Highlights
► NYCBHΔE3L vaccine candidate partially protects macaques from monkeypox virus challenge. ► Potential safer smallpox vaccine. ► Induces 2-fold lower titers of neutralizing antibodies as current smallpox vaccine.
Keywords: Vaccinia virus, E3L, Monkeypox virus, Smallpox
Abstract
The New York City Board of Health (NYCBH) vaccinia virus is the currently licensed vaccine for use in the US against smallpox. The vaccine under investigation in this study has been attenuated by deletion of the innate immune evasion gene, E3L, and shown to be protective in homologous virus mouse challenge and heterologous virus mouse and rabbit challenge models. In this study we compared NYCBH deleted for the E3L gene (NYCBHΔE3L) to NYCBH for the ability to induce phosphorylation of proinflammatory signaling proteins and the ability to protect cynomolgus macaques from heterologous challenge with monkeypox virus (MPXV). NYCBHΔE3L induced phosphorylation of PKR and eIF2α as well as p38, SAPK/JNK, and IRF3 which can lead to induction of proinflammatory gene transcription. Vaccination of macaques with two doses of NYCBHΔE3L resulted in negligible pock formation at the site of scarification in comparison to vaccination using a single dose of NYCBH, but still elicited neutralizing antibodies and protected 75% of the animals from mortality after challenge with MPXV. However, NYCBHΔE3L-vaccinated animals developed a high number of secondary skin lesions and blood viral load similar to that seen in unvaccinated controls. The NYCBHΔE3L-vaccinated animals that survived MPXV challenge were able to show resolution of blood viral load, a decrease in number of skin lesions, and an improved clinical score by three weeks post challenge. These results suggest that although the highly attenuated NYCBHΔE3L allows proinflammatory signal transduction to occur, it does not provide full protection against monkeypox challenge.
1. Introduction
The eradication of smallpox was achieved due to a large-scale worldwide vaccination program using a number of vaccinia virus (VACV) strains [1]. In the Americas and West Africa the New York City Board of Health (NYCBH) VACV strain was used in the vaccination program [1]. This VACV strain (designated as Dryvax®) was manufactured by Wyeth and was grown and harvested from lymph fluid of calf skin that is infected with NYCBH VACV [2]. In order to increase safety from contaminants and adventitious agents that may be present in calf lymph and to decrease the genetic heterogeneity found in Dryvax®, a single clone isolate of NYCBH, named Acambis2000™, was purified in tissue culture conditions and tested in clinical comparison to Dryvax® [3]. Vaccination with either Dryvax® or Acambis2000™ results in equivalent neutralizing antibody and T cell responses, however similar rates of adverse side effects, specifically myocarditis/myopericarditis, are also observed. Therefore Acambis2000™ is considered a risk for other known serious adverse events that include eczema vaccinatum, progressive vaccinia, postvaccinial encephalitis, and generalized vaccinia [3].
Due to the adverse events that occur with the tissue culture-adapted NYCBH VACV strain, an attenuated mutant of NYCBH (Acambis2000™) was made by deletion of the immunomodulatory gene, E3L [4]. The E3L gene codes for proteins that contain a dsRNA binding domain and a Z form nucleic acid binding domain (Zα) [5], [6]. The dsRNA binding domain is required for inhibition of proinflammatory signal transduction and for inhibition of the type I interferon response. Specifically, E3L binds and sequesters dsRNA, a byproduct of viral transcription, and prevents activation of type I interferon-induced protein kinase R (PKR) and oligoadenylate synthetase (OAS) [7], [8], [9]. In addition, E3L inhibits the activation/phosphorylation of IRF3 which is responsible for induction of the IFNβ gene, and this is also due to E3L's ability to bind dsRNA [7], [10]. A specific cytosolic function for the Zα domain in binding Z-form nucleic acid has not yet been determined, although the ability to bind Z DNA in vitro has been correlated with pathogenicity in vivo [11], [12], [13]. Both domains are required for pathogenicity in an animal model, so deletion of the entire E3L gene from VACV results in a nonpathogenic virus that replicates to 3 log lower levels in skin than the parental VACV [4], [14].
Testing of a new candidate smallpox vaccine in multiple animal models using several related orthopoxviruses is required if a new vaccine strain is to be used in the human population for protection against smallpox [15]. The highly attenuated virus, NYCBHΔE3L, has thus far been tested as a vaccine in homologous and heterologous mouse challenge models and in a heterologous rabbit challenge model [4], [16], [17]. In the homologous mouse model a single dose of NYCBHΔE3L fully protected mice from mortality and weight loss following VACV challenge, but neutralizing antibody titers were low. However two doses increased the levels of neutralizing antibody titers [4]. Similarly in the heterologous mouse model a single dose of NYCBHΔE3L fully protected mice from mortality and weight loss following challenge with ectromelia virus (ECTV), and two doses increased neutralizing antibody titers [17]. In contrast, in the rabbit model a single dose of NYCBHΔE3L fully protected rabbits from a low dose lethal challenge with the heterologous rabbitpox virus but the eruption of secondary lesions was higher than that seen upon vaccination with parental NYCBH. However two doses of NYCBHΔE3L protected rabbits from the eruption of secondary lesions after high dose challenges similar to protection seen with NYCBH [16].
Monkeypox virus (MPXV) is a member of the orthopoxvirus genus that includes VACV and variola virus (VARV), the causative agent of smallpox. VACV strains have been historically used to vaccinate against both smallpox and monkeypox due to their antigenic relatedness [18]. MPXV causes disease in humans that mimics the rash/lesions observed with VARV infection, but the fatality rate seen with VARV infection is higher [19], [20], [21]. However, the recent outbreak of MPXV in the US in 2003 supports the continued need for a VACV vaccine [22], [23]. Additionally, with an increasingly immunologically suppressed population due to cancer treatment, organ transplantation, infection with HIV, or for those with atopic skin disorders there is a need for a VACV vaccine that causes a lower rate of complications. This paper describes testing of the attenuated NYCBHΔE3L in a MPXV challenge model using cynomolgus macaques. Results showed that two doses of NYCBHΔE3L protected 75% of the animals from death, however in the surviving animals, breakthrough symptoms including viremia and secondary lesions on the skin were observed which resolved 3 weeks after challenge. These data suggest that NYCBHΔE3L can partially protect MPXV-challenged animals against death but only poorly protect against disease.
2. Materials and methods
2.1. Cell lines and virus stocks
BHK-21 and RK-E3L (rabbit kidney cells stably expressing the VACV E3L gene) (Wong, Denzler, and Jacobs, unpublished results) cells were grown in Minimal Essential Media (MEM, Cellgro) containing 50 μg/ml gentamycin and 5% fetal bovine serum (FBS, Hyclone). HeLa and Vero E6 cells were grown in Dulbecco's MEM (D-MEM, Cellgro) containing 5% FBS (Hyclone) and 50 μg/ml gentamycin, and BSC-40 cells were grown in D-MEM (Cellgro) containing 10% FBS (Hyclone) and 50 μg/ml gentamycin.
VACV strains, NYCBH (ACAM2000™, kindly provided by Acambis), NYCBHΔE3L (derived from ACAM2000™) [4], WR (ATCC), and WRΔE3L [14] were propagated in BHK cells as previously described [14]. Infected cells were processed by three rounds of freeze–thaw followed by sonication and pelleting. Supernate was loaded onto a 36% sucrose pad and virus was partially purified by centrifugation. Viral titers were determined using RK-E3L cells. For cynomolgus macaque vaccination/challenge studies, NYCBH (ACAM2000™, Lot # VV04-003a) was obtained from the Centers for Disease Control and was diluted according to manufacturer's recommendations. MPXV (Zaire strain V79-I-005, Master Seed NR-523) was obtained from the National Institutes of Health Biodefense and Emerging Infections Research Resources Repository. Virus was diluted and titers were determined using Vero E6 cells to calculate the actual challenge dose.
2.2. Western blots
HeLa cells were infected with VACV strains at an MOI of 5. Six hours post infections cell lysates were prepared by harvesting the cells in 1× sodium dodecyl sulfate (SDS) lysis buffer (50 mM Tris–Cl pH 6.8, 10% glycerol, 100 mM β-mercaptoethanol, 2% SDS, 0.1% bromophenol blue) followed by centrifugation through a Qiashredder column (Qiagen). Lysates were separated on SDS-PAGE gels, transfered to nitrocellulose, and blocked in buffer (20 mM Tris–Cl pH 7.8, 180 mM NaCl, 0.02% Na azide) containing 3% nonfat dry milk. Monoclonal antibodies to PKR-P, eIF2α-P, p38-P, SAP/JNK-P, GAPDH (Cell Signaling), and IRF3-P (Epitomics) were diluted according to manufacturer's specifications. Development was performed using anti-rabbit IgG/horseradish peroxidase (Santa Cruz) in the presence of a chemiluminescent substrate (Thermo Scientific).
2.3. Viral genome load
MPXV DNA was measured various days post challenge using Real-Time PCR. Briefly, DNA was extracted from 200 μl whole blood using a DNA mini kit (Qiagen), and primers and probes specific for the HA gene were used to measure viremia using a LightCycler Quantitative Pan-orthopox HA PCR assay [24]. Primers used are as follows: forward primer: OPHA F89 5′-GATGATGCAACTCTATCATGTA-3′, reverse primer: OPHA R219 5′-GTATAATTATCAAAATACAAGACGTC-3′, probe: OPHA-P143S-MGB 6FAM-AGTGCTTGGTATAAGGAG MGBNFQ-3′. The lower limit of detection (LLOD) of the assay is 5000 genome copies/ml blood.
2.4. Animals
Twenty-four (24) adult cynomolgus macaques, Macaca fascicularis, were obtained from Three Spring Scientific (Kaiser, MO). Animals were randomly distributed using SAS/STAT software into three groups of eight animals each by sex and weight. Vaccinations were performed by scarification. Seven weeks later animals were challenged intravenously with 5 × 107 pfu/ml MPXV Zaire strain. Following challenge animals were monitored twice daily for clinical signs of disease including body temperature and weight loss. In addition, an 18 point clinical score was assessed at 3 points per indicator and included depression, weakness, dehydration, dyspnea, anorexia, and edema. Animals were euthanized when they exhibited severe signs of monkeypox disease. Housing and care were carried out in accordance with the American Association for Accreditation of Laboratory Animal Care standards. The study was approved by the Institutional Animal Care and Use Committee at Southern Research Institute.
2.5. Plaque reduction neutralization assay (PRNT)
Sera were obtained from vaccinated animals and stored at −80 °C. Prior to assay, sera were heat inactivated at 56 °C for 30 min followed by preparation of serial dilutions in MEM. A known amount of VACV WR was added to each serial dilution and incubated at 37 °C for 2 h followed by titration in duplicate on RK-E3L cells. The neutralizing antibody titer was determined by calculating the inverse of the serum dilution at which a 50% reduction in plaquing efficiency occurred.
2.6. Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc.). For neutralizing antibody titers, a one-way ANOVA followed by Tukey's multiple comparison test was performed.
3. Results
3.1. NYCBH deleted for the E3L gene allows phosphorylation of proteins in inflammatory pathways
The phosphorylation of various inflammatory pathway proteins in VACV NYCBH infection was compared to VACV WR, a derivative of NYCBH that has been neuroadapted by intracerebral passaging in mice [25]. Infection by wild type VACV WR and VACV NYCBH, which both contain the E3L gene, inhibited phosphorylation of PKR and eIF2α (Fig. 1 ). Deletion of the E3L gene (ΔE3L) from either VACV WR or VACV NYCBH resulted in phosphorylation of both PKR and eIF2α. The presence of E3L during VACV WR and VACV NYCBH infection also inhibited p38 and IRF3 phosphorylation and resulted in low levels of SAPK/JNK phosphorylation, whereas deletion of E3L resulted in phosphorylation of p38 and IRF3 and increased levels of SAPK/JNK phosphorylation (Fig. 1). Therefore, deletion of the E3L gene from NYCBH results in phosphorylation of proteins in several inflammatory cascades, similar to that seen with VACV WR, which may aid in promoting a protective immune response.
Fig. 1.
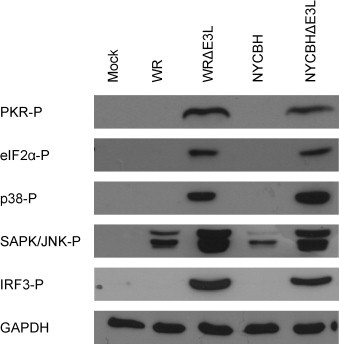
Western blot of signal transduction proteins. HeLa cells were infected with WR, WRΔE3L, NYCBH, NYCBHΔE3L, or were mock infected. Cell lysates were prepared at 6 h post infection and were analysed by Western blot for the detection of phosphorylated proteins. GAPDH was used as a loading control.
3.2. Vaccination of macaques by scarification with NYCBHΔE3L does not induce pock formation
Three groups of 8 macaques were mock vaccinated, vaccinated with an estimated 1 × 106 pfu NYCBHΔE3L on days 0 and 21, or were vaccinated with an estimated 2.5 × 105 pfu NYCBH on day 21. We chose to give two doses of the NYCBHΔE3L vaccine to macaques because two doses increases the neutralizing antibody titers in mice and prevents the development of secondary lesions in rabbits [4], [16]. Every 7 days post-vaccination, the injection sites were photographed. As expected, Fig. 2A shows that vaccination with NYCBH resulted in a visible area of tissue damage with surrounding erythema, whereas vaccination with NYCBHΔE3L did not induce similar lesions, but appeared similar to mock-vaccinated animals. Fig. 2B shows measurements of the surface area of the pocks at various days following scarification. Vaccination with NYCBH resulted in an increase in surface area of the pocks that peaked 7 days after scarification indicating viral replication, whereas the surface area of pocks following vaccination with NYCBHΔE3L did not differ from that observed in mock-vaccinated animals. The median surface area of pocks 7 days after vaccination with NYCBH was 0.39 cm2 in comparison to vaccination with NYCBHΔE3L at 0.05 cm2 (p < 0.05). Therefore, NYCBHΔE3L did not show strong evidence of replication at the vaccination site.
Fig. 2.
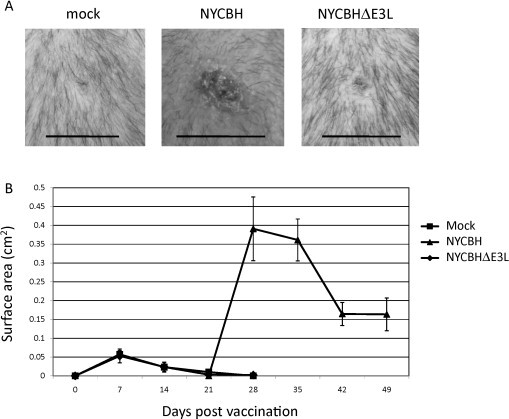
Vaccination of cynomolgus macaques with NYCBH or NYCBHΔE3L. Groups of 8 animals were mock-vaccinated or were vaccinated with NYCBHΔE3L on days 0 and 21, or were vaccinated with NYCBH on day 21 by scarification. (A) Representative photographs of the vaccination sites were taken at 7 days post vaccination. Black lines correspond to 1 cm. (B) Measurements of the surface area (cm2) of the vaccination sites over 4 weeks are shown. The second mock or NYCBHΔE3L vaccinations given at day 21 are not shown.
3.3. Neutralizing antibody titers after vaccination with NYCBHΔE3L
Sera were obtained from animals at 42 days post vaccination, prior to challenge with MPXV. Fig. 3 shows that mock-vaccinated animals displayed a low limit of detection (LLOD) of neutralizing antibody titers (17), whereas animals vaccinated with a single dose of NYCBH had significantly higher titers than mock-vaccinated animals (120, p < 0.05). Animals vaccinated with two doses of NYCBHΔE3L had measurable neutralizing antibody titers (59), however they were 2-fold lower than that seen in animals vaccinated with NYCBH, although this did not reach statistical significance.
Fig. 3.
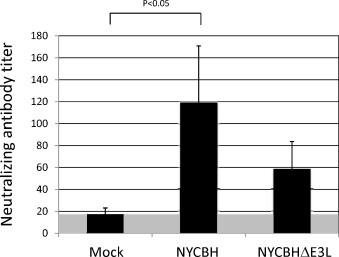
Plaque reduction neutralizing antibody titers (PRNTs) in macaques vaccinated with NYCBH or NYCBHΔE3L. The geometric means of PRNTs from groups of 8 animals that were mock-vaccinated or vaccinated with NYCBHΔE3L on days 0 and 21, or were vaccinated with NYCBH on day 21 were measured. Serum was harvested on day 42, seven days prior to challenge. Titers from individual macaques were determined as the inverse of the serum dilution at which a 50% reduction in plaquing efficiency occurred. Standard error of the mean (SEM) is indicated by error bars. Lower limit of detection is indicated by the gray area.
3.4. NYCBHΔE3L partially protects macaques against challenge with MPXV
Seven weeks after the start of the vaccination scheme (day 49) animals were challenged intravenously with 5 × 107 pfu MPXV. As expected, all eight mock-vaccinated animals developed typical MPXV disease and succumbed to the infection between day 8 and 14 post challenge. In contrast, 100% and 75% of NYCBH and NYCBHΔE3L-vaccinated animals survived lethal MPXV challenge (Fig. 4A). Although notable pock formation did not appear to occur at the injection site following NYCBHΔE3L vaccination, animals were partially protected from mortality following challenge with MPXV.
Fig. 4.
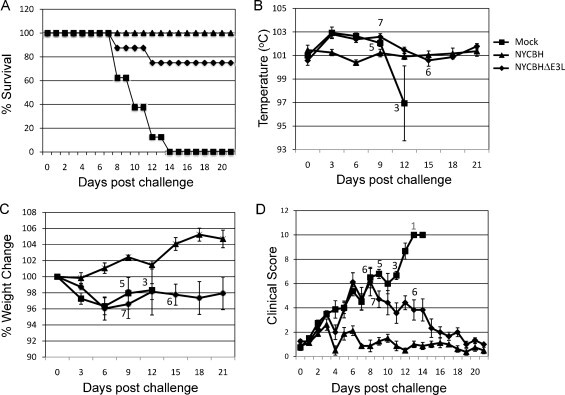
Challenge of vaccinated macaques with MPXV. Groups of 8 animals were mock vaccinated or were vaccinated with NYCBHΔE3L on days 0 and 21, or were vaccinated with NYCBH on day 21. Seven weeks later (day 49) macaques were challenged with 5 × 107 pfu MPXV and monitored for 21 days. (A) Percent survival of animals following challenge with MPXV. (B) Temperature, (C) percent weight change, and (D) clinical scores were monitored during challenge. Numbers indicate the surviving animals at each time point. Standard error of the mean (SEM) is indicated by error bars.
Temperatures, weights, and clinical scores were monitored for 21 days following MPXV challenge. NYCBH-vaccinated animals did not show a significant temperature change over the course of challenge, but mock- and NYCBHΔE3L-vaccinated animals displayed a slight increase in temperature over a period of 9 days (Fig. 4B). While the temperatures of surviving mock-vaccinated animals dropped just prior to death, the temperatures of surviving NYCBHΔE3L-vaccinated animals returned to that seen in NYCBH-vaccinated animals by day 12 post challenge. Steady weight gain was observed in NYCBH-vaccinated animals following challenge with MPXV, while mock- and NYCBHΔE3L-vaccinated animals lost weight through day 6 post challenge (Fig. 4C). Surviving mock-vaccinated animals gained a small percentage of weight until day 12, but by day 15 all mock-vaccinated animals died. Surviving NYCBHΔE3L-vaccinated animals were unable to gain back their prechallenge weights by day 21. Fig. 4D shows that clinical scores for NYCBH-vaccinated animals remained low, whereas mock- and NYCBHΔE3L-vaccinated animals showed similar increases in clinical scores through day 8 post challenge. The scores of the surviving mock-vaccinated animals continued to rise through day 14 until all animals died, whereas clinical scores of surviving NYCBHΔE3L-vaccinated animals decreased to levels seen in NYCBH-vaccinated animals by day 16 post challenge.
The number of secondary lesions and blood viral load were monitored for 21 days following MPXV challenge. NYCBH-vaccinated animals displayed very low numbers of secondary lesions (Fig. 5A). In contrast, mock- and NYCBHΔE3L-vaccinated animals displayed similar high numbers of secondary lesions which peaked at 9 days post challenge. All mock-vaccinated animals displayed high numbers of secondary lesions until they succumbed to infection. While the surviving NYCBHE3L-vaccinated animals also exhibited high secondary pock lesions, these numbers gradually decreased and resolved through day 21.
Fig. 5.
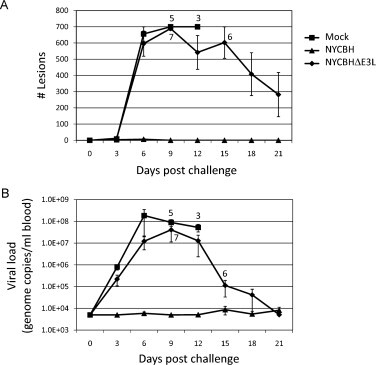
Number of lesions and viral load in vaccinated macaques following challenge with MPXV. Groups of 8 animals were mock vaccinated or were vaccinated with NYCBHΔE3L on days 0 and 21, or were vaccinated with NYCBH on day 21. Seven weeks later (day 49) macaques were challenged with 5 × 107 pfu MPXV and monitored for 21 days. (A) Number of secondary skin lesions and (B) blood viral load were monitored during challenge. Numbers indicate the surviving animals at each time point. Standard error of the mean (SEM) is indicated by error bars.
Consistent with the development of very low numbers of secondary pock lesions, viral load levels in the blood of NYCBH-vaccinated animals were undetectable, low or only transiently detectable following MPXV challenge. By contrast, mock- and NYCBHΔE3L-vaccinated animals showed an increase in viremia following challenge (Fig. 5B). Viral load peaked on day 6 post-challenge in mock-vaccinated animals at 1.8 × 108 genome copies/ml blood, while NYCBHΔE3L-vaccinated animals peaked later at day 9 post challenge with 4 × 107 genome copies/ml blood. While viral load remained high and all mock-vaccinated animals succumbed to infection, the viral loads in the surviving NYCBHΔE3L-vaccinated animals were brought under control and decreased to the limit of detection by day 21 post challenge. In addition, in NYCBHΔE3L-vaccinated animals, a low blood viral load correlated with the development of less severe secondary lesions while two of the NYCBHΔE3L-vaccinated animals that survived had high blood viral loads and developed lesions similar to those seen in mock-vaccinated animals (data not shown).
4. Discussion
NYCBHΔE3L provided protection against mortality in 75% of the macaques tested, however it did not protect against morbidity of MPXV disease. Viral load was readily measurable in the blood of NYCBHΔE3L-vaccinated animals, although the peak viral load occurred 3 days later than the peak viral load in mock-vaccinated animals suggesting vaccination was able to slightly delay viral replication. At the same time, the number of skin lesions observed in mock- and NYCBHΔE3L-vaccinated animals was very similar through day 9 post challenge, and NYCBHΔE3L-vaccinated animals were unable to gain back weight by 21 days post challenge. This suggests that although animals survived the challenge, the vaccination regimen was not able to protect against symptoms of MPXV disease at early times post challenge. However, at later times post challenge, the NYCBHΔE3L-vaccinated survivors recovered from fever and displayed reduced clinical scores, numbers of skin lesions, and blood viral load. In comparison, a single vaccination with a VACV deleted for E3L is able to protect mice from morbidity in both homologous (VACV) and heterologous (ECTV) virus challenge models, but two doses are required to protect rabbits from morbidity in a heterologous (RPV) virus challenge model [4], [16], [17]. Therefore, vaccination with NYCBHΔE3L gave some protection of macaques from death, but it was unable to protect from the morbidity associated with MPXV disease.
The lower level of protection in macaques seen upon vaccination with NYCBHΔE3L may be due to the absence of obvious pock formation which suggests low levels of viral replication at the site of scarification. We have previously shown that VACVΔE3L replicates poorly in the skin of vaccinated mice, and we have found that VACVΔE3L replicates poorly in primary human fibroblasts and keratinocytes (unpublished observations) [4]. In previous studies we have seen protection despite low levels of replication, and we hypothesized that protection was due to the induction of proinflammatory signaling by NYCBHΔE3L [4]. However, a certain threshold of antigen is likely required to obtain an effective repertoire of neutralizing antibodies, and the presence of neutralizing antibodies has been historically shown to be correlated with protection against smallpox [26], [27]. This study measured 2-fold lower levels of antibodies upon vaccination with NYCBHΔE3L in comparison to NYCBH. It is unclear if a drop of this magnitude in antibody levels can be correlated with the incomplete protection observed following challenge with MPXV as well as the morbidity displayed by viremia, skin lesions, and weight loss. However, Chaudhri et al. have shown that an antibody response is important in preventing the formation of secondary skin lesions following challenge with ectromelia virus, a related poxvirus [28]. In contrast, depletion of B cells does not affect the size of the primary skin lesion that develops following vaccination with either Dryvax® or the attenuated, replication competent LC16m8 and suggests that a B cell response is not required to regulate replication following initial vaccination [29].
Infection of human epithelial cells with NYCBHΔE3L resulted in the phosphorylation of several proteins involved in cellular signal transduction. Phosphorylation of PKR and its substrate, eIF2α, were observed at late times post infection in HeLa cells and this has previously been shown to result in an inhibition of protein synthesis [8]. Phosphorylation of PKR and eIF2α at the scarification site could also likely result in an inhibition of viral protein synthesis in the skin which could result in lower levels of viral antigen production. In comparison, low levels of phosphorylation of eIF2α have also been reported in the highly attenuated NYVAC where the viral replication cycle is inhibited at late translation, however eIF2α phosphorylation does not occur in the completely attenuated MVA where replication is blocked at virus assembly [30], [31], [32], [33]. At the same time, infection with NYCBHΔE3L activated PKR-dependent signaling pathways like p38 and SAPK/JNK which lead to activation of nuclear transcription factors ATF-2 and c-jun, or dsRNA-dependent signaling pathways leading to IRF3 activation which initiate transcription of genes that aid in the development of innate immunity [7], [8]. In comparison, NYVAC infection of human epithelial cells or monocytes does not result in IRF3 phosphorylation, whereas MVA infection results in JNK and IRF3 phosphorylation [34], [35]. NYCBHΔE3L is therefore similar to NYVAC for inhibition of protein synthesis in infected cells, but is similar to MVA in activation of proinflammatory signal transduction cascades.
For a highly attenuated vaccine, this study used relatively low doses compared to those used for NYVAC- and MVA-based vaccinations. Two doses of an estimated 1 × 106 pfu NYCBHΔE3L were used for scarification of macaques since two doses have been shown to increase antibody titers in mice and prevent development of secondary lesions in rabbits [4], [16]. Vaccination schemes using NYVAC generally involve one or more doses of 1 × 107–8 pfu given by intramuscular injection [36], [37], [38]. Similarly, MVA vaccinations often use 1 × 108 pfu given by injection at intramuscular or subcutaneous routes [39], [40], [41], [42]. Increased doses of NYCBHΔE3L could provide larger amounts of antigen at the initial site of vaccination which may allow a better antibody response, however retaining a larger dose at the vaccination site may require needle injections rather than the noninvasive scarification which uses a bifurcated needle. To that end, increasing the ability of NYCBHΔE3L to replicate at the scarification site might increase immunogenicity while still allowing for low dose vaccination with a bifurcated needle. NYCBH (Acambis2000™) has a truncation in the soluble IFNα/β binding protein which may allow IFN signaling to occur in surrounding uninfected cells that would render them more resistant to infection [43]. Reinsertion of a full-length IFNα/β binding protein into NYCBHΔE3L could potentially augment viral replication at lower doses of vaccine, thereby allowing an increase in antigen load while simultaneously allowing proinflammatory cascade activation to promote the immune response.
Alternately, mutants of E3L could be constructed in NYCBH resulting in a more replication competent vaccine. NYCBH deleted for the Zα domain of E3L (Δ83N) is attenuated for pathogenesis by 4 logs (data not shown) as compared to NYCBHΔE3L which is attenuated by 6 logs in a newborn CD1 mouse model. However, a VACV with a Δ83N deletion in the E3L gene is pathogenic in SCID mice, and could pose potential problems in an immunocompromised population [4]. Alternately, viruses containing Δ7C or Δ54N deletions in E3L are less pathogenic in SCID mice with Δ54N being less pathogenic [4]. This coincides with the higher levels of replication in the skin seen with Δ7C as compared to Δ54N, although Δ54N gives 2 log higher titers in skin than a ΔE3L mutant (Jentarra and Jacobs, unpublished observations). Construction of one of these deletion mutants of E3L in a NYCBH background could result in a virus that is highly attenuated for safety, but still replication competent enough for antigen production.
Funding
This work was supported by a grant from the National Institute of Health 5UO1AI066326.
References
- 1.Jacobs B.L., Langland J.O., Kibler K.V., Denzler K.L., White S.D., Holechek S.A., et al. Vaccinia virus vaccines: past, present and future. Antiviral Res. 2009;84(October (1)):1–13. doi: 10.1016/j.antiviral.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenthal S.R., Merchlinsky M., Kleppinger C., Goldenthal K.L. Developing new smallpox vaccines. Emerg Infect Dis. 2001;7(November–December (6)):920–926. doi: 10.3201/eid0706.010602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg R.N., Kennedy J.S. ACAM2000: a newly licensed cell culture-based live vaccinia smallpox vaccine. Expert Opin Investig Drugs. 2008;17(April (4)):555–564. doi: 10.1517/13543784.17.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jentarra G.M., Heck M.C., Youn J.W., Kibler K., Langland J.O., Baskin C.R., et al. Vaccinia viruses with mutations in the E3L gene as potential replication-competent, attenuated vaccines: scarification vaccination. Vaccine. 2008;26(June (23)):2860–2872. doi: 10.1016/j.vaccine.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang H.W., Jacobs B.L. Identification of a conserved motif that is necessary for binding of the vaccinia virus E3L gene products to double-stranded RNA. Virology. 1993;194(June (2)):537–547. doi: 10.1006/viro.1993.1292. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y.G., Lowenhaupt K., Oh D.B., Kim K.K., Rich A. Evidence that vaccinia virulence factor E3L binds to Z-DNA in vivo: implications for development of a therapy for poxvirus infection. Proc Natl Acad Sci U S A. 2004;101(February (6)):1514–1518. doi: 10.1073/pnas.0308260100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langland J.O., Kash J.C., Carter V., Thomas M.J., Katze M.G., Jacobs B.L. Suppression of proinflammatory signal transduction and gene expression by the dual nucleic acid binding domains of the vaccinia virus E3L proteins. J Virol. 2006;80(October (20)):10083–10095. doi: 10.1128/JVI.00607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langland J.O., Jacobs B.L. Inhibition of PKR by vaccinia virus: role of the N- and C-terminal domains of E3L. Virology. 2004;324(July (2)):419–429. doi: 10.1016/j.virol.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Perdiguero B., Esteban M. The interferon system and vaccinia virus evasion mechanisms. J Interferon Cytokine Res. 2009;29(September (9)):581–598. doi: 10.1089/jir.2009.0073. [DOI] [PubMed] [Google Scholar]
- 10.Xiang Y., Condit R.C., Vijaysri S., Jacobs B., Williams B.R., Silverman R.H. Blockade of interferon induction and action by the E3L double-stranded RNA binding proteins of vaccinia virus. J Virol. 2002;76(May (10)):251–259. doi: 10.1128/JVI.76.10.5251-5259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valentine R., Smith G.L. Inhibition of the RNA polymerase III-mediated dsDNA-sensing pathway of innate immunity by vaccinia virus protein E3. J Gen Virol. 2010;91(September (Pt 9)):2221–2229. doi: 10.1099/vir.0.021998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marq J.B., Hausmann S., Luban J., Kolakofsky D., Garcin D. The double-stranded RNA binding domain of the vaccinia virus E3L protein inhibits both RNA- and DNA-induced activation of interferon beta. J Biol Chem. 2009;284(September (38)):25471–25478. doi: 10.1074/jbc.M109.018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim Y.G., Muralinath M., Brandt T., Pearcy M., Hauns K., Lowenhaupt K., et al. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc Natl Acad Sci U S A. 2003;100(June (12)):6974–6979. doi: 10.1073/pnas.0431131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandt T.A., Jacobs B.L. Both carboxy- and amino-terminal domains of the vaccinia virus interferon resistance gene, E3L, are required for pathogenesis in a mouse model. J Virol. 2001;75(January (2)):850–856. doi: 10.1128/JVI.75.2.850-856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snoy P.J. Establishing efficacy of human products using animals: the US food and drug administration's animal rule. Vet Pathol. 2010;47(September (5)):774–778. doi: 10.1177/0300985810372506. [DOI] [PubMed] [Google Scholar]
- 16.Denzler KL, Rice AD, MacNeill AL, Fukushima N, Lindsey SF, Wallace G, et al. The NYCBH vaccinia virus deleted for the innate immune evasion gene, E3L, protects rabbits against lethal challenge by rabbitpox virus. doi:10.1016/j.vaccine.2011.07.140. [DOI] [PMC free article] [PubMed]
- 17.Denzler K.L., Schriewer J., Parker S., Werner C., Hartzler H., Hembrador E., et al. The attenuated NYCBH vaccinia virus deleted for the immune evasion gene, E3L, completely protects mice against heterologous challenge with ectromelia virus. Vaccine. 2011 doi: 10.1016/j.vaccine.2011.09.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fenner F., Henderson D.A., Arita I., Jezek Z., Ladnyi I.D. 1st ed. World Health Organization; Geneva, Switzerland: 1988. Smallpox and its eradication. [Google Scholar]
- 19.Arita I., Jezek Z., Khodakevich L., Ruti K. Human monkeypox: a newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. Am J Trop Med Hyg. 1985;34(July (4)):781–789. doi: 10.4269/ajtmh.1985.34.781. [DOI] [PubMed] [Google Scholar]
- 20.Jezek Z., Grab B., Szczeniowski M., Paluku K.M., Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66(4):459–464. [PMC free article] [PubMed] [Google Scholar]
- 21.Jezek Z., Fenner F. Karger; Basel: 1988. Human monkeypox. Monographs in virology. p. 1–140. [Google Scholar]
- 22.Stephenson J. Monkeypox outbreak a reminder of emerging infections vulnerabilities. JAMA. 2003;290(July (1)):23–24. doi: 10.1001/jama.290.1.23. [DOI] [PubMed] [Google Scholar]
- 23.Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(January (4)):342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 24.Kulesh D.A., Baker R.O., Loveless B.M., Norwood D., Zwiers S.H., Mucker E., et al. Smallpox and pan-orthopox virus detection by real-time 3′-minor groove binder TaqMan assays on the roche LightCycler and the Cepheid smart Cycler platforms. J Clin Microbiol. 2004;42(February (2)):601–609. doi: 10.1128/JCM.42.2.601-609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z., Rubin S.A., Taffs R.E., Merchlinsky M., Ye Z., Carbone K.M. Mouse neurotoxicity test for vaccinia-based smallpox vaccines. Vaccine. 2004;22(March (11–12)):1486–1493. doi: 10.1016/j.vaccine.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Downie A.W., McCarthy K. The antibody response in man following infection with viruses of the pox group. III. Antibody response in smallpox. J Hyg (Lond) 1958;56(December (4)):479–487. doi: 10.1017/s0022172400037980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mack T.M., Noble J., Jr., Thomas D.B. A prospective study of serum antibody and protection against smallpox. Am J Trop Med Hyg. 1972;21(March (2)):214–218. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhri G., Panchanathan V., Bluethmann H., Karupiah G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J Virol. 2006;80(July (13)):6339–6344. doi: 10.1128/JVI.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordon S.N., Cecchinato V., Andresen V., Heraud J.M., Hryniewicz A., Parks R.W., et al. Smallpox vaccine safety is dependent on T cells and not B cells. J Infect Dis. 2011;203(April (8)):1043–1053. doi: 10.1093/infdis/jiq162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerra S., Lopez-Fernandez L.A., Pascual-Montano A., Najera J.L., Zaballos A., Esteban M. Host response to the attenuated poxvirus vector NYVAC: upregulation of apoptotic genes and NF-kappaB-responsive genes in infected HeLa cells. J Virol. 2006;80(January (2)):985–998. doi: 10.1128/JVI.80.2.985-998.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Najera J.L., Gomez C.E., Domingo-Gil E., Gherardi M.M., Esteban M. Cellular and biochemical differences between two attenuated poxvirus vaccine candidates (MVA and NYVAC) and role of the C7L gene. J Virol. 2006;80(June (12)):6033–6047. doi: 10.1128/JVI.02108-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludwig H., Suezer Y., Waibler Z., Kalinke U., Schnierle B.S., Sutter G. Double-stranded RNA-binding protein E3 controls translation of viral intermediate RNA, marking an essential step in the life cycle of modified vaccinia virus Ankara. J Gen Virol. 2006;87(May (Pt 5)):1145–1155. doi: 10.1099/vir.0.81623-0. [DOI] [PubMed] [Google Scholar]
- 33.Sancho M.C., Schleich S., Griffiths G., Krijnse-Locker J. The block in assembly of modified vaccinia virus Ankara in HeLa cells reveals new insights into vaccinia virus morphogenesis. J Virol. 2002;76(August (16)):8318–8334. doi: 10.1128/JVI.76.16.8318-8334.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kibler K.V., Gomez C.E., Perdiguero B., Wong S., Huynh T., Holechek S., et al. Improved NYVAC-based vaccine vectors. PLoS One. 2011 doi: 10.1371/journal.pone.0025674. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delaloye J., Roger T., Steiner-Tardivel Q.G., Le Roy D., Knaup Reymond M., Akira S., et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5(June (6)):e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Mooij P., Balla-Jhagjhoorsingh S.S., Beenhakker N., van Haaften P., Baak I., Nieuwenhuis I.G., et al. Comparison of human and rhesus macaque T-cell responses elicited by boosting with NYVAC encoding human immunodeficiency virus type 1 clade C immunogens. J Virol. 2009;83(June (11)):5881–5889. doi: 10.1128/JVI.02345-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mooij P., Balla-Jhagjhoorsingh S.S., Koopman G., Beenhakker N., van Haaften P., Baak I., et al. Differential CD4+ versus CD8+ T-cell responses elicited by different poxvirus-based human immunodeficiency virus type 1 vaccine candidates provide comparable efficacies in primates. J Virol. 2008;82(March (6)):2975–2988. doi: 10.1128/JVI.02216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hel Z., Nacsa J., Tsai W.P., Thornton A., Giuliani L., Tartaglia J., et al. Equivalent immunogenicity of the highly attenuated poxvirus-based ALVAC-SIV and NYVAC-SIV vaccine candidates in SIVmac251-infected macaques. Virology. 2002;304(December (1)):125–134. doi: 10.1006/viro.2002.1722. [DOI] [PubMed] [Google Scholar]
- 39.Earl P.L., Americo J.L., Wyatt L.S., Eller L.A., Whitbeck J.C., Cohen G.H., et al. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428(March (6979)):182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- 40.Nigam P., Earl P.L., Americo J.L., Sharma S., Wyatt L.S., Edghill-Spano Y., et al. DNA/MVA HIV-1/AIDS vaccine elicits long-lived vaccinia virus-specific immunity and confers protection against a lethal monkeypox challenge. Virology. 2007;366(September (1)):73–83. doi: 10.1016/j.virol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Earl P.L., Americo J.L., Wyatt L.S., Espenshade O., Bassler J., Gong K., et al. Rapid protection in a monkeypox model by a single injection of a replication-deficient vaccinia virus. Proc Natl Acad Sci U S A. 2008;105(August (31)):10889–10894. doi: 10.1073/pnas.0804985105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stittelaar K.J., van Amerongen G., Kondova I., Kuiken T., van Lavieren R.F., Pistoor F.H., et al. Modified vaccinia virus Ankara protects macaques against respiratory challenge with monkeypox virus. J Virol. 2005;79(June (12)):7845–7851. doi: 10.1128/JVI.79.12.7845-7851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osborne J.D., Da Silva M., Frace A.M., Sammons S.A., Olsen-Rasmussen M., Upton C., et al. Genomic differences of vaccinia virus clones from Dryvax smallpox vaccine: the Dryvax-like ACAM2000 and the mouse neurovirulent Clone-3. Vaccine. 2007;25(December (52)):8807–8832. doi: 10.1016/j.vaccine.2007.10.040. [DOI] [PubMed] [Google Scholar]


