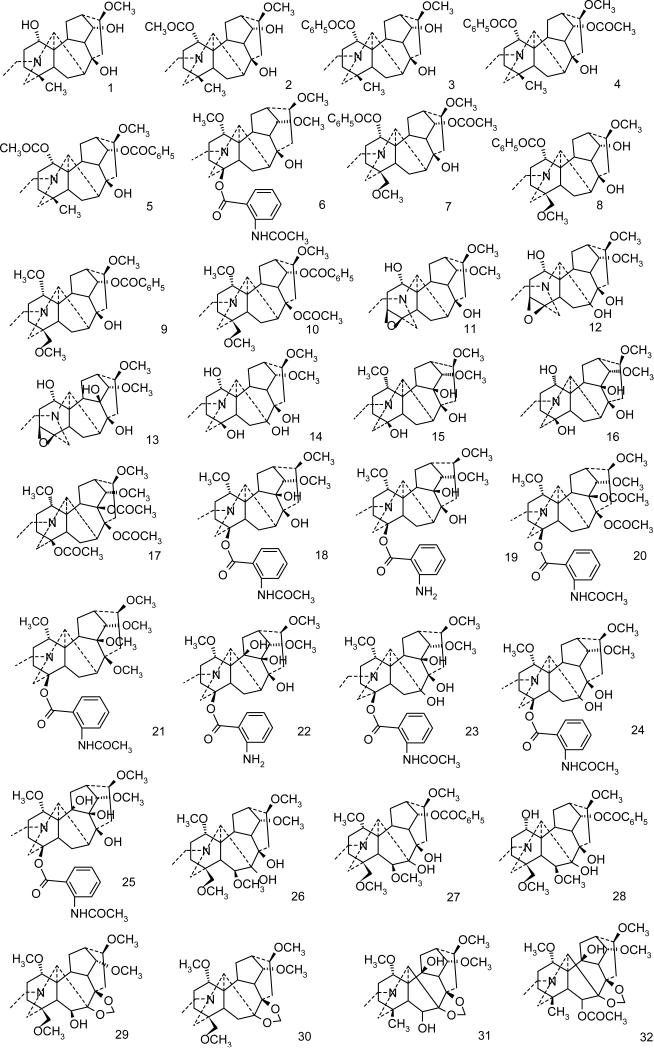
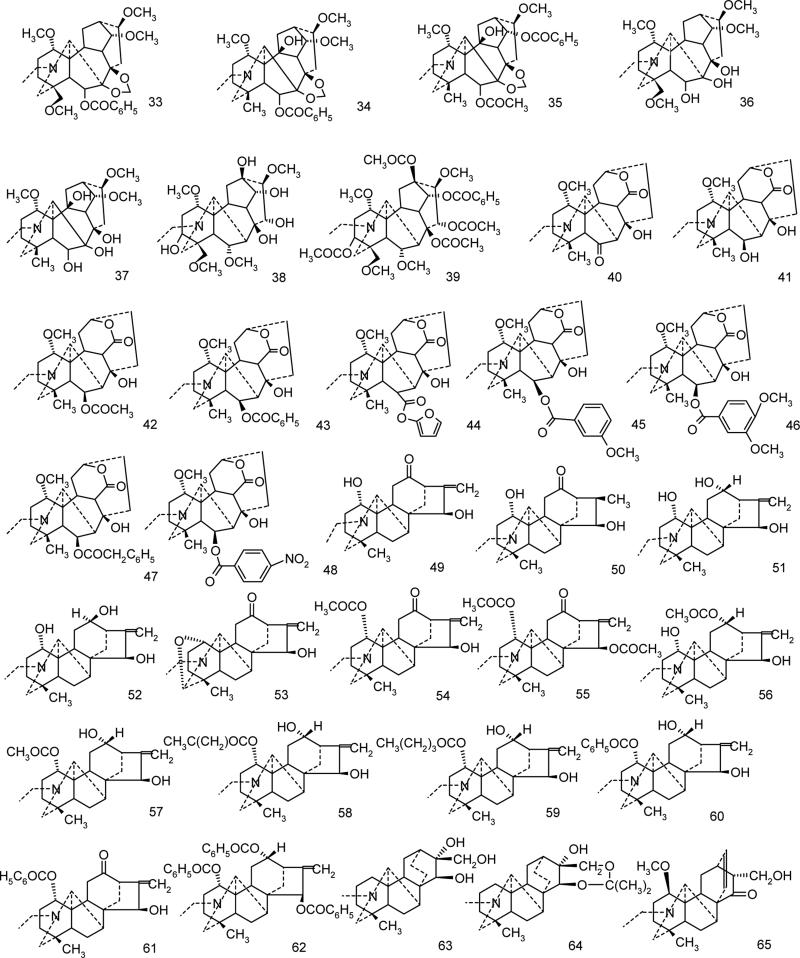
Though Delphinium and Aconitum alkaloids have been known for centuries and applied in folk medicine for their analgesic, antirheumatic and neurological indications, they have received little attention of computational chemists. Hence only recently a QSAR analysis of the analgesic and anesthetic properties for 12 Aconitum alkaloids (Bello-Ramirez et al., 2003, Bello-Ramirez and Nava-Ocampo, 2004, Bello-Ramirez and Nava-Ocampo, 2004) and 19 nAChR antagonists (Turabekova and Rasulev, 2005) have been reported. The toxicity/activity data available for these alkaloids and the intriguing sodium channels modulation exhibited (openers and blockers in one homologous series) makes them very interesting targets for molecular modelling studies. The present work attempts to shed some light on antagonist Na+ channel modulation of Delphinium and Aconitum species alkaloids by predicting structure-activity (toxicity) relationship at electronic structure level. The library of the alkaloids of the following four skeletons: lycoctonine, napelline, heteratizine and denadutine have been the targets of our investigations (Fig. 2).
Figure 2.
Lycoctonine (1), heteratizine (2), napelline (3) and denadutine (4) core skeletons of Aconitum and Delphinium alkaloids
2. Materials and Methods
2.1. Training Compounds
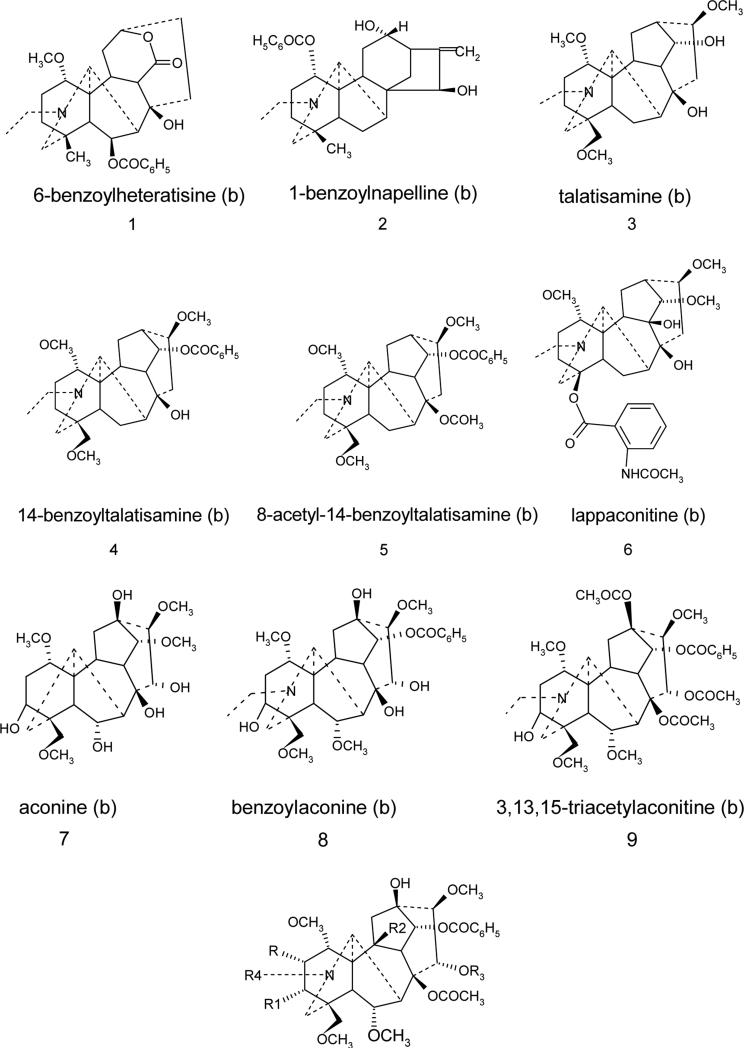
Selection of diterpene alkaloids for AM1/DFT Electronic Structure Investigations has been performed ensuring an equal number of channel blockers and openers in a series (9 openers and 9 blockers). Moreover, the structures maximally related to aconitine alkaloid (strong activator) but with various combinations of three crucial residues were of the first choice. (Fig. 3)
Figure 3.
Aconitum and Delphinium alkaloids selected for AM1/DFT studies.
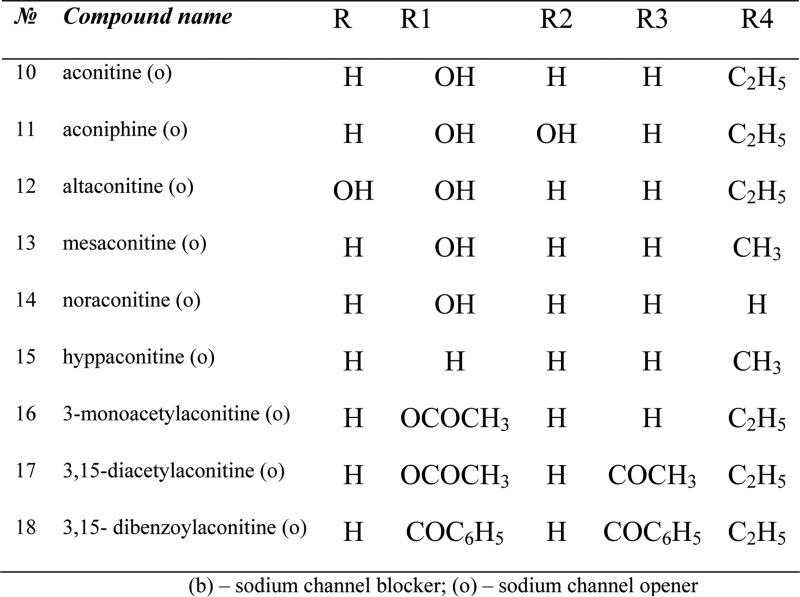
The QSAR studies have been performed for the 65 antiarrhythmic and 63 toxicity data available for the set of sodium channel blocking alkaloids (Figure 4).
Figure 4.
A series of Aconitum and Delphinium alkaloids studied.
2.2. Biological Data
Toxicity and antiarrhythmic activity data used in this study are obtained from the early report (Dzhakhangirov et al., 1997). The data suggest alkaloids inducing sodium channel activation are more toxic than those alkaloids that block Na+ ion permeation through the channels. All original in vivo LD50 toxicity data (mg/kg) were expressed as the logarithm of the inverse molar concentration (log 1/LD50) response variables. Acute intravenous mammalian toxicity data have been applied as it is known to produce QSAR models of better predicting abilities rather than in vitro toxicological assays (Cronin, 2003).
2.3. Molecular Modeling
All molecular models were built using the HyperChem 6.01 software package (HyperChem 6.01 (HyperCube, Inc), 2000). The Molecular Mechanics (MM+) force field was applied for preliminary structure optimization and study of the conformational behavior of each alkaloid. Molecular mechanics has been shown to produce realistic geometry values for the majority of organic molecules owing to the fact of being highly parameterized (Young, 2001). The global minimum-energy conformation for each compound was identified using the following steps: (1) geometry optimization of each molecule to the local minimum-energy conformation using an energy gradient of 0.001 kcal/(mol Å), (2) analysis of conformational space by varying all degrees of freedom (i.e. torsional angles) of side chains by using a Monte Carlo search, (3) geometry re-optimization of the molecules to their lowest minimum-energy conformation using the bond angles established in step two. The next step was a re-optimization of the MM+ optimized structures by using combined technique of AM1/DFT computation in Gaussian03 package (Gaussian 03, 2004). In particular, geometry optimizations were performed using AM1 Hamiltonian followed by single point calculations at gradient-corrected density functional levels of theory. A combination of Becke's three-parameter adiabatic connection exchange functional with Lee-Yang-Parr correlation (B3LYP/ 6-31G(d,p)) was employed in order to obtain reliable energetics and accurate data on electronic properties of molecules. This technique simplifies the ab initio procedure of calculation of quite large molecules by saving time due to AM1 geometry optimization. As can be seen from the Figure 3 the molecular structures of the alkaloids are quite rigid. Nevertheless, it is known that the variability of the molecular orbitals and of the energies is highly dependent on the conformation.
2.4. QSAR and Statistical Software
Preliminary models selection was performed by means of GA-MLRA (Devillers, 1996) technique as implemented in the BuildQSAR (de Oliveira and Gaudio, 2000) program. This approach allows selection of the models with the following characteristics for the better performance: high correlation coefficient R, low standard deviation S and the least number of descriptors involved. Next, the NCSS98 (NCSS98, 1998) professional software package was applied for detailed statistical analysis of the models obtained. Thus, the high F-ration coefficient, non-collinear descriptors and the significance level P variable served as additional selection parameters.
The correlation coefficients for all pair of descriptor variables used in the models were evaluated in order to identify highly correlated descriptors and to detect redundancy in the data set. Any type of redundancy might lead to an overexploitation of a chemical property in the explanation of the dependent variable. Hence, some highly correlated and constant descriptors (cross-correlation r2>0.9) were removed from the further consideration. Furthermore, at the process of each model building (i.e. inside of each model) the descriptors with cross-correlation coefficient more than 0.6 are avoided.
A final set of QSARs was identified by applying the “leave-one-out” technique with its predicting ability being evaluated and confirmed by cross validation coefficient q2 based on predictive error sum of squares (SPRESS). Physicochemical descriptors used in this study have been calculated applying the DRAGON program (Todeschini and Consonni, 2003).
3. Results and Discussion
3.1. AM1/DFT Electronic Structure Calculations
All 18 compounds included in this study are shown in Fig.3 of which 9 are blockers (1-9) and other 9 (10-18) compounds are openers of sodium ion channel. AM1 geometry optimization followed by single point B3LYP/6-31G(d, p) calculations were carried out for the detailed investigations of frontier orbitals in order to reveal whether electronic features of a molecule would reflect the modulation effect nature of a particular alkaloid. Aiming to understand better the experimental results, the energies of HOMO (highest occupied molecular orbital, a measure of nucleophilicity), and LUMO (lowest unoccupied molecular orbital, a measure of electrophilicity) of the compounds were calculated. Four most important molecular orbitals were analyzed including HOMO, second highest molecular orbital (HOMO-1), LUMO and second lowest unoccupied molecular orbital (LUMO+1).
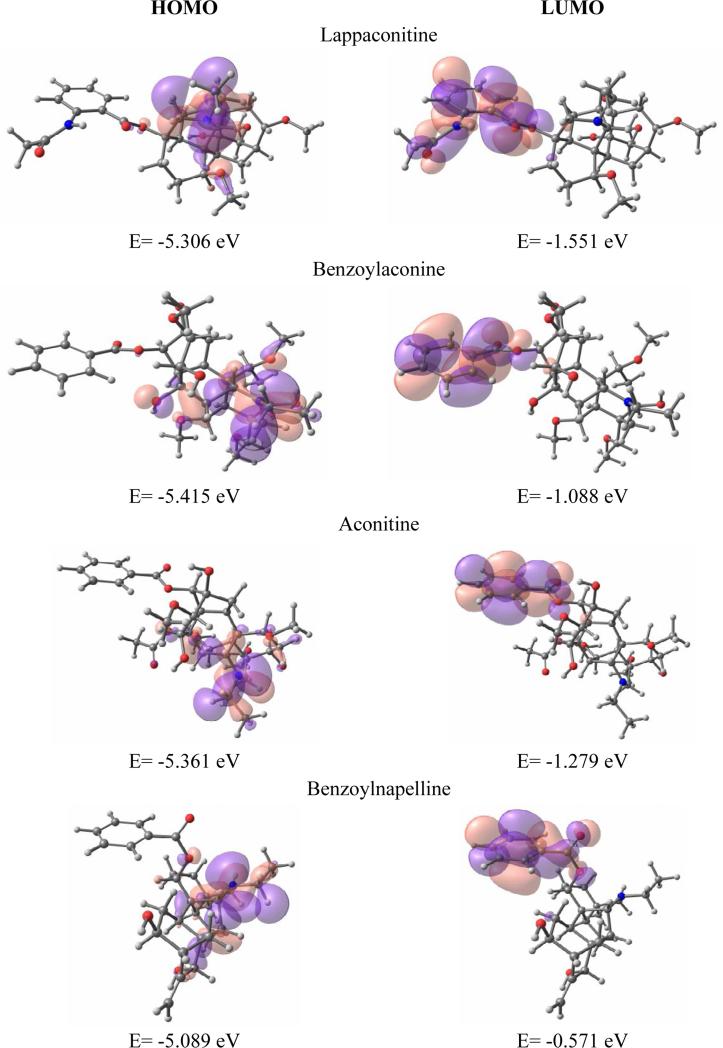
Thus, well definite separation of functional groups was observed in distribution of HOMO and LUMO. They are located in two distinct parts of each molecule. Also, further molecular orbitals analysis showed two distinct location sites for each HOMO: the HOMOs were mainly represented by contributions of lycoctonine carcass nitrogen atom orbitals, while all the HOMO-1s were comprised of benzoylester's atom orbitals. LUMOs and LUMO+1s were located mostly on benzyl/benzoylester group apart from talatizamine and aconine alkaloids that do not possess such side chain. No contribution from nitrogen atom orbitals has been predicted for these orbitals. Interestingly, the HOMO (and HOMO-1) geometries were almost identical for each alkaloid of a set and therefore no difference induced at physicochemical level is to be expected. Likewise, after having a close look at LUMOs no substantial features have been identified that would help to distinguish whether particular structure is activator or blocker of Na+ channels. The Figure 5 shows LUMOs plotted for benzoylnapelline (strong blocker), benzoylaconine (blocker), aconitine (strong activator) and lappaconitine (strong blocker) that are distributed on benzoylester group irrespectively of its attachment position. Moreover, it clearly demonstrates that three essential functional groups do not participate altogether in this molecular orbital formation. The exception is benzoylester group (about 90-95% contribution) which however can not be solely responsible for opening the sodium ion channels.
Figure 5.
Ground state: LUMO/HOMO isosurfaces of lappaconitine 6, benzoylaconine 8, aconitine 10 and benzoylnapelline 2 (positive –pink; negative – violet)
Toxicity and/or therapeutic action of many medicinal agents strongly depend on solubility of a molecule which subsequently defines its bioavailability. The tertiary nitrogen atom present in each molecule would be a centre with the highest proton affinity once drug is in water.
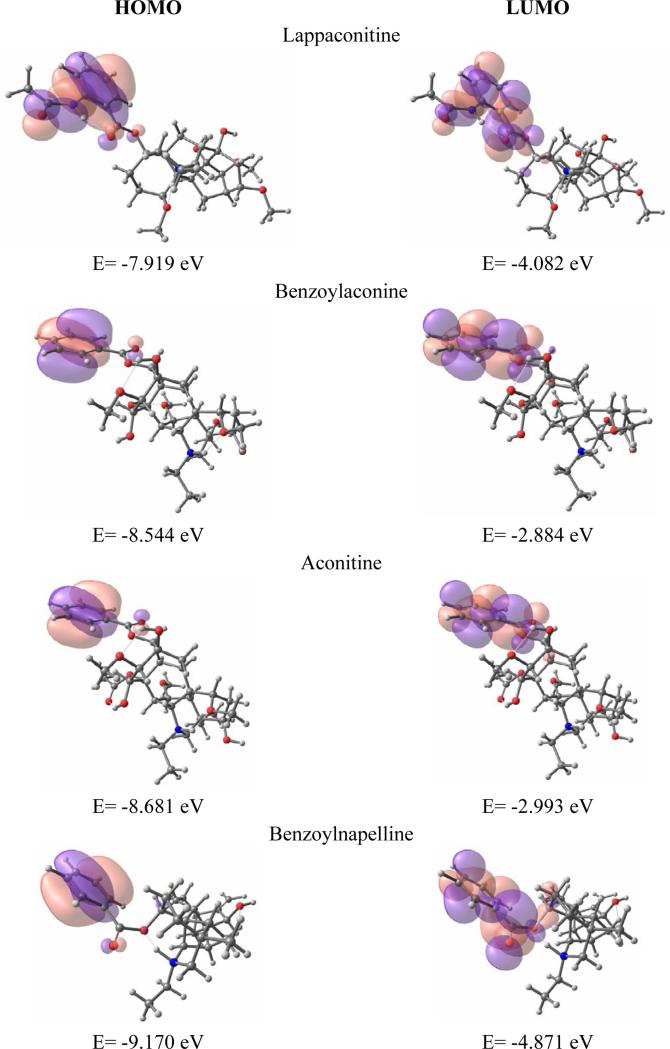
The next logical step involves the exploration of re-arranged electronic structure of alkaloids induced by protonation of the tertiary nitrogen. The careful examination of molecular orbitals revealed that the orbitals corresponding to the nitrogen atom have acquired a lower energy as the result of its protonation. Hence, the HOMO has moved to the group of inner orbitals, while LUMO has now appeared in the group of frontier orbitals (Fig.6). Again, close values of LUMO and LUMO+1 have been observed for protonated forms of alkaloids.
Figure 6.
Protonated state: LUMO/HOMO isosurfaces of benzoylnapelline 2, benzoylaconine 8, aconitine 10 and lappaconitine 6 (positive – pink; negative – violet)
As we already mentioned Na+ channel activators and blockers have several common features and it was well summarized (D.B. Tikhonov et al., 2005). First, the structures are not vastly different. Second, both activators and blockers bind in use-dependent manner. Third, both activators and blockers can prevent channel closure and shift gating equilibrium of the channel. Authors also pointed that action of activators and blockers on the channel conductance is obviously different, but the difference is quantitative rather then qualitative. Practically all activators decrease the unitary current, having an efficacy <1. In extreme, the activators having a zero efficacy are blockers. In other words, the binding of blockers completely abolishes channel conductance, whereas activators allow ions to flow, but at reduced rate.
Based on previously stated, it is clear that both activators and blockers share common structural features and differ only in efficacy. Therefore, the energetic properties can play important role in activity profile of these alkaloids. For supporting hypothesis about importance of energetic properties, the following correlation observed - the correlation between HOMO-LUMO gap values that distinguish activators and blockers. For ground state we have not seen any essential difference in HOMO-LUMO gap values for all four compounds (lappaconitine (3.76 eV), benzoylaconine (4.33 eV), aconitine (4.08 eV) and benzoylnappeline (4.52eV )), whereas for protonated state we can see the difference (3.84, 5.66, 5.69, 4.30, respectively). I.e. aconitine (activator) has the highest value of HOMO-LUMO gap, while for the rest compounds (blockers) these values slowly decreasing as blockers increase in strength (benzoylaconine (weak blocker)<benzoylnapelline<lappaconitine (strong blocker)).
The location of LUMO on benzoylester group (for ground and protonated states) suggests that this group plays an important role in interacting with the binding site of sodium channel together with HOMO (which is active in protonated state), i.e. the benzoylester group and its position determine the interaction with the binding site on the sodium channel and the later observation was confirmed by a number of experimental papers (Valeev et al. 1990). Additionally, it is very likely that benzoylester group defines the toxicity of the compound due to its activity (high contribution to LUMO and HOMO from this group). The later is also supported by the fact that those compounds, which lack of benzoylester group, are much less toxic.
3.2. QSAR Analysis of Na+ channel blocker alkaloids
The next step applied in this study was QSAR analysis. Aiming to identify which of the physicochemical descriptors (molar refractivity MR, molecular weight, MW, lipophilicity MlogP etc.) or quantum-chemical characteristics (energy of HOMO, energy of LUMO, HOMO and LUMO energy gap (Todeschini, 2000) calculated for both ground and protonated compounds applying AM1 Hamiltonian) correlates best with toxicity and antiarrhythmic activity data we built several QSAR models. The statistical significance of each model is evaluated by the correlation coefficient r, standard error s, adjusted r-squared r2adj, F-test value, significance level of the model P, leave-one-out cross-validation coefficient q2 and predictive error sum of squares SPRESS. The descriptors used to build QSARs are collected in Tables 1.
Table 1.
The list of descriptors applied in this study
| No. | AA | TOX | LUMO | nOHt | nHDon | logP | nNH2 | nCO | HLgap | IBEG |
|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 4.36 | 3.87 | 2.55 | 1 | 3 | 0.48 | 0 | 0 | 11.72 | 0 |
| 02 | 4.38 | 3.52 | 1.17 | 1 | 2 | 0.61 | 0 | 0 | 10.07 | 0 |
| 03 | 5.2 | 4.36 | −0.32 | 1 | 2 | 1.76 | 0 | 0 | 8.59 | 1 |
| 04 | 5.46 | 4.16 | −0.28 | 1 | 1 | 1.89 | 0 | 0 | 8.62 | 1 |
| 05 | 6.05 | 4.45 | −0.34 | 1 | 1 | 1.89 | 0 | 0 | 8.53 | 1 |
| 06 | 6.45 | - | −0.56 | 1 | 2 | −1.03 | 0 | 0 | 8.21 | 1 |
| 07 | 5.7 | 4.01 | −0.32 | 1 | 1 | 1.17 | 0 | 0 | 8.67 | 1 |
| 08 | 5.3 | 4.31 | −0.36 | 1 | 2 | 1.04 | 0 | 0 | 8.67 | 1 |
| 09 | 6.3 | 4.32 | −0.31 | 1 | 1 | 1.32 | 0 | 0 | 8.47 | 1 |
| 10 | 5.85 | 4.58 | −0.23 | 0 | 0 | 1.44 | 0 | 0 | 8.53 | 1 |
| 11 | 4 | 3.19 | 2.12 | 1 | 2 | −0.97 | 0 | 0 | 11.48 | 0 |
| 12 | 4.3 | 2.91 | 2.16 | 2 | 3 | −1.75 | 0 | 0 | 11.36 | 0 |
| 13 | 4.16 | 3.5 | 2.12 | 2 | 3 | −1.68 | 0 | 0 | 11.48 | 0 |
| 14 | 4.27 | 2.98 | 2.54 | 3 | 4 | −1.47 | 0 | 0 | 11.63 | 0 |
| 15 | 4.28 | 3.34 | 2.44 | 3 | 3 | −1.12 | 0 | 0 | 11.24 | 0 |
| 16 | 4.36 | 3.32 | 2.43 | 3 | 4 | −1.40 | 0 | 0 | 11.66 | 0 |
| 17 | 4.62 | 3.58 | 1.24 | 0 | 0 | −0.73 | 0 | 0 | 10.08 | 0 |
| 18 | 7.07 | 5.0 | −0.54 | 2 | 3 | −1.73 | 0 | 0 | 8.33 | 1 |
| 19 | 7.04 | 4.87 | −0.09 | 2 | 3 | −1.56 | 1 | 0 | 8.48 | 1 |
| 20 | 5.52 | 4.42 | −0.65 | 0 | 1 | −1.47 | 0 | 0 | 8.35 | 1 |
| 21 | 4.97 | 3.47 | −0.51 | 0 | 1 | −1.18 | 0 | 0 | 8.25 | 1 |
| 22 | 7.42 | 4.53 | −0.13 | 3 | 4 | −2.44 | 1 | 0 | 8.47 | 1 |
| 23 | 7.07 | 4.99 | −0.53 | 3 | 4 | −2.51 | 0 | 0 | 8.08 | 1 |
| 24 | 6.36 | - | −0.55 | 2 | 3 | −1.8 | 0 | 0 | 8.05 | 1 |
| 25 | 6.93 | 4.6 | −0.58 | 3 | 4 | −2.65 | 0 | 0 | 8.29 | 1 |
| 26 | 4.07 | 3.68 | 2.71 | 2 | 2 | −0.88 | 0 | 0 | 11.29 | 0 |
| 27 | 5.94 | 4.51 | −0.11 | 2 | 2 | 0.12 | 0 | 0 | 8.50 | 1 |
| 28 | 5.49 | 4.2 | −0.12 | 2 | 3 | −0.16 | 0 | 0 | 8.93 | 1 |
| 29 | 4.5 | 3.62 | 2.11 | 0 | 1 | −0.41 | 0 | 0 | 10.90 | 0 |
| 30 | 4.7 | 4.0 | 2.29 | 0 | 0 | 0.28 | 0 | 0 | 11.00 | 0 |
| 31 | 4.26 | 3.3 | 2.02 | 1 | 2 | −0.45 | 0 | 0 | 10.79 | 0 |
| 32 | 4.7 | 3.57 | 1.22 | 1 | 1 | −0.41 | 0 | 0 | 9.97 | 0 |
| 33 | 5.46 | 4.11 | −0.27 | 0 | 0 | 0.87 | 0 | 0 | 8.58 | 1 |
| 34 | 5.92 | 4.55 | −0.20 | 1 | 1 | 0.74 | 0 | 0 | 8.64 | 1 |
| 35 | 5.86 | 3.43 | −0.18 | 1 | 1 | 0.59 | 0 | 0 | 8.78 | 1 |
| 36 | 4.36 | 3.59 | 2.65 | 2 | 3 | −1.16 | 0 | 0 | 11.27 | 0 |
| 37 | 4.18 | 3.29 | 2.48 | 3 | 4 | −1.29 | 0 | 0 | 11.08 | 0 |
| 38 | 3.92 | 3.4 | 2.39 | 2 | 5 | −2.60 | 0 | 0 | 11.24 | 0 |
| 39 | 5.14 | 3.71 | −0.26 | 0 | 0 | −0.81 | 0 | 0 | 8.47 | 1 |
| 40 | 4.29 | 3.37 | 0.45 | 1 | 1 | 1.16 | 0 | 1 | 9.73 | 0 |
| 41 | 4.5 | 3.34 | 1.10 | 1 | 2 | 0.63 | 0 | 0 | 10.08 | 0 |
| 42 | 4.93 | 3.38 | 1.13 | 1 | 1 | 0.75 | 0 | 0 | 10.27 | 0 |
| 43 | 7.15 | 5.0 | −0.38 | 1 | 1 | 1.90 | 0 | 0 | 8.71 | 1 |
| 44 | 6.84 | 4.48 | −0.27 | 1 | 1 | 0.17 | 0 | 0 | 8.78 | 0 |
| 45 | 7.02 | 4.94 | −0.38 | 1 | 1 | 0.91 | 0 | 0 | 8.71 | 1 |
| 46 | 5.44 | 4.12 | −0.39 | 1 | 1 | −0.08 | 0 | 0 | 8.68 | 1 |
| 47 | 5.45 | 3.93 | 0.04 | 1 | 1 | 1.84 | 0 | 0 | 9.08 | 1 |
| 48 | 6.44 | 4.3 | −1.25 | 1 | 1 | 1.02 | 0 | 0 | 7.98 | 1 |
| 49 | 4.7 | 3.4 | 0.64 | 0 | 2 | 1.99 | 0 | 1 | 9.77 | 0 |
| 50 | 4.47 | 3.47 | 0.98 | 0 | 2 | 2.31 | 0 | 1 | 10.04 | 0 |
| 51 | 4.56 | 3.61 | 0.89 | 0 | 3 | 1.62 | 0 | 0 | 9.87 | 0 |
| 52 | 4.65 | 3.64 | 0.85 | 0 | 3 | 1.62 | 0 | 0 | 9.89 | 0 |
| 53 | 4.63 | 3.47 | 0.58 | 0 | 1 | 2.43 | 0 | 1 | 9.82 | 0 |
| 54 | 4.42 | 3.42 | 0.50 | 0 | 1 | 2.12 | 0 | 1 | 9.24 | 0 |
| 55 | 4.39 | 3.53 | 0.42 | 0 | 0 | 2.25 | 0 | 1 | 9.27 | 0 |
| 56 | 4.42 | 3.6 | 0.64 | 0 | 2 | 1.75 | 0 | 0 | 9.70 | 0 |
| 57 | 4.42 | 3.6 | 0.74 | 0 | 2 | 1.75 | 0 | 0 | 9.34 | 0 |
| 58 | 4.23 | 3.63 | 0.68 | 0 | 2 | 2.78 | 0 | 0 | 9.28 | 0 |
| 59 | 4.34 | 3.83 | 0.74 | 0 | 2 | 3.17 | 0 | 0 | 9.34 | 0 |
| 60 | 6.28 | 4.19 | 0.01 | 0 | 2 | 2.90 | 0 | 0 | 8.59 | 1 |
| 61 | 5.75 | 4.05 | −0.20 | 0 | 1 | 3.27 | 0 | 1 | 8.53 | 1 |
| 62 | 4.53 | 3.58 | −0.56 | 0 | 0 | 5.46 | 0 | 0 | 8.09 | 1 |
| 63 | 4.36 | 3.35 | 2.83 | 1 | 3 | 1.52 | 0 | 0 | 11.539 | 0 |
| 64 | 4.36 | 3.93 | 2.22 | 1 | 1 | 2.90 | 0 | 0 | 10.87 | 0 |
| 65 | 4.37 | 3.35 | 0.72 | 0 | 0 | 2.51 | 0 | 1 | 9.30 | 0 |
Extensive QSAR (including 3D-QSAR) studies on various groups of sodium channel blockers have been reported (Li and Harte, 2002, Gupta, 1998). Pharmacophore models have been generated by analyzing structure-activity relationship and mapping common structural features of small-molecules of different sodium channel modulator classes (Li and Harte, 2002). One of these models suggested an aromatic centre, electron donor atom and hydrogen-bond acceptor/donor unit to be the key features for the voltage-gated sodium channel blockers (Unverferth et al., 1998). The analysis of the other applied model has also revealed an importance of one or two phenyl groups for the manifestation of sodium channel modulation effect (Chung-Chin Kuo et al., 2000).
A great number of molecular descriptors including molecular size descriptors (molecular weight, molar refractivity, molecular volume, van-der-Waals volume etc.), hydrogen bonding, hydrophobicity, quantum-chemical, indicator variables descriptors etc. were also identified as one of the most influential descriptors [see reviews (Li and Harte, 2002, Gupta, 1998) for more references].
Our QSAR studies carried out for 9 channel openers available within the studied set of alkaloids have also indicated the importance of molecular size and lipophilicity descriptors (Turabekova et al., submitted). Thus we have identified MR, TPSA, MW and MlogP descriptors as those affecting the toxicity exhibited by the alkaloids (Turabekova and Rasulev, 2005). Here we present QSAR models obtained only for antiarrhythmic activity and the toxicity of the alkaloids that block the sodium ion channel (i.e. blockers).
3.2.1. QSAR models related to antiarrhythmic activity
As it was stated before, to date, there have been very few publications concerning QSAR studies on antiarrhythmic activity of alkaloids. The recent review of SAR studies on antiarrhythmic drugs (Gupta, 1998) describes QSAR models developed for the antiarrhythmic and/or local anesthetic agents. The activity of these type of compounds has been found to depend on their lipid solubility described via logP or π (hydrophobic constant of the aryl ring substituent). The electronic parameters have also demonstrated strong correlation with activity data confirming the importance of the coupling of electronic levels of considered compounds and the related receptor sites.
In our study several runs of GA-MLRA technique have identified the following three-descriptor models as the best ones correlating with antiarrhythmic activity of the alkaloids:
| (1) |
| (2) |
| (3) |
| (4) |
As can be seen all of the models contain quantum-chemical descriptor – the HOMO-LUMO energies gap (HLgap) calculated with the AM1 level of theory for protonated states. The descriptors nOHt (number of tertiary aliphatic alcohols), nHDon (number of donor N, O atoms for H-bond), logP, nNH2 (number of primary aliphatic amines) and IBEG (indicator descriptor for presence of benzoylester group) results in good models when combined with the HOMO-LUMO energy gap. Interesting to note, that variable describing the HOMO-LUMO energy gap for protonated molecules turned out as the most important property affecting the antiarrhythmic activity. It is also important to mention that HOMO energy for ground states absolutely does not correlate with antiarrhythmic activity (r=0.065), while for protonated states the situation dramatically changes, the correlation is quite good (r=0.710). This is in agreement with computational studies discussed earlier in part 3.1. The HOMO visibly changes to active position in protonated states, while the LUMO energy becomes inactive when transferred from ground to protonated state. Earlier, the energy of LUMO (as important part of HOMO-LUMO difference) was identified as one of the most influential descriptors for the set of hydantoin analogues that bind to the neuronal voltage-gated sodium channel (Thenmozhiyal et al., 2004). The correlation observed (Thenmozhiyal et al., 2004) might be the consequence of the fact that Authors used LUMO energies for ground state of studied compounds. However, our present study confirms that application of energies of protonated states is a more correct approach.
It is well known that the HOMO-LUMO energy difference describes the reactivity of compounds and the models containing it are straightforward to explain. Our model shows smaller HOMO-LUMO energy difference favoring reactivity properties is associated with the high antiarrhythmic activity. The correlation observed between HOMO-LUMO gap and the activity data suggests that formation of a charge transfer complex underlies the drug-receptor interactions.
Equations 1 and 2 are of equivalently good statistical fit and exhibit the same trend in structure-activity relationship. Thus, equation 1 confirms the importance of –OH functional groups for the stronger antiarrhythmic activity likely due to formation of H-bonds between ligand and the receptor site. The descriptor nHDon might be considered as the general case of OHt descriptor and also confirms that the antiarrhythmic activity is improved as the number of H-bond donors gets larger. The third model confirms the importance of lipophilicity that is in a good agreement with a number of earlier reports (Thenmozhiyal et al., 2004). The equation (3) with hydrophobicity descriptor LogP suggests binding of alkaloids occurs on the surface of the binding site where partial desolvation might occur (Burger, 2003). To all appearance, the benzoylester group plays an important role in ligand-receptor interaction process of studied alkaloids. We can see a quite strong correlation between the presence of benzoylester group and antiarrhythmic activity.
Remarkably, the descriptors chosen are well-fitted with the molecular structure features requested to be present in a compound of antiarrhythmic activity. These are: 1. a lipophilic aromatic group; 2. tertiary, secondary and in some cases primary amino group; 3. an ester (amide or hydroxyalkyl group capable of forming hydrogen bonds (Gupta, 1998). Descriptors OHt (eq.1) and nHDon (eq.2) are responsible for the ability of an alkaloid to participate in hydrogen bond formation, while lipophilicity is determined mainly by the benzene ring present. Tertiary nitrogen is already present in the each alkaloid of the set chosen and the model 4 indicates that primary amino groups are also necessary for the stronger manifestation of antiarrhythmic properties.
3.2.2. QSAR models related to toxicity
Early experimental studies performed by J. Friese et al. (1997) have demonstrated that the affinities of these alkaloids to sodium channels correlate with their effective doses (ED50) determined for acute toxicity. The values of therapeutic index (LD50/ED50) are in the range from 1 to 6. The authors of the report on QSAR toxicity analysis performed for the 12 Aconitum alkaloids have also observed significant relationship between log LD50 and analgesic log ED50 (r=0.96) with the therapeutic effect ranging 5.9-5.0 (Bello-Ramirez and Nava-Ocampo, 2004). However, the LD50/ED50 ratio obtained for local anesthetic activity appeared to be considerably higher (between 40.4 and 318). Correlation coefficient was also much lower (r=0.71). Based on their results, the authors of the second report concluded that analgesic effects of the set of alkaloids are secondary to their toxicity, while alkaloids of lappaconitine-type can further be pursued as local anesthetics. In our additional QSAR toxicity studies the set of 104 Aconitum and Delphinium alkaloids have been divided into “drugs” and “non-drugs” against a number of “drug-likeness” descriptors (Turabekova et al., submitted). We suggested curariform and antiarrhythmic alkaloids are more drug-like compounds, while arrhythmogenic alkaloids (aconitine-type) are all likely to be classified as “non-drugs”.
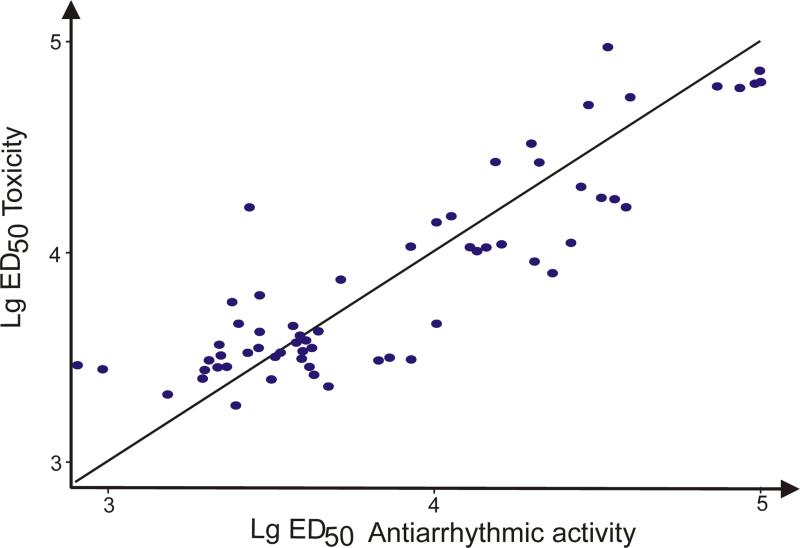
In order to check if there is any relationship between antiarrhytmic activity and toxicity we used the linear regression analysis. As it was expected, the regression analysis resulted in high correlation coefficient between antiarrhythmic and toxicity data available for 63 alkaloids (Eq.5, Fig.7). According to equation, we can observe antiarrhythmic activity positively correlates with LD50 toxicity of alkaloids. Most likely, such correlation reflects a common property of antiarrhythmic compounds – the positive antiarrhythmic potency is very close to LD50 toxicity due to similar nature of action. Structurally and energetically, this correlation can be explained by the presence of benzoylester groups and the descriptor energy of HOMO-LUMO gap. Both characteristics equally contribute to antiarrhythmic activity and LD50 toxicity. The benzoylester group plays an important role in ligand-receptor interactions leading to Na+ channel blockade and at the same time different locations of benzoylester group alters interaction of the compounds with Na+ channel resulting in higher toxicity properties. The later fact is confirmed by the Eq. 8. Again, HLgap descriptor has been selected as the best one by the GA-MLRA technique. The correlation of HLgap with toxicity is obvious, since more reactive compounds are normally more toxic ones. Presence of HLgap descriptor in both cases (for antiarrhythmic activity and for toxicity) once again confirms the fact that there is a very thin energetic layer between compounds with antiarrhythmic properties (blockers) and arrhythmogenic properties (activators). Ability of a compound to cross the layer results in changing of current conductance of Na+ channel and consequently in high toxic effect of it. Interestingly, the new descriptor (nCO) contributing to the alteration of toxicity of the alkaloids has emerged. nCO indicates the number of aliphatic ketons present in a structure of an alkaloid. An increase in the number of aliphatic ketons seems to lower toxicity. Too many keton groups worsen binding affinity of a ligand as the receptor site is likely to have hydrogen bond acceptor atoms according to the model 7.
| (5) |
| (6) |
| (7) |
Figure 7.
Linear relationship between toxicity and antiarrhythmic activity data
Equations 6 and 7 have been modeled excluding a few outlier compounds: 21, 35, 43 and 62. The bad fitting to correlation line for those compounds can be explained by possible errors in estimating of experimental toxicity data for explored compounds.
4. Conclusions
An antagonist modulation of voltage-gated sodium channels exhibited by Aconitum and Delphinium plant species alkaloids has been investigated by means of two computational approaches: analysis of frontier MOs generated at B3LYP/6-31G(d, p) level and QSAR study. An examination of HOMO (HOMO-1) and LUMO (LUMO+1) for both ground and protonated forms of each molecule has been carried out in order to estimate contributions of three crucial residues responsible for channel opening activity into these orbitals. It was shown that HOMO (ground state) and LUMO (protonated state) were mainly comprised of nitrogen atom orbitals, while LUMO and HOMO for ground and protonated states respectively were located on benzyl/benzoyl side chain for majority of the alkaloids. The contribution values of –OH and –OCOCH3 groups into frontier orbitals were found to be negligibly low (about 1% in total). The results obtained from this research have confirmed the experimental findings suggesting neurotoxins acting at type 2 receptor site of voltage-dependent sodium channel are activators and blockers with common structural features and differ only in efficacy. The energetic properties play an important role in activity profile of these compounds. The energetic tendency of HOMO-LUMO energy gap helps to distinguish between activators and blockers. It was shown the presence of benzoylester group and its position play essential role for the interaction of the target molecule with the binding site at sodium ion channel. This group makes compound more toxic because of its activity (high density of HOMO and LUMO on this group) and compounds, which lack benzoylester group sharply diminish in toxicity.
QSAR models generated separately for antiarrhythmic activity and toxicity data have confirmed the importance of a number of descriptors altering the activity of the alkaloids. Thus, HOMO-LUMO energy gap descriptor present in each QSAR model has confirmed the crucial role of charge transfer in receptor-ligand interactions. Other descriptors were: number of tertiary aliphatic alcohol nOHt, number of donors for hydrogen bond formation (-NH, -OH) nHDon, IBEG (presence of benzyl ring), logP, nNH2 and nCO (identified for toxicity). Presence of the nHDon, nOHt and nCO descriptors in the models suggest that there are functional residues in the receptor site containing acceptor atoms for H-bonds (for example –OC(O) – groups). Lipophilicity determined mostly by the aromatic ring and the presence of aliphatic amino groups have appeared to favor antiarrhythmic activity. Also, a reasonable correlation has been observed between antiarrhythmic activity and toxicity exhibited by the alkaloids of the series. Most likely, such correlation reflects a common property of antiarrhythmic compounds – the positive antiarrhythmic potency is very close to LD50 toxicity due to similar nature of action. Structurally and energetically, this correlation can be explained by the presence of benzoylester groups and the parameter HOMO-LUMO energy gap.
Acknowledgments
The authors would like to thank Dr. F. Dzhakhangirov and Dr. M. Faskhutdinov for useful discussions and helpful comments on the manuscript. One of the authors (M.A.T.) also would like to thank School of Chemistry, Birmingham University (UK) for providing informational support.
This work was supported in part by National Science Foundation through RISE grant No. HRD-0401730 and National Institute of Health SCORE grant No.3S06 GM008047-31S1. We thank the Mississippi Center for Supercomputer Research (Oxford, MS) for a generous allotment of computer time.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ameri A. Structure-dependent differences in the effects of the Aconitum alkaloids lappaconitine, N-desacetyllappaconitine and lappaconidine in rat hippocampal slices. Brain Research. 1997;769:36. doi: 10.1016/s0006-8993(97)00664-1. [DOI] [PubMed] [Google Scholar]
- Ameri. A. The effects of Aconitum alkaloids on the central nervous system. Prog. Neurobiol. 1998;56:211. doi: 10.1016/s0301-0082(98)00037-9. [DOI] [PubMed] [Google Scholar]
- Ameri A, Simmet T. Interaction of the structurally related aconitum alkaloids, aconitine and 6-benzoylheteratisine, in the rat hippocampus. Eur. J. Pharmacol. 1999;386:187. doi: 10.1016/s0014-2999(99)00692-5. [DOI] [PubMed] [Google Scholar]
- Anger T, Madge DJ, Mulla M, Riddal D. Medicinal chemistry of neuronal voltage-gated sodium channel blockers. J. Med. Chem. 2001;44:115. doi: 10.1021/jm000155h. [DOI] [PubMed] [Google Scholar]
- Baker MD, Wood JN. Involvement of Na+ Channels in Pain Pathways. TiPS. 2001;22:27. doi: 10.1016/s0165-6147(00)01585-6. [DOI] [PubMed] [Google Scholar]
- Bello-Ramirez AM, Buendia-Orozco J, Nava-Ocampo AA. A QSAR analysis to explain the analgesic properties of Aconitum alkaloids. Fundam. Clin. Pharmacol. 2003;17:575. doi: 10.1046/j.1472-8206.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- Bello-Ramirez AM, Nava-Ocampo AA. The local anesthetic activity of Aconitum alkaloids can be explained by their structural properties. A QSAR analysis. Fundam. Clin. Pharmacol. 2004;18:157. doi: 10.1111/j.1472-8206.2004.00222.x. [DOI] [PubMed] [Google Scholar]
- Bello-Ramirez AM, Nava-Ocampo AA. A QSAR analysis of toxicity of Aconitum alkaloids. Fundam. Clin. Pharmacol. 2004;18:699. doi: 10.1111/j.1472-8206.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- Benn MN, Jacyno JM. The toxicology and pharmacology of diterpenoid alkaloids. In: Pelletier SW, editor. Alkaloids: chemical and biological perspectives. Vol. 1. John Willey; New-York: 1984. pp. 153–376. [Google Scholar]
- Burger A, Abraham DJ. Burger Med. Chem. and Drug Discovery. 6th edition Vol. 1. John Wiley & Sons, Inc; 2003. p. 36. [Google Scholar]
- Catteral WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu. Rev. Pharmacol. Toxicol. 1980;20:15. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Catterall WA. A 3D view of sodium channels. Nature. 2001;409:988. doi: 10.1038/35059188. [DOI] [PubMed] [Google Scholar]
- Cestele S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- Chung-Chin Kuo, Huang RC, Lou BS. Inhibition of Na+ current by diphenhydramine and other diphenyl compounds: Molecular determinants of selective binding to the inactivated channels. Mol. Pharmacol. 2000;57:135. [PubMed] [Google Scholar]
- Clare JJ, Tate SN, Nobbs M, Romanos M. Voltage-gated sodium channels as therapeutic targets. DDT. 2000;5:506. doi: 10.1016/s1359-6446(00)01570-1. [DOI] [PubMed] [Google Scholar]
- Cronin MTD. Computer-aided prediction of drug toxicity and metabolism. In: Hillisch A, Hilgenfeld R, editors. Modern methods of drug discovery. Birkhauser Verlag; Basel: 2003. pp. 259–278. [DOI] [PubMed] [Google Scholar]
- de Oliveira DB, Gaudio AC. BuildQSAR: A new computer program for QSAR analysis. Quant. Struct.-Act. Relat. 2000;19:599. [Google Scholar]
- Devillers J. Genetic Algorithms in Molecular Modeling. Academic Press Ltd; London: 1996. [Google Scholar]
- Dzhakhangirov FN, Sultankhodzhaev MN, Tashkhodzhaev B, Salimov BT. Diterpenoid alkaloids as a new class of antiarrhythmic agents. Structure-activity relationship. Chem. Nat. Comp. 1997;33:190. [Google Scholar]
- Friese J, Gleitz J, Guster UT, Heubach JF, Matthiesen T, Wilffert B, Selve N. Aconitum sp. alkaloids: the modulation of voltage-dependent Na+ channels, toxicity and antinociceptive properties. Eur. J. Pharmacol. 1997;337:165. doi: 10.1016/s0014-2999(97)01268-5. [DOI] [PubMed] [Google Scholar]
- Heubach JF, Schule A. Cardiac effects of lappaconitine and N-deacetyllappaconitine, two diterpenoid alkaloids from plants of the Aconitum and Delphinium. Planta Med. 2000;64:22. doi: 10.1055/s-2006-957359. [DOI] [PubMed] [Google Scholar]
- HyperChem 6.01. HyperCube, Inc; 2000. [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Jr., Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck D, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA. Gaussian 03, Revision C.02. Gaussian, Inc.; Wallingford CT: 2004. [Google Scholar]
- Gupta SP. Quantitative structure-activity relationships of antiarrhythmic drugs. Curr. Pharm. Des. 1998;4:455. [PubMed] [Google Scholar]
- Li Y, Harte WE. A review of molecular modeling approaches to pharmacophore models and structure-activity relationships of ion channel modulators in CNS. Curr. Pharm. Des. 2002;8:99. doi: 10.2174/1381612023396546. [DOI] [PubMed] [Google Scholar]
- Mozahayeva GN, Naumov AP, Negulayev YA, Nosyreva ED. The permeability of aconitine-modified sodium channels to univalent cations in myelinated nerve. Biochim. Biophys. Acta. 1977;466:461. doi: 10.1016/0005-2736(77)90339-x. [DOI] [PubMed] [Google Scholar]
- Statistical analysis software NCSS98. 1998.
- Taylor CP, Meldrum BS. Na+ channels as targets for neuroprotective drugs. TiPS. 1995;16:309. doi: 10.1016/s0165-6147(00)89060-4. [DOI] [PubMed] [Google Scholar]
- Thenmozhiyal JC, Wong PT-H, Chui W-K. Anticonvulsant activity of phenylmethylenehydantoins: A structure-activity relationship study. J. Med. Chem. 2004;47:1527. doi: 10.1021/jm030450c. [DOI] [PubMed] [Google Scholar]
- Tikhonov DB, Zhorov BS. Sodium channel activators: Model of binding inside the pore and a possible mechanism of action. FEBS Lett. 2005;579:4207. doi: 10.1016/j.febslet.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Todeschini R, Consonni V. Handbook of Molecular Descriptors. In: Mannhold R, Kubinyi H, Timmerman H, editors. Methods and Principles in Medicinal Chemistry. Vol. 11. Wiley –VCH Verlag GmbH; Weinheim: 2000. [Google Scholar]
- Todeschini R, Consonni V. DRAGON software for the Calculation of Molecular Descriptors. Web version 3.0 for Windows. 2003.
- Turabekova MA, Rasulev BF. QSAR analysis of the structure—toxicity relationship of Aconitum and Delphinium diterpene alkaloids. Chem.Nat.Comp. 2005;41:213. [Google Scholar]
- Turabekova MA, Rasulev BF, Dzhakhangirov FN. Aconitum and Delphinium alkaloids. “Drug-likeness” descriptors related to toxic mode of action. Environ. Tox. Pharmacol. 2007 doi: 10.1016/j.etap.2007.10.035. submitted. [DOI] [PubMed] [Google Scholar]
- Unverferth K, Engel J, Hofgen N, Rostock A, Gunther R, Lankau HJ, Menzer M, Rolfs A, Leibscher J, Muller B, Hofmann HJ. Synthesis, anticonvulsant activity, and structure activity relationships of sodium channel blocking 3-aminopyrroles. J. Med. Chem. 1998;41:63. doi: 10.1021/jm970327j. [DOI] [PubMed] [Google Scholar]
- Young D. Computational chemistry: A practical guide for applying techniques to real world problems. John-Wiley & Sons; New York: 2001. [Google Scholar]
- Valeev AE, Verkhratskii AN, Dzhakhangirov FN. Effects of allapinine on sodium currents in neurons isolated from the trigeminal ganglion and cardiomyocytes. Neirofiziologiya. 1990;22:201. [PubMed] [Google Scholar]
- Wang S-Y, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipidsoluble neurotoxins. Cellular Signalling. 2003;15:151. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Wright SN. Irreversible block of human heart (hH1) sodium channels by the plant alkaloid lappaconitine. Mol. Pharmacol. 2001;59:183. doi: 10.1124/mol.59.2.183. [DOI] [PubMed] [Google Scholar]