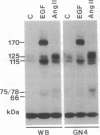
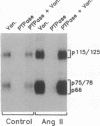
Abstract
The cellular effects of numerous hormones and neurotransmitters, including the vasoactive agents angiotensin II (AngII) and [Arg8]vasopressin, are mediated in part by protein-serine threonine kinases activated by increase of cytosolic Ca2+ concentration. In this study, we have tested the ability of Ca(2+)-mobilizing agents to activate cellular tyrosine kinases. Treatment of intact GN4 liver epithelial cells with AngII rapidly (less than or equal to 15 sec) increased tyrosine kinase activity measured either in unfractionated cell lysates or in anti-phosphotyrosine immune complexes from detergent-solubilized cells. Increased phosphorylation of the exogenous substrate poly(Glu80Tyr20) (3- to 4-fold over control) by immunoprecipitated kinases closely paralleled the time- and dose-dependence of the appearance of tyrosine phosphoproteins in intact cells. This effect of AngII was mimicked by thapsigargin, a Ca(2+)-elevating tumor promoter. The ability of AngII, but not epidermal growth factor, to increase tyrosine kinase activity was blocked in cells loaded with the Ca2+ chelator bis-(O-aminophenoxy)-ethane-N,N,N',N'-tetraacetic acid. Dephosphorylation of immunoprecipitated proteins by tyrosine phosphatase treatment was accompanied by a 60-70% loss in in vitro kinase activity, suggesting that the AngII-sensitive kinase(s) are activated by phosphorylation in intact cells. These findings demonstrate a link between two widely occurring signaling pathways, the tyrosine kinases and the Ca2+ second-messenger system, and suggest the possible involvement of Ca(2+)-activated tyrosine kinases in the endocrine actions of AngII and [Arg8]vasopressin.
Full text
PDF

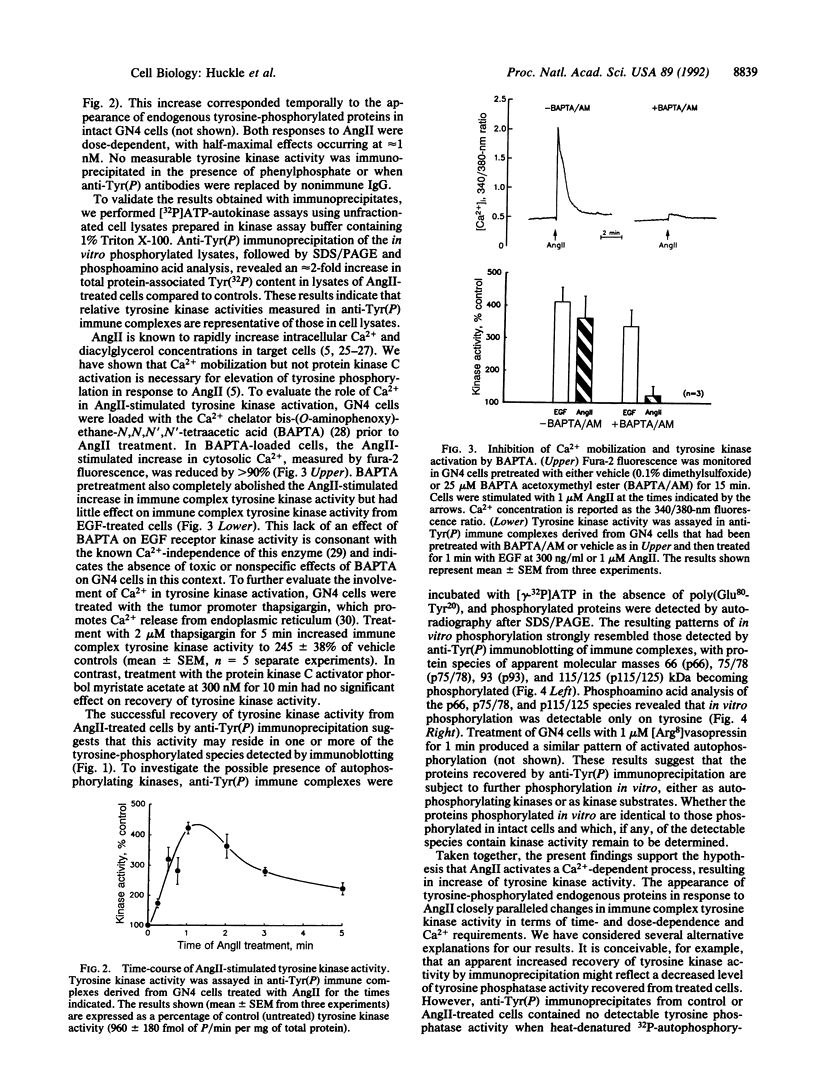

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bading H., Greenberg M. E. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991 Aug 23;253(5022):912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- Bertics P. J., Gill G. N. Self-phosphorylation enhances the protein-tyrosine kinase activity of the epidermal growth factor receptor. J Biol Chem. 1985 Nov 25;260(27):14642–14647. [PubMed] [Google Scholar]
- Braun S., Raymond W. E., Racker E. Synthetic tyrosine polymers as substrates and inhibitors of tyrosine-specific protein kinases. J Biol Chem. 1984 Feb 25;259(4):2051–2054. [PubMed] [Google Scholar]
- Cheng S. H., Espino P. C., Marshall J., Harvey R., Merrill J., Smith A. E. Structural elements that regulate pp59c-fyn catalytic activity, transforming potential, and ability to associate with polyomavirus middle-T antigen. J Virol. 1991 Jan;65(1):170–179. doi: 10.1128/jvi.65.1.170-179.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. D., Reimann E. M. Assay of cyclic AMP-dependent protein kinases. Methods Enzymol. 1974;38:287–290. doi: 10.1016/0076-6879(74)38044-5. [DOI] [PubMed] [Google Scholar]
- Fava R. A., Cohen S. Isolation of a calcium-dependent 35-kilodalton substrate for the epidermal growth factor receptor/kinase from A-431 cells. J Biol Chem. 1984 Feb 25;259(4):2636–2645. [PubMed] [Google Scholar]
- Golden A., Nemeth S. P., Brugge J. S. Blood platelets express high levels of the pp60c-src-specific tyrosine kinase activity. Proc Natl Acad Sci U S A. 1986 Feb;83(4):852–856. doi: 10.1073/pnas.83.4.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cambronero J., Wang E., Johnson G., Huang C. K., Sha'afi R. I. Platelet-activating factor induces tyrosine phosphorylation in human neutrophils. J Biol Chem. 1991 Apr 5;266(10):6240–6245. [PubMed] [Google Scholar]
- Griendling K. K., Berk B. C., Socorro L., Tsuda T., Delafontaine P., Alexander R. W. Secondary signalling mechanisms in angiotensin II-stimulated vascular smooth muscle cells. Clin Exp Pharmacol Physiol. 1988 Feb;15(2):105–112. doi: 10.1111/j.1440-1681.1988.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harper J. F., Sussman M. R., Schaller G. E., Putnam-Evans C., Charbonneau H., Harmon A. C. A calcium-dependent protein kinase with a regulatory domain similar to calmodulin. Science. 1991 May 17;252(5008):951–954. doi: 10.1126/science.1852075. [DOI] [PubMed] [Google Scholar]
- Huckle W. R., Hepler J. R., Rhee S. G., Harden T. K., Earp H. S. Protein kinase C inhibits epidermal growth factor-dependent tyrosine phosphorylation of phospholipase C gamma and activation of phosphoinositide hydrolysis. Endocrinology. 1990 Oct;127(4):1697–1705. doi: 10.1210/endo-127-4-1697. [DOI] [PubMed] [Google Scholar]
- Huckle W. R., Prokop C. A., Dy R. C., Herman B., Earp S. Angiotensin II stimulates protein-tyrosine phosphorylation in a calcium-dependent manner. Mol Cell Biol. 1990 Dec;10(12):6290–6298. doi: 10.1128/mcb.10.12.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Kmiecik T. E., Johnson P. J., Shalloway D. Regulation by the autophosphorylation site in overexpressed pp60c-src. Mol Cell Biol. 1988 Oct;8(10):4541–4546. doi: 10.1128/mcb.8.10.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kojima I., Kojima K., Rasmussen H. Characteristics of angiotensin II-, K+- and ACTH-induced calcium influx in adrenal glomerulosa cells. Evidence that angiotensin II, K+, and ACTH may open a common calcium channel. J Biol Chem. 1985 Aug 5;260(16):9171–9176. [PubMed] [Google Scholar]
- Martin T. F. Receptor regulation of phosphoinositidase C. Pharmacol Ther. 1991;49(3):329–345. doi: 10.1016/0163-7258(91)90062-q. [DOI] [PubMed] [Google Scholar]
- McCune B. K., Earp H. S. The epidermal growth factor receptor tyrosine kinase in liver epithelial cells. The effect of ligand-dependent changes in cellular location. J Biol Chem. 1989 Sep 15;264(26):15501–15507. [PubMed] [Google Scholar]
- Migliaccio A., Rotondi A., Auricchio F. Calmodulin-stimulated phosphorylation of 17 beta-estradiol receptor on tyrosine. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5921–5925. doi: 10.1073/pnas.81.19.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard H. L., Trowbridge I. S. Negative regulation of CD45 protein tyrosine phosphatase activity by ionomycin in T cells. Science. 1991 Sep 20;253(5026):1423–1425. doi: 10.1126/science.1654595. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T. J. Characterization of a bovine brain magnesium-dependent phosphotyrosine protein phosphatase that is inhibited by micromolar concentrations of calcium. Biochem Biophys Res Commun. 1990 Mar 16;167(2):621–627. doi: 10.1016/0006-291x(90)92070-g. [DOI] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Takayama H., Nakamura T., Yanagi S., Taniguchi T., Nakamura S., Yamamura H. Ionophore A23187-induced protein-tyrosine phosphorylation of human platelets: possible synergism between Ca2+ mobilization and protein kinase C activation. Biochem Biophys Res Commun. 1991 Jan 31;174(2):922–927. doi: 10.1016/0006-291x(91)91506-8. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao M. S., Grisham J. W., Chou B. B., Smith J. D. Clonal isolation of populations of gamma-glutamyl transpeptidase-positive and -negative cells from rat liver epithelial cells chemically transformed in vitro. Cancer Res. 1985 Oct;45(10):5134–5138. [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Tsuda T., Kawahara Y., Shii K., Koide M., Ishida Y., Yokoyama M. Vasoconstrictor-induced protein-tyrosine phosphorylation in cultured vascular smooth muscle cells. FEBS Lett. 1991 Jul 8;285(1):44–48. doi: 10.1016/0014-5793(91)80721-e. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Veillette A., Fournel M. The CD4 associated tyrosine protein kinase p56lck is positively regulated through its site of autophosphorylation. Oncogene. 1990 Oct;5(10):1455–1462. [PubMed] [Google Scholar]
- Vostal J. G., Jackson W. L., Shulman N. R. Cytosolic and stored calcium antagonistically control tyrosine phosphorylation of specific platelet proteins. J Biol Chem. 1991 Sep 5;266(25):16911–16916. [PubMed] [Google Scholar]
- Weinmaster G., Zoller M. J., Smith M., Hinze E., Pawson T. Mutagenesis of Fujinami sarcoma virus: evidence that tyrosine phosphorylation of P130gag-fps modulates its biological activity. Cell. 1984 Jun;37(2):559–568. doi: 10.1016/0092-8674(84)90386-6. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]
- Zander N. F., Lorenzen J. A., Cool D. E., Tonks N. K., Daum G., Krebs E. G., Fischer E. H. Purification and characterization of a human recombinant T-cell protein-tyrosine-phosphatase from a baculovirus expression system. Biochemistry. 1991 Jul 16;30(28):6964–6970. doi: 10.1021/bi00242a022. [DOI] [PubMed] [Google Scholar]