Abstract
Purpose
To investigate the clinicopathological features, survival and prognostic factors of primary intestinal extranodal natural killer/T-cell lymphoma, nasal type (PI-ENKTCL).
Methods
Clinical and histological characteristics of PI-ENKTCL cases were retrospectively evaluated. Immunohistochemical phenotype and status of Epstein-Barr virus (EBV) and T-cell receptor (TCR) gene rearrangement were examined. The overall survival and prognostic parameters were also analyzed.
Results
Fifty-five (2.7%) cases with PI-ENKTCL were identified out of 2017 archived ENKTCL cases, with a median age of 39 years and a male to female ratio of 2.1:1. The most common symptom was abdominal pain (90.9%), accompanied frequently with fever and less commonly with intestinal perforation or B symptoms. Small intestine (50.9%) was the most common site to be involved. 47.3% and 36.4% cases presented with stage I and II diseases, respectively. Histologically, most cases displayed characteristic morphologic changes of ENKTCL. Cytoplasmic CD3, TIA-1 and CD56 expression was found in 100%, 94.5% and 89.1% of cases, respectively. In situ hybridization detection for EBV demonstrated positive results in all cases. Monoclonal TCR gene rearrangement was found in 52.9% of tested cases. Chemotherapy with a DICE or L-asparaginase/peg-asparginase-containing regimen was most often employed. Both advanced tumor stage and B symptoms were independent inferior prognostic factors (p = 0.001 and p = 0.010). Noticeably, 6 cases demonstrated a CD4-positive phenotype. These cases featured a relatively older median age (58 years), predominance of small/medium-sized neoplastic cells, a higher rate of TCR rearrangement and slightly favorable outcome.
Conclusion
We reported by far the largest series of PI-ENKTCL, and demonstrated its heterogeneity, aggressive clinical behavior and unsatisfying response to the current therapeutic strategies. Those CD4-positive cases might represent a unique subtype of PI-ENKTCL or distinct entity. Further investigations are required for the better understanding and management of this unusual disease.
Introduction
Extranodal natural killer (NK)/T-cell lymphoma, nasal type (ENKTCL), a rare distinct malignancy that comprises 3–8% of all lymphomas, is most prevalent in Asian and Central and South American populations [1]. This tumor predominantly involves extranodal sites, and features pathologically vascular invasion, prominent necrosis, cytotoxic phenotype and association with Epstein-Barr virus (EBV) [2, 3]. While most ENKTCL cases derived from NK cells, some show a cytotoxic T-cell phenotype [3, 4]. Approximately 80% of ENKTCL cases occur in the upper aerodigestive tract, with the nasal cavity being mostly affected [5, 6], other preferential sites of involvement include the skin, soft tissue, gastrointestinal (GI) tract and the gonad [7, 8]. Primary intestinal ENKTCL (PI-ENKTCL) is rare, which accounts for 3.1% of all intestinal non-Hodgkin lymphoma cases according to the literature [9]. Due to its rarity, the clinical and pathological features of intestinal ENKTCL have not been well illustrated, which may lead to dilemmas not only in the diagnosis but also the treatment of this disease. Furthermore, data regarding the therapeutic strategies and prognostic factors of this peculiar lymphoma is still limited. We thus retrospectively analyzed by far the largest series of PI-ENKTCL, for the purpose of better understanding the clinicopathological features of this rare tumor, which is of paramount importance for the accurate diagnosis and appropriate treatment.
Materials and Methods
Case selection
Altogether 55 cases with PI-ENKTCL, diagnosed between January 2007 and August 2015, were retrieved from the files of Department of Pathology, Fudan University Shanghai Cancer Center (Shanghai, China). For all cases, a primary intestinal manifestation of the disease was confirmed by a precise staging work-up through a computed tomography (CT) staging and/or PET-CT scan. And those with a probable secondary involvement of the intestine were not included. Pathological diagnosis was made by two of the authors (BHY and XQL) according to the criteria described in the WHO classification of tumors of the haematopoietic and lymphoid tissues [2]. Clinical data including the follow up information were also collected and analyzed.
Histology and immunohistochemistry
Archival formalin-fixed paraffin-embedded tumor tissues were recut for a routine hematoxylin and eosin (H&E) stain and the immunohistochemical procedure. Histological characteristics, including the cytological details of the tumor, the presence of necrosis and ulceration, angiocentricity and angiodestruction, admixed inflammatory infiltrates, and the depth of the neoplastic infiltration, were reviewed and assessed by three of the authors (BHY, XYZ and XQL) under a multi-headed microscope.
The immunohistochemical study was performed using a Ventana Bench Mark ultra autostainer (Ventana Medical System Inc., Roche Tuson, AZ, USA) and the Ventana ultra view universal DAB detection kit. The primary antibodies against CD20, CD2, cytoplasmic CD3 [CD3 (epsilon)], CD4, CD5, CD7, CD8, CD30, CD56, T-cell-restricted intracellular antigen-1 (TIA-1), granzyme B (GrB), perforin, ALK1 and Ki-67 were employed in the present study. Except for TIA-1 (Dako, Glostrup, Denmark), all of the above-mentioned antibodies were commercial products by Roche Ventana. For each stain, a parallel stain using appropriate positive and negative controls was performed. The Ki-67 labeling index was estimated to the closest decile.
In situ hybridization (ISH) detection for EBV-encoded small RNA (EBER)
The status of EBV infection was assessed by an ISH detection for EBER on paraffin-embedded tissue sections using fluorescein-labeled oligonucleotide probes (INFROM EBER Probe, Ventana), as previously described [10]. The visualization system used was the Bench Mark XT with enzymatic digestion (ISH Protease 2, Ventana) and the iVIEW Blue v3 detection kit (Ventana). Appropriate positive and negative control sections were included for each run.
Polymerase chain reaction (PCR) assays for T-cell receptor (TCR) gene rearrangement
Genomic DNA was extracted from formalin-fixed paraffin-embedded tissues, using the QIAamp mini kit (Qiagen, GmbH, Germany), and the concentration of DNA was measured by a spectrophotometer. Rearrangement of TCR-β, γ, δ genes was detected by multiplex PCR assays according to standard techniques, as described previously [11]. Amplifiability of the DNA was confirmed by concurrent PCR amplification of the β-globin sequence. Each PCR study was carried out in duplicate and included positive, negative, and no-template controls. The PCR products were analyzed by capillary electrophoresis as previously documented [11], using the ABI PRISM 310 Genetic Analyzer (Applied Biosystems, CA, USA).
Statistical analysis
Overall survival (OS) was defined as the interval from the initial diagnosis to the date of death from any cause or the last contact. The OS was estimated using the Kaplan-Meier method and was compared by means of the log-rank test. Multivariate analyses were also carried out using the Cox proportional hazard regression model to identify prognostic factors. The clinicopathologic parameters for assessment included age, tumor location, stage of disease, B symptoms [including fever (temperature >38°C) for 3 consecutive days, night sweats, and/or weight loss exceeding 10% of body weight in 6 months], intestinal perforation, size and immunophenotype of tumor cells, as well as TCR gene rearrangement status. All the statistical analyses were conducted using the SPSS software package (SPSS version 19.0; SPSS Inc., Chicago IL, USA). A p value of <0.05 was considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of Fudan University Shanghai Cancer Center (Shanghai Cancer Center Ethical Committee). The patient records/information was anonymized and de-identified prior to analysis.
Results
Clinical findings
Altogether 2017 ENKTCL cases were documented in our laboratory database during the period between 2007 and 2015, among which only 55 (2.7%) were proved to present with primary intestinal lesions. There were 37 male and 18 female patients, with a male-to-female ratio of 2.1:1. The average and median age at diagnosis was 43 and 39 years, respectively (range, 14–75 years). The most common symptom at diagnosis was abdominal pain (50 patients, 90.9%). Thirty-one patients (56.4%) had fever, and 10 (18.2%) presented with lower GI bleeding or fecal occult blood. Intestinal perforation and B symptoms were observed in 18 (32.7%) and 19 (34.5%) patients, respectively. Other concomitant clinical manifestations included diarrhea, nausea, vomiting, abdominal mass, intestinal obstruction and constipation.
With regard to the anatomic sites of involvement, 28 (50.9%) tumors occurred in the small intestine (including the duodenum, jejunum and ileum). Eleven (20.0%) and 13 (23.6%) were located in the ileocecal junction and colon, respectively. And the remaining 3 (5.5%) had multifocal lesions involving at least two different intestinal segments. Mesentery lymph node involvement was observed in 14 (36.8%) out of 38 cases. According to the Lugano staging system, 26 cases (47.3%) in the current series presented with stage I diseases, and 20 (36.4%) and 9 (16.4%) with stage II and advanced stage (stage III/IV) diseases, respectively.
Histological findings
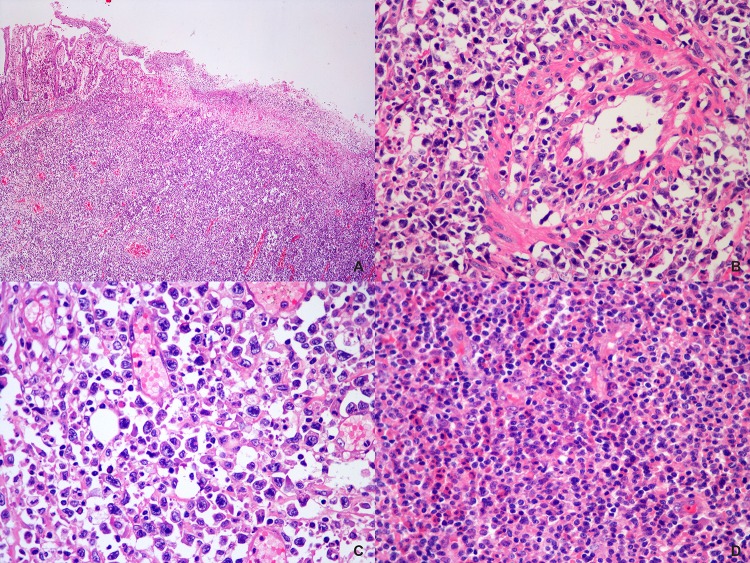
Overall, the specimens from the 55 cases comprised 42 resected tumors and 13 endoscopical biopsies. Ulceration and necrosis of the overlying mucosa, ranging from focal to extensive, were noticed in all cases. The atypical lymphoid cells infiltrated and effaced the mucosal architecture, which is more easily to be appreciated in the resected tumors (Fig 1A). Geographic necrosis was also frequently observed. Angiocentric /angiodestructive growth pattern of the tumor cells was another common feature noticed in these specimens (Fig 1B). Of the 42 cases with resected specimens, tumor invaded muscularis propria in 2 (4.8%), subserosa in 30 (71.4%), and penetrated the intestinal wall and spread beyond in 10 (23.8%) cases. Cytologically, the neoplastic lymphoid cells exhibited a broad spectrum in their size and appearance. Twenty-four cases (43.6%) displayed a mixed population of small, medium to large cells, 18 cases (32.7%) were composed predominantly of small to medium-sized cells, 12 cases (21.8%) predominated by medium-sized cells, and the remaining one (1.8%) was composed of uniform small cells. The small and intermediate cells often had oval or slightly irregular convoluted nuclei, hyperchromatic or granulated chromatin and inconspicuous nucleoli. The larger cells might have exceedingly irregular nuclei with vesicular or coarsely clumped chromatin and prominent nucleoli. Moderate to abundant, clear or lightly eosinophilic cytoplasm was seen in most cases. In occasional cases, the tumor cells showed a striking atypia with noticeable horseshoe- or kidney-shaped nuclei (Fig 1C). In addition, mononucleated or multinucleated giant cells with striking bizarre appearance were identified in 5 (9.1%) cases. Mitotic figures and varying amounts of apoptotic bodies were easily identified in most cases. Inflammatory infiltrates, consisting of small lymphocytes, plasma cells, histiocytes and eosinophils, were admixed with the tumor cell populations in variable proportions from case to case (Fig 1D). In one case, deposits of calcified schistosome ova were noticed in the intestinal wall and lymph nodes.
Fig 1. Histological features of PI-ENKTCL.
(A) A low power view of the H&E stained section showed the overlying mucosa was partially effaced and the neoplastic cells extensively infiltrated the muscular wall. (B) A blood vessel with invasion by the neoplastic cells was shown. (C) A high power view demonstrated that the tumor was composed of a mixture of small, medium and large atypical lymphoid cells, with some hallmark cells identified. (D) Numerous eosinophils and plasma cells were intermingled with the tumor cells.
Immunohistochemistry, ISH for EBER, and TCR gene rearrangement
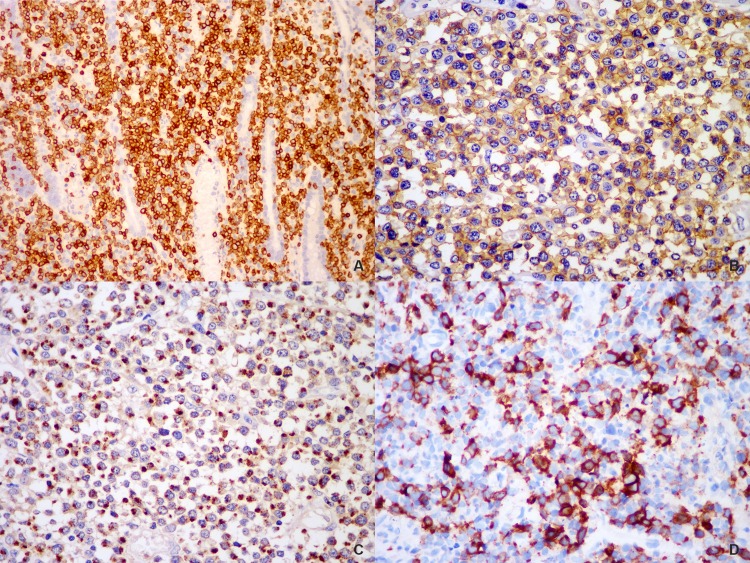
Immunohistochemically, tumor cells of all cases were positive, either diffusely or partially, for cytoplasmic CD3. Positivity for CD4 and CD8 of tumor cells was found in 14.0% (6/43) and 2.4% (1/41) of the tested cases, respectively, but none expressed both antigens. Other T or NK markers, such as CD2, CD5 and CD7, were expressed in a variable proportion of all cases. Positive CD56 immunostaining was observed in 89.1% (49/55) of cases. Tumor cells were consistently positive for the cytotoxic marker TIA-1 (52/55, 94.5%), less frequently, the expression of GrB (32/45, 71.1%) and perforin (31/42, 73.8%) was noticed. CD30 was expressed, at least partially, in 40.6% (13/32) of the cases. Ki-67 proliferation index varied from 50% to 90%, with an average of 72.7%. In all cases, the tumor cells expressed neither ALK1 nor CD20. (Fig 2)
Fig 2. Immunophenotypic features of PI-ENKTCL.
The neoplastic cells were positive for CD3 (A), CD56 (B) and TIA-1 (C). Positivity for CD30 (D) was seen in some larger atypical cells.
Positive ISH signals of EBER were identified in tumor cells in all cases. Of the 17 tested cases, 9 (52.9%) demonstrated monoclonal TCR gene rearrangement, with a phenotype of TCR γδ+, αβ+ and αβ/γδ+ in 3 patients each.
Treatment and outcome
Forty-two patients received intestinal segment resection. A total of 35 patients (63.6%) were treated with systemic chemotherapy. Another one (1.8%) received chemoradiotherapy and subsequent autologous hematopoietic stem cell transplantation. Eleven (20.0%) failed to receive any intervention due to poor health condition. And no information was available for the remaining 8 (14.6%) patients. The chemotherapy regimens varied considerably, whereas a DICE regimen (dexamethasone, ifosfamide, cisplatin and etoposide) or L-asparaginase/peg-asparginase-containing regimens, such as SMILE (dexamethasone, methotrexate, ifosfamide, L-asparaginase and etoposide) and P-GEMOX (peg-asparginase, gemcitabine and oxaliplatin), were most frequently employed. Other regimens including the CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone), CVAD (cyclophosphamide, vincristine, doxorubicin and dexamethasone), MINE (mesna, ifosfamide, mitoxantrone and etoposide) and EPOCH (etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin) were variably adopted.
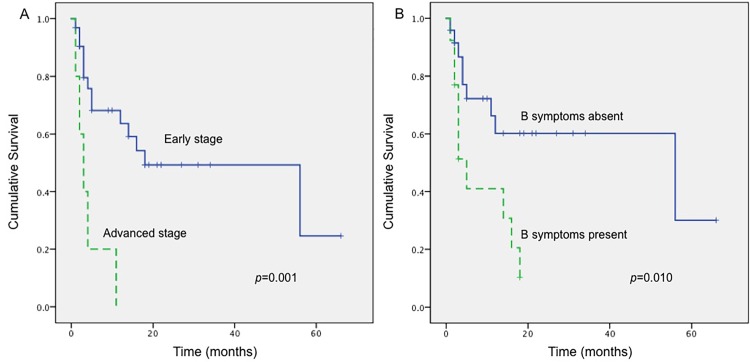
The follow-up information was available in 37 patients with a follow-up time averaged 12.7 months (range, 1–66 months). Three cases relapsed 16–20 months after diagnosis, 19 patients died of the disease and 18 were alive at the last contact. Kaplan-Meier analysis revealed that the stage of disease at diagnosis and B symptoms were statistically associated with the OS (p = 0.001, p = 0.010, respectively) (Fig 3). Patients at earlier stage and those without B symptoms had relatively longer OS. Further multivariate analysis confirmed that both the stage and B symptoms were independent prognostic indicators. In addition, the prognosis of patients with intestinal perforation or old age (≧40 years) tended to be less favorable. Interestingly, patients with a T-lineaged tumor tended to have a better survival. Nevertheless, none of the above-mentioned findings could be proven statistical significant (p>0.05). Other parameters, including the tumor location, size of tumor cells, CD30 or CD56 positivity and Ki-67 index, did not show any correlation with the prognosis.
Fig 3. Kaplan-Meier survival curves of patients with PI-ENKTCL.
(A) Patients with early stage disease had superior survival compared with those with advanced stage disease (p = 0.001). (B) Patients with B symptoms were associated with a much poorer survival than those without B symptoms (p = 0.010).
A distinct subgroup of intestinal CD4-positive ENKTCL
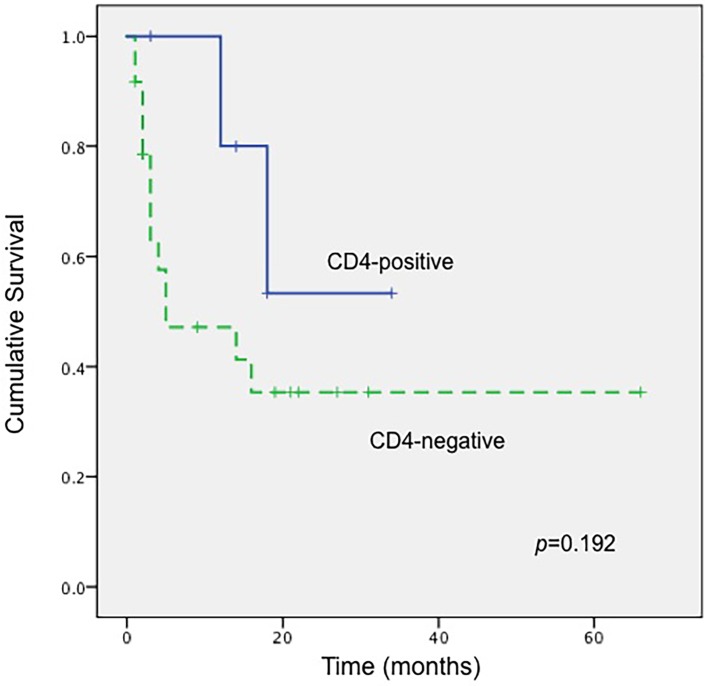
Worthy to be noticed, 6 tumors in the current series demonstrated a CD4-positive phenotype, characterized by a diffuse and intensive positive staining for this marker in almost all the tumor cells (Table 1, Fig 4). All tumors arose from the small intestine with one exception that involved the ileocecal region. Four patients were male and 2 were female, with a median age of 58 years (range, 37–65 years). Morphologically, these tumors were composed predominantly of a proliferation of small to medium-sized atypical lymphoid cells with mild pleomorphism. Large cells were rarely seen, usually accounting for less than 10% of the tumor cell population. All the cases were positive for CD56, cytotoxic molecules and EBER, and clonal TCR gene rearrangements were demonstrated in all tested cases. Two patients died of disease at 18 and 12 months after diagnosis, respectively, and the remaining 4 were alive at the last contact. The one-year OS of these CD4-positive cases was 80.0%, better than that of CD4-negative ones (47.1%). Kaplan-Meier analysis revealed a superior OS of these CD4-positive ENKTCL cases compared with those CD4-negative ones, although the difference did not reach statistical significance (p = 0.192, Fig 5).
Table 1. The clinicopathological features of primary intestinal CD4+ ENKTCL.
| Case No. | Sex/Age (years) | Site | Stage | Status | Follow-up time (months) | Tumor cell size | CD4 | CD8 | CD56 | Ki-67 (%) | EBER | TCR gene rearrangement | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Female/37 | Ileum | IIE | Alive | 3 | Small | + | - | + | 70 | + | Monoclonal | Resection +chemotherapy |
| 2 | Male/62 | Ileum | IE | Alive | 14 | Medium | + | - | + | 60 | + | Monoclonal | Resection +chemotherapy |
| 3 | Male/55 | Small intestine | IE | Alive | 18 | Small/medium | + | - | + | 80 | + | ND | Resection +chemotherapy |
| 4 | Male/65 | Small intestine | IE | Dead | 12 | Small/medium, scattered large cells | + | - | + | 60 | + | Monoclonal | Resection* |
| 5 | Male/60 | Duodenum | IE | Alive | 34 | Small/medium, scattered large cells | + | - | + | 80 | + | Monoclonal | Chemotherapy |
| 6 | Female/39 | ileocecal region | IIE | Dead | 18 | Small/medium, scattered large cells | + | - | + | 70 | + | ND | Resection+chemotherapy |
ND, not done.
*, information on subsequent treatment of this patient is unavailable.
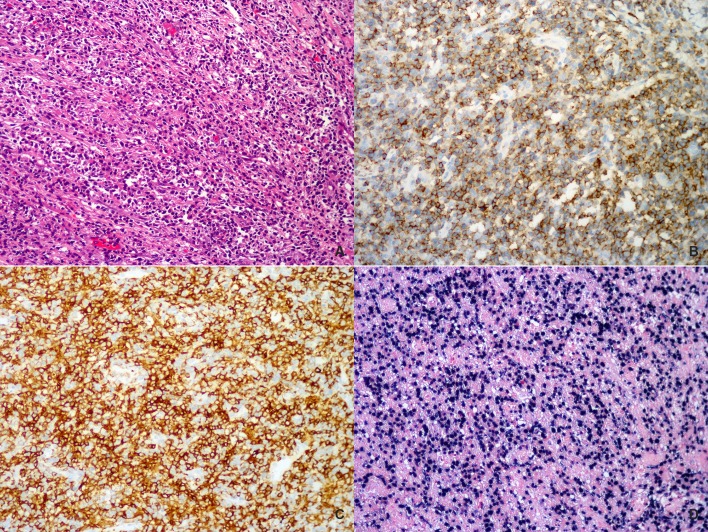
Fig 4. A representative case of primary intestinal CD4+ ENKTCL involving the ileum.
(A) The neoplastic cells were predominantly small to medium-sized ones with slightly irregular nuclei. Tumor cells were diffusely positive for (B) CD56 and (C) CD4. (D) In situ hybridization for EBER demonstrated positive signals.
Fig 5. Kaplan-Meier survival curves of patients with PI-ENKTCL according to CD4 expression.
Patients with a CD4-positive tumor had a slightly better OS compared with those with CD4-negative ones, although the difference was not statistically significant (p = 0.192).
Discussion
The GI tract is one of the most common extranodal sites that can be involved by lymphomas [12]. However, ENKTCL originating from the intestine is extremely rare. So far only few studies with limited number of cases were documented in the English literature [13, 14]. Due to its rarity, the clinical and pathological features of PI-ENKTCL are less well defined. We thus collected a large cohort of cases to observe the clinicopathological characteristics of this uncommon disease. To the best of our knowledge, the current data set may represent by far the largest one focusing on intestinal ENKTCL.
Based on our findings, PI-ENKTCL more frequently affected middle-aged adults in their forties with a male predominance, which is in consistent with several previous studies [12, 15]. Regarding the sites of involvement, small intestine is most commonly affected, followed by colon and the ileocecal junction. This result is in good concordance with the findings by Kim et al [16], but differs with some other studies, which demonstrated that large intestine is much more commonly involved by this type of lymphoma [12, 17]. The most common presenting symptom is abdominal pain, with or without fever, as same as Fang et al indicated before [13]. Endoscopically, most intestinal ENKTCL presents as ulcerative lesions, being prone to perforation, rather than a polypoid mass, suggesting a potential more aggressive behavior of this tumor [12].
The variety of histologic appearance of intestinal ENKTCL might have reflected the striking heterogeneity of this tumor. In terms of immunophenotype, CD56 appears to be a useful marker aiding in the diagnosis, with an expression rate varying between 60% and 100% in different series [3, 8, 18]. It has been proposed that CD56 might be expressed more frequently in NK-derived tumors than T-cell-originated ones [4]. Our results, together with those of some other studies, however, did not reveal such a difference [3, 8, 18]. The expression of one or more cytotoxic molecules, including TIA-1, GrB and perforin, is invariably present in every case, with TIA-1 expression being more frequently detected [4, 18]. As a marker related to cell activation, CD30 was frequently expressed by the tumor cells in the current series, which presumably reflected the fact that some ENKTCL cases feature an activated phenotype. The relatively high positivity for CD30 in intestinal ENKTCL is also in good keeping with that a higher percentage of CD30 expression was noticed in extra-nasal ENKTCL lesions than those nasal ones [4, 19].
ENKTCL is almost constantly associated with EBV, which is suspected to play an important role in the oncogenesis of this disease [20]. Identification of EBV-encoded EBER is therefore essential for the establishment of a diagnosis of ENKTCL. In the current series, nearly half of the intestinal ENKTCL cases that submitted for TCR gene rearrangement detection demonstrated a germline configuration of the TCR genes, confirming a genuine NK-cell origin of the tumor, however, T-cell-derived tumors accounted for 52.9% of all tested cases, an incidence slightly higher than those previously reported ranging from 0%-46% [4, 5, 10, 14, 19, 21–23]. The clinical significance of cell origin of an intestinal ENKTCL remains largely unknown, although in general, ENKTCL cases of different lineage do not show distinct clinopathological features including the survival [4, 10, 19]. We noticed a trend of a better survival in patients with T-cell-derived intestinal ENKTCLs than those with NK-lineaged ones, although the survival advantage was not statistically significant. Pongpruttipan et al seemed to have similar findings although the tumors in their series were mostly located in the upper aerodigestive tract instead of the intestine [4]. Chuang and colleagues also noticed a superior survival of those EBV-related cytotoxic T-cell lymphomas arising in the intestine compared with their NK-cell counterparts, but they referred to the former as “NK-like peripheral T-cell lymphomas”, possibly an entity distinct from the genuine PI-ENKTCLs or enteropathy-associated T-cell lymphomas (EATL) [24].
Intestinal ENKTCL must be distinguished from its morphological mimics. It is not uncommon that some intestinal ENKTCL cases are misdiagnosed as peripheral T-cell lymphoma, not otherwise specified (PTCL, NOS) due to CD3 positivity and a high proliferation rate of the tumor cells. However, the later usually lacks the characteristic massive necrosis and angiodestructive growth pattern. In general, EBER is absent in PTCL, NOS. CD56 expression is also less commonly seen in this tumor compared with ENKTCL. Type II EATL might be confused with intestinal ENKTCL due to their common CD56+ and cytotoxic phenotype [4]. Nevertheless, the former is composed of monotonous medium-sized tumor cells with marked epitheliotropism. Necrosis is uncommon in type II EATL, too. The tumor cells typically co-express CD8 but lack EBER [25], although it still remains controversial that whether some EATL-appearing but EBER-positive intestinal T-cell lymphomas can be labeled as an ENKTCL. Occasionally, PI-ENKTCLs consisting mainly of large or anaplastic cells may show CD30 positivity and thus, might be potentially misinterpreted as anaplastic large cell lymphoma (ALCL) or the classical form of EATL. Detection for EBER is critical for the distinction between these diseases. Rare examples of indolent T- or NK-cell lymphoproliferative disorders of the GI tract, such as the NK-cell lymphomatoid gastroenteropathy and indolent T-cell lymphoproliferative disorder of the GI tract, may morphologicaly or phenotypically mimic ENKTCL, too [26, 27]. These lesions, however, differ from ENKTCL by their characteristic indolent clinical course and an absence of EBV in the lesional cells.
Given the rarity of PI-ENKTCL and its clinicohistological heterogeneity, there is currently no consensus approached on the proper treatment strategy [16, 28]. A timely surgical operation might be necessary since it has been found that patients receiving surgery before perforation usually feature an improved outcome compared to those undergoing surgery later or without surgery [12]. Moreover, surgery with subsequent chemotherapy might be more beneficial than initially treatment with chemotherapy for patients eligible for surgery [16]. Nevertheless, Fang et al presumed that operation might accelerate deterioration of the disease [13]. On the other hand, so far there is no standard chemotherapeutic regimen for ENKTCL [29]. It has been reported that patients with GI ENKTCL who received nonanthracycline-based or intensified regimens did not show a significant survival difference compared with those who received CHOP or CHOP-like regimens regardless of surgery [16]. Recently, the efficacy of L-asparaginase or peg-asparginase in the treatment of ENKTCL has been confirmed, and clinical trials using L-asparaginase or peg-asparginase-based chemotherapeutic regimens have achieved promising results [29]. Yet, about half of our patients died during the follow-up period irrespective of the administration of comprehensive treatment. Novel effective approaches are thus warranted to improve the survival of patients with this rare but fatal disease.
Both our study and some previous ones had demonstrated a large proportion of patients with intestinal ENKTCL presented with early stage diseases [13], these patients, however, tended to exhibit highly aggressive behavior and thus, conferred an inferior prognosis as compared to their nasal counterparts [16]. For such a contradiction, one reasonable explanation lies that the overt extra-nasal tumors in some patients might represent actually the extra-nasal dissemination of an occult nasal lesion. And the primary nasal lesions are easily to be missed or overlooked only because of their small sizes [3]. Therefore, a PET-CT scan, nasal panendoscopy and careful imaging of the aerodigestive tract, with biopsy when necessary, are mandatory for each new case of ENKTCL, irrespective of the initial presentation sites, as indicated previously [1, 3, 30]. However, all cases enrolled in the current study have been submitted for a precise staging work-up and hence secondary onset can be excluded. Thus there might be some other reasons accounting for the poor outcome of PI-ENKTCL. It has been suggested that old age, intestinal perforations, B symptoms are independent prognostic factors indicating a poor outcome of patients with GI-ENKTCL, and surgery prior to the perforation is another key factor that may influence the survival [12]. In our study, the results have confirmed that both disease stage and B symptoms are significant independent prognostic factors for this rare type of tumor. The prognostic significance concerning CD56 expression in ENKTCL is controversial [31, 32]. We have failed to find any correlations between CD56 expression and OS, probably due to the limited number of CD56-negative cases.
Of interest, we noticed and described for the first time 6 cases of intestinal T-cell lymphomas characterized by their predilection for small intestine involvement, predominance of small to medium-sized tumor cells, a CD4+, CD56+, EBER+ T-cell phenotype and importantly, relatively favorable outcome. We tentatively labeled these lesions as ENKTCL mainly based on their cytotoxic T-cell phenotype and EBV positivity. CD4-positive ENKTCL is extremely rare. In a large cohort of 67 cases with ENKTCL, Pongpruttipan et al identified only one CD4-positive case, which might be of αβ T-cell origin [4]. We suppose these peculiar CD4+, CD56+, EBER+ tumors of the small intestine may represent a special variant of ENKTCL or even novel entity due to their unique clinical and pathological features. Recognition of these CD4-positive tumors may aid in predicting the prognosis and tailoring a personalized treatment strategy to the patients. Further work is needed to elucidate the biological nature of the disease and its relationship with the classical form of intestinal ENKTCL.
In conclusion, PI-ENKTCL is a rare condition. The disease predominantly affects middle-aged males and features frequently aggressive clinical course and poor outcome. Independent inferior prognostic indicators may include the stage of disease and B symptoms. A subgroup of cases with a CD4-positive phenotype might represent a unique variant or clinicopathological entity. Further molecular and clinical studies are encouraged for the better characterization and management of the disease.
Supporting Information
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hawkes EA, Wotherspoon A, Cunningham D. Diagnosis and management of rare gastrointestinal lymphomas. Leuk Lymphoma. 2012; 53:2341–2350. 10.3109/10428194.2012.695780 [DOI] [PubMed] [Google Scholar]
- 2.Chan JKC, Quintanilla-Martinez L, Ferry JA, Peh SC. Extranodal NK/T-cell lymphoma, nasal type In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. , editors. World Health Organization Classification of Tumours Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008. pp. 285–288. [Google Scholar]
- 3.Jhuang JY, Chang ST, Weng SF, Pan ST, Chu PY, Hsieh PP, et al. Extranodal natural killer/T-cell lymphoma, nasal type in Taiwan: a relatively higher frequency of T-cell lineage and poor survival for extranasal tumors. Human Pathology. 2015; 46:313–321. 10.1016/j.humpath.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 4.Pongpruttipan T, Sukpanichnant S, Assanasen T, Wannakrairot P, Boonsakan P, Kanoksil W, et al. Extranodal NK/T-cell lymphoma, nasal type, includes cases of natural killer cell and αβ, γδ, and αβ/γδ T-cell origin: a comprehensive clinicopathologic and phenotypic study. Am J Surg Pathol. 2012;36:481–499. 10.1097/PAS.0b013e31824433d8 [DOI] [PubMed] [Google Scholar]
- 5.Takata K, Hong ME, Sitthinamsuwan P, Loong F, Tan SY, Liau JY, et al. Primary cutaneous NK/T-cell lymphoma, nasal type and CD56-positive peripheral T-cell lymphoma: a cellular lineage and clinicopathologic study of 60 patients from Asia. Am J Surg Pathol. 2015;39:1–12. 10.1097/PAS.0000000000000312 [DOI] [PubMed] [Google Scholar]
- 6.Bautista-Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J Gastrointest Oncol. 2012;3:209–225. 10.3978/j.issn.2078-6891.2012.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aozasa K, Zaki MA. Epidemiology and pathogenesis of nasal NK/T-cell lymphoma: a mini-review. Scientific World Journal. 2011; 14:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gualco G, Domeny-Duarte P, Chioato L, Barber G, Natkunam Y, Bacchi CE. Clinicopathologic and molecular features of 122 Brazilian cases of nodal and extranodal NK/T-cell lymphoma, nasal type, with EBV subtyping analysis. Am J Surg Pathol. 2011;35:1195–1203. 10.1097/PAS.0b013e31821ec4b5 [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Choi CW, Mun YC, Oh SY, Kang HJ, Lee SI, et al. Multicenter retrospective analysis of 581 patients with primary intestinal non-hodgkin lymphoma from the Consortium for Improving Survival of Lymphoma (CISL). BMC Cancer. 2011;29: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richendollar BG, Hsi ED, Cook JR. Extramedullary plasmacytoma-like posttransplantation lymphoproliferative disorders: clinical and pathologic features. Am J Clin Pathol. 2009;132:581–588. 10.1309/AJCPX70TIHETNBRL [DOI] [PubMed] [Google Scholar]
- 11.Ng SB, Lai KW, Murugaya S, Lee KM, Loong SL, Fook-Chong S, et al. Nasal-type extranodal natural killer/T-cell lymphomas: a clinicopathologic and genotypic study of 42 cases in Singapore. Mod Pathol. 2004; 17:1097–1107. [DOI] [PubMed] [Google Scholar]
- 12.Jiang M, Chen X, Yi Z, Zhang X, Zhang B, Luo F, et al. Prognostic characteristics of gastrointestinal tract NK/T-cell lymphoma: an analysis of 47 patients in China. J Clin Gastroenterol. 2013;47:e74–e79. 10.1097/MCG.0b013e31829e444f [DOI] [PubMed] [Google Scholar]
- 13.Fang JC, Xia ZX, Wang CN and Li Z. Clinicopathologic and Immunophenotypic features of primary intestinal extranodal NK/T-cell lymphoma, nasal type. Int J Surg Pathol. 2015;23:609–616. 10.1177/1066896915595863 [DOI] [PubMed] [Google Scholar]
- 14.Zheng S, Ouyang Q, Li G, Xu H, Jiang M, Cui D, et al. Primary intestinal NK/T cell lymphoma: a clinicopathologic study of 25 Chinese cases. Arch Iran Med. 2012;15:36–42. doi: 012151/AIM.0011 [PubMed] [Google Scholar]
- 15.Gou HF, Zang J, Jiang M, Yang Y, Cao D, Chen XC. Clinical prognostic analysis of 116 patients with primary intestinal non-Hodgkin lymphoma. Med Oncol. 2012; 29: 227–234. 10.1007/s12032-010-9783-x [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Jung HA, Chuang SS, Hong H, Guo CC, Cao J, et al. Extranodal natural killer/T-cell lymphoma involving the gastrointestinal tract: analysis of clinical features and outcomes from the Asia Lymphoma Study Group. J Hematol Oncol. 2013;6:86 10.1186/1756-8722-6-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun j, Lu Z, Yang D, Chen J. Primary intestinal T-cell and NK-cell lymphomas: a clinicopathological and molecular study from China focused on type II enteropathy-associated T-cell lymphoma and primary intestinal NK-cell lymphoma. Mod Pathol. 2011;24:983–992. 10.1038/modpathol.2011.45 [DOI] [PubMed] [Google Scholar]
- 18.Pongpruttipan T, Kummalue T, Bedavanija A, Khuhapinant A, Ohshima K, Arakawa F, et al. Aberrant antigenic expression in extranodal NK/T-cell lymphoma: a multi-parameter study from Thailand. Diagn Pathol. 2011; 25:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009; 113:3931–3937. 10.1182/blood-2008-10-185256 [DOI] [PubMed] [Google Scholar]
- 20.Aozasa K, Zaki MA. Epidemiology and pathogenesis of nasal NK/T-cell lymphoma: a mini-review. Scientific World Journal. 2011; 14:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko YH, Cho EY, Kim JE, Lee SS, Huh JR, Chang HK, et al. NK and NK-like T-cell lymphoma in extranasal sites: a comparative clinicopathological study according to site and EBV status. Histopathology. 2004;44:480–489. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Feng X, Li T, Zhang S, Zuo Z, Lin P, et al. Extranodal NK/T-cell lymphoma, nasal type: a report of 73 cases at MD Anderson Cancer Center. Am J Surg Pathol. 2013; 37: 14–23. 10.1097/PAS.0b013e31826731b5 [DOI] [PubMed] [Google Scholar]
- 23.Suzuki R, Suzumiya J, Yamaguchi M, Nakamura S, Kameoka J, Kojima H, et al. Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol. 2010;21:1032–1040. 10.1093/annonc/mdp418 [DOI] [PubMed] [Google Scholar]
- 24.Chuang SS, Chang ST, Chuang WY, Huang WT, Hsieh PP, Tsou MH, et al. NK-cell lineage predicts poor survival in primary intestinal NK-cell and T-cell lymphomas. Am J Surg Pathol. 2009; 33: 1230–1240. 10.1097/PAS.0b013e3181a95c63 [DOI] [PubMed] [Google Scholar]
- 25.Medeiros LJ, Miranda RN, Wang SA, Vega F, Muzzafar T, Yin CC, et al. Diagnostic Pathology: Lymph Nodes and Spleen with Extranodal Lymphomas. Altona: Amirsys; 2011. [Google Scholar]
- 26.Takeuchi K, Yokoyama M, Ishizawa S, Terui Y, Nomura K, Marutsuka K,et al. lymphomatoid gastropathy: a distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation. Blood. 2010;116:5631–5637. 10.1182/blood-2010-06-290650 [DOI] [PubMed] [Google Scholar]
- 27.Perry AM, Warnke RA, Hu Q, Gaulard P, Copie-Bergman C, Alkan S, et al. Indolent T-cell lymphoproliferative disease of thegastrointestinal tract. Blood.2013;122:3599–3606. 10.1182/blood-2013-07-512830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jo JC, Yoon DH, Kim S, Lee BJ, Jang YJ, Park CS, et al. Clinical features and prognostic model for extranasal NK/T-cell lymphoma. Eur J Haematol. 2012; 89:103–110. 10.1111/j.1600-0609.2012.01796.x [DOI] [PubMed] [Google Scholar]
- 29.Yong W. Clinical study of l-asparaginase in the treatment of extranodal NK/T-cell lymphoma, nasal type. Hematol Oncol. 2015. April 21 10.1002/hon.2207 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013;121: 4997–5005. 10.1182/blood-2013-01-453233 [DOI] [PubMed] [Google Scholar]
- 31.Li YX, Wang H, Feng XL, Liu QF, Wang WH, Lv N, et al. Immunophenotypic characteristics and clinical relevance of CD56+ and CD56- extranodal nasal-type natural killer/T-cell lymphoma. Leuk Lymphoma. 2011;52:417–424. 10.3109/10428194.2010.543718 [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Wang Z, Xia ZJ, Lu Y, Huang HQ, Zhang YJ. CD56-negative extranodal NK/T cell lymphoma should be regarded as a distinct subtype with poor prognosis. Tumor Biol. 2015; 36:7717–7723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.