Abstract
The New York City Board of Health (NYCBH) vaccinia virus (VACV) vaccine strain was deleted for the immune evasion gene, E3L, and tested for its pathogenicity and ability to protect mice from heterologous challenge with ectromelia virus (ECTV). NYCBHΔE3L was found to be highly attenuated for pathogenicity in a newborn mouse model and showed a similar attenuated phenotype as the NYVAC strain of vaccinia virus. Scarification with one or two doses of the attenuated NYCBHΔE3L was able to protect mice equally as well as NYCBH from death, weight loss, and viral spread to visceral organs. A single dose of NYCBHΔE3L resulted in low poxvirus-specific antibodies, and a second dose increased levels of poxvirus-specific antibodies to a level similar to that seen in animals vaccinated with a single dose of NYCBH. However, similar neutralizing antibody titers were observed following one or two doses of NYCBHΔE3L or NYCBH. Thus, NYCBHΔE3L shows potential as a candidate for a safer human smallpox vaccine since it protects mice from challenge with a heterologous poxvirus.
Introduction
The New York City Board of Health (NYCBH) vaccine strain which was manufactured by Wyeth and designated as Dryvax® is a heterologous mixture of VACVs that was historically used to vaccinate populations in the Americas and West Africa during the smallpox eradication program [1]. A single plaque isolate of Dryvax®, named Acambis 2000™, was purified in tissue culture conditions in order to increase safety from contaminants and was tested clinically in comparison to Dryvax® [2]. Vaccination with either Dryvax® or Acambis 2000™ results in equivalent neutralizing antibody and T cell responses, however similar adverse reactions, specifically myocarditis/myopericarditis, are observed [2]. Due to the potential for other known serious side effects such as eczema vaccinatum, progressive vaccinia, postvaccinial encephalitis, and generalized vaccinia, we sought to attenuate further the NYCBH VACV strain (Acambis 2000™) and test its ability to protect against challenge in established animal models of poxvirus infection.
The NYCBH VACV was attenuated by deletion of the immunomodulatory gene, E3L (NYCBHΔE3L). The E3L gene is expressed early during VACV infection and codes for proteins that contain an N-terminal Z nucleic acid binding domain (Zα) and a C-terminal dsRNA-binding domain [3,4,5]. The dsRNA-binding domain inhibits the activation of type I interferon (IFN)-induced proteins such as protein kinase R (PKR) and oligoadenylate synthetase (OAS) by binding and sequestering activator dsRNA, a byproduct of viral transcription [6,7]. The ability to bind dsRNA is also responsible for inhibition of proinflammatory signaling in VACV-infected cells [8]. Both the dsRNA-binding and Zα domains are required for optimal pathogenicity in mice, so deletion of the entire E3L gene results in a nonpathogenic virus that replicates to levels in skin 3 logs lower than wild type VACV [3,9,10].
NYCBHΔE3L has been previously tested as a vaccine in a mouse model. Tail scarification using NYCBHΔE3L successfully protected mice from a homologous challenge with VACV WR [10]. This study has extended these observations by testing the NYCBHΔE3L vaccine in a heterologous poxvirus challenge model using ectromelia virus (ECTV). ECTV is the causative agent of mousepox, a disease that mimics smallpox in humans due to its infection of the respiratory tract, ensuing blood viral load, and characteristic skin rash [11,12]. This paper shows that mice immunized with one dose of NYCBHΔE3L were protected from death and weight loss when intranasally challenged with a lethal dose of ECTV. In addition, viral spread to visceral organs was substantially inhibited .
Materials and Methods
Cell lines and virus stocks
Baby hamster kidney (BHK-21) cells and rabbit kidney-E3L (RK-E3L) cells stably expressing the VACV E3L gene (Wong, Denzler, and Jacobs, unpublished results) were grown in minimal essential media (MEM, Cellgro) containing 5% fetal bovine serum (FBS, HyClone) and 50 μg/ml gentamycin. Murine fibroblasts (L929) and African green monkey kidney (BSC-1) cells were grown in Dulbecco’s-MEM (DMEM, Lonza) containing 10% heat-inactivated fetal calf serum (FCII, HyClone).
NYCBH (ACAM2000™, kindly provided by Acambis), NYCBHΔE3L[10], MVA (kindly provided by Sanofi Pasteur), and NYVAC (kindly provided by Sanofi Pasteur) were propagated in BHK cells as previously described [9]. Infected cells were freeze-thawed three times followed by sonication and pelleting. Supernatant was partially purified by centrifugation through a 36% sucrose pad. Viral titers were determined by plaque assay using BHK cells for pathogenesis studies or on RK-E3L cells for vaccination studies. ECTV-Moscow was propagated in L929 cells, processed and partially purified through a sucrose pad as described above, and titrated using BSC-1 cells [13]. VACV WR was similarly propagated on HeLa-S3 cells and titrated on BSC-1 cells.
Pathogenesis and vaccination/challenge of mice
For pathogenesis studies, pregnant CD1 mice were obtained from Charles River Laboratories at two weeks gestation and were housed one mouse per cage for delivery of pups. Newborns at 48-72 hours of age were given intracranial injections with the indicated doses of each virus resuspended in Tris-buffered saline (TBS) using a 27-gauge needle to deliver 5μl of virus preparation. Mice were monitored daily for 2 weeks for morbidity and mortality. All procedures were approved by the Institutional Animal Care and Use Committee at Arizona State University.
Female A/Ncr mice at 5-6 weeks of age were purchased from The Jackson Laboratory (Bar Harbor, Maine). Animals were allowed to acclimate for 1 week prior to vaccination and were distributed into groups with <20% variation in weights. Vaccinations were performed at day 0 or days 0 and 28 by tail scarification using 1 × 106 pfu in a 2.5 μl volume of virus diluted in phosphate buffered saline (PBS) without Ca2+ and Mg2+ which was distributed into 15 punctures on the tail skin using a bifurcated needle. Mice were anesthetised with a ketamine HCl/ xylazine mix (9 mg/ml/1 mg/ml) by intraperitoneal injection using 0.1 ml per 10 g body weight. Mice were challenged on day 56 by intranasal infection using 5 μl virus diluted in PBS without Ca2+ and Mg2+ by pipetting the diluted virus into each naris. Animal procedures were approved by the St. Louis University School of Medicine Institutional Animal Care and Use Committee.
Tissue titers
Mice were euthanized on day 7 post challenge followed by resection of tissues (kidney, liver, lung, spleen). Processing of tissues was performed using glass grinders by pulverizing the tissue at 10% wt/vol in PBS/1% serum, performing freeze/thaw for three cycles, and sonicating in an ice water bath. Virus infectivity levels were assayed using BSC-1 cells. The horizontal dashed line represents the limit of detection of the assay.
Antibody Titers
Blood was obtained from mice at various times during the vaccination schedule via the submandibular vein into microtainer serum separator tubes. Individual samples of serum were separated by centrifugation and stored at -20°C. A direct anti-vaccinia virus ELISA was performed using a lysate from BSC-1 cells infected with VACV-WR. The clarified cell lysate was diluted in 50 mM carbonate-bicarbonate buffer pH 9.6 and used to coat 96-well microtiter ELISA plates (Immulon-2 HB) at 4°C overnight. Plates were blocked with PBS pH 7.2, 0.05% Tween20, 2% normal goat serum (Vector) at room temperature for 30 minutes. Mouse serum was serially diluted in PBS pH 7.2, 0.05% Tween20 (PBST) and incubated for one hour at room temperature. Wells were washed with PBST and bound antibody was detected using biotin-conjugated goat anti-mouse IgG (Caltag) incubated one hour followed by a wash with PBST and the application of streptavidin-HRP (Zymed) for thirty minutes. Wells were washed and O-phenylenediamine dihydrochloride (0.4 mg/ml) in 50 mM citrate buffer (pH 5.0) and 0.05% hydrogen peroxide was added for fifteen minutes, then the reaction was stopped with 3N HCl. Optical density was measured at 490 nm. Titers were determined by calculating the inverse of the serum dilution at which the optical density exceeded the background value of 0.1 as measured with PBST. For measurement of neutralizing antibody titers, serum was thawed and serially diluted in D-MEM/2% serum. A known amount (50 plaque forming units) of ECTV was added to each dilution and incubated at 37°C for two hours followed by titration in BSC-1 cells. The neutralizing antibody titer of each sample was calculated as the inverse of the serum dilution at which a 50% reduction in plaquing efficiency occurred.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 5, GraphPad Software, Inc. Weights were analyzed by two way ANOVA followed by Bonferroni posttests to determine differences between vaccination groups. Virus titers in tissues and antibody titers were analyzed by one way ANOVA followed by Tukey’s multiple comparison test.
Results
NYCBHΔE3L is highly attenuated for pathogencity in newborn mice
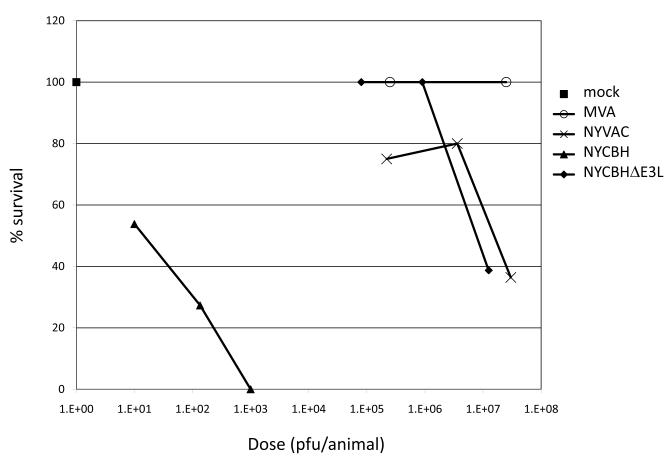
The pathogenicity of NYCBHΔE3L was tested in the highly sensitive newborn CD1 mouse model and compared to NYVAC and MVA [14]. Increasing doses of each VACV strain were injected intracranially and the LD50 was assessed. The wild type NYCBH vaccine was pathogenic at low doses with an LD50 of 20 pfu (Fig. 1). Deletion of the E3L gene from NYCBH resulted in a virus (NYCBHΔE3L) that was attenuated by nearly 6 logs and had an LD50 of 1 × 107 pfu. NYCBHΔE3L was similar in pathogenicity to NYVAC and MVA, two highly attenuated VACV strains [15, 16].
Fig. 1.
CD1 mouse pathogenesis. Groups of 10-15 newborn mice were infected by intracranial injection with increasing doses of MVA, NYVAC, NYCBH, and NYCBHΔE3L or were mock infected with TBS. Percent survival was determined after 14 days.
Vaccination with NYCBHΔE3L fully protects mice from challenge with ECTV
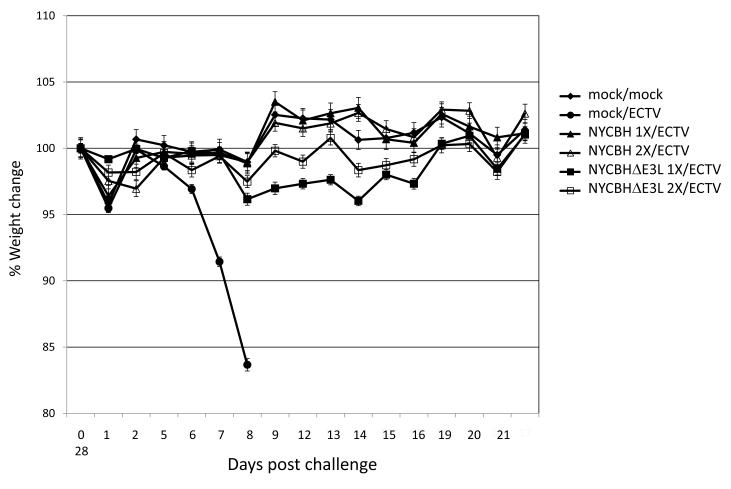
Six groups of 9-10 mice per group were were vaccinated by scarification with a single dose of 1 × 106 pfu NYCBH or NYCBHΔE3L at day 0, two doses of NYCBH or NYCBHΔE3L at days 0 and 28, or were mock vaccinated with PBS. Four weeks after vaccination (day 56) mice were challenged intranasally with 5.8 pfu (15 × LD50) ECTV. All the mock-vaccinated mice died at 8-9 days post challenge while all vaccinated mice, whether singly or doubly vaccinated with NYCBH or NYCBHΔE3L survived challenge (data not shown). The weights of the mice were recorded for 28 days following challenge with ECTV. Mock-vaccinated ECTV-challenged mice lost weight from day 5 post challenge until death (Fig. 2). Mice vaccinated with one dose of NYCBH or NYCBHΔE3L or two doses of NYCBHΔE3L maintained weights similar to mock-vaccinated, unchallenged mice, although there appeared to be a trend of weight loss in mice vaccinated with NYCBHΔE3L, but this did not reach statistical significance (Fig. 2).
Fig. 2.
Percent weight change of A/Ncr mice vaccinated with NYCBH or NYCBHΔE3L following ECTV challenge. Groups of 9-10 mice were mock-vaccinated or were vaccinated by scarification with one dose of NYCBH or NYCBHΔE3L on day 0 or with two doses of NYCBH or NYCBHΔE3L on days 0 and 28. On day 56 mice were challenged intranasally with ECTV. Percent weight change was monitored for 28 days. There were no statistically significance differences between the mock/mock group as compared to either of the NYCBH- or NYCBHΔE3L–vaccinated groups. Standard error of the mean (SEM) is indicated by error bars.
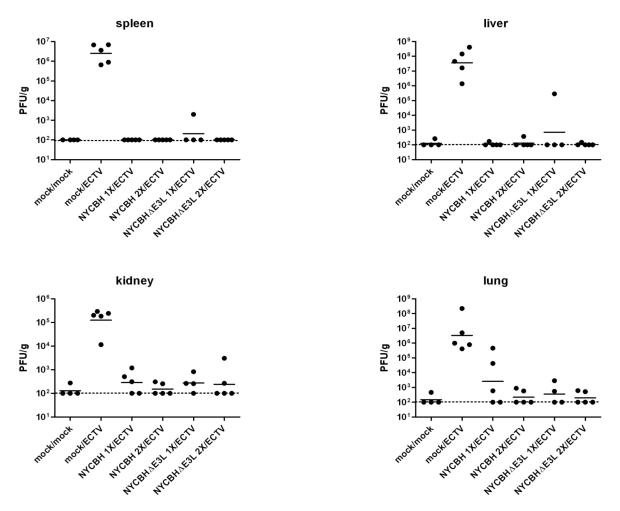
Six groups of 4-5 mice per group were similarly mock-vaccinated or were vaccinated with one or two doses of NYCBH or NYCBHΔE3L, and mice were challenged intranasally on day 56 with ECTV. At day 7 post challenge, tissues were assayed for viral load. Mock-vaccinated mice showed high mean ECTV titers in liver (3.6 × 108pfu/g), lung (3.3 × 106 pfu/g), spleen (2.5 × 106 pfu/g), and kidney (1.3 × 105 pfu/g), whereas mice vaccinated either singly or doubly with NYCBH or NYCBHΔE3L controlled virus replication in all tested tissues (p<0.05) (Fig. 3). Two animals vaccinated with one dose of NYCBH showed some breakthrough (in lung), whereas two animals vaccinated with one dose of NYCBHΔE3L showed some evidence of breakthrough (one in liver and one in spleen), although this did not reach statistical significance.
Fig. 3.
ECTV titers in tissues. Groups of 4-5 mice were mock-vaccinated or were vaccinated by scarification with one dose of NYCBH or NYCBHΔE3L on day 0 or with two doses of NYCBH or NYCBHΔE3L on days 0 and 28. On day 56 mice were challenged intranasally with ECTV. On day 7 post challenge tissues (spleen, liver, kidney, and lung) were harvested and assayed for ECTV by plaque assay. Statistical significance in mean titers (p<0.05) was observed between the mock/ECTV group in comparison to all other vaccination groups, and this was observed in all tissues.
Induction of VACV-specific antibody titers and neutralizing antibody titers in vaccinated mice
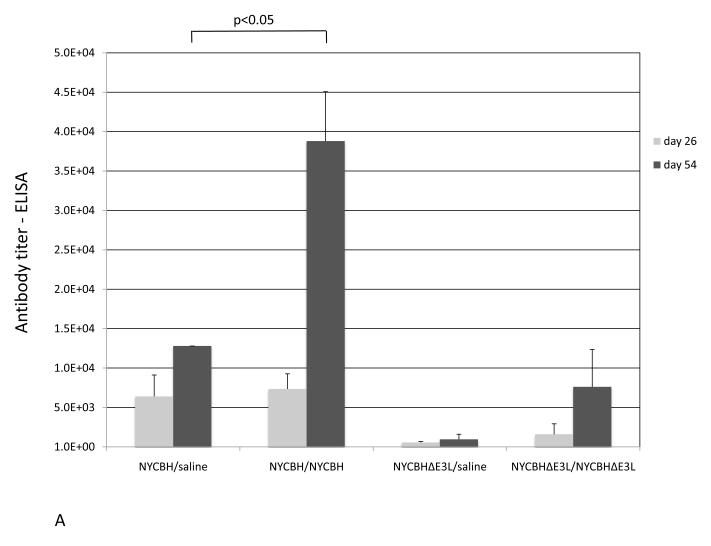
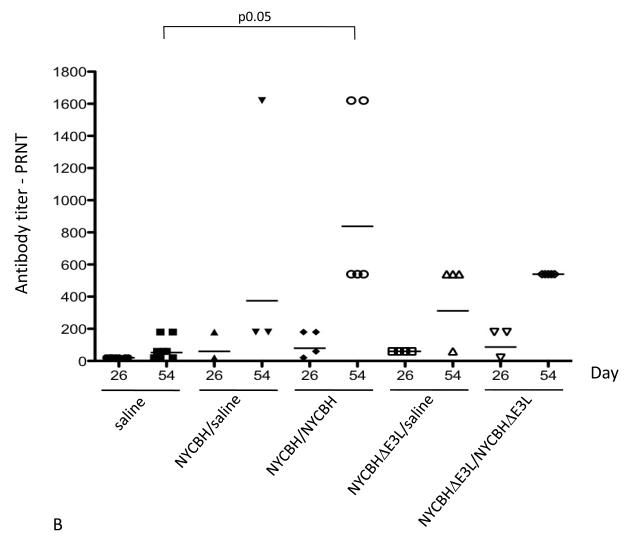
Serum from mice vaccinated with one or two doses of NYCBH or NYCBHΔE3L or from mock-vaccinated mice was collected from the submandibular vein on days 26 and 54, prior to challenge with ECTV. VACV-specific antibody titers were analyzed by ELISA and neutralizing antibodies were measured by plaque reduction neutralization assay (PRNT). Fig. 4A shows that a single vaccination with NYCBH resulted in antibody titers of 6,400 at day 26 and 12,800 at day 54. Administration of a boost with NYCBH at day 28 resulted in an increase in titers up to 38,802 at day 54. A single vaccination with NYCBHΔE3L resulted in lower titers of 656 and 951 at days 26 and 54, respectively, while a boost with NYCBHΔE3L administered at day 28 caused an increase in titers up to 7611, similar to titers seen after a single vaccination with NYCBH. Figure 4B shows that a single vaccination with NYCBH resulted in neutralizing antibody titers of 60 at day 26 and 374 by day 54. A boost with NYCBH resulted in an increase in neutralizing antibodies to 838 by day 54. Similarly, a single vaccination with NYCBHΔE3L resulted in a PRNT of 60 at day 26 and 312 by day 54. A boost with NYCBHΔE3L resulted in an increase up to 540 by day 54 which is not statistically different from titers seen with two doses of NYCBH at day 54 (p>0.05).
Fig. 4.
Antibody titers in serum following vaccination with NYCBH or NYCBHΔE3L. Groups of 3-5 mice were mock-vaccinated or were vaccinated by scarification with one dose of NYCBH or NYCBHΔE3L on day 0 or with two doses of NYCBH or NYCBHΔE3L on days 0 and 28. Serum samples were harvested prior to challenge at days 26 and 54 from each group of mice and were assayed individually. (A) Geometric means of VACV-specific antibodies were measured by an ELISA. Statistical significance in titers was observed at day 54 between the NYCBH/saline and NYCBH/NYCBH groups, but not between the NYCBHΔE3L/saline and NYCBHΔE3L/NYCBHΔE3L groups. (B) Neutralizing antibodies against ECTV were measured by a plaque reduction neutralization assay (PRNT). Individual PRNTs are shown for each vaccination group, and geometric means are shown as horizontal lines. Statistical significance in titers was observed at day 54 between the saline and NYCBH/NYCBH group only.
Discussion
The testing of vaccinia virus (VACV) vaccine strains in animal models of poxvirus infection is necessary to determine the potential efficacy against related, but distinct, human pathogens such as monkeypox virus (MPXV) or variola virus [17]. In this study we tested the ability of the current NYCBH vaccine deleted for the immunomodulatory E3L gene to protect mice from a lethal challenge with ECTV, which like variola virus and MPXV shows ~95% amino acid sequence identity with VACV [18,19].
Vaccination with the highly attenuated NYCBHΔE3L was equally as effective as NYCBH in protecting mice from death and weight loss following intranasal challenge with ECTV. Additionally, vaccination with NYCBHΔE3L protected mice from the accumulation of high levels of serum IFNγ (data not shown). ECTV infection of unvaccinated mice results in high levels of serum IFNγ at later times post challenge [11]. A single dose vaccination with NYCBHΔE3L prevented the increase in IFNγ levels at day 7 post challenge equally as well as a single dose of NYCBH (data not shown).
Vaccination with NYCBHΔE3L also protected mice from viral spread to internal organs similar to protection seen with NYCBH. Mock-vaccinated ECTV-challenged mice showed high titers in liver and spleen as well as showing titers in kidney, and lung [20]. While the average liver and spleen titers in single-dosed NYCBHΔE3L-vaccinated mice were low, one out of four mice had measurable ECTV titers in these organs, although the titers were two and three logs lower, respectively, than average titers in mock-vaccinated mice. Two doses of NYCBHΔE3L completely prevented measurable titers in liver and spleen in the five mice tested. In addition, two out of five mice vaccinated once with NYCBH showed viral titers in the lung, while two doses of NYCBH resulted in low titers in all five mice tested. Although these titers did not show statistical significance, this suggests that two doses of either vaccine may be more efficacious at preventing potential “breakthrough” of viral replication. Similarly, some breakthrough of viral titers following a single dose of NYCBHΔE3L was also observed in rabbits upon challenge with rabbitpox virus even though all rabbits survived challenge with no weight loss, fever, or other clinical indicators (Denzler et al., submitted). This suggests that although a single dose of NYCBHΔE3L completely protects animals from rabbitpox virus challenge, two doses are more effective at preventing potential breakthrough of viral replication. In contrast, two doses of NYCBHΔE3L were not able to protect macaques from morbidity that includes viral replication or the development of skin lesions following challenge with MPXV (Denzler et al., submitted). The ability of two doses of NYCBHΔE3L to protect against morbidity associated with heterologous poxvirus challenge appears to depend on the poxvirus with protection being effective against ECTV and RPV but less effective against MPXV.
NYCBHΔE3L showed a high level of attenuation in a newborn CD1 model which suggests its potential for safety as a vaccine. The attenuation seen with NYCBHΔE3L is similar to that seen with NYVAC, a VACV-based vaccine that has been attenuated by deletion of 18 open reading frames, and MVA, a VACV strain attenuated by 570 passages in chick embryo fibroblast cells resulting in loss of 30 Kb of the genome [15,16,21]. MVA has been previously tested for its ability to protect mice against challenge by ECTV [22]. A single dose of 1 × 106 pfu was not able to protect against weight loss, while 1 × 107 pfu was protective. In this study we found that one dose of 1 × 106 pfu of NYCBHΔE3L was able to protect against significant weight loss, similar to the parental NYCBH, although in comparison, the previous study challenged mice using a higher dose of ECTV [22]. Therefore, it is possible that a lower level of protection with NYCBHΔE3L could be observed with a higher challenge dose.
While protection of mice from heterologous virus challenge using a single dose of NYCBHΔE3L is effective, total serum VACV-specific antibody titers, as detected by ELISA, were low with a single dose. A boost with NYCBHΔE3L yielded an increase in VACV-specific antibody titers to levels similar to that observed after a single dose of NYCBH. In contrast, a single vaccination with either NYCBH or NYCBHΔE3L resulted in similar neutralizing antibody titers at day 26 that continued to rise to similar levels by day 54, and both vaccines completely protected mice from challenge with the heterologous ECTV. A boost with either vaccine resulted in an increase in neutralizing antibody titers with no statistical difference between endpoint titers at day 54 between NYCBH or NYCBHΔE3L. The reason for the discrepancy of ELISA and neutralization titers from comparable sera of mice immunized with NYCBH or NYCBHΔE3L is not clear. In comparison, a previous study has shown that a single vaccination with either NYCBH or NYCBHΔE3L is able to fully protect mice from weight loss and death following challenge with the homologous virus, VACV WR, despite the fact that a single dose of either virus induces low levels of neutralizing antibody titers [10]. However a second dose of NYCBHΔE3L induces a nearly 6-fold increase in titers. Similarly in this study, a second dose of NYCBHΔE3L or NYCBH caused a 6.3- or 10.6-fold increase, respectively, in neutralizing antibody titers. Even if serum antibody levels are low following a single vaccination, it is likely that memory B cells can mount an effective antibody response following challenge resulting in full protection of mice. Panchanathan et al. have shown that an intact B cell response is in fact responsible for protection of mice from a secondary ECTV infection while CD4 or CD8 cells are not required, further augmenting the importance of a B cell-mediated antibody response [23]. In addition, Spriggs et al. have shown that VACV-vaccinated β2- microglobulin deficient mice which lack CD8+ cells are also able to survive homologous VACV challenge, and this has been verified in our lab using CD8 knockout mice (data not shown)[24]. This is in contrast to the study by Jentarra et al. which shows that NYCBHΔE3L-vaccinated B cell knockout mice survive homologous VACV challenge with no associated morbidity [10]. This suggests that in the homologous virus challenge model, either a T or B cell response can protect mice from secondary infection, while in the heterologous ECTV model a B cell response is necessary. Similarly, in VACV-vaccinated macaques, recovery from heterologous challenge with monkeypox virus requires B cells or antibodies [25]. The difference in immunological requirements for protection from ECTV challenge in comparison to VACV is not completely understood, however ECTV is a natural mouse pathogen and requires low doses to initiate a robust infection, similar to the low dose that is estimated to cause variola infection in humans [26]. It is possible that a rapid B cell response is required following challenge of an immunized subject with a highly robust naturally-occurring pathogen whereas either a T or B cell response is protective against a laboratory-induced pathogen like VACV.
Highlights.
>NYCBHΔE3L vaccine candidate protects mice from ectromelia virus challenge.
>Potential safer smallpox vaccine.
>Induces similar titers of neutralizing antibodies as current smallpox vaccine.
Acknowledgments
Funding : This work was supported by a grant from the National Institute of Health 5UO1AI066326.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- [1].Jacobs BL, Langland JO, Kibler KV, Denzler KL, White SD, Holechek SA, et al. Vaccinia virus vaccines: past, present and future. Antiviral Res. 2009 Oct;84(1):1–13. doi: 10.1016/j.antiviral.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Greenberg RN, Kennedy JS. ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine. Expert Opin Investig Drugs. 2008 Apr;17(4):555–64. doi: 10.1517/13543784.17.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim YG, Muralinath M, Brandt T, Pearcy M, Hauns K, Lowenhaupt K, et al. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc Natl Acad Sci U S A. 2003 Jun 10;100(12):6974–9. doi: 10.1073/pnas.0431131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Watson JC, Chang HW, Jacobs BL. Characterization of a vaccinia virus-encoded double-stranded RNA-binding protein that may be involved in inhibition of the double-stranded RNA-dependent protein kinase. Virology. 1991 Nov;185(1):206–16. doi: 10.1016/0042-6822(91)90768-7. [DOI] [PubMed] [Google Scholar]
- [5].Chang HW, Jacobs BL. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology. 1993 Jun;194(2):537–47. doi: 10.1006/viro.1993.1292. [DOI] [PubMed] [Google Scholar]
- [6].Langland JO, Jacobs BL. Inhibition of PKR by vaccinia virus: role of the N- and C-terminal domains of E3L. Virology. 2004 Jul 1;324(2):419–29. doi: 10.1016/j.virol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- [7].Perdiguero B, Esteban M. The interferon system and vaccinia virus evasion mechanisms. J Interferon Cytokine Res. 2009 Sep;29(9):581–98. doi: 10.1089/jir.2009.0073. [DOI] [PubMed] [Google Scholar]
- [8].Langland JO, Kash JC, Carter V, Thomas MJ, Katze MG, Jacobs BL. Suppression of proinflammatory signal transduction and gene expression by the dual nucleic acid binding domains of the vaccinia virus E3L proteins. J Virol. 2006 Oct;80(20):10083–95. doi: 10.1128/JVI.00607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brandt TA, Jacobs BL. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J Virol. 2001 Jan;75(2):850–6. doi: 10.1128/JVI.75.2.850-856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jentarra GM, Heck MC, Youn JW, Kibler K, Langland JO, Baskin CR, et al. Vaccinia viruses with mutations in the E3L gene as potential replication-competent, attenuated vaccines: scarification vaccination. Vaccine. 2008 Jun 2;26(23):2860–72. doi: 10.1016/j.vaccine.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].SAM Parker S., Painter G, Schriewer J, Buller RM. Ectromelia Virus Infections of Mice as a Model to Support the Licensure of Anti-Orthopoxvirus Therapeutics. Viruses. 2010;2(9):1918–32. doi: 10.3390/v2091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Parker S, Schriewer J, Oberle C, Robertson A, Lanier R, Painter G, et al. Using biomarkers to stage disease progression in a lethal mousepox model treated with CMX001. Antivir Ther. 2008;13(7):863–73. [PMC free article] [PubMed] [Google Scholar]
- [13].Chen W, Drillien R, Spehner D, Buller RM. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology. 1992 Apr;187(2):433–42. doi: 10.1016/0042-6822(92)90445-u. [DOI] [PubMed] [Google Scholar]
- [14].Li Z, Rubin SA, Taffs RE, Merchlinsky M, Ye Z, Carbone KM. Mouse neurotoxicity test for vaccinia-based smallpox vaccines. Vaccine. 2004 Mar 29;22(11-12):1486–93. doi: 10.1016/j.vaccine.2003.10.022. [DOI] [PubMed] [Google Scholar]
- [15].Tartaglia J, Perkus ME, Taylor J, Norton EK, Audonnet JC, Cox WI, et al. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992 May;188(1):217–32. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- [16].Sutter G, Staib C. Vaccinia vectors as candidate vaccines: the development of modified vaccinia virus Ankara for antigen delivery. Curr Drug Targets Infect Disord. 2003 Sep;3(3):263–71. doi: 10.2174/1568005033481123. [DOI] [PubMed] [Google Scholar]
- [17].Snoy PJ. Establishing efficacy of human products using animals: the US food and drug administration’s “animal rule”. Vet Pathol. 2010 Sep;47(5):774–8. doi: 10.1177/0300985810372506. [DOI] [PubMed] [Google Scholar]
- [18].Chen N, Danila MI, Feng Z, Buller RM, Wang C, Han X, et al. The genomic sequence of ectromelia virus, the causative agent of mousepox. Virology. 2003 Dec 5;317(1):165–86. doi: 10.1016/s0042-6822(03)00520-8. [DOI] [PubMed] [Google Scholar]
- [19].Gubser C, Hue S, Kellam P, Smith GL. Poxvirus genomes: a phylogenetic analysis. J Gen Virol. 2004 Jan;85(Pt 1):105–17. doi: 10.1099/vir.0.19565-0. [DOI] [PubMed] [Google Scholar]
- [20].Esteban DJ, Buller RM. Ectromelia virus: the causative agent of mousepox. J Gen Virol. 2005 Oct;86(Pt 10):2645–59. doi: 10.1099/vir.0.81090-0. [DOI] [PubMed] [Google Scholar]
- [21].Antoine G, Scheiflinger F, Dorner F, Falkner FG. The complete genomic sequence of the modified vaccinia Ankara strain: comparison with other orthopoxviruses. Virology. 1998 May 10;244(2):365–96. doi: 10.1006/viro.1998.9123. [DOI] [PubMed] [Google Scholar]
- [22].Coulibaly S, Bruhl P, Mayrhofer J, Schmid K, Gerencer M, Falkner FG. The nonreplicating smallpox candidate vaccines defective vaccinia Lister (dVV-L) and modified vaccinia Ankara (MVA) elicit robust long-term protection. Virology. 2005 Oct 10;341(1):91–101. doi: 10.1016/j.virol.2005.06.043. [DOI] [PubMed] [Google Scholar]
- [23].Panchanathan V, Chaudhri G, Karupiah G. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J Virol. 2006 Jul;80(13):6333–8. doi: 10.1128/JVI.00115-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Spriggs MK, Koller BH, Sato T, Morrissey PJ, Fanslow WC, Smithies O, et al. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6070–4. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005 Jul;11(7):740–7. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- [26].Nicas M, Hubbard AE, Jones RM, Reingold AL. The infectious dose of variola (smallpox) virus. Applied Biosafety. 2004;9(3):118–27. [Google Scholar]