Abstract
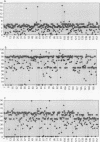
AIMS: To analyse osteoblast function in 153 cases of established osteoporosis as previous work has indicated that osteoporosis is a heterogeneous condition characterised by different patterns of osteoclast and osteoblast dysfunction. METHODS: Histomorphometric data from 153 cases with established osteoporosis was used to analyse osteoblast function, using the following parameters: osteoblast number was assessed using the ratio of osteoblast surface to bone surface (ObS:BS); the percentage of active osteoblasts was assessed by using mineralising surface as a proportion of osteoid surface (sLS + dLS/OS); and the efficiency of active osteoblasts was assessed using the ratio of double to total labelled surface (dLS:tLS). The values of each parameter were standardised using age and sex matched control data and a three dimensional matrix was used to identify groups of patients with similar patterns of altered function. RESULTS: The largest group (60 cases) showed a reduction in all three parameters, while a small group (9 cases) had normal osteoblast function. However, one group showed reduction in osteoblast number only (23 cases), while another group showed a normal number of osteoblasts but both reduced percentage and efficiency of activity (14 cases). The results also suggest that efficiency of activity falls first and that this eventually leads to exit from the active pool. CONCLUSIONS: These results demonstrate the presence of heterogeneity of osteoblast dysfunction in osteoporosis, indicating that the disease is caused by interference at a variety of target sites along the pathway of osteoblast proliferation, differentiation, and activation. Greater understanding of this pathway and of the variety of alterations in the pathway that can occur in osteoporosis may allow more focused therapy for different patient groups identified on the basis of histomorphometric analysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandre C. Diagnosis and treatment of osteoporosis. Curr Opin Rheumatol. 1995 May;7(3):240–242. doi: 10.1097/00002281-199505000-00015. [DOI] [PubMed] [Google Scholar]
- Aubin J. E., Liu F., Malaval L., Gupta A. K. Osteoblast and chondroblast differentiation. Bone. 1995 Aug;17(2 Suppl):77S–83S. doi: 10.1016/8756-3282(95)00183-e. [DOI] [PubMed] [Google Scholar]
- Beresford J. N. Osteogenic stem cells and the stromal system of bone and marrow. Clin Orthop Relat Res. 1989 Mar;(240):270–280. [PubMed] [Google Scholar]
- Bruder S. P., Fink D. J., Caplan A. I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994 Nov;56(3):283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston J. E., Mellish R. W., Croucher P., Newcombe R., Garrahan N. J. Structural mechanisms of trabecular bone loss in man. Bone Miner. 1989 Jul;6(3):339–350. doi: 10.1016/0169-6009(89)90039-1. [DOI] [PubMed] [Google Scholar]
- Eriksen E. F., Hodgson S. F., Eastell R., Cedel S. L., O'Fallon W. M., Riggs B. L. Cancellous bone remodeling in type I (postmenopausal) osteoporosis: quantitative assessment of rates of formation, resorption, and bone loss at tissue and cellular levels. J Bone Miner Res. 1990 Apr;5(4):311–319. doi: 10.1002/jbmr.5650050402. [DOI] [PubMed] [Google Scholar]
- Garabédian M. Genetic aspects of osteoporosis. Curr Opin Rheumatol. 1995 May;7(3):237–239. doi: 10.1097/00002281-199505000-00014. [DOI] [PubMed] [Google Scholar]
- Heaney R. P., Recker R. R., Saville P. D. Menopausal changes in bone remodeling. J Lab Clin Med. 1978 Dec;92(6):964–970. [PubMed] [Google Scholar]
- Kleerekoper M., Villanueva A. R., Stanciu J., Rao D. S., Parfitt A. M. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif Tissue Int. 1985 Dec;37(6):594–597. doi: 10.1007/BF02554913. [DOI] [PubMed] [Google Scholar]
- Lian J. B., Stein G. S. Development of the osteoblast phenotype: molecular mechanisms mediating osteoblast growth and differentiation. Iowa Orthop J. 1995;15:118–140. [PMC free article] [PubMed] [Google Scholar]
- Manolagas S. C., Bellido T., Jilka R. L. New insights into the cellular, biochemical, and molecular basis of postmenopausal and senile osteoporosis: roles of IL-6 and gp130. Int J Immunopharmacol. 1995 Feb;17(2):109–116. doi: 10.1016/0192-0561(94)00089-7. [DOI] [PubMed] [Google Scholar]
- Marie P. J. Human osteoblastic cells: a potential tool to assess the etiology of pathologic bone formation. J Bone Miner Res. 1994 Dec;9(12):1847–1850. doi: 10.1002/jbmr.5650091202. [DOI] [PubMed] [Google Scholar]
- Mundy G. R. No bones about fluoride. Nat Med. 1995 Nov;1(11):1130–1131. doi: 10.1038/nm1195-1130. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M. Age-related structural changes in trabecular and cortical bone: cellular mechanisms and biomechanical consequences. Calcif Tissue Int. 1984;36 (Suppl 1):S123–S128. doi: 10.1007/BF02406145. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987 Dec;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Parfitt A. M., Mathews C. H., Villanueva A. R., Kleerekoper M., Frame B., Rao D. S. Relationships between surface, volume, and thickness of iliac trabecular bone in aging and in osteoporosis. Implications for the microanatomic and cellular mechanisms of bone loss. J Clin Invest. 1983 Oct;72(4):1396–1409. doi: 10.1172/JCI111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel N., Eastell R. ABC of rheumatology. Osteoporosis. BMJ. 1995 Apr 15;310(6985):989–992. doi: 10.1136/bmj.310.6985.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman M. T., Hoyland J. A., Denton J., Freemont A. J. Age related histomorphometric changes in bone in normal British men and women. J Clin Pathol. 1994 Jun;47(6):529–534. doi: 10.1136/jcp.47.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman M. T., Hoyland J. A., Denton J., Freemont A. J. Histomorphometric classification of postmenopausal osteoporosis: implications for the management of osteoporosis. J Clin Pathol. 1995 Mar;48(3):229–235. doi: 10.1136/jcp.48.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard D. J., Kassem M., Hefferan T. E., Sarkar G., Spelsberg T. C., Riggs B. L. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 1996 Mar;11(3):312–324. doi: 10.1002/jbmr.5650110305. [DOI] [PubMed] [Google Scholar]
- Riis B. J., Rødbro P., Christiansen C. The role of serum concentrations of sex steroids and bone turnover in the development and occurrence of postmenopausal osteoporosis. Calcif Tissue Int. 1986 Jun;38(6):318–322. doi: 10.1007/BF02555743. [DOI] [PubMed] [Google Scholar]
- Rydén I. C., Cobbs C. G. Respiratory infections. Med Times. 1981 Mar;109(3):80-93, 95. [PubMed] [Google Scholar]
- Silver J. J., Einhorn T. A. Osteoporosis and aging. Current update. Clin Orthop Relat Res. 1995 Jul;(316):10–20. [PubMed] [Google Scholar]
- Steiniche T. Bone histomorphometry in the pathophysiological evaluation of primary and secondary osteoporosis and various treatment modalities. APMIS Suppl. 1995;51:1–44. [PubMed] [Google Scholar]
- Vesterby A., Gundersen H. J., Melsen F. Star volume of marrow space and trabeculae of the first lumbar vertebra: sampling efficiency and biological variation. Bone. 1989;10(1):7–13. doi: 10.1016/8756-3282(89)90140-3. [DOI] [PubMed] [Google Scholar]
- Ziegler R., Scheidt-Nave C., Scharla S. Pathophysiology of osteoporosis: unresolved problems and new insights. J Nutr. 1995 Jul;125(7 Suppl):2033S–2037S. doi: 10.1093/jn/125.suppl_7.2033S. [DOI] [PubMed] [Google Scholar]